Abstract
Tofacitinib is an oral Janus kinase inhibitor that is being investigated for psoriasis and psoriatic arthritis. Japanese patients aged 20 years or more with moderate to severe plaque psoriasis and/or psoriatic arthritis were double‐blindly randomized 1:1 to tofacitinib 5 or 10 mg b.i.d. for 16 weeks, open‐label 10 mg b.i.d. for 4 weeks, then variable 5 or 10 mg b.i.d. to Week 52. Primary end‐points at Week 16 were the proportion of patients achieving at least a 75% reduction in Psoriasis Area and Severity Index (PASI75) and Physician's Global Assessment of “clear” or “almost clear” (PGA response) for psoriasis, and 20% or more improvement in American College of Rheumatology criteria (ACR20) for patients with psoriatic arthritis. Safety was assessed throughout. Eighty‐seven patients met eligibility criteria for moderate to severe plaque psoriasis (5 mg b.i.d., n = 43; 10 mg b.i.d., n = 44), 12 met eligibility criteria for psoriatic arthritis (5 mg b.i.d., n = 4; 10 mg b.i.d., n = 8) including five who met both criteria (10 mg b.i.d.). At Week 16, 62.8% and 72.7% of patients achieved PASI75 with tofacitinib 5 and 10 mg b.i.d., respectively; 67.4% and 68.2% achieved PGA responses; all patients with psoriatic arthritis achieved ACR20. Responses were maintained through Week 52. Adverse events occurred in 83% of patients through Week 52, including four (4.3%) serious adverse events and three (3.2%) serious infections (all herpes zoster). No malignancies, cardiovascular events or deaths occurred. Tofacitinib (both doses) demonstrated efficacy in patients with moderate to severe plaque psoriasis and/or psoriatic arthritis through 52 weeks; safety findings were generally consistent with prior studies.
Keywords: Japan, kinase inhibitor, plaque psoriasis, psoriatic arthritis, tofacitinib
Introduction
Psoriasis is a chronic, immune‐mediated disease, characterized by prominent skin lesions, and affects 0.1–3% of the global population.1, 2 Psoriasis can cause significant impairment to quality of life (QoL)3 and requires long‐term treatment. The prevalence of psoriasis in the Japanese population is estimated to be lower (0.3%)4 than in countries with a mostly Caucasian population (up to 3.6%).5, 6, 7 However, more than 400 000 patients with psoriasis have been recently identified in Japan.4 The most common type of psoriasis is plaque psoriasis, with a prevalence of nearly 90% among patients with psoriasis in Japan.8
Psoriatic arthritis is an inflammatory arthropathy associated with psoriasis. Its prevalence was estimated to be approximately 3% in Japanese patients with psoriasis,8 but the most recent survey indicates an even higher prevalence of 14.3%.9
Treatment options for Japanese patients with moderate to severe psoriasis include oral systemic therapies, such as cyclosporin and etretinate, as well as phototherapy. Four injectable biologic agents, adalimumab, infliximab, ustekinumab and recently secukinumab, have each demonstrated considerable efficacy in this patient population,10, 11, 12, 13 and are approved in Japan for moderate to severe psoriasis following an inadequate response to systemic therapy.14 However, all of these treatments have limitations in addition to their clinical benefit. Long‐term use of systemic therapies is limited by safety considerations, including organ toxicity, infection and malignancy,15, 16 and teratogenicity with systemic retinoids, such as etretinate.17 Phototherapy is associated with increased risk of skin malignancies,18, 19 while some patients do not respond to phototherapy or have disease recurrence, and multiple office visits are required. The efficacy of biologic therapies may diminish over time owing in part to immunogenicity and use is restricted by parenteral administration.20, 21 Given the potentially serious toxicities associated with long‐term use of orally available systemic therapies and phototherapy and the drawbacks of biologic therapy, benefit : risk and patient preference need to be considered for each individual patient with psoriasis.22 Furthermore, patient dissatisfaction with available treatments is common in psoriasis, and both physician and patient perspectives should be considered in disease management.23, 24 Consequently, there is a need for new therapies for moderate to severe psoriasis, especially p.o. administrated therapies that offer sustained efficacy and manageable long‐term safety, as oral treatment options are currently limited and patients with psoriasis have reported that injections required with biologics are burdensome.25
Tofacitinib is an oral Janus kinase inhibitor that is being investigated for psoriasis and psoriatic arthritis. Phase 2 and 3 studies have demonstrated tofacitinib efficacy with a manageable safety profile.26, 27, 28, 29 Here, we present results from a 52‐week, phase 3 study conducted at Japanese centers evaluating the efficacy, safety and tolerability of oral tofacitinib for the treatment of Japanese patients with moderate to severe plaque psoriasis (psoriasis vulgaris) and/or psoriatic arthritis. In addition, this study is the first to report outcomes with tofacitinib treatment for psoriatic arthritis in Japanese patients.
Methods
Patients
Patients were aged 20 years or older. Patients with moderate to severe plaque psoriasis were required to have a diagnosis of plaque‐type psoriasis for 12 months or more prior to baseline, a Psoriasis Area and Severity Index (PASI) score of 12 or more, a Physician's Global Assessment (PGA) of 3 (moderate) or 4 (severe) (on a 5‐point scale), and an affected body surface area of 10% or more, at baseline. Patients with psoriatic arthritis were required to have a diagnosis of psoriatic arthritis 6 months or more prior to baseline, meet the Classification Criteria for Psoriatic Arthritis,30 have active arthritis as defined as three or more tender/painful joints (68‐joint count) and three or more swollen joints (66‐joint count) and active plaque psoriasis with a qualifying lesion of 2 cm or more in diameter confirmed by a dermatologist. Patients were to be considered candidates for systemic therapy or phototherapy for psoriasis (either treatment‐naive or ‐experienced). Exclusion criteria included evidence of active, latent or inadequately treated Mycobacterium tuberculosis infection, non‐plaque forms or drug‐induced psoriasis, other skin conditions that would interfere with psoriasis evaluation, inability to discontinue systemic, topical or phototherapies, concomitant oral or injectable corticosteroids, a history of disseminated herpes zoster or disseminated herpes simplex or recurrent localized dermatomal herpes zoster, a history of infection requiring hospitalization or parenteral microbial therapy (or other infection deemed clinically significant) within 6 months of baseline, a history or symptoms of any lymphoproliferative disorders, or a history of malignancies except for adequately treated or excised non‐metastatic basal cell or squamous cell skin cancer or cervical carcinoma in situ without recurrence for 3 years. Patients with psoriatic arthritis were excluded if they had a history of another autoimmune rheumatic disease, fibromyalgia or rheumatic inflammatory disease aside from psoriatic arthritis, unless agreed with the sponsor.
Washout periods for prior medications were 7 days or more or five half‐lives (whichever was longer) prior to first dose of study drug. A washout period of 2 weeks or more was applied for topical medications and ultraviolet B phototherapy, 4 weeks or more for etanercept, psoralen plus ultraviolet A therapy and non‐biologic systemic therapies, 8 weeks or more for adalimumab and infliximab, 10 weeks or more for golimumab, and 12 weeks or more for ustekinumab, tocilizumab and investigational/experimental agents. Topical hydrocortisone/hydrocortisone acetate of 1% or less were allowed (after 16 weeks for scalp), as were tar and salicylic acid preparations for the scalp.
The study was conducted in compliance with the ethical principles of the Declaration of Helsinki, and in accordance with the International Conference on Harmonization Good Clinical Practice guidelines. The study protocol was approved by the Institutional Review Boards and/or Independent Ethics Committees at each investigational center. All patients provided written informed consent at the first screening visit and before initiation of any washout period of prior medications.
Study design and treatment
This was a 52‐week, phase 3, multisite, randomized, double‐blind study conducted in 16 centers in Japan (clinicaltrials.gov NCT01519089; Pfizer protocol A3921137). The study comprised an initial 16‐week, double‐blind period, in which patients were randomized to tofacitinib 5 or 10 mg b.i.d. At Week 16, all patients received open‐label tofacitinib 10 mg b.i.d. for 4 weeks, after which the dose could be adjusted to tofacitinib 5 or 10 mg b.i.d. at the investigator's discretion, to Week 52 (Fig. 1). The visit window at Week 52 had a 7‐day allowance, so patients could receive tofacitinib up to Week 53 (day 372). A follow‐up visit was performed 2–4 weeks after the patient's last dose.
