Summary
Objective
We examined parental and early‐life variables in order to identify risk factors for adulthood overweight and obesity in offspring. We report here on the longitudinal prevalence of overweight and obesity in Australian children born between 1989 and 1991 and followed from birth to age 22.
Methods
Data were analysed on 1355 participants from the Western Australian Pregnancy Cohort (Raine) Study, with anthropometry collected during pregnancy, at birth, one year and at three yearly intervals thereafter. Multivariate analyses and cross‐sectional logistic regression quantified the timing and contribution of early‐life risk factors for overweight and obesity in young‐adulthood.
Results
At five years of age 12.6% of children were overweight and 5.2% were obese. By early adulthood, the prevalence of obesity had increased to 12.8%, whilst overweight remained relatively stable at 14.2% (range from early childhood to adulthood 11–16%). Parental pre‐pregnancy body mass index (BMI) was the strongest determinant of adult offspring BMI. Although rapid first year weight gain was associated with increased offspring BMI, the impact of first year weight‐gain diminished over childhood, whilst the impact of parental BMI increased over time.
Conclusions
Parental pre‐pregnancy BMI and rapid early‐life weight gain predispose offspring to obesity in adulthood.
Keywords: BMI, early‐life, Raine cohort
Introduction
Obesity and overweight are significant contributors to the burden of chronic non‐communicable diseases 1. Weight trajectories established in childhood often persist into adulthood 2. The prevalence of childhood and adolescent obesity in the USA almost tripled between the early 1960s and 2000s reaching 37.5% in adults and 16.9% in children by 2010 3. Similar trends have been observed in Western Europe, Australia and Japan 4, but relative changes in prevalence are greatest for low‐income and middle‐income countries 5, 6. Recent US data indicate that incident obesity declines over childhood, suggesting that interventions should target early life 7.
Implementation of appropriate prevention strategies requires identification of early‐life risk factors 4, 8, 9. Studies looking at risk factors for childhood obesity have identified maternal smoking 10 pre‐pregnancy parental obesity 11, heavy TV usage and short sleep duration prior to age three as well as rapid weight gain in the first year of life 4. Exclusive breastfeeding has been found to have a protective effect in some studies 12 but not others 13. Increased risk of adolescent obesity has been associated with maternal smoking during pregnancy14, higher birth weight 15, post‐natal catch‐up growth 4, 16 and upward centile crossing of the body mass index (BMI) throughout childhood 17.
Our report here represents the first comprehensive analysis of a large cohort from birth to young adulthood. We aimed to examine parental and early‐life variables to identify risk factors for adulthood overweight and obesity. We also analysed longitudinal BMI profiles from offspring with obesity in an attempt to identify an age when their BMI curves diverged from the norm.
A large contemporary Australian birth cohort born between 1989 and 1991 was evaluated. Mothers and offspring were prospectively followed from early pregnancy with direct anthropometric measurements of offspring at 12 time points: birth, 1, 2, 3, 5, 8, 10, 14, 17, 18, 20 and 22 years. These data provided a unique opportunity to interrogate BMI trajectories from infancy to adulthood and to explore the relative importance of contributing early‐life factors.
Methods
Western Australian Pregnancy Cohort (Raine) Study
The Raine Study recruited 2,900 pregnancies from King Edward Memorial Hospital (KEMH) between 1989 and 1991 as part of a randomized controlled trial, evaluating repeated ultrasounds in pregnancy 18, 19. Detailed data were collected throughout pregnancy and at follow‐up, based on the 2868 live‐births 18, 20.
Ethics statement
Ethics approval was obtained from the Human Ethics Committees at KEMH, Princess Margaret Hospital, The University of Western Australia and Curtin University. All participants and guardians provided written consent.
Study population
At 20 years of age, 2,076 participants remained enrolled in the cohort and 1,423 provided anthropometric measurements at either the 20‐year or 22‐year follow‐up or at both. The analysis excluded participants with congenital anomalies (5 individuals excluded) and those with eligible older siblings (63 younger siblings excluded), leaving 1,355 participants eligible for analysis. Of these, 83% had two parents of European descent and 9% had one such parent. At the 17‐year follow‐up, demographic characteristics of the cohort were similar to the Western Australian population of families with 15‐ to 17‐year‐old children 21.
Anthropometric measurements and evaluation of data
Measures on the children were taken in a clinic setting (KEMH, Princess Margaret Hospital, Sir Charles Gairdner Hospital) or a research institute (Telethon Institute for Child Health, The University of Western Australia). Measures were performed by a trained research nurse (years 1 to 5) or a trained research assistant according to set protocols. For more details of the weight, height and length at each follow‐up see the Supporting Information. The protocol for the most recent follow‐up (year 22) has been described by Straker et al 2015 22. BMI was calculated as weight in kilograms (kg) divided by squared height in metres (m). To account for age‐related and sex‐related variation, BMI was standardized producing z‐scores, using the 2000 Center for Disease Control and Prevention growth charts 23. Overweight was defined as BMI z‐score ≥85th centile, obesity as BMI z‐score ≥95th centile 24. In adulthood, these equate to BMIs of 25 and 30 kg m−2, respectively.
Risk factors investigated included: sex, highest maternal education level attained (paternal education level not available), maternal and paternal pre‐pregnancy BMI (based on recalled weight recorded at 18 weeks' gestation), maternal smoking during pregnancy (indicating ‘no exposure throughout pregnancy’ and ‘exposure at any point during the pregnancy’), maternal anemia and diabetes during pregnancy (pre‐existing or gestational), gestational weight gain (change in weight from pre‐pregnancy to 18 weeks and 34 weeks' gestation), cesarean section delivery, prematurity, offspring birth‐weight, first‐year weight‐gain and duration of breast‐feeding. Continuous variables were re‐coded as categorical for summary purposes in Table 1 but not in the longitudinal analyses.
