Abstract
The safety and efficacy of LY2963016 insulin glargine (LY IGlar) and Lantus® insulin glargine (IGlar), products with identical primary amino acid sequences, were assessed in subgroups of patients with type 1 (T1D, n = 452) or type 2 diabetes (T2D, n = 299) reporting prestudy IGlar treatment in 52‐week open‐label (ELEMENT‐1) and 24‐week double‐blind (ELEMENT‐2) studies. At randomization, patients transitioned from their prestudy IGlar to equivalent doses of LY IGlar or IGlar. Primary efficacy (change in glycated haemoglobin from baseline to 24 weeks), other efficacy and select safety outcomes of LY IGlar were compared with those of IGlar. Continuous data were analysed using analysis of covariance, categorical data by Fisher's exact test, and treatment comparisons for hypoglycaemia by Wilcoxon test. No statistically significant treatment differences were identified for efficacy and safety outcomes except for weight change (T1D), overall incidence of detectable insulin antibodies (T2D), and serious adverse events (T2D). These differences were neither consistently observed across both studies nor observed in the total study populations, and their magnitude suggests they were not clinically meaningful. LY IGlar and IGlar show similar efficacy and safety profiles in patients reporting prestudy IGlar treatment.
Keywords: biosimilar insulin, insulin glargine, LY2963016 insulin glargine
Introduction
Lantus® [insulin glargine (rDNA origin); Sanofi‐Aventis, Paris, France; IGlar), a protein product manufactured using recombinant DNA technology, was the first long‐acting basal insulin analogue approved for the treatment of type 1 (T1D) and type 2 diabetes (T2D) 1, 2. LY2963016 IGlar (LY IGlar), the first biosimilar insulin to receive marketing authorization in the European Union 3 and Japan 4, has an identical primary amino acid sequence to that of IGlar. Biosimilars, which are complex molecules produced through different manufacturing processes, cannot be considered identical to their reference products 5. Non‐clinical, phase I and phase III studies (ELEMENT‐1 and ELEMENT‐2) demonstrated LY IGlar and IGlar are highly similar 6, 7, 8, 9.
In ELEMENT‐1, patients with T1D reported prestudy IGlar (prior IGlar) or other basal insulin use 8. In ELEMENT‐2, patients with T2D were either insulin naïve or had prior IGlar use 9. Results for both full analysis set (FAS) populations 8, 9 and prior IGlar and insulin‐naïve subgroups (T2D) were previously reported 9; however, this paper examines whether any treatment differences exist in clinical outcomes between LY IGlar and IGlar in the prior IGlar subgroups. Such data may be relevant in clinical practice.
Materials and Methods
Methods for both studies, carried out according to ethical principles described in the International Conference on Harmonisation on Good Clinical Practices and Declaration of Helsinki 10, have been reported 8, 9. At randomization, patients received an LY IGlar or IGlar dose that was equivalent to their prestudy IGlar dose. Prefilled pen devices 9 or covered vials and syringes 9 were used to deliver study drug. Both studies used treat‐to‐target approaches to achieve protocol‐specified glycaemic goals 8, 9.
In each study, separate analyses compared LY IGlar to IGlar for the prior IGlar subgroup. Prespecified analyses included change from baseline in glycated haemoglobin (HbA1c) at 24 weeks (primary efficacy outcome), change in body weight, hypoglycaemia (total, severe and nocturnal), serious adverse events (SAEs), treatment‐emergent adverse events (TEAEs), adverse events (AEs) leading to discontinuation, allergic events, injection site reactions and insulin antibodies as a categorical outcome [treatment‐emergent antibody response (TEAR)] 11. Post hoc analyses included proportion of patients achieving HbA1c targets, basal insulin dose, prandial insulin dose (ELEMENT‐1), percent weight change from baseline (ELEMENT‐1), fasting plasma glucose (FPG), prior IGlar insulin antibody levels and documented symptomatic hypoglycaemia.
Continuous data (HbA1c change, weight change) were analysed using an analysis of covariance model with treatment, country, time of basal insulin injection, sulphonylurea use (ELEMENT‐2 only) as fixed effects and baseline value of response variable as a covariate, and the subgroup (prior IGlar at study entry: yes, no), and subgroup‐by‐treatment interaction. Treatment comparisons were made within the subgroup of patients who reported prestudy treatment with IGlar. Treatment comparisons for insulin antibody levels and hypoglycaemia rates were carried out using the Wilcoxon test. Hypoglycaemia incidence and the proportion of patients achieving HbA1c targets were analysed using the Mantel‐Haenszel test. Categorical data (detectable antibodies, TEAR and AEs) were analysed using Fisher's exact test.