Figure 1.
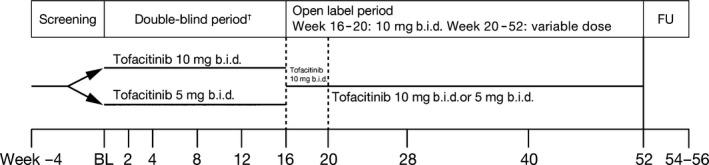
Study design. †Stratified by type of psoriasis at randomization. BL, baseline; FU, follow‐up.
Patients were randomized 1:1 to tofacitinib 5 or 10 mg b.i.d. using a computer‐generated randomization schedule; patients were registered by the investigator in a central randomized management system, and a stratification factor of psoriatic arthritis was employed.
Tofacitinib was supplied as 5 mg tablets with a corresponding matching placebo. Patients and study staff were unable to determine from the packaging which treatment group the patient was assigned to. Patients, investigators, study teams, the contract research organization and the sponsor remained blinded throughout the study period.
Study assessments
The primary efficacy end‐points at Week 16 for plaque psoriasis were the proportion of patients achieving at least a 75% reduction in PASI score (PASI75) from baseline at Week 16 and the proportion of patients achieving a PGA response of “clear” or “almost clear”. The primary efficacy end‐point at Week 16 for psoriatic arthritis was the proportion of patients with 20% or more improvement in American College of Rheumatology criteria (ACR20), calculated as a 20% or more improvement in tender/painful and swollen joint counts and improvements in three of Patient Global Assessment of arthritis, PGA of arthritis, patient pain assessment, Health Assessment Questionnaire–Disability Index and an acute‐phase reactant.
Secondary end‐points in plaque psoriasis included PASI75 and 90% or more improvement in PASI from baseline (PASI90) through Week 52, and the proportion of patients with PASI of 125% or more of baseline score at any time point. Nail Psoriasis Severity Index (NAPSI) score,31 change from baseline in NAPSI and patient‐reported outcomes (PRO) were also measured through Week 52. PRO included the Itch Severity Item (ISI), a single‐item, horizontal numeric rating scale to assess itching due to psoriasis with response options ranging from 0 (no itching) to 10 (worst possible itching) and the Dermatology Life Quality Index (DLQI), a 10‐item questionnaire that assesses the impact of chronic skin conditions on health‐related QoL (HRQoL).32 For psoriatic arthritis, ACR20, 50 and 70 were measured through Week 52.
Safety assessments
Safety was assessed according to the incidence and severity of treatment‐emergent adverse events (AEs), clinical laboratory abnormalities, physical examination changes, vital sign abnormalities and electrocardiogram (ECG) changes for all patients who received one or more dose of study drug (safety analysis set; SAS). Serious AEs were defined as those resulting in death, were life‐threatening, required hospitalization or prolongation of existing hospitalization, led to persistent or significant disability, or resulted in congenital abnormalities. Serious infections were defined as treated infections requiring parenteral antimicrobial therapy or hospitalization, or met other criteria for a serious AE. Patients with serious infections were discontinued from the study. Cardiovascular and opportunistic infection events were adjudicated by a prospectively defined, sponsor‐independent, masked safety event adjudication committee. All biopsies of potentially malignant tumors were confirmed by a central laboratory pathologist over‐read of local histology data. Pooled data for psoriasis and psoriatic arthritis are presented for Weeks 0–16 by treatment group, and for all tofacitinib‐treated patients through Week 52.
Statistical analyses
The treatment groups (tofacitinib 5 mg b.i.d. and 10 mg b.i.d.) were defined based on the initial tofacitinib dose received during Weeks 0–16. The full analysis set included all randomized patients who received one or more dose of study drug. No formal statistical hypotheses were tested to investigate efficacy and safety between treatment groups; although there was statistical testing applied at a significance level of 0.05 (two‐sided), statistical analysis was descriptive in nature. A sample size of 88 patients was chosen to allow 80% probability to observe a positive point estimate of a difference between treatment groups (10 mg b.i.d. to 5 mg b.i.d.) in PASI75 and PGA end‐points at Week 16. Of these 88 patients, the target number of patients with psoriatic arthritis was 10, with five expected responders based on data on tofacitinib in rheumatoid arthritis (RA) clinical studies. For primary end‐points, response rates were calculated. Missing values were treated as non‐responders (non‐responder imputation).
Secondary end‐points involving binary data were summarized as for the primary end‐points, above. For continuous data, descriptive statistics were calculated.
Results
Patient disposition and demographics
Between March and December 2012, 95 patients were randomized and 94 treated with tofacitinib 5 and 10 mg b.i.d. (Fig. 2). Of these, 87 (91.6%) had moderate to severe plaque psoriasis and 12 (12.6%) had active psoriatic arthritis. Five of these 12 patients also had a diagnosis of moderate to severe plaque psoriasis (all in tofacitinib 10 mg b.i.d. group); these patients were included in both the plaque psoriasis and psoriatic arthritis populations. Eighteen patients with plaque psoriasis and four with psoriatic arthritis discontinued from the study (Fig. 2). Mean [SD] age was 48.7 [11.5] years, 83.0% were male, and the mean [SD] body mass index was 24.0 [4.1] kg/m2. Baseline demographics were similar across groups (Table 1). Patients with plaque psoriasis had a longer duration of disease than those with psoriatic arthritis. Patients with psoriatic arthritis in the tofacitinib 10 mg b.i.d. group had a higher mean proportion of body surface area affected by psoriasis compared with those in the tofacitinib 5 mg b.i.d. group, likely due to the five patients in the 10 mg b.i.d. group who also had psoriasis.
Figure 2.
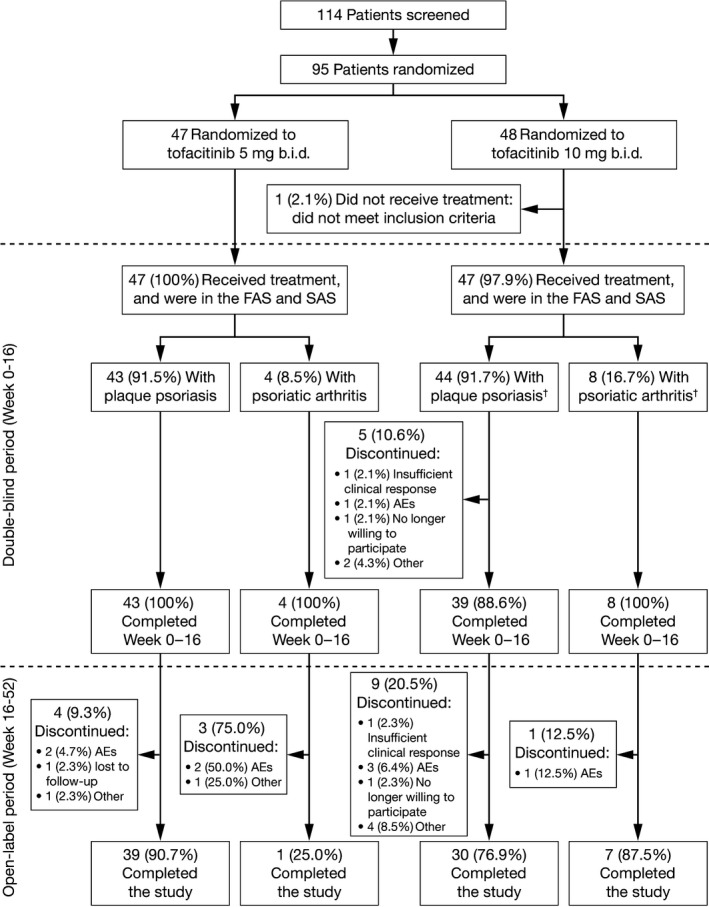
Patient disposition. †Five patients in the tofacitinib 10 mg b.i.d. group had both plaque psoriasis and psoriatic arthritis. AEs, adverse events; FAS, full analysis set; SAS, safety analysis set.