Table 1.
Prevalence of obesity among Australian children of European descent between the ages of 3 and 22 years (1989–2013) for selected early‐life factors
| Prevalence (%) of obesity (95% CI) | |||||
|---|---|---|---|---|---|
| Variable | No. of Children eligible at any timepoint | Aged 3 n = 916a | Aged 5 n = 1190a | Aged 14 n = 1146a | Aged 22 n = 1015a |
| All children | 1355 | 5.0 (3.6, 6.4) | 5.2 (3.9, 6.5) | 9.4 (7.7, 11.1) | 12.8 (10.7, 14.9) |
| Male participants | 688 | 6.1 (3.9, 8.3) | 6.1 (4.2, 8.0) | 10.3 (7.8, 12.8) | 10.9 (8.2, 13.6) |
| Female participants | 667 | 3.9 (2.1, 5.7) | 4.3 (2.7, 5.9) | 8.6 (6.3, 10.9) | 14.6 (11.5, 17.7) |
| Maternal education | |||||
| High school qualification | 569 | 3.1 (1.3, 4.9) | 5.2 (3.2, 7.2) | 11.7 (8.8, 14.6) | 17.2 (13.6, 20.8) |
| Trade qualification | 567 | 6.5 (4.1, 8.9) | 5.8 (3.8, 7.8) | 8.3 (5.9, 10.7) | 10.2 (7.3, 13.1) |
| University degree or higher | 178 | 7 (2.6, 11.4) | 4.2 (1.1, 7.3) | 7.7 (3.5, 11.9) | 9.9 (5.2, 14.6) |
| Maternal smoker | 314 | 4.3 (1.4, 7.2) | 7.3 (4.1, 10.5) | 15.4 (10.9, 19.9) | 21.5 (16.1, 26.9) |
| Maternal non‐smoker | 1035 | 5.3 (3.7, 6.9) | 4.7 (3.3, 6.1) | 7.8 (6.0, 9.6) | 10.5 (8.4, 12.6) |
| Maternal weight gain to 18 weeks' gestation | |||||
| <5 kg | 706 | 6.0 (3.9, 8.1) | 5.1 (3.4, 6.8) | 10.2 (7.8, 12.6) | 12.2 (9.4, 15.0) |
| ≥5 and <10 kg | 440 | 3.4 (1.3, 5.5) | 4.5 (2.4, 6.6) | 6.5 (4.0, 9.0) | 12.3 (8.8, 15.8) |
| ≥10 kg | 134 | 6.5 (1.5, 11.5) | 10.3 (4.8, 15.8) | 18.8 (11.6, 26.0) | 23.7 (15.1, 32.3) |
| Maternal BMI pre‐pregnancy | |||||
| <25 | 1088 | 3.5 (2.2, 4.8) | 4.2 (2.9, 5.5) | 6.0 (4.5, 7.5) | 8.7 (6.8, 10.6) |
| ≥25 and <30 | 146 | 11.0 (5.1, 16.9) | 8.3 (3.6, 13.0) | 22.6 (15.2, 30.0) | 30.5 (22.2, 38.8) |
| ≥30 | 80 | 17.0 (6.3, 27.7) | 15.7 (7.2, 24.2) | 38.1 (26.1, 50.1) | 40.0 (27.1, 52.9) |
| Paternal BMI pre‐pregnancy | |||||
| <25 | 709 | 4.0 (2.2, 5.8) | 3.8 (2.3, 5.3) | 6.1 (4.2, 8.0) | 10.7 (8.1, 13.3) |
| ≥25 and <30 | 341 | 6.5 (3.4, 9.6) | 6.9 (4.0, 9.8) | 14.9 (10.8, 19.0) | 14.7 (10.4, 19.0) |
| ≥30 | 59 | 4.9 (0.0, 11.5) | 7.7 (0.5, 14.9) | 19.2 (8.5, 29.9) | 31 (17.0, 45.0) |
| Birth weight | |||||
| ≤2.5 kg | 97 | 4.4 (0.0, 9.2) | 5.1 (0.4, 9.8) | 8.8 (2.7, 14.9) | 12.6 (5.0, 20.2) |
| >2.5 and <4 kg | 1128 | 4.2 (2.8, 5.6) | 2.4 (1.4, 3.4) | 7.2 (5.6, 8.8) | 13.7 (11.4, 16.0) |
| ≥4 kg | 127 | 11.4 (4.8, 18.0) | 8.0 (3.0, 13.0) | 16.5 (9.5, 23.5) | 14.3 (7.1, 21.5) |
| Breastfeeding | |||||
| <3 months | 274 | 5.0 (1.8, 8.2) | 6.8 (3.6 10.0) | 14.4 (9.7, 18.9) | 19.7 (14.0, 25.4) |
| 3 to <6 months | 194 | 6.7 (2.5, 10.9) | 7.0 (3.2, 10.8) | 12.3 (7.2, 17.4) | 16.2 (10.0, 22.4 |
| 6 to <9 months | 172 | 5.4 (1.2, 9.6) | 7.1 (3.1, 11.1) | 9.1 (4.6, 13.6) | 14.1 (8.1, 20.1) |
| 9+ months | 475 | 5.3 (2.9, 7.7) | 3.9 (2.1, 5.7) | 7.2 (4.7, 9.7) | 9.2 (6.3, 12.1) |
| Offspring weight gain in first year | |||||
| <5 kg | 46 | 0 (0.0, 0.0) | 0 (0.0, 0.0) | 7.3 (0.0, 15.3) | 14.7 (2.8, 26.6) |
| ≥5 and <7 kg | 638 | 1.4 (0.3, 2.5) | 3.1 (1.7, 4.5) | 6.7 (4.6, 8.8) | 11.5 (8.6, 14.4) |
| ≥7 and <9 kg | 509 | 7.5 (4.8, 10.2) | 6.7 (4.4, 9.0) | 11.7 (8.7, 14.7) | 11.7 (8.5, 14.9) |
| ≥9 kg | 62 | 29.3 (15.4, 43.2) | 26.4 (14.5, 38.3) | 19.2 (8.5, 29.9) | 22.7 (10.3, 35.1) |
Because of the longitudinal nature of this pregnancy cohort not all children were involved in every assessment. Data for the prevalence of obesity with other early‐life factors such as maternal diabetes, anemia, prematurity and mode of delivery are presented in the Supporting Information, Table S1.