The study was registered at ClinicalTrials.gov with trial numbers NCT01421147 and NCT01421459.
Results
Of the 535 patients in the FAS with T1D, 452 (84.5%) reported prestudy IGlar treatment. Of the 756 patients in the FAS with T2D, 299 (39.6%) reported prestudy IGlar treatment. In both studies, baseline characteristics were generally similar between both groups and consistent with the FAS, except for race in patients with T2D. As expected, patients with T2D who reported prior IGlar had slightly lower baseline HbA1c and FPG than the FAS (Table S1).
In patients with T1D, no statistically significant treatment differences were observed for the primary efficacy measure, change in HbA1c from baseline to the 24‐week endpoint [last observation carried forward (LOCF)], and 52‐week endpoint (LOCF; Figure 1). No statistically significant treatment differences were observed for the proportions of patients achieving HbA1c targets at 52 weeks (LOCF; Figure S1). Increases in basal and prandial insulin doses (U/kg/day) from baseline to the 52‐week endpoint (LOCF) were similar for both treatments (Figure 1). Daily mean blood glucose and FPG at 52 weeks were similar between the two groups (Table S2). A small, statistically significant treatment difference was observed for weight change, where LY IGlar‐treated patients gained more weight (Figure S1) with minimal least squares mean percent change from baseline (<2%) [LY IGlar: 1.81 ± 0.42; IGlar: 0.41 ± 0.39; p = 0.035].
Figure 1.
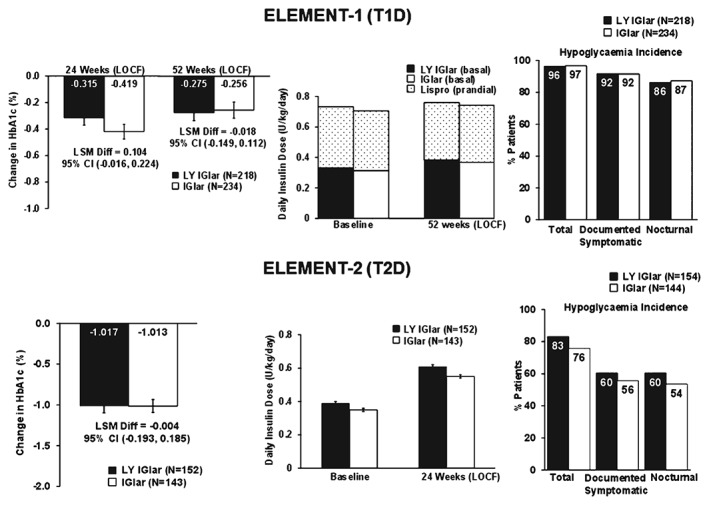
Efficacy and safety outcomes in the prior IGlar subgroup of patients with type 1 diabetes (T1D) and type 2 diabetes (T2D). All p values >0.05. CI, confidence interval; IGlar, insulin glargine; LOCF, last observation carried forward (endpoint); LSM Diff, least‐squares mean difference; LY IGlar, LY2963016 insulin glargine.
In patients with T2D, no significant treatment differences were observed for change in HbA1c from baseline to endpoint (LOCF; Figure 1), the proportion of patients achieving glycaemic targets (Figure S1), mean FPG and daily mean blood glucose (Table S2), basal insulin dose (Figure 1) and weight change (Figure S1).
In patients with T1D, the overall incidence of total, documented symptomatic, nocturnal (Figure 1) and severe hypoglycaemia (LY IGlar: 5%; IGlar: 4%, p = 0.816) was similar for both groups throughout the 52‐week study. Likewise, overall 1‐year adjusted hypoglycaemia rates were similar (Figure S1). Severe hypoglycaemia rates were not statistically compared because of the low number of events in each treatment arm.
In patients with T2D, the incidence of total, documented symptomatic and nocturnal hypoglycaemia was similar for both groups during the 24‐week study (Figure 1). No patients reported severe hypoglycaemia. The 1‐year adjusted hypoglycaemia rates were similar in the two groups (Figure S1).
No significant treatment differences were observed in the proportion of patients with T1D with detectable antibodies at the 52‐week endpoint and overall (Figure 2A). Median insulin antibody levels were also similar and low (<5%) for both groups at 52 weeks (LOCF; Figure 2B) 12. Likewise, the incidence of TEAR was similar at the endpoint [LOCF; LY IGlar: 15 patients (7%), IGlar: nine patients (4%); p = 0.148) and overall [LY IGlar: 21 patients (10%), IGlar: 21 patients (9%); p = 0.798].