Table 1.
Baseline demographics and disease characteristics
| Plaque psoriasis | Psoriatic arthritis | |||
|---|---|---|---|---|
| Tofacitinib 5 mg b.i.d. (n = 43) | Tofacitinib 10 mg b.i.d. (n = 44) | Tofacitinib 5 mg b.i.d. (n = 4) | Tofacitinib 10 mg b.i.d. (n = 8) | |
| Mean age, years (range) | 50.9 (28–72) | 46.6 (28–69) | 51.0 (41–64) | 48.5 (43–59) |
| Male, n (%) | 35 (81.4) | 36 (81.8) | 4 (100) | 7 (87.5) |
| Mean weight, kg (SD) | 67.0 (13.3) | 66.6 (13.6) | 76.4 (13.9) | 74.4 (18.4) |
| Mean BMI, kg/m2 (SD) | 23.8 (4.0) | 23.6 (4.0) | 26.4 (4.5) | 25.7 (5.0) |
| Mean duration of disease, years (SD) | 13.4 (10.5) | 13.9 (8.2) | 4.0 (3.1) | 7.5 (6.5) |
| Mean PASI score (SD) | 26.9 (11.3) | 26.7 (11.8) | 6.7 (2.2) | 15.2 (12.6) |
| PGA, n (%) | ||||
| Almost clear | 0 | 0 | 1 (25.0) | 2 (25.0) |
| Mild | 0 | 0 | 1 (25.0) | 0 |
| Moderate | 34 (79.1) | 37 (84.1) | 2 (50.0) | 6 (75.0) |
| Severe | 9 (20.9) | 7 (15.9) | 0 | 0 |
| Total % psoriatic BSA, mean (SD) | 43.0 (21.3) | 43.1 (23.6) | 6.8 (1.9) | 24.3 (22.8) |
| Nail psoriasis, n (%) | 30 (69.8) | 29 (65.9) | 2 (50.0) | 5 (62.5) |
| Psoriatic arthritis, n (%) | 0 | 5 (11.4)† | 4 (100) | 8 (100) |
| Tender/painful joint count, mean (SD) | NA | NA | 15.0 (9.3) | 15.0 (11.9) |
| Swollen joint count, mean (SD) | NA | NA | 7.8 (7.6) | 10.1 (4.9) |
| DLQI total score, mean (SD) | 11.3 (6.3) | 8.6 (5.9) | 5.0 (2.8) | 7.8 (3.1) |
| FAS | Tofacitinib 5 mg b.i.d. (n = 47) | Tofacitinib 10 mg b.i.d. (n = 47) | ||
|---|---|---|---|---|
| Mean NAPSI score (SD)‡ | 29.3 (20.8) | 24.3 (17.8) | ||
| Mean ISI score (SD) | 6.0 (2.7) | 5.3 (2.9) | ||
| Mean DLQI score (SD) | 10.7 (6.3) | 8.4 (5.8) |
| SAS | Tofacitinib 5 mg b.i.d. (n = 47) | Tofacitinib 10 mg b.i.d. (n = 47) | ||
|---|---|---|---|---|
| Prior therapies, n (%) | ||||
| Topical | 47 (100) | 47 (100) | ||
| Biologics§ | 6 (12.8) | 9 (19.1) | ||
| Non‐biologics¶ | 30 (63.8) | 30 (63.8) | ||
| Inadequate response or intolerance to TNFi | 4 (8.5) | 4 (8.5) | ||
| Inadequate response or intolerance to MTX, cyclosporin, etretinate or phototherapy | 21 (44.7) | 24 (51.1) | ||
| Concomitant therapies used by ≥5% of patients in any group, n (%) | ||||
| Hydrocortisone in Eurax lotion | 4 (8.5) | 5 (10.6) | ||
| Fexofenadine hydrochloride | 3 (6.4) | 4 (8.5) | ||
| Betamethasone butyrate propionate | 2 (4.3) | 4 (8.5) | ||
| Levocetirizine dihydrochloride | 1 (2.1) | 4 (8.5) | ||
| Celecoxib | 0 | 4 (8.5) | ||
| Total | 13 (27.7) | 20 (42.6) | ||
†These five patients had both plaque psoriasis and psoriatic arthritis and were included in both populations. ‡ n = 34 for tofacitinib 5 mg b.i.d. and n = 31 for tofacitinib 10 mg b.i.d. (patient numbers differed from those with nail psoriasis according to plaque psoriasis and psoriatic arthritis, as patients diagnosed with both psoriasis and psoriatic arthritis were included in both population groups). §Irrespective of previous use of conventional systemic or phototherapy. ¶Including conventional systemic or phototherapy. BMI, body mass index; BSA, body surface area; DLQI, Dermatology Life Quality Index; FAS, full analysis set; ISI, Itch Severity Item; MTX, methotrexate; NA, not applicable; NAPSI, Nail Psoriasis Severity Index; SAS, safety analysis set; SD, standard deviation; TNFi, tumor necrosis factor inhibitor.
Between Weeks 20 and 52, 8/47 patients initially assigned tofacitinib 5 mg b.i.d. and 11/42 patients initially assigned 10 mg b.i.d. underwent dose changes from the 10 mg b.i.d. dose given during Weeks 16 to 20. Most patients initially assigned to tofacitinib 5 mg b.i.d. were exposed to 10 mg b.i.d. from Weeks 20 to 52. Therefore, formal comparisons between initial dose groups after Week 16 were not appropriate. From Weeks 20 to 52 the median (inter‐quartile range) daily doses received by patients initially assigned to 5 and 10 mg b.i.d. were 19.8 (18.4, 19.9) mg and 19.7 (17.6, 19.9) mg, respectively.
Efficacy
At Week 16, with tofacitinib 5 and 10 mg b.i.d., 27 (62.8%, standard error; SE 7.4) and 32 (72.7%; SE 6.7) patients with plaque psoriasis, respectively, achieved a PASI75 response, and 29 [67.4%; SE 7.1] and 30 [68.2%; 7.0] patients achieved a PGA response (Table 2).
Table 2.
Clinical responses at Week 16 (double‐blind period; NRI)†
| Tofacitinib 5 mg b.i.d., n (%) (SE) | Tofacitinib 10 mg b.i.d., n (%) (SE) | |
|---|---|---|
| Moderate to severe plaque psoriasis population | n = 43 | n = 44 |
| PASI75 | 27 (62.8) (7.4) | 32 (72.7) (6.7) |
| PASI90 | 16 (37.2) (7.4) | 24 (54.5) (7.5) |
| PGA response | 29 (67.4) (7.1) | 30 (68.2) (7.0) |
| Psoriatic arthritis population | n = 4 | n = 8 |
| ACR20 | 4 (100) (0) | 8 (100) (0) |
| ACR50 | 3 (75.0) (21.7) | 7 (87.5) (11.7) |
| ACR70 | 2 (50) (25.0) | 5 (62.5) (17.1) |
†No statistically significant difference was observed between dose groups for any end‐point at Week 16. ACR, American College of Rheumatology response criteria; NRI, non‐responder imputation; PASI, Psoriasis Area and Severity Index; PGA, Physician's Global Assessment; SE, standard error.
Clinical response rates increased throughout time during the double‐blind period (Fig. 3); although responses with tofacitinib 10 mg b.i.d. appeared higher than with 5 mg b.i.d., actual differences between the doses were relatively small and the only statistically significant difference between doses was observed at Week 4 with PASI75. Response rates of PASI75 in the tofacitinib 5 mg b.i.d. group increased from 27/43 (62.8%; SE 7.4) at Week 16 to 29/43 (67.4%; SE 7.1) at Week 20 upon advancement to 10 mg b.i.d., and response rates remained stable from Week 20 to 52. In patients who achieved a PASI75 response at Week 16 with tofacitinib 10 mg b.i.d., 20/32 (62.5%; SE 8.6) maintained their response to Week 52. Similarly, 18/30 (60.0%; SE 8.9) patients initially assigned tofacitinib 10 mg b.i.d. maintained their Week 16 PGA response to Week 52. Upon treatment cessation, response rates dropped for all measures during the follow‐up period (Fig. 3).
