Abstract
AIM
To investigate Japanese traditional (Kampo) medicine’s effectiveness on cancer chemotherapy-induced peripheral neuropathy (CIPN), we carried out this retrospective study.
METHODS
By searching our outpatient database of 3154 patients who consulted our outpatient clinic of Japanese-Oriental (Kampo) Medicine at Chiba University Hospital from November 2005 to December 2010, a total of 281 patients diagnosed with cancer were identified. Twenty-four patients out of the 281 patients identified met the following three conditions and were eligible for further investigation of the effectiveness of Kampo treatment: At least one course of cancer chemotherapy had been administered; numbness and pain appeared after the chemotherapy; and CIPN was diagnosed before they were given Kampo treatment.
RESULTS
The 24 patients included 6 males and 18 females and ranged in age from 39 to 86 (mean 61.2 ± 11.5) years old. Kampo formulas were individually chosen by Kampo expert doctors based on Kampo-specific diagnostics. Beneficial outcomes were obtained by Kampo treatment in 20 out of the 24 cases (83.3%). Nine out 20 cases had a major response (the numbness and pain showed improvement or reduction by 50% or more), with 7 of 9 cases showing a more than 70% symptom reduction. Eleven out of 20 cases showed a minor response (less than 50% symptom reduction), and 4 out of the 24 cases had no beneficial response. The most frequently used formula was goshajinkigan (GJG), followed by hachimijiogan (HJG) and keishibukuryogan. Thirteen of the 24 cases (54.2%) were prescribed aconite root-containing formulas including GJG and HJG. Aconite root has “warming” effects and ameliorates pain and numbness; 21 out of 24 cases (87.5%) in total used warming formulas such as aconite root-containing formulas to reduce CIPN.
CONCLUSION
Our current study suggested that Kampo formulas chosen based on Kampo-specific diagnostics could be for treating CIPN that is refractory to conventional medicine.
Keywords: Asian medicine, Traditional Chinese medicine, Goshajinkigan, Keishibukuryogan, Hachimijiogan
Core tip: Our single-institution 5-year retrospective case series study revealed that Japanese traditional (Kampo) medicine was beneficial in 83.3% of patients with cancer chemotherapy-induced peripheral neuropathy that is refractory to conventional medicine. The most frequently used formula was goshajinkigan, followed by hachimijiogan and keishibukuryogan.
INTRODUCTION
Peripheral neuropathy is one of the most serious side effects in cancer chemotherapies. Chemotherapy-induced peripheral neuropathy (CIPN) is well known to occur due to taxanes, platinum analogues, vinca alkaloids, and molecular target drugs such as bortezomib[1]. A few of the mechanisms by which CIPN is caused by these chemotherapeutic agents are known[2-6], but most of the mechanisms remain unclear, which makes CIPN difficult to control. Calcium and magnesium infusions, glutathione, and anticonvulsants (e.g., carbamazepine) are used to prevent CIPN, but their effects are limited. In addition, once CIPN appears, anticonvulsants, tricyclic antidepressants, alpha-lipoic acid, and opioids often fail to improve it[7,8]. The most serious problem is that the abrogation of chemotherapy is required to prevent the exacerbation of CIPN symptoms, even when the chemotherapy is effectively treating the cancer.
Japanese traditional (Kampo) medicine, which originated in China and has been uniquely modified in Japan for 1500 years, can be used to treat a wide variety of diseases based on Kampo-specific diagnostics, regardless of the pathogenesis determined on the basis of conventional medicine. The Japanese Ministry of Health, Labor, and Welfare has approved 294 Kampo formulas for clinical use, which means that these formulas are also approved for reimbursement by Japan’s national health insurance system. Of these 294 formulas, 148 have extract granule-type (freeze-dried extracts of herbs) formulas and are manufactured by several licensed pharmaceutical companies in Japan, whereas all 294 formulas are also available as decoctions. Recently, Kampo medicine has received renewed attention because it provides a valid approach to treating symptoms refractory to conventional medicine and because one Kampo formula is often effective for treating a patient’s various complaints by improving the patient’s physical and mental balance as a whole; it is also likely that Kampo medicine decreases the inflated medical costs that result from the over-prescription of medications to treat refractory symptoms and many other physical and mental complaints[9].
CIPN has been treated with Kampo medicine in Japan’s clinical practice for decades, and clinical trials of Kampo medicine for the prevention or treatment of CIPN have been conducted using goshajinkigan (GJG, Niu Che Shen Qi Wan in Chinese)[10-14], shakuyakukanzoto[15,16], and keishikajutsubuto[17]. However, most studies were of an anteroposterior comparative nature using Kampo formulas that were not chosen by Kampo expert doctors but by oncologists, which means that the Kampo formulas were not properly chosen based on Kampo-specific diagnostics. There have been only a few reports done by Kampo expert doctors themselves. Kampo expert doctors are conventional medical school graduates who start training to become Kampo experts after becoming certified physicians or specialists in another medical field, such as surgery or pediatrics, which means that Kampo experts are also conventional medical experts. When Kampo medicine is prescribed by non-Kampo experts, its effectiveness can be limited, while it shows remarkable effectiveness, especially when chosen based on a Kampo-specific diagnosis irrespective of the conventional diagnosis.