BMI = body mass index, CI = confidence interval.
Statistical analysis
Obesity or overweight prevalence was calculated as the proportion of affected children in each age group. Measurements are only included for the assessment age at which they were recorded. Prevalence was stratified by the factors outlined previously. There were insufficient mothers and fathers whose pre‐pregnancy BMI was classified as underweight to accurately determine the prevalence of obesity in their offspring, therefore, underweight and normal weight parents were grouped together. A sensitivity analysis indicated that this did not skew results. Simple logistic regression was used to compare the unadjusted risk of offspring obesity between maternal and paternal pre‐pregnancy BMI categories at ages 1, 3, 5, 14 and 22 years. Longitudinal BMI z‐scores were modelled separately for male and female participants using linear mixed effects, including fixed effects for a quartic polynomial function of age. Fixed effects were also included in the sex‐specific models based on backwards elimination for main effects and time‐dependent interactions for the previously mentioned early‐life factors. Random effects for intercept and slope and a compound symmetry correlation structure for the errors were included in the models, using the statistical package nlme in R 25, 26. Mean predicted BMI z‐scores, with 95% confidence intervals, were estimated using these models. A sensitivity analysis was performed including only those eligible individuals with two parents of European descent. A stringent significance level of 0.5% was used in the analyses; however, no adjustment was made for multiple testing as the associations between these factors and childhood obesity have been previously reported.
Results
The overall prevalence of obesity in this sample was 5.2% at 5 years of age, steadily increasing to 12.8% by age 22 (Table 1).The prevalence of overweight remained relatively constant at 11–16% from early childhood through to adulthood. Although the prevalence of obesity or overweight was higher in females compared with males at 22 years (and lower prior to that), the differences did not reach statistical significance.
Obesity prevalence at age 22 was higher in offspring of mothers who had no tertiary education, smoked during pregnancy, gained more than 10 kg in the first 18 weeks of pregnancy or had higher pre‐pregnancy BMIs. It was also higher in those whose fathers' were obese, those breast‐fed for a shorter duration and those with greater weight‐gain by age one. Birth‐weight greater than 4 kg appeared to increase the risk of obesity throughout childhood but the effect was not sustained into adulthood (Table 1). There was limited evidence that maternal diabetes, anemia or cesarean section delivery affected the prevalence of offspring obesity (Supporting Information, Table S1).
Of the factors mentioned previously, maternal and paternal BMI exerted the strongest influence on offspring BMI, particularly in adolescence and early adulthood. At ages 14, and 22, compared with offspring of parents who were not overweight, maternal overweight increased offspring obesity risk over fourfold, whilst maternal obesity resulted in a sevenfold to 10‐fold increase. Paternal overweight almost tripled obesity risk at age 14 but was not significant at age 22, whilst paternal obesity resulted in an almost fourfold increased risk of offspring obesity at both ages (Table 2). Maternal pre‐pregnancy BMI appeared to have a slightly greater magnitude of effect on the adult BMI of both male and female offspring compared with paternal BMI.
Table 2.
Age‐specific risk of offspring obesity compared with offspring from parents with pre‐pregnancy BMI z‐score < 85th percentile (<25 kg m−2)
| Maternal overweight | Paternal overweight | Maternal obesity | Paternal obesity | |
|---|---|---|---|---|
| Obese at year 1 | 1.06 (0.54, 1.88) | 1.76 (1.12, 2.74) | 1.44 (0.65, 2.85) | 1.87 (0.74, 4.11) |
| Odds ratio (95% CI) | P = 0.86 | P = 0.01 | P = 0.33 | P = 0.15 |
| P‐value | ||||
| Obese at year 3 | 3.36 (1.59, 6.75) | 1.67 (0.83, 3.31) | 5.58 (2.24, 12.66) | 1.23 (0.19, 4.44) |
| Odds ratio (95% CI) | P = 9 × 10−4 | P = 0.14 | P = 8 × 10−5 | P = 0.79 |
| P‐value | ||||
| Obese at year 5 | 2.08 (1.00, 4.04) | 1.88 (1.02, 3.44) | 4.27 (2.00, 8.51) | 2.11 (0.60, 5.73) |
| Odds ratio (95% CI) | P = 0.04 | P = 0.04 | P =7x10‐5 | P = 0.18 |
| P‐value | ||||
| Obese at year 14 | 4.61 (2.76, 7.56) | 2.70 (1.70, 4.31) | 9.72 (5.42, 17.25) | 3.66 (1.63, 7.64) |
| Odds ratio (95% CI) | P = 2 × 10−9 | P = 3 × 10−5 | P = 1 × 10−14 | P = 9 × 10−4 |
| P‐value | ||||
| Obese at year 22 | 4.58 (2.87, 7.24) | 1.44 (0.92, 2.23) | 6.96 (3.81, 12.52) | 3.75 (1.80, 7.50) |
| Odds ratio (95% CI) | P = 1 × 10−10 | P = 0.11 | P = 1 × 10−10 | P = 3 × 10−4 |
| P‐value |
Analyses not adjusted for covariates. BMI = body mass index, CI = confidence interval.