Figure 2.
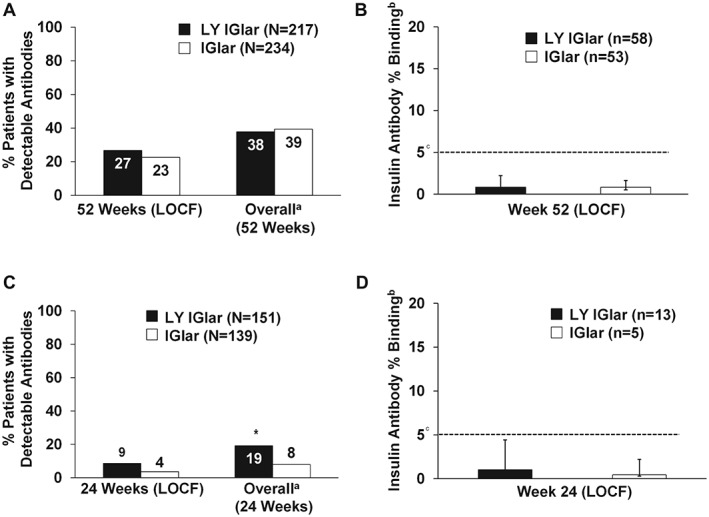
(A) Proportion of patients with detectable antibodies in the prior IGlar subgroup of patients with type 1 diabetes (T1D). All p values >0.05. Only patients with detected or non‐detected insulin antibody levels at baseline and post‐baseline were included in the analysis. (B) Level of insulin antibodies (percent binding) in patients with detectable antibodies in the prior IGlar subgroup of patients with T1D. aMeasurements taken at any time/visit postbaseline. bMedian + interquartile range. cFive percent binding level in the screening assay approximately equates to 250 ng/ml (250–500 ng/ml US Food and Drug Administration (FDA)‐recommended screening assay sensitivity correlated with clinical events) 12. Insulin antibody values depicted in the graph are from determinations following screening 11. All p values >0.05. (C) Proportion of patients with detectable antibodies in the prior IGlar subgroup of patients with type 2 diabetes (T2D). *p = 0.01; all other p values >0.05. Only patients with detected or non‐detected insulin antibody levels at baseline and post‐baseline were included in the analysis. (D) Level of insulin antibodies (percent binding) in patients with detectable antibodies in the prior IGlar subgroup of patients with T2D. aMeasurements taken at any time/visit post‐baseline. bMedian + interquartile range. cFive percent binding level in the screening assay approximately equates to 250 ng/ml (250–500 ng/ml FDA‐recommended screening assay sensitivity correlated with clinical events) 12. Insulin antibody values depicted in the graph are from determinations following screening 11. All p values >0.05. IGlar, insulin glargine; LOCF, last observation carried forward (endpoint); LY IGlar, LY2963016 insulin glargine.
The overall proportion of detectable antibodies in LY IGlar‐treated patients with T2D was statistically significantly higher than IGlar‐treated patients (Figure 2C), which may be the result of more patients with detectable antibodies being randomly assigned to the LY IGlar group at baseline 11; however, the incidence of patients with detectable antibodies was similar at the 24‐week endpoint 11. Median insulin antibody levels were low (<5% binding) and similar between the two groups at the 24‐week‐endpoint (LOCF; Figure 2D) 12. Endpoint and overall incidence of TEAR were similar 11.
The incidence of AEs and SAEs, injection site reactions and allergic events was similar for both groups in both studies (Table S3); however, significantly fewer LY IGlar‐treated patients with T2D experienced ≥1 SAE than IGlar‐treated patients. The incidence of SAEs in both treatment groups was <1% for all preferred terms with no significant treatment differences.
Discussion
The results of the present study were intended to address questions about clinical outcomes when LY IGlar is compared with IGlar in the prior IGlar subgroup of patients with T1D or T2D. No treatment differences were observed for the primary and other clinical outcomes, except as noted.
A significant treatment difference in weight change was noted for patients with T1D. The weight gain for LY IGlar was somewhat higher in the prior IGlar subgroup than in the FAS 9. This trend is reversed for IGlar in the FAS 8. The weight gain with LY IGlar falls within reported weight changes with insulin glargine 13, 14. Moreover, a widespread weight change was observed in both groups (LY IGlar: median = 0.85, range −7.10 to 15.20; IGlar: median = 0.10, range −14.20 to 18.40). This treatment difference was not observed in either the FAS [8, 9] or in the prior IGlar subgroup of patients with T2D 9, suggesting this observed difference is probably a chance finding.