We herein performed a retrospective case series study which examined the effectiveness of Kampo medicines chosen by Kampo expert doctors in patients with CIPN.
MATERIALS AND METHODS
Patients
A total of 281 patients diagnosed with cancer were identified by searching our outpatient database of 3154 patients who consulted our outpatient clinic of Japanese-Oriental (Kampo) Medicine at Chiba University Hospital from when our outpatient clinic was established in November 2005 to December 2010. Twenty-four patients out of the 281 patients identified met the following three conditions and were eligible for further investigation of the effectiveness of Kampo treatment: At least one course of cancer chemotherapy had been administered; numbness and pain appeared after the chemotherapy; and CIPN was diagnosed before they were given Kampo treatment.
Kampo treatment
In this study, Kampo formula was individually chosen by Kampo expert doctors in our outpatient clinic based on Kampo-specific diagnostics and was prescribed in the form of decoctions or extract granules according to each patient’s preference and each doctor’s decision (see the content of each Kampo formula at: http://dentomed.u-toyama.ac.jp/en/medical_information_on_kampo_formulas/).
Endpoints and evaluation
The 24 patients’ medical records were examined retrospectively. The checklist used to conduct the examination included the following items: Patient background (age, sex, and cancer type), contents of chemotherapy, period from onset of CIPN to the start of the Kampo treatment, and the effectiveness of the Kampo treatment.
The effectiveness of the Kampo treatment was self-evaluated by the patients in this study. They described their unpleasantness mixed with pain, numbness, and pins-and-needles sensation and those symptoms were prone to change into each other even during the Kampo treatment. The patients in the clinical records, therefore, described the improvement of symptoms how many percent of symptom reduction or how many points of the 11-Point Numerical Rating Scale for Pain Intensity was gained in terms of the total unpleasantness including pain, numbness, and pins-and-needles sensation comparing before and after the Kampo treatment. In this retrospective study covering 5 years, their improvement of symptoms was equally defined as signifying a “major response” when the whole unpleasantness showed reduction by 50% or more, a “minor response” when the reduction was less than 50%, and “no response” when they felt no positive response, comparing before and after the Kampo treatment. When the pain, numbness, and pins-and-needles sensation were present at two or more sites, we used an averaged evaluation. We evaluated the improvement in other cancer- and cancer-chemotherapy-associated symptoms in addition to CIPN in all cases because Kampo treatment is known to often be effective for other symptoms as well as the target symptom, as described earlier.
Our current study was approved by the Ethics Committee of Chiba University Hospital (Identification No. 1555).
RESULTS
Patient characteristics
The 24 cases consisted of 18 females and 6 males, and their ages ranged from 39 to 86 years (mean 61.2 ± 11.5 years) (Table 1). Eleven out of the 24 cases involved breast cancer (45.8%), 4 cases involved gynecological cancer (16.7%), 3 cases involved hematological tumors (12.5%), 3 cases involved gastrointestinal carcinoma (12.5%), and 3 cases involved other tumors (12.5%).
Table 1.
Patient demographic characteristics
| n (%) | ||
| Sex | Male | 6 (25.0) |
| Female | 18 (75.0) | |
| Age | Mean (range) | 61.2 ± 11.5 (39-86) |
| Primary tumor | Breast | 11 (45.8) |
| Gynecological | 4 (16.7) | |
| Hematological | 3 (12.5) | |
| Gastrointestinal | 3 (12.5) | |
| Others | 3 (12.5) | |
| Responsible chemotherapeutic agents (overlapping, see Table 4) | Taxanes | 15 (62.5) |
| Platinum analogues | 7 (29.2) | |
| Vinca alkaloids | 3 (12.5) | |
| Bortezomiib | 3 (12.5) | |
| Duration until starting Kampo medicine after responsible chemotherapy | During chemotherapy | 6 (25.0) |
| < 6 mo after | 8 (33.3) | |
| 7-12 mo after | 4 (16.7) | |
| > 13 mo after | 6 (25.0) | |
Taxanes (15 cases, 62.5%) were the most-used among the chemotherapies responsible for peripheral neuropathy, followed by platinum analogues including oxaliplatin and cisplatin (7 cases, 29.2%), vinca alkaloids (3 cases, 12.5%), bortezomib (3 cases, 12.5%). The responsible chemotherapeutic agents overlapped in 4 cases.
Table 1 also shows the duration from the end of the responsible chemotherapy to the start of the Kampo treatment. Six cases (25.0%) received Kampo treatment during chemotherapy, 8 cases (33.3%) received it within 6 mo after the chemotherapy was ended, 4 cases (16.7%) received it 7-12 mo after the chemotherapy was ended, and 6 cases (25.0%) received it more than 13 mo after the final chemotherapy.