To further explore longitudinal BMI profiles of offspring who were obese, we reviewed the subgroups of individuals who were obese at ages 1, 3, 5, 10, 14 and 22 (Table 3). For comparison, children with BMI <85th centile at ages one and five were also reviewed: 43.5% (95% CI 33.0–54.0%) of 1‐year olds who were obese and 77.6% (95% CI 65.9–89.3%) of 5‐year olds who were obese, became overweight or obese in adulthood. In contrast, 18.8% (95% CI 16.0–21.6%) of subjects with BMI <85th centile at five were overweight or obese at 22. Amongst 22‐year olds who were obese, 36.0% (95% CI 27.2–44.8%) were already overweight or obese at age one, increasing to 53.5% (95% CI 44.3–62.7%) at 5 years.
Table 3.
Percentage obese or overweight over childhood based on age‐specific BMI subgroups
| Age‐specific BMI subgroup | Percent overweight or obese (BMI >85th centile) at | ||||
|---|---|---|---|---|---|
| 1 year | 5 years | 10 years | 14 years | 22 years | |
| % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | |
| Obese at 1 year: n = 114 | 100 | 60 (50–70) | 47 (37–56) | 41 (31–51) | 44 (33–54) |
| Non‐overweight at 1 year: n = 929 | 0 | 10 8, 9, 10, 11, 12 | 21 18, 19, 20, 21, 22, 23, 24 | 20 17, 18, 19, 20, 21, 22, 23 | 24 21, 22, 23, 24, 25, 26, 27 |
| Obese at 3 years: n = 46 | 82 (70–93) | 86 (76–96) | 70 (56–84) | 65 (50–80) | 68 (52–83) |
| Obese at 5 years: n = 62 | 55 (42–67) | 100 | 92 (84–99) | 82 (71–92) | 78 (66–89) |
| Non‐overweight at 5 years: n = 978 | 19 16, 17, 18, 19, 20, 21 | 0 | 14 12, 13, 14, 15, 16 | 14 12, 13, 14, 15, 16, 17 | 19 16, 17, 18, 19, 20, 21, 22 |
| Obese at 10 years: n = 113 | 46 (37–56) | 80 (72–88) | 100 | 93 (88–98) | 83 (75–91) |
| Obese at 14 years: n = 108 | 44 (34–54) | 68 (59–77) | 96 (92–100) | 100 | 89 (82–96) |
| Obese at 22 years: n = 130 | 36 (27–45) | 54 (44–63) | 77 (69–84) | 82 (75–90) | 100 |
BMI = body mass index, CI = confidence interval.
Sex‐specific multivariate analyses were performed on offspring BMI z‐scores (see Figure 1 for female trajectories and Figure 2 for male trajectories and Table S2 for regression results). After adjustment, maternal and paternal pre‐pregnancy BMIs were the only factors to have increasing strength of association with offspring BMI over time (Figures 1a,b and 2a,b). By adulthood both maternal and – to a lesser degree – paternal BMI strongly influenced offspring BMI. In contrast, birth‐weight and first‐year weight gain both had relatively strong associations with BMI in early childhood, which diminished with age (Figures 1c,d and 2c,d). Duration of exclusive breastfeeding did not maintain significance after adjustment for confounders. Maternal smoking during pregnancy demonstrated a constant association throughout childhood, increasing BMI z‐score by an average 0.25 SD and 0.19 SD at all ages for female and male participants, respectively (Figures 1e, 2e, and Table S2). Excess gestational weight gain at 18 weeks, but not at 34 weeks, was associated with increased BMI in females (Figure 1f), whereas no associations were detected for males. Socio‐economic status markers in the form of postcode, income (collected as a categorical variable with large band width) and maternal education level were not associated with offspring BMI after adjustment for confounders. Sensitivity analyses performed on 1125 individuals, excluding the 17% of individuals who had one or both parents of non‐European descent, produced similar results, and no evidence was detected of strong co‐linearity between the regression estimates for these early‐life factors.
Figure 1.