Figure 3.
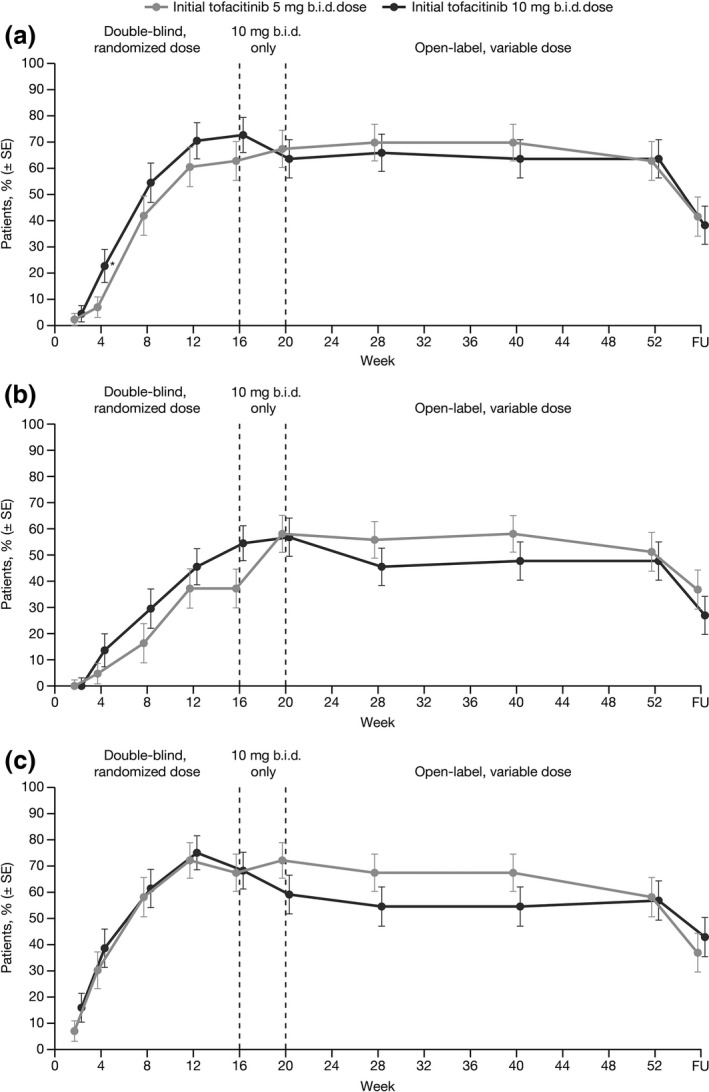
Proportion of patients with moderate to severe plaque psoriasis achieving (a) PASI75, (b) PASI90 and (c) PGA response of “clear” or “almost clear” over time, according to initially randomized dose group (NRI). *P < 0.05 versus tofacitinib 5 mg b.i.d.; FU, follow‐up; NRI, non‐responder imputation; PASI, Psoriasis Area and Severity Index; PGA, Physician's Global Assessment; SE, standard error.
All 12 patients with psoriatic arthritis achieved an ACR20 response at Week 16, and at least half achieved ACR50 or ACR70 (Table 2). Responses were maintained with tofacitinib treatment through Week 52 and dropped upon treatment cessation (Fig. 4). However, small patient numbers meant that SE were wide.
Figure 4.
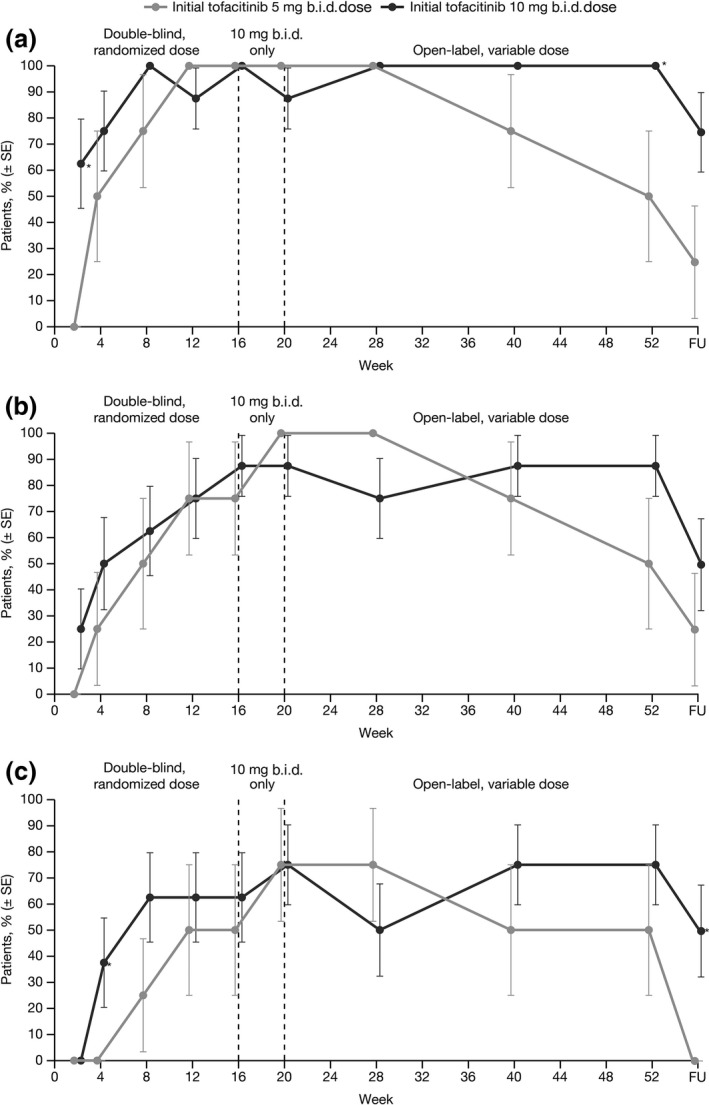
Proportion of patients with psoriatic arthritis who achieved (a) ACR20, (b) ACR50 and (c) ACR70 response, according to initially randomized dose group (NRI). *P < 0.05 versus tofacitinib 5 mg b.i.d. ACR, American College of Rheumatology response criteria; FU, follow‐up; NRI, non‐responder imputation; SE, standard error.
At Week 16, 16 (37.2%) patients receiving tofacitinib 5 mg b.i.d., and 24 (54.5%) receiving tofacitinib 10 mg b.i.d., achieved a PASI90 response (Table 2), which followed a similar pattern to PASI75 through 52 weeks (Fig. 3). Improvements in nail psoriasis were observed with both tofacitinib doses by Week 16, which continued to improve through Week 52 (Table 3). Patients also reported reduced itching with tofacitinib at Week 16 irrespective of dose, which was maintained through Week 52. More than half of patients who had a baseline ISI score of more than 1 reported little or no itching (score up to 1) at Week 16 and through Week 52 (Table 3); as early as the first post‐dose visit at Week 2, 13.0% (SE 5.0) and 18.2% (SE 5.8) of patients reported little or no itching with tofacitinib 5 and 10 mg b.i.d., respectively. Similarly, mean DLQI scores decreased by Week 16, representing an improvement in HRQoL, which was maintained to Week 52. Most patients with a baseline DLQI score of more than 1 achieved a score of up to 1, representing no effect of the skin condition (e.g, psoriasis) on HRQoL through Week 52. Improvements in ISI and DLQI were not dose‐related.
Table 3.