Efficacy of Kampo treatments
Figure 1 shows that 9 cases had a major response, with 7 of these cases showing a more than 70% symptom reduction and 2 cases showing a 50%-70% symptom reduction. Eleven cases showed a minor response (less than 50% symptom reduction), and 4 out of the 24 cases had no beneficial response. Thus, a beneficial (both major and minor) response was obtained in 20 (83.3%) out of the 24 cases in total by Kampo treatment, although the CIPN in these cases had been refractory to conventional medicine.
Figure 1.
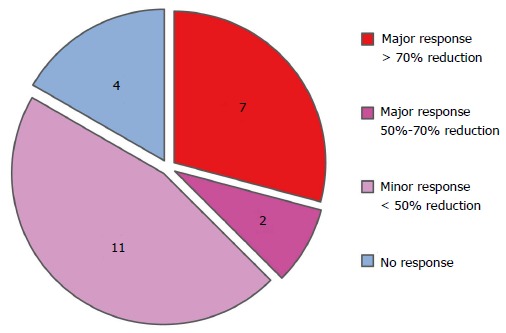
Efficacy of Kampo treatments.
No adverse effects due to the Kampo treatment were observed in this study.
Effective Kampo formulas
Table 2 shows the Kampo formulas used in our study and their response rates. The GJG-related formulas were the most frequently used (6 cases, 25.0%). Among these GJG-treated patients, 2 patients used the extract granule-type (abbreviated to “E” in Table 2) of GJG alone, but the other 2 patients used the decoction-type (abbreviated to “D” in Table 2) of GJG or a combination therapy with the extract granule-type of other Kampo formulas. The second-most frequently used Kampo formulas were hachimijiogan (HJG, Mai-men-dong-tang in Chinese) and keishibukuryogan (KBG, Gui-zhi-fu-ling-wan in Chinese) (3 cases, 12.5%, for each). GJG and HJG are categorized into the same group in our current study because they are very similar formulas, which made HJG the top KBG in the classification given in Table 2, as will be discussed later. All other Kampo formulas were used in 2 cases or fewer.
Table 2.
Kampo formulas and response rates
| Kampo formulas | Major response | Minor response | No response | Total (%) |
| GJG-related formulas | 3 | 3 | 0 | 6 (25.0) |
| GJG alone | 3 (D; 1, E; 2) | 1 (D) | 0 | 4 |
| Combination with | ||||
| + Hangebyakujutsutemmato (E) | 0 | 1 (E) | 0 | 1 |
| + Nijutsuto (E) | 0 | 1 (E) | 0 | 1 |
| HJG-related formulas | 1 | 1 | 1 | 3 (12.5) |
| HJG alone | 1 (E) | 0 | 0 | 1 |
| Combination with | ||||
| + KBG (E) | 0 | 1 (E) | 0 | 1 |
| + Juzentaihoto (D) | 0 | 0 | 1 (E) | 1 |
| Others | 1 | 2 | 1 | 4 (16.7) |
| Keishikaryojutsubuto (E) | 1 (E) | 0 | 0 | 1 |
| + Bukuryoingohangekobokuto (E) | ||||
| Uzukeishito (D) | 0 | 1 (E) | 0 | 1 |
| Shimbuto (E) | 0 | 1 (E) | 0 | 1 |
| Juzentaihoto-ka-bushi (E) | 0 | 0 | 1 (E) | 1 |
| Total (aconite root-containing formulas) | 5 | 6 | 2 | 13 (54.2) |
| KBG (D) + Nichinto (D) | 1 (D) | 1 (D) | 0 | 2 |
| Tokishigyakukagoshuyushokyoto (D, E) | 0 | 1 (D) | 1 (E) | 2 |
| Keihito (D) | 1 (D) | 0 | 0 | 1 |
| Boiogito (D) + Tokishakuyakusan (E) | 1 (D + E) | 0 | 0 | 1 |
| Ryokyojutsukanto (E) + Unkeito (E) | 0 | 1 (E) | 0 | 1 |
| Ryokyojutsukanto (E) | 0 | 0 | 1 (E) | 1 |
| Total (warming formulas without aconite roots) | 3 | 3 | 2 | 8 (33.3) |
| Seinetsuhokito (D) | 1 (D) | 0 | 0 | 1 |
| Hochuekkito (D) | 0 | 1 (D) | 0 | 1 |
| Goreisan (D) | 0 | 1 (D) | 0 | 1 |
| Total (formulas without warming effects nor aconite roots) | 1 | 2 | 0 | 3 (12.5) |
| Total (all formulas) (%) | 9 (37.5) | 11 (45.8) | 4 (16.7) | 24 (100.0) |
D: Decoction-type; E: Extract granule-type; GJG: Goshajinkigan; HJG: Hachimijiogan; KBG: Keishibukuryogan.