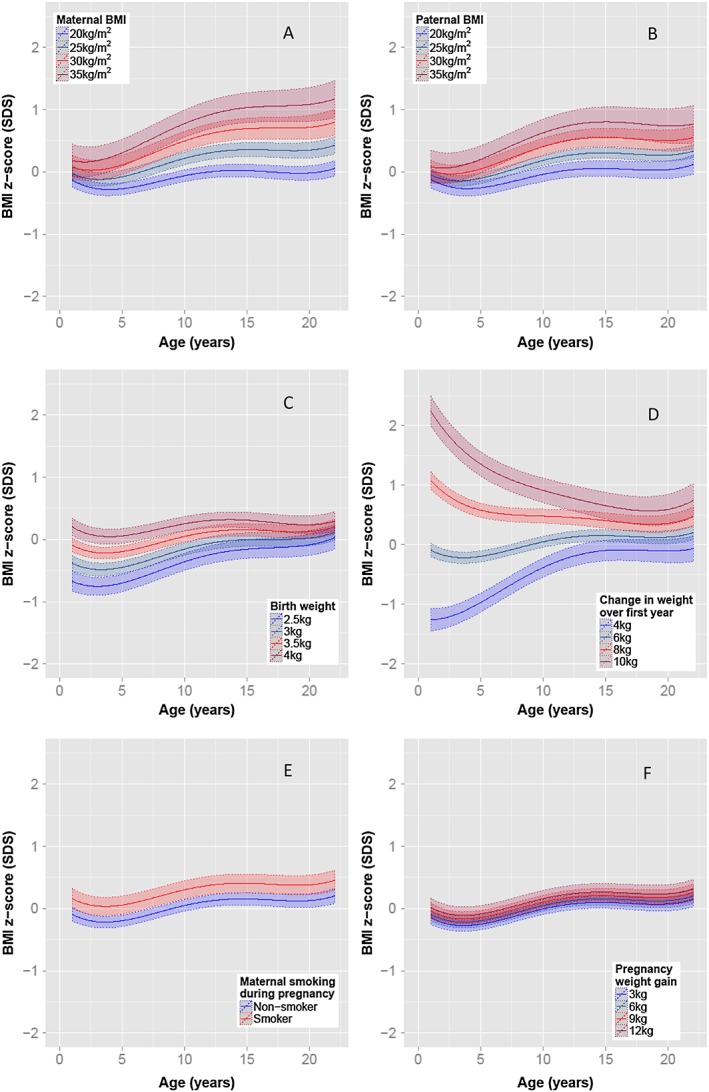
Adjusted female BMI z‐score trajectories based on early‐life risk factors. Unless otherwise stated, average female BMI trajectories with 95% confidence intervals based on offspring with a 3.5 kg birth weight and 6 kg weight gain in the first year of life, parental BMI of 22 kg m−2 and maternal characteristics of non‐smoker with a gestational weight gain of 6 kg by 18 weeks.
Figure 2.
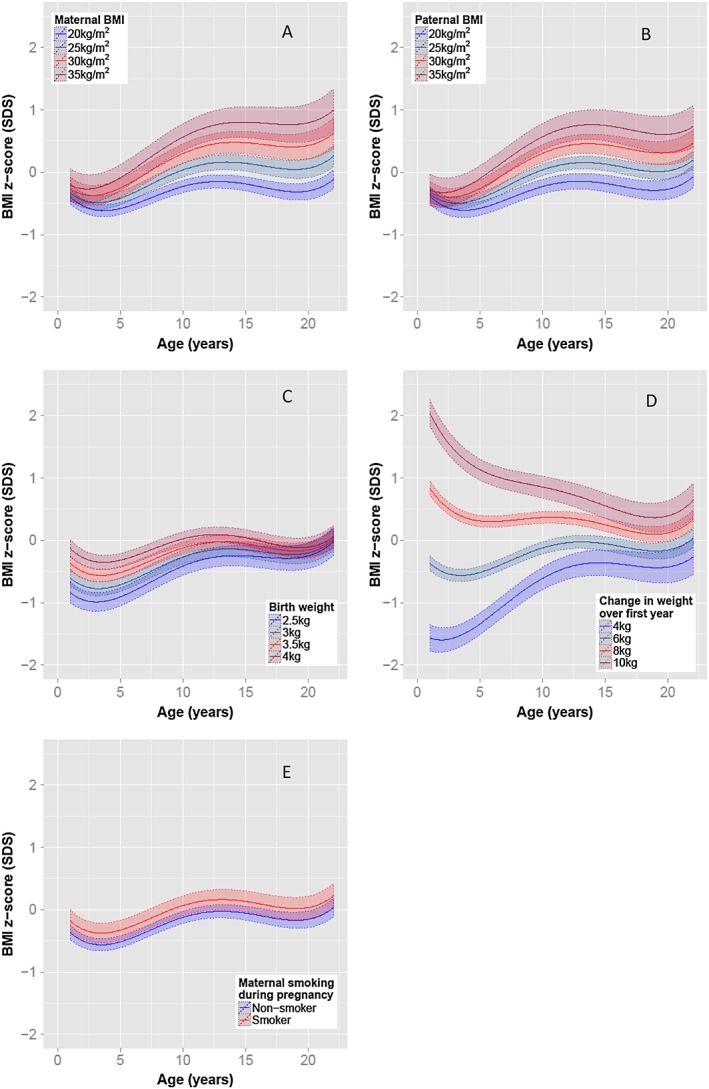
Adjusted male BMI z‐score trajectories based on early‐life risk factors. Unless otherwise stated, average male BMI trajectories with 95% confidence intervals based on offspring with a 3.5 kg birth weight and 6 kg weight gain in the first year of life, parental BMI of 22 kg m−2 and maternal characteristics of a non‐smoker
The combined impact of parental BMI and first‐year weight‐gain is further elucidated in Figure 3: Offspring of parents with a BMI of 25 kg m−2 who gained 8 kg in the first year of life (i.e. 2 kg greater than required to maintain a 3.5 kg infant on the 50th centile), initially had a higher average BMI than offspring who gain 6 kg, irrespective of their maternal and paternal pre‐pregnancy BMI. The difference in the trajectories that resulted in adult obesity compared with healthy adult weight was the speed at which BMI increased following the early childhood nadir (indicated by rectangles on Figure 3). The greatest acceleration in this period was observed for the offspring of parents who were obese pre‐pregnancy, irrespective of healthy or excessive weight gain in the first year of life.
Figure 3.