Summary of secondary efficacy end‐points at Weeks 16, 20 and 52 according to initial randomized dose at baseline (FAS)
| Initial tofacitinib 5 mg b.i.d. | Initial tofacitinib 10 mg b.i.d. | |
|---|---|---|
| Mean change from baseline in NAPSI score† (observed cases) (SD) | ||
| Week 16 | −11.3 (14.9) | −10.2 (13.2) |
| Week 20 | −15.1 (15.0) | −14.6 (13.1) |
| Week 52 | −20.6 (15.2) | −16.3 (15.3) |
| Patients achieving NAPSI75 response, n/n (%) (NRI) | ||
| Week 16 | 5/34 (14.7) | 6/31 (19.4) |
| Week 20 | 8/34 (23.5) | 13/31 (41.9) |
| Week 52 | 19/34 (55.9) | 17/31 (54.8) |
| Mean change from baseline in ISI score (observed cases) (SD) | ||
| Week 16 | −4.4 (3.3) | −4.5 (3.1) |
| Week 20 | −5.1 (3.1) | −4.1 (3.3) |
| Week 52 | −4.6 (3.5) | −4.2 (3.1) |
| Proportion of patients with ISI score of ≤1‡, n/n (%) (NRI) | ||
| Week 16 | 31/46 (67.4) | 31/44 (70.5) |
| Week 20 | 35/46 (76.1) | 25/44 (56.8) |
| Week 52 | 30/46 (65.2) | 24/44 (54.5) |
| Mean change from baseline in DLQI score (observed cases) (SD) | ||
| Week 16 | −8.5 (6.4) | −6.7 (5.8) |
| Week 20 | −8.8 (6.7) | −6.8 (5.6) |
| Week 52 | −9.7 (6.4) | −6.1 (5.2) |
| Proportion of patients with DLQI score of ≤1‡, n/n (%) (NRI) | ||
| Week 16 | 29/45 (64.4) | 26/44 (59.1) |
| Week 20 | 30/45 (66.7) | 24/44 (54.5) |
| Week 52 | 27/45 (60.0) | 25/44 (56.8) |
†In patients with nail psoriasis at baseline. ‡In patients with a baseline score of more than 1. DLQI, Dermatology Life Quality Index; FAS, full analysis set (both plaque psoriasis and psoriatic arthritis populations); ISI, Itch Severity Item; NAPSI, Nail Psoriasis Severity Index; NRI, non‐responder imputation; SD, standard deviation.
Safety
In the SAS, the median duration of tofacitinib exposure was 365 days (Table 4). During the double‐blind phase, AEs were similar between treatment groups; most common AEs are presented in Table 4. Throughout the study, four serious AEs were reported: one case of vertigo with tofacitinib 10 mg b.i.d. during the double‐blind period and three cases of herpes zoster in the open‐label variable‐dose period (one receiving 5 mg b.i.d. and two receiving 10 mg b.i.d. at onset of herpes zoster). Two additional serious AEs were reported during follow‐up after Week 52: one case of rapid progression of psoriasis on post‐dose Day 14, and impetigo and erythrodermic psoriasis in the same patient on post‐dose Day 7; this patient was receiving tofacitinib 10 mg b.i.d. at the earliest onset of impetigo. Patients with serious AEs were permanently withdrawn from the study; all serious AEs, including those occurring during follow‐up, resolved, except the erythrodermic psoriasis that was still resolving at the time of this report.
Table 4.
Summary of all‐causality AEs (SAS)
| Tofacitinib 5 mg b.i.d., n (%) | Tofacitinib 10 mg b.i.d., n (%) | |
|---|---|---|
| Median duration of exposure, days (range) | 365 (139–372) | 365 (7–370) |
| Week 0–16 | n = 47 | n = 47 |
|---|---|---|
| AEs | 27 (57.4) | 28 (59.6) |
| Serious AEs | 0 | 1 (2.1) |
| Discontinuations due to AEs | 0 | 1 (2.1) |
| Most common AEs (≥5% of patients in any group) | ||
| Nasopharyngitis | 9 (19.1) | 8 (17.0) |
| Headache | 1 (2.1) | 3 (6.4) |
| Influenza | 3 (6.4) | 0 |
| Decreased hemoglobin | 0 | 3 (6.4) |
| Weeks 0–52† | Total n = 94 | |
|---|---|---|
| AEs | 78 (83.0) | |
| Serious AEs | 4 (4.3) | |
| Discontinuations due to AEs | 8 (8.5) | |
| Most common AEs (≥5% of patients) | ||
| Nasopharyngitis | 28 (29.8) | |
| Herpes zoster (serious and non‐serious) | 16 (17.0)‡ | |
| Psoriasis | 9 (9.6) | |
| Tinea pedis | 9 (9.6) | |
| Influenza | 6 (6.4) | |
| Increased blood creatine phosphokinase | 5 (5.3) | |
| Decreased hemoglobin | 5 (5.3) | |
†AEs occurring up to day 372 are included. ‡Three cases were serious. AEs, adverse events; SAS, safety analysis set.
Overall, herpes zoster occurred in 16 patients, all but two events (neither considered serious) occurred during the open‐label variable‐dosing period. Tofacitinib doses at onset of herpes zoster were 5 mg b.i.d. in two patients, 10 mg b.i.d. in 13 patients, and one case occurred during follow‐up after the patient had been receiving 10 mg b.i.d. One case was considered severe, and no cases of multidermatomal, disseminated, systemic or ophthalmic herpes zoster were reported (serious cases are described above). Commonly occurring infections included nasopharyngitis, influenza and tinea pedis (Table 4), none of which were serious.
No serious infections other than herpes zoster were reported during the double‐blind period. In the open‐label period, further to the three herpes zoster cases described above, one serious infection of impetigo was reported, as described above.
Discontinuations due to AEs included one patient with worsening psoriasis receiving tofacitinib 10 mg b.i.d. in the double‐blind period. In the open‐label period three patients with serious herpes zoster described above, two with worsening psoriasis and two with AEs relating to liver function test abnormalities discontinued the study.
No malignancies, cardiovascular events, or deaths were reported in this study.
Changes in laboratory parameters throughout time are summarized in Figure 5. Neutrophil levels decreased with initiation of tofacitinib, but remained stable through the study and returned to baseline upon treatment cessation (Fig. 5a). Lymphocyte levels initially rose with tofacitinib treatment, then, gradually decreased to baseline levels at Week 52 (Fig. 5b). Decreased hemoglobin levels were reported with tofacitinib 10 mg b.i.d. (Table 4); however, levels remained stable for the duration of the study (Fig. 5c). There were no confirmed cases (two consecutive measurements or occurring at the final visit) of patients with neutrophil counts <0.5 × 103/mm3, lymphocyte counts <0.5 × 103/mm3, hemoglobin levels <10 g/dL, or aspartate aminotransferase levels ≥3× upper limit of normal (ULN). Two patients with normal baseline alanine aminotransferase levels had confirmed post‐dose levels ≥3× ULN (one in each initial dose group). Creatine phosphokinase levels initially increased in the first 16 weeks, and remained stable for the remainder of the study (Fig. 5d). No rhabdomyolysis events were reported, and no potential Hy's Law cases were found. Levels of low‐density lipoprotein cholesterol (LDL‐C) and high‐density lipoprotein cholesterol (HDL‐C) increased upon treatment initiation and returned to baseline levels upon treatment cessation (Fig. 5e, f); the LDL‐C/HDL‐C ratio remained stable throughout the study (Fig. 5g).
Figure 5.