All Kampo formulas are categorized into 3 groups depending on whether those formulas tend to have “warming” effects or not (Table 2). For example, aconite roots are supposed to have the strongest warming effects among the Kampo herbs used to improve local coldness and cold intolerance and to ameliorate pain, numbness, and body fluid metabolism by means of these warming effects. Thirteen out of 24 cases (54.2%, colored pink) used Kampo formulas containing aconite roots. Eight out of 24 cases (33.3%, colored orange) used Kampo formulas composed of herbs other than aconite roots that have warming effects. Three cases (12.5%, colored in blue) gained beneficial effects from Kampo formulas that had no warming effects nor contained aconite roots.
Configuration of Kampo formula and response rates
Decoction-type formulas tend to be superior to extract granule-type formulas in therapeutic efficacy (Table 3). In our study, the decoction-type (“D” in Table 3) was used in 10 cases, the extract granule-type (“E” in Table 3) in 12 cases, and a combination of the decoction- and extract granule-types in 2 cases. Beneficial effects (major and minor responses) were seen in all (10 out of 10) cases treated with decoction-type formulas, while 9 out of 12 cases treated with extract granule-type formulas showed beneficial effects.
Table 3.
Configuration of Kampo formula and response rates
| Major response (%) | Minor response (%) | No response (%) | Total (%) | |
| D | 4 (40.0) | 6 (60.0) | 0 | 10 (100.0) |
| E | 4 (33.3) | 5 (41.7) | 3 (25.0) | 12 (100.0) |
| Combination of D and E | 1 (50.0) | 0 | 1 (50.0) | 2 (100.0) |
D: Decoction-type; E: Extract granule-type.
Responsible chemotherapeutic agents
Table 4 shows the correlation between the chemotherapeutic agents responsible for peripheral neuropathy and the response rates to Kampo treatment. The response rates were 100.0% (5 out of 5 cases) in cases treated with platinum analogues (oxaliplatin in 2 cases and cisplatin in 3 cases) and 84.6% (11 out of 13 cases) in those treated with taxanes. On the other hand, cases that underwent chemotherapy that included vinca alkaloids had lower response rates than the other cases.
Table 4.
Responsible chemotherapeutic agents and response rates to Kampo treatment
| Major response (%) | Minor response (%) | No response (%) | Total (%) | |
| Taxanes | 5 (38.5) | 6 (46.1) | 2 (15.4) | 13 (100.0) |
| Platinum analogues | ||||
| Oxaliplatin | 0 | 2 (100.0) | 0 | 2 (100.0) |
| Cisplatin | 2 (66.7) | 1 (33.3) | 0 | 3 (100.0) |
| Taxanes + cisplatin | 2 (100.0) | 0 | 0 | 2 (100.0) |
| Bortezomib | 0 | 1 (100.0) | 0 | 1 (100.0) |
| Vinca alkaloids | 0 | 0 | 1 (100.0) | 1 (100.0) |
| Bortezomib + vinca alkaloids | 0 | 1 (50.0) | 1 (50.0) | 2 (100.0) |
Timing of Kampo treatment after responsible chemotherapy
We also investigated the correlation between the response rates and the time elapsed from the end of the responsible chemotherapy to the start of the Kampo treatment (Table 5). Beneficial effects were observed in 5 out of 6 cases (83.3%) in which the Kampo treatment was started during chemotherapy, in 7 out of 8 cases (87.5%) in which the Kampo treatment was started within 6 mo after the chemotherapy ended, and in 4 out of 4 cases (100.0%) in which Kampo treatment was started between 6 and 12 mo after the chemotherapy ended. Four out of 6 cases (66.6%) in which more than 12 mo had passed after the responsible chemotherapy ended still showed positive effects.
Table 5.
Timing of Kampo treatment after responsible chemotherapy
| Major response (%) | Minor response (%) | No response (%) | Total (%) | |
| During chemotherapy | 2 (33.3) | 3 (50.0) | 1 (16.7) | 6 (100.0) |
| ≤ 6 m | 4 (50.0) | 3 (37.5) | 1 (12.5) | 8 (100.0) |
| > 6 m, ≤ 12 m | 1 (25.0) | 3 (75.0) | 0 | 4 (100.0) |
| > 12 m | 2 (33.3) | 2 (33.3) | 2 (33.3) | 6 (100.0) |
DISCUSSION
Conventional medical doctors in Japan introduce patients to Kampo experts when the Kampo medicines prescribed by them are not effective enough or when conventional medicine cannot improve patients’ symptoms[18]. Quality control of the herbs used in Kampo has been established for both extract granules and decoctions, and the safety and reliability of Kampo have been well-established through the strict monitoring of side effects under the control of the Japanese Ministry of Health, Labor, and Welfare in the same way as conventional medicines are used[19]. In addition, the clinical efficacy of some Kampo medicines, especially daikenchuto and yokukansan, has been demonstrated in many clinical trials done in the United States and Japan by conventional medical doctors using the standard methods of conventional medicine[18,20-23].