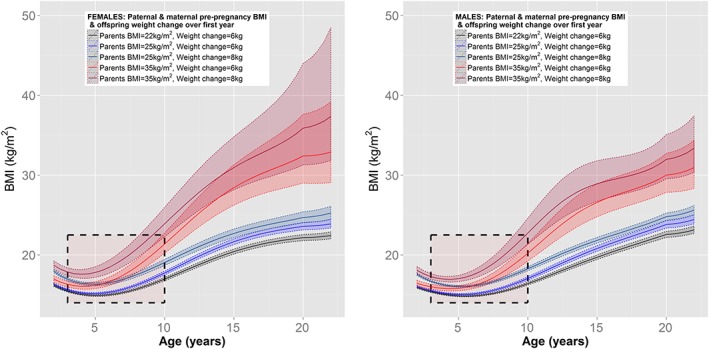
Adjusted sex‐specific BMI mean trajectories based on early‐life risk factors. Boxes indicate the period of acceleration in BMI following the early‐childhood nadir. Unless stated otherwise, average BMI trajectories with 95% confidence intervals based on offspring with 3.5 kg birth weight, maternal characteristics of non‐smoker and for females only, gestational weight gain of 6 kg by 18 weeks.
Discussion
Parental – particularly maternal – pre‐pregnancy BMI increases the risk of childhood obesity 8, 11. Retrospective data from a cohort born between 1965 and 1971 27 demonstrated that parental obesity increased the risk of adulthood obesity for children under 10 years; however, they did not find an association beyond that age. Our longitudinal analyses of prospective data from a cohort born almost 20 years later (1989–1991) revealed that maternal obesity and paternal obesity were the most powerful early‐life risk factor for adult obesity. Multivariate analyses showed that greater first‐year weight‐gain and maternal smoking in pregnancy also contributed to increasing adulthood BMI, but the impact of maternal overweight and obesity was profound, even after adjustment for potential confounding factors. These findings are expected to be generalizable beyond the West Australian population because the Raine cohort has childhood BMI trajectories comparable to the contemporary UK‐based Avon Longitudinal Study of Parents and Children cohort 28 and other contemporary Australian cohorts 29. BMI is highly correlated with both total body fat and fat mass index in the Raine cohort at age 20 (refer to Supporting Information). Although we did not observe a sex difference in obesity rate at ages 20 or 22, a previous study identified a sex difference in body composition at 20 years in 1183 participants of the Raine Study. Male participants had significantly higher lean mass and lean mass index, but significantly lower fat mass and fat mass index compared with female participants30. It is therefore likely that obesity is more prevalent amongst young adult female participants than male participants.
Genetic predisposition to obesity may contribute to offspring BMI but its expression is likely affected by environmental and epigenetic influences 8. Our results implicate maternally mediated causative factors which may include the intra‐uterine environment and its effect on offspring epigenetic programming as well as post‐natal exposures, given that mothers who are overweight and obese are likely to provide offspring with an obesogenic environment 8, 9.
Animal studies demonstrate that maternal over‐nutrition prior to conception and during gestation, increases adiposity of mature offspring exposed to a 12‐week feeding challenge 31. Also, early fetal and post‐natal nutrition levels correlate with susceptibility to obesity 32. These findings accord with the Barker hypothesis that intra‐uterine and early‐life adverse exposures program epigenetic modifications in offspring, resulting in life‐long increased morbidity 33, highlighting the importance of pre‐pregnancy BMI.
Rapid first‐year weight‐gain and both maternal and paternal BMI were associated with increased offspring BMI (Figure 3), but the impact of first‐year weight‐gain – in early childhood a stronger determinant of offspring BMI than parental weight – diminished over childhood, whilst the impact of parental BMI increased over time. Pre‐pregnancy obesity rates in Australia rose from 6% in 1990 (Raine) to 17% in 2006 34, whilst maternal obesity rates in the USA increased from 13% in 1993–1994 to 22% in 2002–2003 35. Given that the 6% of infants born to mothers who are obese in the Raine cohort comprised 17.3% of the obese adult cohort, rising pre‐pregnancy maternal weight may contribute disproportionately to increasing adult obesity rates.
Pre‐pregnancy education programmes and interventions aimed at reducing parental and particularly maternal BMI may help reduce adult offspring obesity rates. This is supported by the finding that maternal bariatric surgery with sustained weight reduction, lowered obesity prevalence and improved cardiometabolic indices in offspring born subsequent to surgery 36. In addition, rapid weight gain in the first year of life is an independent risk factor for childhood obesity 4, 37 and has been shown to have both environmental and genetic components 38. Monitoring first‐year weight‐gain to identify that those crossing multiple centile lines would further assist in determining families likely to benefit from early intervention.
The trajectory to adulthood overweight and obesity has been described as commencing by age five 7. Our data supports this, as demonstrated in Table 3. Over a third of 22‐year olds with obesity had a BMI >85th centile by year one, increasing to 54% by 5 years of age. This effect may be due in part to environmental factors during early childhood, including first‐year weight gain, but maternal factors are also at play prior to conception and during pregnancy. Table 3 illustrates the weight trajectory of age‐specific subgroups of children. For children with obesity, the older the subgroup the greater the risk of overweight and obesity in adulthood. An increase in risk of overweight is apparent over the first 5 years: 5‐year olds who were obese were at over three times the risk of overweight in adulthood compared with 5‐year olds who were not overweight, whilst 1‐year olds who were obese were less than double the risk compared with 1‐year olds who were not overweight. Consistent with other studies 39, 40, this indicates that the early years comprise an important window for intervention.
Excessive gestational weight gain was identified as a risk factor for childhood obesity and, in a large Australian cohort, for early adulthood obesity 41, even when adjusted for pre‐pregnancy maternal BMI and other potential confounders. On the other hand, as with our cohort, pre‐pregnancy maternal BMI confounded the effect of gestational weight gain in the Generation R Cohort 42, casting uncertainty on the utility of targeting the latter as a means to reduce adult offspring obesity.