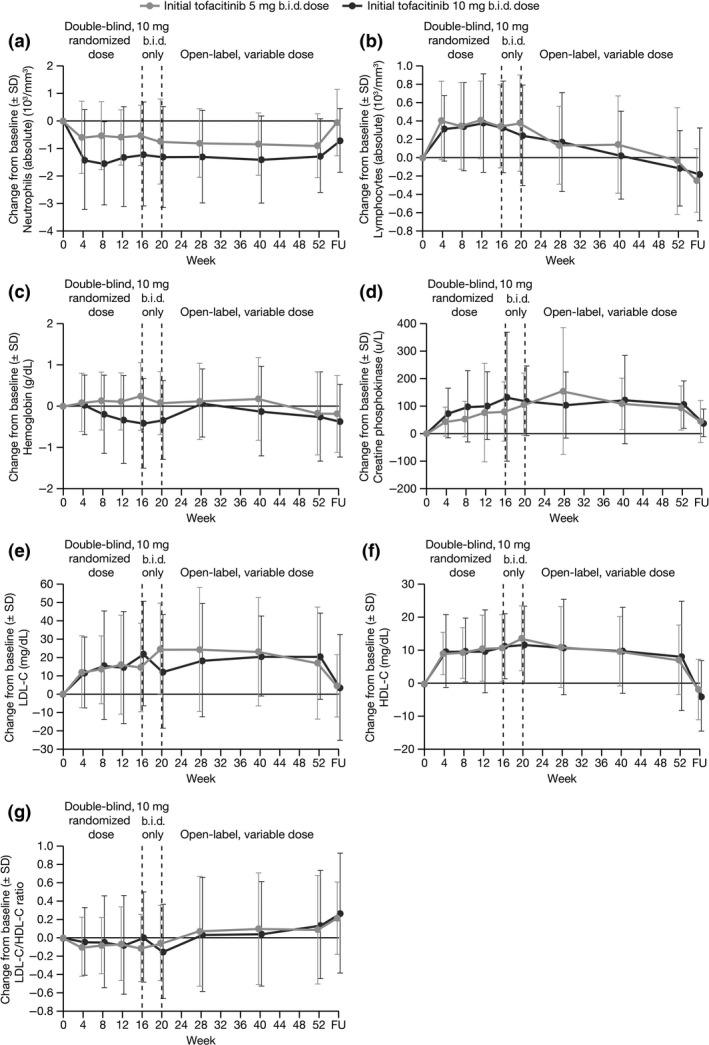
Change from baseline in laboratory parameters through follow‐up, for (a) neutrophil counts, (b) lymphocyte counts, (c) hemoglobin levels, (d) creatine phosphokinase, (e) LDL cholesterol, (f) HDL cholesterol and (g) LDL/HDL ratio, by initially randomized dose group (safety analysis set). HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SD, standard deviation.
No notable changes in blood pressure, ECG, or physical examination were observed at any time point, or between treatment groups.
Discussion
This phase 3 study demonstrated short‐term efficacy of tofacitinib at both 5 and 10 mg b.i.d. doses and maintenance of efficacy for 52 weeks with a manageable safety profile in Japanese patients with moderate to severe plaque psoriasis and/or active psoriatic arthritis.
This study population exhibited a considerable disease burden at baseline; all had previously used topical therapies and approximately half had previously had inadequate responses to, or were intolerant to, commonly used conventional systemic or phototherapy. In addition, 16% of patients had previously received biologics. Patient HRQoL was also affected by their disease, as evidenced by mean DLQI scores of 8.4–10.7 at baseline corresponding to a moderate to large effect on the patient's HRQoL.
Clinical responses in patients with plaque psoriasis at Week 16 were slightly higher than those reported in one phase 2 study and four global phase 3 studies of 12–24 weeks of tofacitinib treatment in patients with moderate to severe plaque psoriasis.26, 27, 28, 29 In this study, mean PASI75 responses with tofacitinib at Week 16 in patients with plaque psoriasis were numerically higher with tofacitinib 10 mg b.i.d. (72.7%) than with 5 mg b.i.d. (62.8%), and mean PGA responses were similar with 10 mg b.i.d. (68.2%) and 5 mg b.i.d. (67.4%). However, this apparent difference was narrower than in the global studies, which reported PASI75 rates of 59–68% with 10 mg b.i.d. and 40–46% with 5 mg b.i.d. after 12–24 weeks.26, 27, 28, 29 Week 16 mean PGA responses in our study were similar between doses (68% with 10 mg b.i.d. and 67% with 5 mg b.i.d.), again in contrast with the above global studies which showed a marked difference between doses: 59–68% with 10 mg b.i.d. and 41–47% with 5 mg b.i.d. after 12–24 weeks.
PASI75 response rates previously reported in global studies of patients with psoriasis after 12–16 weeks of treatment with other oral systemic agents have ranged from 10 to 41% with methotrexate33 and apremilast.34, 35 PASI75 responses after 10–24 weeks of treatment with biologics have ranged from 53 to 82% with infliximab, adalimumab, ustekinumab, and secukinumab in global studies36, 37, 38, 39, 40 and were similar in Japanese patients.10, 11, 12, 13 PASI75 responses with tofacitinib in this study appeared to be higher than those reported with oral systemic therapies and were in the range for those with biologics, but direct comparisons cannot be made due to differences in study designs and patient populations between studies; head‐to‐head studies would be required. The slight increase in PASI75 response rates observed with tofacitinib in Japanese patients in the present study compared with the global tofacitinib studies was not readily apparent in the above studies of biologics in global and Japanese patient populations.
In the present study, all 12 patients with psoriatic arthritis achieved an ACR20 response by Week 16 with both tofacitinib dose groups; rates were maintained with tofacitinib treatment to Week 52, although interpretation of the response is limited by low patient numbers. This is the first study to show efficacy of tofacitinib in psoriatic arthritis. ACR20 rates in global studies of patients with psoriatic arthritis after 12–16 weeks of apremilast treatment were 31–44%.41, 42 In global studies with biologics, ACR20 rates have ranged from 44 to 59% after 12–24 weeks of treatment with golimumab,43 adalimumab,44 ustekinumab,45 etanercept (US only),46 and infliximab47 in patients with psoriatic arthritis. Tofacitinib showed promising efficacy in the context of the above studies, although direct comparisons cannot be drawn, as patient numbers were low in the present study and study populations and designs differed.
Tofacitinib has been evaluated in multiple phase 2 and 3 studies in RA, including one phase 2 study in a Japanese RA population.48 Week 12 ACR20 rates were 73% and 85% with tofacitinib 5 and 10 mg b.i.d., respectively, which were in line with the high response rates observed in the present study. However, caution must be used in comparing efficacy in patients with psoriatic arthritis and RA, due to differences in the background diseases and demographics for each patient population.
Patients in the present study reported improvements in their nail psoriasis, itch and dermatology‐related QoL for the duration of the study; most patients (54.5–65.2%) reported little or no itching following 52 weeks of tofacitinib treatment with some reporting improvements as early as Week 2. The mean change from baseline in the DLQI total score (−9.7 in patients initially assigned tofacitinib 5 mg b.i.d., −6.1 in patients initially assigned 10 mg b.i.d.) was greater than the estimated minimally important difference of 4 points,49 demonstrating clinically meaningful improvements in HRQoL with tofacitinib by Week 16, which were maintained through Week 52. These results are consistent with those seen in Japanese patients with moderate to severe plaque psoriasis treated with ustekinumab11 or adalimumab.10 Nail psoriasis can be difficult to treat and can affect patients' QoL.50 In addition, itching has been identified as one of the most bothersome symptoms of psoriasis and psoriatic arthritis with skin involvement.25 Therefore, effective treatment of these conditions, as indicated here, is an important factor in disease management.
The safety profile of tofacitinib did not raise any new safety signals compared with the global phase 3 tofacitinib studies; rates of AEs, serious AEs and discontinuations were similar to those previously reported with tofacitinib in plaque psoriasis,27, 28, 29 except for herpes zoster and tinea pedis, discussed below. In addition, 52‐week rates for AEs were similar to those reported in Japanese patients with moderate to severe plaque psoriasis treated with ustekinumab11 or secukinumab,12 and in global studies of methotrexate33 and apremilast34 in moderate to severe plaque psoriasis. AEs with tofacitinib were fewer than those in a study of Japanese patients with moderate to severe plaque psoriasis treated with 24 weeks of adalimumab, which reported AEs in 91–97% of patients,10 although direct comparisons would require head‐to‐head studies. The most common AEs reported here were similar to those previously reported with biologics with the exception of tinea pedis, which occurred in 10% of patients in the present study. Tinea pedis was not reported as a common AE (<2–12% of patients) in the above studies of biologics,10, 11, 13, 36, 37, 38, 39, 40, 43, 44, 45, 46, 47 except for a subanalysis in Japanese patients of a phase 3 study of the interleukin (IL)‐17 inhibitor secukinumab in patients with psoriasis, which reported tinea pedis in 6% of patients.12 This was not reported as a common AE in the parent study.40 IL‐17 levels have been shown to decrease in patients treated with tofacitinib,51 but tinea pedis was not identified as a common AE in the tofacitinib global studies.26, 27, 28, 29 Further research would be necessary to identify any link between tofacitinib and tinea pedis in Japanese patients with psoriasis.