CIPN is one of the most frequent reasons for the early cessation of cancer treatment and can result in an increased risk of recurrence and a decreased survival rate[7]. This is the case simply because there is no medication for CIPN that has shown strong evidence of effectiveness in clinical trials[7,8]. In our study, a beneficial response was observed in 83.3% of cases treated with Kampo medicine; the severity of CIPN was reduced by more than half in 9 out of 24 cases (37.5%, shown in Figure 1). In addition, those patients with CIPN had already tried conventional medicine to reduce the severity of their symptoms but had seen no positive effect before consulting our clinic. The present result strongly suggests that Kampo formulas, when chosen by Kampo expert doctors based on Kampo diagnostics, are effective for treating CIPN refractory to conventional medicine.
In the past several years, there have been many reports on Kampo medicine’s effectiveness in treating CIPN[10-17]. In particular, peripheral neuropathy caused by oxaliplatin was reported to be preventable by the prophylactic use of GJG[10,11], and GJG was found not to decrease the anti-tumor effect of oxaliplatin[11]. Clinical trials have shown not only GJG’s prophylactic efficacy in treating CIPN but also its effectiveness in treating CIPN that has developed after chemotherapy[12-14]. GJG was the most frequently used Kampo formula in the present study, even when Kampo expert doctors chose the appropriate Kampo formula for each patient based on Kampo-specific diagnostics. GJG is composed of 10 herbs, and the mechanisms by which it improves pain and numbness are not fully understood. Some of these mechanisms are known, such as stimulation of the intraspinal κ opioid receptor[24], promotion of the production of carbon monoxide (NO) in the algesthesia perception site, and increased peripheral blood flow[25]. Some of these effects were reported to be mainly caused by the aconite root included in GJG[26], but the effects of GJG are superior to those of aconite root alone[24].
Aconite root has been used to treat hypofunctional state and cold intolerance in Kampo medicine and traditional Chinese medicine for thousands of years[27]. Aconite root contains many alkaloids such as aconitine and mesaconitine, which are known to have cardiotonic, analgesic, and vasodilator actions[28-31]. Moreover, when a Kampo formula is partly effective, the addition of processed aconite root sometimes augments the formula’s efficacy among patients with pain exacerbated by coldness[15,27,32]. In addition to GJG, the Kampo prescriptions used in this study mostly contained aconite roots and had warming effects. Intriguingly, 13 out of 24 cases (54.2%) were prescribed aconite-root-containing formulas, which is a remarkably high rate given the fact that there are only 25 aconite-root-containing formulas among 294 Kampo formulas. Given that 8 out of 24 cases were prescribed other formulas with warming effects, a total of 21 out of 24 cases (87.5%) were prescribed formulas with warming effects to reduce their symptoms.
Decoction-type formulas showed a higher response rate than extract granule-type formulas (Table 3), implying that decoction-type formulas may be more efficacious than extract granule-type formulas. Extract granule-type formulas, however, were also effective for treating refractory CIPN in our study and were especially beneficial because of the small amounts (generally 6-9 g/d) required and their portability. In addition, if a Kampo formula improves a patient’s physical and mental imbalance as a whole, this single formula can often treat a wide variety of symptoms, even if the formula is of the extract granule-type. In fact, in our study, symptoms were also observed after Kampo treatment was initiated (data not shown). The improved symptoms included edema in 6 cases, fatigue and constipation in 4 cases each, and other symptoms in 8 cases. A previous study also reported that the benefit of Kampo treatment includes reducing peripheral neuropathy but also extends to ameliorating the side effects of cancer therapy or the symptoms of the cancer itself[33].
The major response rate in patients who started Kampo treatment during chemotherapy was not high (33.3%, as shown in Table 5). This means that the damage caused by ongoing chemotherapy is stronger than Kampo’s therapeutic effect, although Kampo may contribute to the patients’ ability to continue the chemotherapy by alleviating some side effects[10,11]. CIPN has been known to spontaneously resolve over time in some cases[34], and this may have happened in some patients in our study as well. However, it is not likely that CIPN would naturally go into remission during a repeated administration of the responsible chemotherapy or after more than a year had passed since the last responsible chemotherapy in cases in which CIPN had not been reduced at all until the start of Kampo treatment. In several previous reports, Kampo treatment was started after chemotherapy began or after CIPN occurred and was shown to have beneficial effects[10-16].
CIPN caused by taxanes and platinum analogues showed a high therapeutic response to Kampo treatment, and CIPN caused by vinca-alkaloid-containing chemotherapies showed a low response, indicating that Kampo’s effectiveness differed depending on the responsible chemotherapeutic agent. There is also a possibility that the mechanism by which CIPN develops and that by which Kampo formulas have their effects are different depending on the chemotherapeutic agent responsible.