Birth weight has been reported to play an important role in BMI at age 14 with a disproportionate representation of high birth weight babies in the cohort who were obese at that age 7. Although our data support this finding, the prevalence of obesity in the high birth weight group declined substantially with age, and by 22 it was no longer a clinically significant contributor to obesity risk.
The size of this cohort may limit the identification of risk factors with small effect sizes or those associated with less common exposures such as maternal diabetes. For example, the cohort is primarily of European descent, potentially reducing inter‐ethnic generalizability. Socio‐economic status measures collected prospectively were not sufficiently sensitive to detect an association. In addition, data relating specifically to the prevalence of gestational diabetes and alcohol consumption during pregnancy were not available. Larger, specialized cohorts are required to further explore the impact of these variables. Further studies are required to determine the utility of targeted family based early‐life interventions. Parental lifestyle choices may be one of the few modifiable variables that could be effectively targeted pre‐conception and in early life to reduce the prevalence of adulthood obesity in offspring. Our analysis of BMI trajectories suggests that the first 5 years of life may also provide an important window for intervention. Future studies analysing early‐life biological specimens collected prospectively in the Raine study and similar cohorts, such as placental tissue, sera and stool sample, may shed more light on the role of the intra‐uterine environment and the establishment of the gut microbiome in determining adulthood BMI.
Conclusions
This rigorous multivariate analysis identified maternal and paternal pre‐pregnancy BMI as strong determinants of adult offspring BMI, with weight‐gain in the first year of life and smoking during pregnancy also contributing. Interventions aimed at reducing adult obesity rates could also target at‐risk parents prior to conception and families of children who gain excess weight early in life.
Funding
This study was supported by Telethon New Children's Hospital Research Fund and Princess Margaret Hospital Foundation. The funders had no role in the study design, data collection, analysis, interpretation of the data, writing of the report or decision to submit the paper for publication.
Disclosure
The authors have nothing to disclose.
Author contributions
SRR, JAM, JPN, KZ, HCA, JM, WHO, PDS, CEP and CSC contributed to data acquisition and/or management. JAM performed the statistical analyses and prepared the tables and figures. SRR and JAM wrote the manuscript and prepared the figures and tables. JAM, CSC and CEP had access to the full raw dataset. CSC oversaw all aspects of this report and edited the manuscript. All co‐authors contributed to interpretation of the findings, critical revision of the manuscript and approved of its final content.
Supporting information
Supporting info item
Acknowledgements
This work is supported by Telethon New Childrens' Hospital Research Fund (to CSC), and a fellowship from the Princess Margaret Hospital Foundation (SRR). We acknowledge the Raine Study participants and their families, the Raine Study Team for cohort co‐ordination and data collection and the NH&MRC for their long‐term contribution to funding the study over the last 20 years. We acknowledged the following for providing funding for Core Management of the Raine Study: The University of Western Australia (UWA), The Telethon Kids Institute, Raine Medical Research Foundation, UWA Faculty of Medicine, Dentistry and Health Sciences, Women's and Infant's Research Foundation, Curtin University and Edith Cowan University. We thank Gillian Northcott from Medical illustrations at Princess Margaret Hospital for the assistance with digital artwork.
Rath, S. R. , Marsh, J. A. , Newnham, J. P. , Zhu, K. , Atkinson, H. C. , Mountain, J. , Oddy, W. H. , Hughes, I. P. , Harris, M. , Leong, G. M. , Cotterill, A. M. , Sly, P. D. , Pennell, C. E. , and Choong, C. S. (2016) Parental pre‐pregnancy BMI is a dominant early‐life risk factor influencing BMI of offspring in adulthood. Obesity Science & Practice, 2: 48–57. doi: 10.1002/osp4.28.
References
- 1. Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011; 378: 815–825. [DOI] [PubMed] [Google Scholar]
- 2. Serdula MK, Ivery D, Coates RJ, et al Do obese children become obese adults? A review of the literature. Prev Med 1993; 22: 167–177. [DOI] [PubMed] [Google Scholar]
- 3. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief, no 82. Hyattsville, MD: National Center for Health Statistics; 2012. [PubMed] [Google Scholar]
- 4. Lakshman R, Elks CE, Ong KK. Childhood obesity. Circulation 2012; 126: 1770–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 2012; 307: 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr 2010; 92: 1257–1264. [DOI] [PubMed] [Google Scholar]
- 7. Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med 2014; 370: 1660–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lawlor DA, Relton C, Sattar N, Nelson SM. Maternal adiposity‐‐a determinant of perinatal and offspring outcomes? Nat Rev Endocrinol 2012; 8: 679–688. [DOI] [PubMed] [Google Scholar]
- 9. Ojha S, Fainberg HP, Sebert S, Budge H, Symonds ME. Maternal health and eating habits: metabolic consequences and impact on child health. Trends Mol Med 2015; 9: 126–133. [DOI] [PubMed] [Google Scholar]
- 10. Timmermans SH, Mommers M, Gubbels JS, et al. Maternal smoking during pregnancy and childhood overweight and fat distribution: the KOALA Birth Cohort Study. Pediatr Obes 2014; 9: e14–25. [DOI] [PubMed] [Google Scholar]
- 11. Bammann K, Peplies J, De Henauw S, et al. Early life course risk factors for childhood obesity: the IDEFICS case‐control study. PLoS One 2014; 9: e86914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chivers P, Hands B, Parker H, et al. Body mass index, adiposity rebound and early feeding in a longitudinal cohort (Raine Study). Int J Obes (Lond) 2010; 34: 1169–1176. [DOI] [PubMed] [Google Scholar]