Previous global studies in psoriasis and RA with tofacitinib identified an increased incidence rate of herpes zoster in patients from Japan and South Korea compared with the rest of the study population.52, 53 In the current study, herpes zoster was reported in 17% of patients. Most patients who developed herpes zoster were receiving the 10 mg b.i.d. dose. A 56‐week phase 3 global study of tofacitinib in patients with moderate to severe plaque psoriasis reported eight patients (1.1%) with herpes zoster.28 In RA, higher incidence rates of herpes zoster with tofacitinib have been reported in Japanese patients versus global study populations (8.0 vs 4.4 events/100 patient‐years).52, 54 In addition, analysis of data from global phase 2, 3 and long‐term extension studies of tofacitinib in patients with psoriasis revealed that Asian race (mostly Japanese) was found to be independently associated with the development of herpes zoster versus non‐Asian race (hazard ratio, 5.87; 95% confidence interval, 3.06–11.26).53 Further analysis of incidence rates in Japanese and global study populations with tofacitinib is required to understand the potential difference in herpes zoster rates between these patient populations. The incidence rate of herpes zoster in Japanese patients with psoriasis has been estimated at 4.15/1000 patient‐years.55
This study had a number of limitations. Between Weeks 16 and 20, all patients received tofacitinib 10 mg b.i.d. and many patients remained on tofacitinib 10 mg b.i.d. thereafter, so comparisons between doses were not feasible after Week 16. Although there were a low number of patients with psoriatic arthritis, the study did show signs of tofacitinib efficacy in these patients, and phase 3 studies are ongoing to further evaluate tofacitinib in patients with psoriatic arthritis (clinicaltrials.gov NCT01882439 and NCT01877668). Furthermore, there was no placebo control.
In this study, oral tofacitinib demonstrated efficacy for the treatment of patients with moderate to severe plaque psoriasis and/or psoriatic arthritis during a 52‐week period, with a manageable safety profile that was generally consistent with the global population. Many patients had received prior systemic therapy, which reflects clinical practice where many patients have previously received systemic therapies. Furthermore, the open‐label period of the study reflected what may take place in clinical practice, where physicians could increase the dose from 5 to 10 mg b.i.d. in cases of inadequate response. Differences in clinical response between the 5 and 10 mg b.i.d. doses were small in this study compared with global studies; however, the proportion of patients with PASI75 response showed a small increase from 62.8% to 67.4% when patients switched from 5 to 10 mg b.i.d. from Week 16 to 20. These responses were maintained through Week 52 in the majority of patients, which may suggest a patient could be treated with tofacitinib 5 mg b.i.d. initially, and that tofacitinib 10 mg b.i.d. could be a feasible option for patients who do not achieve a sufficient response at the 5 mg b.i.d. dose. However, higher rates of herpes zoster seen with tofacitinib 10 mg b.i.d. must be taken into account, and further research with larger patient numbers is required to confirm this conclusion.
This study adds to a growing body of evidence of tofacitinib efficacy and safety in plaque psoriasis and supports the continued investigation of tofacitinib in the management of moderate to severe plaque psoriasis in Japan.
Conflict of interest
This study was sponsored by Pfizer Inc. A. A., A. I., S. I., H. S. and M. O. have received consultancy fees from Pfizer Inc. Y. S., Y. T., S. T. and M. N. are employees of Pfizer Japan Inc. T. E. has nothing to disclose.
Supporting information
Appendix S1. Principal study investigators at each center.
Acknowledgments
The authors would like to thank the investigators and patients of the A3921137 study. This study was sponsored by Pfizer Inc. Medical writing support under the guidance of the authors was provided by Kate Silverthorne, Ph.D., at Complete Medical Communications and was funded by Pfizer Inc.
References
- 1. Gudjonsson JE, Elder JT. Psoriasis In: Goldsmith LA, Katz SI, Gilchrest BA, Paller A, Leffell DJ, Wolff K, eds. Fitzpatrick's Dermatology in General Medicine, 8th edn New York: McGraw‐Hill Medical, 2012; 197–232. [Google Scholar]
- 2. Schön MP, Boehncke WH. Psoriasis. N Engl J Med 2005; 352: 1899–1912. [DOI] [PubMed] [Google Scholar]
- 3. Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 1999; 41: 401–407. [DOI] [PubMed] [Google Scholar]
- 4. Kubota K, Kamijima Y, Sato T et al Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open 2015; 5: e006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gelfand JM, Stern RS, Nijsten T et al The prevalence of psoriasis in African Americans: results from a population‐based study. J Am Acad Dermatol 2005; 52: 23–26. [DOI] [PubMed] [Google Scholar]
- 6. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133: 377–385. [DOI] [PubMed] [Google Scholar]
- 7. Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol 2014; 70: 512–516. [DOI] [PubMed] [Google Scholar]
- 8. Takahashi H, Nakamura K, Kaneko F, Nakagawa H, Iizuka H. Analysis of psoriasis patients registered with the Japanese Society for Psoriasis Research from 2002‐2008. J Dermatol 2011; 38: 1125–1129. [DOI] [PubMed] [Google Scholar]
- 9. Ohara Y, Kishimoto M, Takizawa N et al Prevalence and Clinical Characteristics of Psoriatic Arthritis in Japan. J Rheumatol 2015; 42: 1439–1442. [DOI] [PubMed] [Google Scholar]
- 10. Asahina A, Nakagawa H, Etoh T, Ohtsuki M. Adalimumab in Japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a Phase II/III randomized controlled study. J Dermatol 2010; 37: 299–310. [DOI] [PubMed] [Google Scholar]
- 11. Igarashi A, Kato T, Kato M, Song M, Nakagawa H. Efficacy and safety of ustekinumab in Japanese patients with moderate‐to‐severe plaque‐type psoriasis: long‐term results from a phase 2/3 clinical trial. J Dermatol 2012; 39: 242–252. [DOI] [PubMed] [Google Scholar]
- 12. Ohtsuki M, Morita A, Abe M et al Secukinumab efficacy and safety in Japanese patients with moderate‐to‐severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo‐controlled, phase 3 study. J Dermatol 2014; 41: 1039–1046. [DOI] [PubMed] [Google Scholar]
- 13. Torii H, Nakagawa H. Infliximab monotherapy in Japanese patients with moderate‐to‐severe plaque psoriasis and psoriatic arthritis. A randomized, double‐blind, placebo‐controlled multicenter trial. J Dermatol Sci 2010; 59: 40–49. [DOI] [PubMed] [Google Scholar]
- 14. Ohtsuki M, Terui T, Ozawa A et al Japanese guidance for use of biologics for psoriasis (the 2013 version). J Dermatol 2013; 40: 683–695. [DOI] [PubMed] [Google Scholar]
- 15. Hsu S, Papp KA, Lebwohl MG et al Consensus guidelines for the management of plaque psoriasis. Arch Dermatol 2012; 148: 95–102. [DOI] [PubMed] [Google Scholar]
- 16. Weger W. Current status and new developments in the treatment of psoriasis and psoriatic arthritis with biological agents. Br J Pharmacol 2010; 160: 810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Halioua B, Saurat JH. Risk: benefit ratio in the treatment of psoriasis with systemic retinoids. Br J Dermatol 1990; 122(Suppl 36): 135–150. [DOI] [PubMed] [Google Scholar]
- 18. Stern RS, Nichols KT, Vakeva LH. Malignant melanoma in patients treated for psoriasis with methoxsalen (psoralen) and ultraviolet A radiation (PUVA). The PUVA Follow‐Up Study. N Engl J Med 1997; 336: 1041–1045. [DOI] [PubMed] [Google Scholar]
- 19. Studniberg HM, Weller P. PUVA, UVB, psoriasis, and nonmelanoma skin cancer. J Am Acad Dermatol 1993; 29: 1013–1022. [DOI] [PubMed] [Google Scholar]