Our current study has many limitations due to the small study population and the retrospective methodology. Another limitation of this study is the potential for gender bias (the study included 16 females and 8 males as shown in Table 1), which reflects that the use of complementary and alternative medicine (CAM) is more prevalent among women[35] and that many more females than males come to our outpatient clinic for the treatment of other diseases as well. However, despite these limitations, our current study shows that the use of Kampo formulas for treating CIPN is promising and can be effective, especially when the Kampo formulas are chosen by Kampo expert doctors based on Kampo-specific diagnostics. This is a preliminary retrospective case series study that we expect will contribute to the design of a future clinical trial in which Kampo-specific diagnostics will be used to choose Kampo formulas to investigate Kampo’s effectiveness in ameliorating CIPN.
There are few valid approaches to treating refractory CIPN; thus, CIPN often leads to the discontinuation of chemotherapy and the deterioration of the patient’s quality of life. Our results showed that Kampo formulas can reduce the severity of CIPN, even if a long interval has passed since the last chemotherapy, or during the repeated administration of chemotherapy. Kampo formulas chosen based on Kampo-specific diagnostics can be effective, and Kampo treatment should be considered as an alternative approach to treating CIPN that is refractory to conventional medicine.
COMMENTS
Background
Cancer chemotherapy-induced peripheral neuropathy (CIPN) is often refractory to conventional medicine and results in the discontinuation of treatment with potentially effective anticancer agents. Kampo medicine has been reported to be effective in CIPN but Kampo formulas were not chosen by Kampo expert doctors but by non-Kampo expert oncologists in most those studies.
Research frontiers
Aconite, which was included in 13 out of 24 cases (54.2%) in the authors’ study, has been recently reported to show an analgesic activity by attenuating the hypersensitivity of neural cells. The prophylactic use of goshajinkigan (GJG), one of representative Kampo formulae including aconite, was reported to be preventable for peripheral neuropathy caused by oxaliplatin and GJG was found not to decrease the anti-tumor effect of oxaliplatin.
Innovations and breakthroughs
There have been many reports on Kampo medicine’s effectiveness in treating CIPN lately and the effectiveness of a single Kampo prescription were examined in each report. This is the first study performed in Kampo-specialized clinic showing what Kampo medicine is often chosen by Kampo expert doctors.
Applications
Most Kampo prescriptions chosen by Kampo expert doctors contained aconite roots which treat hypofunctional state and cold intolerance. In addition, decoction-type formulas, which in Japan are mostly prescribed by Kampo expert doctors, showed a higher response rate than extract granule-type formulas. These findings in this current study suggest that Kampo prescriptions including aconite roots, especially decoctions chosen by Kampo expert doctors, have more beneficial effects even on refractory CIPN to conventional medicine.
Terminology
CIPN occurs due to taxanes, platinum analogues, vinca alkaloids, and molecular target drugs such as bortezomib and is often refractory to conventional medicine. The most serious problem is that the abrogation of chemotherapy is required to prevent the exacerbation of CIPN symptoms, even when the chemotherapy is effectively treating the cancer.
Peer-review
The paper is about an important issue in oncology, it is well written and the results are interesting.
Footnotes
Institutional review board statement: This case report was exempt from the Ethics Committee of Chiba University Hospital.
Informed consent statement: This is a retrospective case series study. There was no way to get touch with all patients involved in this study so we had been notifying them of this study on our department’s website.
Conflict-of-interest statement: Kimata Y, Ogawa K, Okamoto H, and Namiki T got a grant as Research Support from Tsumua Co. Atsushi Chino has no conflict of interests regarding the publication of this paper.
Data sharing statement: No additional data are available.
Manuscript source: Invited manuscript
Specialty Type: Medicine, research and experimental
Country of Origin: Japan
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: April 28, 2016
First decision: June 16, 2016
Article in press: August 8, 2016