The statistically significant difference in overall antibody incidence of LY IGlar‐treated patients with T2D could be explained by an imbalance in baseline antibody levels. The difference was not observed at the 24‐week endpoint (LOCF) 11. The incidence of detectable antibodies was notably lower at endpoint than for overall, which included patients who were positive for antibodies at any time during the study but may have had subsequent non‐detectable antibody levels. Furthermore, both groups had similar median insulin antibody levels and similar proportions of patients with TEAR. No differences were observed in the prior IGlar subgroup of patients with T1D or in the FAS for both studies [8, 9].
Finally, data from both FAS populations of patients show no evidence that these antibodies had any impact on efficacy and safety outcomes 11. Although significantly fewer LY IGlar‐treated patients with T2D experienced ≥1 SAE, there were no significant differences in preferred terms (Table S4).
One limitation of the present results is that neither study was designed to prospectively compare LY IGlar and IGlar in patients with diabetes reporting prestudy IGlar treatment and the results should not be considered sufficient to support interchangeability. The decision to transition patients from one type of insulin to another should be made by the prescribing healthcare professional and according to existing product labels 1, 6 and country‐specific guidelines 15.
In conclusion, our results show that patients reporting prestudy IGlar treatment who were randomized to LY IGlar have similar efficacy and safety outcomes to those of patients receiving IGlar. The majority of our findings were consistent with those of the FAS of patients with T1D or T2D 8, 9. Data from these subgroup analyses suggest LY IGlar is a well tolerated and effective treatment option when prescribed for patients with T1D and T2D who have had prior treatment with IGlar.
Conflict of Interest
I. H., L. B. L., R. K. P., C. L. C. and L. L. I., are employees of and hold stock in Eli Lilly and Company. D. D. has no conflicts of interest to disclose.
I. H. participated in the interpretation and discussion of the research, and in writing the manuscript. D. D. contributed to the interpretation and discussion of the research, in conducting the study and reviewed and edited the manuscript. L. B. L. and L. L. I. participated in the study design, in conducting the study, in the data analysis, in the interpretation and discussion of the research and in writing the manuscript. R. K. P. participated in conducting the study, in the data analysis, in the interpretation and discussion of the research and in writing the manuscript. C. L. C. participated in the interpretation and discussion of the research and in writing the manuscript. All authors approved the version to be published. R. K. P is the guarantor of this work and, as such, takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript. Eileen Girten (non‐author) prepared the draft manuscript and provided editorial support.
Supporting information
Figure S1. Efficacy and safety outcomes in the prior IGlar subgroup of patients with type 1 diabetes (T1D) and type 2 diabetes (T2D). *p = 0.016 for LY IGlar vs IGlar; all other p‐values >0.05. Abbreviations: IGlar, insulin glargine; LSM, least squares mean; LY IGlar, LY2963016 insulin glargine; SD, standard deviation; SE, standard error.
Table S1. Patient demographics and baseline characteristics for the total study population and prior IGlar subgroup in patients with type 1 or type 2 diabetes.
Table S2. Fasting plasma glucose and daily mean glucose in the prior IGlar subgroup of patients with type 1 or type 2 diabetes.
Table S3. Adverse events summary for prior IGlar subgroup of patients with type 1 or type 2 diabetes.
Table S4. Summary of serious adverse events for prior IGlar patients with type 2 diabetes.
Acknowledgements
The authors would like to thank Elemer Balogh, MD, Eli Lilly and Company and Andelene Asaro‐Harris (employee of Eli Lilly and Company at the time of the Diabetes UK disclosure) for their content, writing and editorial contributions to the Diabetes UK disclosure. The authors also acknowledge Eileen Girten, MS, of inVentiv Health Clinical, for assistance with the preparation of this manuscript. This work was previously published as an abstract presented at the Diabetes UK Professional Conference, London, UK, 11–13 March 2015 (P456) and at the 2015 European Association for the Study of Diabetes Annual Conference, Stockholm, Sweden, 14–18 September (P969).
This study was funded by Eli Lilly and Company and Boehringer‐Ingelheim.
References
- 1. European Medicines Agency . Lantus. 2015. Available from URL: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000284/human_med_000882.jsp&mid=WC0b01ac058001d124. Accessed 18 May 2015.