- 20. Weinberg JM. An overview of infliximab, etanercept, efalizumab, and alefacept as biologic therapy for psoriasis. Clin Ther 2003; 25: 2487–2505. [DOI] [PubMed] [Google Scholar]
- 21. Zisapel M, Zisman D, Madar‐Balakirski N et al Prevalence of TNF‐alpha Blocker Immunogenicity in Psoriatic Arthritis. J Rheumatol 2015; 42: 73–78. [DOI] [PubMed] [Google Scholar]
- 22. Naldi L, Griffiths CE. Traditional therapies in the management of moderate to severe chronic plaque psoriasis: an assessment of the benefits and risks. Br J Dermatol 2005; 152: 597–615. [DOI] [PubMed] [Google Scholar]
- 23. Umar N, Schaarschmidt M, Schmieder A, Peitsch WK, Schollgen I, Terris DD. Matching physicians' treatment recommendations to patients' treatment preferences is associated with improvement in treatment satisfaction. J Eur Acad Dermatol Venereol 2013; 27: 763–770. [DOI] [PubMed] [Google Scholar]
- 24. Torii H, Nakagawa H. Questionnaire survey of perceived satisfaction with treatment of patients with psoriasis. Jpn J Dermatol 2013; 123: 1935–1944. [Google Scholar]
- 25. Lebwohl MG, Bachelez H, Barker J et al Patient perspectives in the management of psoriasis: results from the population‐based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol 2014; 70: 871–881. [DOI] [PubMed] [Google Scholar]
- 26. Papp KA, Menter A, Strober B et al Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a Phase 2b randomized placebo‐controlled dose‐ranging study. Br J Dermatol 2012; 167: 668–677. [DOI] [PubMed] [Google Scholar]
- 27. Bachelez H, van de Kerkhof PCM, Strohal R et al Tofacitinib versus etanercept or placebo in moderate‐to‐severe chronic plaque psoriasis: a phase 3 randomised non‐inferiority trial. Lancet 2015; 386: 552–561. [DOI] [PubMed] [Google Scholar]
- 28. Bissonnette R, Iversen L, Sofen H et al Tofacitinib withdrawal and retreatment in moderate‐to‐severe chronic plaque psoriasis: a randomized controlled trial. Br J Dermatol 2015; 172: 1395–1406. [DOI] [PubMed] [Google Scholar]
- 29. Papp KA, Menter A, Abe M et al Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo‐controlled, phase III trials. Br J Dermatol 2015; 173: 949–961. [DOI] [PubMed] [Google Scholar]
- 30. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006; 54: 2665–2673. [DOI] [PubMed] [Google Scholar]
- 31. Rich P, Scher RK. Nail Psoriasis Severity Index: a useful tool for evaluation of nail psoriasis. J Am Acad Dermatol 2003; 49: 206–212. [DOI] [PubMed] [Google Scholar]
- 32. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 33. Saurat JH, Stingl G, Dubertret L et al Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol 2008; 158: 558–566. [DOI] [PubMed] [Google Scholar]
- 34. Papp K, Cather JC, Rosoph L et al Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet 2012; 380: 738–746. [DOI] [PubMed] [Google Scholar]
- 35. Papp KA, Kaufmann R, Thaci D, Hu C, Sutherland D, Rohane P. Efficacy and safety of apremilast in subjects with moderate to severe plaque psoriasis: results from a phase II, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group, dose‐comparison study. J Eur Acad Dermatol Venereol 2013; 27: e376–e383. [DOI] [PubMed] [Google Scholar]
- 36. Reich K, Nestle FO, Papp K et al Infliximab induction and maintenance therapy for moderate‐to‐severe psoriasis: a phase III, multicentre, double‐blind trial. Lancet 2005; 366: 1367–1374. [DOI] [PubMed] [Google Scholar]
- 37. Gordon KB, Langley RG, Leonardi C et al Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double‐blind, randomized controlled trial and open‐label extension study. J Am Acad Dermatol 2006; 55: 598–606. [DOI] [PubMed] [Google Scholar]
- 38. Leonardi CL, Kimball AB, Papp KA et al Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 76‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 1). Lancet 2008; 371: 1665–1674. [DOI] [PubMed] [Google Scholar]
- 39. Papp KA, Langley RG, Lebwohl M et al Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 52‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 2). Lancet 2008; 371: 1675–1684. [DOI] [PubMed] [Google Scholar]
- 40. Langley RG, Elewski BE, Lebwohl M et al Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med 2014; 371: 326–338. [DOI] [PubMed] [Google Scholar]
- 41. Kavanaugh A, Mease PJ, Gomez‐Reino JJ et al Treatment of psoriatic arthritis in a phase 3 randomised, placebo‐controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis 2014; 73: 1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schett G, Wollenhaupt J, Papp K et al Oral apremilast in the treatment of active psoriatic arthritis: results of a multicenter, randomized, double‐blind, placebo‐controlled study. Arthritis Rheum 2012; 64: 3156–3167. [DOI] [PubMed] [Google Scholar]
- 43. Kavanaugh A, McInnes I, Mease P et al Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty‐four‐week efficacy and safety results of a randomized, placebo‐controlled study. Arthritis Rheum 2009; 60: 976–986. [DOI] [PubMed] [Google Scholar]
- 44. Mease PJ, Gladman DD, Ritchlin CT et al Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double‐blind, randomized, placebo‐controlled trial. Arthritis Rheum 2005; 52: 3279–3289. [DOI] [PubMed] [Google Scholar]
- 45. Ritchlin C, Rahman P, Kavanaugh A et al Efficacy and safety of the anti‐IL‐12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non‐biological and biological anti‐tumour necrosis factor therapy: 6‐month and 1‐year results of the phase 3, multicentre, double‐blind, placebo‐controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 2014; 73: 990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mease PJ, Kivitz AJ, Burch FX et al Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 2004; 50: 2264–2272. [DOI] [PubMed] [Google Scholar]
- 47. Kavanaugh A, Krueger GG, Beutler A et al Infliximab maintains a high degree of clinical response in patients with active psoriatic arthritis through 1 year of treatment: results from the IMPACT 2 trial. Ann Rheum Dis 2007; 66: 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tanaka Y, Takeuchi T, Yamanaka H, Nakamura H, Toyoizumi S, Zwillich S. Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: a 12‐week, randomized, phase 2 study. Mod Rheumatol 2015; 25: 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Basra MK, Salek MS, Camilleri L, Sturkey R, Finlay AY. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Br J Dermatol 2015; 230: 27–33. [DOI] [PubMed] [Google Scholar]
- 50. Gupta AK, Cooper EA. Psoriatic nail disease: quality of life and treatment. J Cutan Med Surg 2009; 13(Suppl 2): S102–S106. [DOI] [PubMed] [Google Scholar]
- 51. Krueger J, Suarez‐Farinas M, Fuentes‐Duculan J et al Pathologic immune pathways in psoriasis are rapidly attenuated by tofacitinib treatment. Br J Dermatol 2014; 171: e120. [Google Scholar]
- 52. Winthrop KL, Yamanaka H, Valdez H et al Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol 2014; 66: 2675–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Winthrop K, Lebwohl M, Cohen AD et al Herpes zoster and tofacitinib therapy in patients with psoriasis. J Invest Dermatol 2015; 135: S1. [Google Scholar]
- 54. Yamaoka K, Tanaka Y, Morishima Y et al Herpes zoster and tofacitinib therapy in Japanese patients with rheumatoid arthritis. Japan College of Rheumatology – 59th Annual Scientific Meeting 2015; 25: S96. [Google Scholar]
- 55. Toyama N, Shiraki K. Epidemiology of herpes zoster and its relationship to varicella in Japan: a 10‐year survey of 48 388 herpes zoster cases in Miyazaki prefecture. J Med Virol 2009; 81: 2053–2058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Principal study investigators at each center.