P- Reviewer: Mocellin S, Tang ZP, Vetvicka V S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Sioka C, Kyritsis AP. Central and peripheral nervous system toxicity of common chemotherapeutic agents. Cancer Chemother Pharmacol. 2009;63:761–767. doi: 10.1007/s00280-008-0876-6. [DOI] [PubMed] [Google Scholar]
- 2.Rowinsky EK, Donehower RC. Paclitaxel (taxol) N Engl J Med. 1995;332:1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 3.LoMonaco M, Milone M, Batocchi AP, Padua L, Restuccia D, Tonali P. Cisplatin neuropathy: clinical course and neurophysiological findings. J Neurol. 1992;239:199–204. doi: 10.1007/BF00839140. [DOI] [PubMed] [Google Scholar]
- 4.Pasetto LM, D’Andrea MR, Rossi E, Monfardini S. Oxaliplatin-related neurotoxicity: how and why? Crit Rev Oncol Hematol. 2006;59:159–168. doi: 10.1016/j.critrevonc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291:1–9. doi: 10.1016/j.tox.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood. 2008;112:1593–1599. doi: 10.1182/blood-2008-04-149385. [DOI] [PubMed] [Google Scholar]
- 7.Kaley TJ, Deangelis LM. Therapy of chemotherapy-induced peripheral neuropathy. Br J Haematol. 2009;145:3–14. doi: 10.1111/j.1365-2141.2008.07558.x. [DOI] [PubMed] [Google Scholar]
- 8.Pachman DR, Barton DL, Watson JC, Loprinzi CL. Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clin Pharmacol Ther. 2011;90:377–387. doi: 10.1038/clpt.2011.115. [DOI] [PubMed] [Google Scholar]
- 9.Sato Y, Hanawa T, Arai M, Cyong JC, Fukuzawa M, Mitani K, Ogihara Y, Sakiyama T, Shimada Y, Toriizuka K, et al. Introduction to Kampo: Chapter II. Diagnosis and treatment, The Japan Society for Oriental Medicine, Tokyo: Elsevier Japan K.K; 2005. pp. 20–21. [Google Scholar]
- 10.Nishioka M, Shimada M, Kurita N, Iwata T, Morimoto S, Yoshikawa K, Higashijima J, Miyatani T, Kono T. The Kampo medicine, Goshajinkigan, prevents neuropathy in patients treated by FOLFOX regimen. Int J Clin Oncol. 2011;16:322–327. doi: 10.1007/s10147-010-0183-1. [DOI] [PubMed] [Google Scholar]
- 11.Ushio S, Egashira N, Sada H, Kawashiri T, Shirahama M, Masuguchi K, Oishi R. Goshajinkigan reduces oxaliplatin-induced peripheral neuropathy without affecting anti-tumour efficacy in rodents. Eur J Cancer. 2012;48:1407–1413. doi: 10.1016/j.ejca.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Kono T, Mamiya N, Chisato N, Ebisawa Y, Yamazaki H, Watari J, Yamamoto Y, Suzuki S, Asama T, Kamiya K. Efficacy of goshajinkigan for peripheral neurotoxicity of oxaliplatin in patients with advanced or recurrent colorectal cancer. Evid Based Complement Alternat Med. 2011;2011:418481. doi: 10.1093/ecam/nep200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto T, Murai T, Ueda M, Katsuura M, Oishi M, Miwa Y, Okamoto Y, Uejima E, Taguchi T, Noguchi S, et al. Clinical features of paclitaxel-induced peripheral neuropathy and role of Gosya-jinki-gan. Gan To Kagaku Ryoho. 2009;36:89–92. [PubMed] [Google Scholar]
- 14.Kaku H, Kumagai S, Onoue H, Takada A, Shoji T, Miura F, Yoshizaki A, Sato S, Kigawa J, Arai T, et al. Objective evaluation of the alleviating effects of Goshajinkigan on peripheral neuropathy induced by paclitaxel/carboplatin therapy: A multicenter collaborative study. Exp Ther Med. 2012;3:60–65. doi: 10.3892/etm.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hidaka T, Shima T, Nagira K, Ieki M, Nakamura T, Aono Y, Kuraishi Y, Arai T, Saito S. Herbal medicine Shakuyaku-kanzo-to reduces paclitaxel-induced painful peripheral neuropathy in mice. Eur J Pain. 2009;13:22–27. doi: 10.1016/j.ejpain.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Fujii K, Okamoto S, Saitoh K, Sasaki N, Takano M, Tanaka S, Kudoh K, Kita T, Tode T, Kikuchi Y. The efficacy of Shakuyaku-Kanzo-to for peripheral nerve dysfunction in paclitaxel combination chemotherapy for epithelial ovarian carcinoma. Gan To Kagaku Ryoho. 2004;31:1537–1540. [PubMed] [Google Scholar]
- 17.Yamada T, Kan H, Matsumoto S, Koizumi M, Sasaki J, Tani A, Yokoi K, Uchida E. Reduction in oxaliplatin-related neurotoxicity by the administration of Keishikajutsubuto(TJ-18)and powdered processed aconite root. Gan To Kagaku Ryoho. 2012;39:1687–1691. [PubMed] [Google Scholar]
- 18.Kono T, Kanematsu T, Kitajima M. Exodus of Kampo, traditional Japanese medicine, from the complementary and alternative medicines: is it time yet? Surgery. 2009;146:837–840. doi: 10.1016/j.surg.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Cameron S, Reissenweber H, Watanabe K. Asian medicine: Japan’s paradigm. Nature. 2012;482:35. doi: 10.1038/482035a. [DOI] [PubMed] [Google Scholar]