- 2. Hilgenfeld R, Seipke G, Berchtold H, Owens DR. The evolution of insulin glargine and its continuing contribution to diabetes care. Drugs 2014; 74: 911–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eli Lilly and Company Press Release . European Commission grants Lilly and Boehringer Ingelheim's insulin glargine product marketing authorisation in Europe. 2014. Available from URL: http://lilly.mediaroom.com/index.php?s=9042&item=137348. Accessed 18 May 2015.
- 4. Pharmaceuticals and Medical Devices Agency . New drugs approved in FY 2014. Available from URL: http://www.pmda.go.jp/files/000206818.pdf. Accessed 10 December 2015.
- 5. European Medicines Agency . Questions and answers on biosimilar medicines (similar biological medicinal products). 2012. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2009/12/WC500020062.pdf. Accessed 16 June 2015.
- 6. European Medicines Agency . Abasaglar (formerly Abrasria). 2015. Available from URL: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002835/human_med_001790.jsp&mid=WC0b01ac058001d124. Accessed 18 May 2015.
- 7. Linnebjerg H, Lam EC, Seger ME et al. Comparison of the pharmacokinetics and pharmacodynamics of LY2963016 insulin glargine and EU‐ and US‐approved versions of Lantus insulin glargine in healthy subjects: three randomized euglycemic clamp studies. Diabetes Care 2015; 38: 2226–2233. [DOI] [PubMed] [Google Scholar]
- 8. Blevins TC, Dahl D, Rosenstock J et al. Efficacy and safety of LY2963016 insulin glargine compared with insulin glargine (Lantus®) in patients with type 1 diabetes in a randomized controlled trial: the ELEMENT 1 study. Diabetes Obes Metab 2015; 17: 726–733. [DOI] [PubMed] [Google Scholar]
- 9. Rosenstock J, Hollander P, Bhargava A et al. Similar efficacy and safety of LY2963016 insulin glargine and insulin glargine (Lantus®) in patients with type 2 diabetes who were insulin‐naïve or previously treated with insulin glargine: a randomized, double‐blind controlled trial (the ELEMENT 2 study). Diabetes Obes Metab 2015; 17: 734–741. [DOI] [PubMed] [Google Scholar]
- 10. World Medical Association Declaration of Helsinki . Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997; 277: 925–926. [PubMed] [Google Scholar]
- 11. Ilag LL, Deeg MA, Costigan T et al. Evaluation of immunogenicity of LY2963016 insulin glargine compared with Lantus® insulin glargine in patients with type 1 diabetes mellitus or type 2 diabetes mellitus. Diabetes Obes Metab 2016; 18: 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Food and Drug Administration . Guidance for industry assay development for immunogenicity testing of therapeutic proteins. Draft guidance. 2009. Available from URL: http://www.fda.gov/downloads/Drugs/…/Guidances/UCM192750.pdf. Accessed 16 December 2014.
- 13. Pieber TR, Treichel HC, Hompesch B et al. Comparison of insulin detemir and insulin glargine in subjects with Type 1 diabetes using intensive insulin therapy. Diabet Med 2007; 24: 635–642. [DOI] [PubMed] [Google Scholar]
- 14. Fulcher GR, Gilbert RE, Yue DK. Glargine is superior to neutral protamine Hagedorn for improving glycated haemoglobin and fasting blood glucose levels during intensive insulin therapy. Intern Med J 2005; 35: 536–542. [DOI] [PubMed] [Google Scholar]
- 15. Diabetes UK . Position statement on Biosimilar insulins. 2013. Available from URL: https://www.diabetes.org.uk/Documents/Position%20statements/diabetes‐uk‐position‐statement‐biosimilar‐insulins‐1013.pdf. Accessed 25 June 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Efficacy and safety outcomes in the prior IGlar subgroup of patients with type 1 diabetes (T1D) and type 2 diabetes (T2D). *p = 0.016 for LY IGlar vs IGlar; all other p‐values >0.05. Abbreviations: IGlar, insulin glargine; LSM, least squares mean; LY IGlar, LY2963016 insulin glargine; SD, standard deviation; SE, standard error.
Table S1. Patient demographics and baseline characteristics for the total study population and prior IGlar subgroup in patients with type 1 or type 2 diabetes.
Table S2. Fasting plasma glucose and daily mean glucose in the prior IGlar subgroup of patients with type 1 or type 2 diabetes.
Table S3. Adverse events summary for prior IGlar subgroup of patients with type 1 or type 2 diabetes.
Table S4. Summary of serious adverse events for prior IGlar patients with type 2 diabetes.