- 13. Martin RM, Patel R, Kramer MS, et al. .Effects of promoting longer‐term and exclusive breastfeeding on adiposity and insulin‐like growth factor‐I at age 11.5 years: a randomized trial. JAMA 2013; 309: 1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Power C, Jefferis BJ. Fetal environment and subsequent obesity: a study of maternal smoking. Int J Epidemiol 2002; 31: 413–419. [PubMed] [Google Scholar]
- 15. Yu ZB, Han SP, Zhu GZ, et al. Birth weight and subsequent risk of obesity: a systematic review and meta‐analysis. Obesity Reviews 2011; 12: 525–542. [DOI] [PubMed] [Google Scholar]
- 16. Stettler N, Zemel BS, Kumanyika S, Stallings VA. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics 2002; 109: 194–199. [DOI] [PubMed] [Google Scholar]
- 17. Cole TJ. Children grow and horses race: is the adiposity rebound a critical period for later obesity? BMC Pediatr 2004; 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Newnham JP, Evans SF, Michael CA, Stanley FJ, Landau LI. Effects of frequent ultrasound during pregnancy: a randomised controlled trial. Lancet 1993; 342: 887–891. [DOI] [PubMed] [Google Scholar]
- 19. Williams LA, Evans SF, Newnham JP. Prospective cohort study of factors influencing the relative weights of the placenta and the newborn infant. BMJ 1997; 314: 1864–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Evans S, Newnham J, MacDonald W, Hall C. Characterisation of the possible effect on birthweight following frequent prenatal ultrasound examinations. Early Hum Dev 1996; 45: 203–214. [DOI] [PubMed] [Google Scholar]
- 21. Beales DJ, Smith AJ, O'Sullivan PB, Straker LM. Low back pain and comorbidity clusters at 17 years of age: a cross‐sectional examination of health‐related quality of life and specific low back pain impacts. J Adolesc Health 2012; 50: 509–516. [DOI] [PubMed] [Google Scholar]
- 22. Straker LM, Hall GL, Mountain J, et al. Rationale, design and methods for the 22 year follow‐up of the Western Australian Pregnancy Cohort (Raine) Study. BMC Public Health 2015; 15: 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Flegal KM, Cole TJ. Construction of LMS parameters for the Centers for Disease Control and Prevention 2000 growth charts. National health statistics reports no 63. Hyattsville, MD: National Center for Health Statistics; 2013. [PubMed] [Google Scholar]
- 24. Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007; 120: S164–192. [DOI] [PubMed] [Google Scholar]
- 25. Pinheiro J, Bates D, DebRoy S, Sarkar D, Team. RDC . nlme: linear and nonlinear mixed effects models. R package version 31‐111 2013.
- 26. R Core Team. R: a language and environment for statistical computing . R Foundation for Statistical Computing, 2013.
- 27. Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 1997; 337: 869–873. [DOI] [PubMed] [Google Scholar]
- 28. Warrington NM, Howe LD, Wu YY, et al. Association of a body mass index genetic risk score with growth throughout childhood and adolescence. PLoS One 2013; 8: e79547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hughes I, Harris M, Cotterill A , et al. Comparison of Centers for Disease Control and Prevention and World Health Organization references/standards for height in contemporary Australian children: analyses of the Raine Study and Australian National Children's Nutrition and Physical Activity cohorts. J Paediatr Child Health 2014; 50: 895–901. [DOI] [PubMed] [Google Scholar]
- 30. Zhu K, Briffa K, Smith A, et al. Gender differences in the relationships between lean body mass, fat mass and peak bone mass in young adults. Osteoporos Int 2014; 25: 1563–1570. [DOI] [PubMed] [Google Scholar]
- 31. Long NM, George LA, Uthlaut AB, et al. Maternal obesity and increased nutrient intake before and during gestation in the ewe results in altered growth, adiposity, and glucose tolerance in adult offspring. J Anim Sci 2010; 88: 3546–3553. [DOI] [PubMed] [Google Scholar]
- 32. Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev 2014; 94: 1027–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gillman MW. Developmental origins of health and disease. N Engl J Med 2005; 353: 1848–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Callaway LK, Prins JB, Chang AM, McIntyre HD. The prevalence and impact of overweight and obesity in an Australian obstetric population. Med J Aust 2006; 184: 56–59. [DOI] [PubMed] [Google Scholar]
- 35. Leddy MA, Power ML, Schulkin J. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol 2008; 1: 170–178. [PMC free article] [PubMed] [Google Scholar]
- 36. Smith J, Cianflone K, Biron S, Hould FS, Lebel S, Marceau S , et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab 2009; 94: 4275–4283. [DOI] [PubMed] [Google Scholar]
- 37. Druet C, Stettler N, Sharp S, et al. Prediction of childhood obesity by infancy weight gain: an individual‐level meta‐analysis. Paediatr Perinat Epidemiol 2012; 26: 19–26. [DOI] [PubMed] [Google Scholar]
- 38. Elks CE, Heude B, de Zegher F, et al. Associations between genetic obesity susceptibility and early postnatal fat and lean mass: an individual participant meta‐analysis. JAMA Pediatr 2014; 168: 1122–1130. [DOI] [PubMed] [Google Scholar]
- 39. Lobstein T, Jackson‐Leach R, Moodie ML, et al. Child and adolescent obesity: part of a bigger picture. Lancet 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hawkes C, Smith TG, Jewell J, Wardle J, Friel S, RA Hammond, Smart food policies for obesity prevention. Lancet 2015. [DOI] [PubMed] [Google Scholar]
- 41. Mamun AA, O'Callaghan M, Callaway L, et al Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation 2009; 119: 1720–1727. [DOI] [PubMed] [Google Scholar]
- 42. Gaillard R, Durmus B, Hofman A, Mackenbach et al Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity (Silver Spring) 2013; 21: 1046–1055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