- 20.Iturrino J, Camilleri M, Wong BS, Linker Nord SJ, Burton D, Zinsmeister AR. Randomised clinical trial: the effects of daikenchuto, TU-100, on gastrointestinal and colonic transit, anorectal and bowel function in female patients with functional constipation. Aliment Pharmacol Ther. 2013;37:776–785. doi: 10.1111/apt.12264. [DOI] [PubMed] [Google Scholar]
- 21.Manabe N, Camilleri M, Rao A, Wong BS, Burton D, Busciglio I, Zinsmeister AR, Haruma K. Effect of daikenchuto (TU-100) on gastrointestinal and colonic transit in humans. Am J Physiol Gastrointest Liver Physiol. 2010;298:G970–G975. doi: 10.1152/ajpgi.00043.2010. [DOI] [PubMed] [Google Scholar]
- 22.Iwasaki K, Satoh-Nakagawa T, Maruyama M, Monma Y, Nemoto M, Tomita N, Tanji H, Fujiwara H, Seki T, Fujii M, et al. A randomized, observer-blind, controlled trial of the traditional Chinese medicine Yi-Gan San for improvement of behavioral and psychological symptoms and activities of daily living in dementia patients. J Clin Psychiatry. 2005;66:248–252. doi: 10.4088/jcp.v66n0214. [DOI] [PubMed] [Google Scholar]
- 23.Mizukami K, Asada T, Kinoshita T, Tanaka K, Sonohara K, Nakai R, Yamaguchi K, Hanyu H, Kanaya K, Takao T, et al. A randomized cross-over study of a traditional Japanese medicine (kampo), yokukansan, in the treatment of the behavioural and psychological symptoms of dementia. Int J Neuropsychopharmacol. 2009;12:191–199. doi: 10.1017/S146114570800970X. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki Y, Goto K, Ishige A, Komatsu Y, Kamei J. Antinociceptive effect of Gosha-jinki-gan, a Kampo medicine, in streptozotocin-induced diabetic mice. Jpn J Pharmacol. 1999;79:169–175. doi: 10.1254/jjp.79.169. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki Y, Goto K, Ishige A, Komatsu Y, Kamei J. Effects of gosha-jinki-gan, a kampo medicine, on peripheral tissue blood flow in streptozotocin-induced diabetic rats. Methods Find Exp Clin Pharmacol. 1998;20:321–328. doi: 10.1358/mf.1998.20.4.485687. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Arita H, Hayashida M, Zhang L, Sekiyama H, Hanaoka K. Pain-relieving effects of processed Aconiti tuber in CCI-neuropathic rats. J Ethnopharmacol. 2006;103:392–397. doi: 10.1016/j.jep.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 27.Sato Y, Hanawa T, Arai M, Cyong JC, Fukuzawa M, Mitani K, Ogihara Y, Sakiyama T, Shimada Y, Toriizuka K, et al. Introduction to Kampo: Chapter III. Materia medica, The Japan Society for Oriental Medicine, Tokyo: Elsevier Japan K.K; 2005. pp. 82–83. [Google Scholar]
- 28.Shu H, Arita H, Hayashida M, Sekiyama H, Hanaoka K. Effects of processed Aconiti tuber and its ingredient alkaloids on the development of antinociceptive tolerance to morphine. J Ethnopharmacol. 2006;103:398–405. doi: 10.1016/j.jep.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Murayama M, Mori T, Bando H, Amiya T. Studies on the constituents of Aconitum species. IX. The pharmacological properties of pyro-type aconitine alkaloids, components of processed aconite powder ‘kako-bushi-matsu’: analgesic, antiinflammatory and acute toxic activities. J Ethnopharmacol. 1991;35:159–164. doi: 10.1016/0378-8741(91)90068-o. [DOI] [PubMed] [Google Scholar]
- 30.Yamada K, Suzuki E, Nakaki T, Watanabe S, Kanba S. Aconiti tuber increases plasma nitrite and nitrate levels in humans. J Ethnopharmacol. 2005;96:165–169. doi: 10.1016/j.jep.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 31.Mitamura M, Boussery K, Horie S, Murayama T, Van de Voorde J. Vasorelaxing effect of mesaconitine, an alkaloid from Aconitum japonicum, on rat small gastric artery: possible involvement of endothelium-derived hyperpolarizing factor. Jpn J Pharmacol. 2002;89:380–387. doi: 10.1254/jjp.89.380. [DOI] [PubMed] [Google Scholar]
- 32.Nakanishi M, Arimitsu J, Kageyama M, Otsuka S, Inoue T, Nishida S, Yoshikawa H, Kishida Y. Efficacy of traditional Japanese herbal medicines-Keishikajutsubuto (TJ-18) and Bushi-matsu (TJ-3022)-against postherpetic neuralgia aggravated by self-reported cold stimulation: a case series. J Altern Complement Med. 2012;18:686–692. doi: 10.1089/acm.2010.0745. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa K, Ogawa M, Motoo Y. Tokyo: Nova Science; 2013. Kampo for Cancer Care: Significance as Supportive Measures; In Motoo Y, editor. Traditional Medicine: New Research; pp. 1–13. [Google Scholar]
- 34.Weiss RB. Miscellaneous Toxicities. Cancer Principles & Practice of Oncology 7th ed. In: Devita VT, Hellman S, Rosenberg SA, editors. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 2602–2614. [Google Scholar]
- 35.Tindle HA, Davis RB, Phillips RS, Eisenberg DM. Trends in use of complementary and alternative medicine by US adults: 1997-2002. Altern Ther Health Med. 2005;11:42–49. [PubMed] [Google Scholar]


