Abstract
Cell growth and differentiation are often driven by subtle changes in gene expression. Many challenges still exist in detecting these changes, particularly in the context of a complex, developing tissue. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) allows relatively high-throughput evaluation of multiple genes and developmental time points. Proper quantification of gene expression levels by qRT-PCR requires normalization to one or more reference genes. Traditionally, these genes have been selected based on their presumed “housekeeping” function, with the implicit assumption that they are stably expressed over the entire experimental set. However, this is rarely tested empirically. Here we describe the identification of novel reference genes for the mouse mammary gland based on their stable expression in published microarray datasets. We compared eight novel candidate reference genes (Arpc3, Clock, Ctbp1, Phf7, Prdx1, Sugp2, Taf11 and Usp7) to eight traditional ones (18S, Actb, Gapdh, Hmbs, Hprt, Rpl13a, Sdha and Tbp) and analysed all genes for stable expression in the mouse mammary gland from pre-puberty to adulthood using four different algorithms (GeNorm, DeltaCt, BestKeeper and NormFinder). Prdx1, Phf7 and Ctbp1 were validated as novel and reliable, tissue-specific reference genes that outperform traditional reference genes in qRT-PCR studies of postnatal mammary gland development.
One of the challenges in developmental biology is to understand how subtle changes in gene expression drive growth and differentiation of complex tissues. The mouse mammary gland serves as a prime example of a dynamic and complex tissue that harbours many different cell types. During embryonic development, the mammary placode develops into a rudimentary mammary epithelial tree1,2. After birth, this epithelial tree remains relatively quiescent until the onset of puberty, when the epithelium grows out via branching morphogenesis and invades the surrounding fat pad. By the time the mouse reaches adulthood, the ducts have reached the end of the fat pad. However, dynamic growth and differentiation do not stop at this stage. Adult mice undergo a complete estrus cycle every 4–5 days, and during this time the epithelium also expands and regresses. Furthermore, during pregnancy the gland forms large alveolar structures that will produce milk during lactation once fully differentiated. After lactation, the mammary gland involutes and is reshaped back to a virgin-like state. This cycle can repeat itself multiple times throughout the reproductive life of an animal. As a result, during postnatal development the mammary gland does not only dramatically change in size, but also in cell type composition and differentiation status3,4.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) is a highly sensitive method for measuring changes in gene expression across multiple experimental conditions. However, even with current technologies it remains challenging to compare different developmental timepoints5,6,7,8,9,10,11, due to changes in tissue composition. To control for technical errors, individual samples are typically normalized against the expression of one or more internal reference genes. The ideal reference gene shows stable expression across the entire experimental dataset and is not influenced by any of the experimental parameters. This ensures that changes in reference gene expression are only due to technical variation such as pipetting errors and differences in sample input. When analysing genes that show subtle expression differences, as is the case for many developmental regulators, even small changes in reference gene expression can lead to aberrant results. It is therefore important to use the most stably expressed reference possible. Including more than one reference gene for the analyses further minimizes the effect of individual reference gene expression variation. While not yet commonplace, the use of at least three bona-fide reference genes is advised for proper normalization12.
Reference genes have historically been picked based on their presumed “housekeeping” function, ensuring that they would be abundantly expressed and easily detectable. However, traditionally used reference genes such as Gapdh, 18S and Actb can change expression in response to experimental treatment and might therefore not be appropriate to use under all circumstances13. Attempts have been made to identify universal reference genes, which could be applied to any sample of interest irrespective of its developmental origin, by comparing published datasets of multiple different human tissues and cell lines14,15,16,17,18. However, so far this has failed to yield a consistent list of candidate genes, raising the question whether such universal references exist at all19. Therefore, finding the best performing reference genes requires a dedicated effort focussing on the specific tissue or organism of interest5,7,20,21,22,23,24.
Because of the dynamic growth and differentiation properties of the mammary gland, it is particularly challenging to find genes that can serve as reliable reference genes across different stages of postnatal development. In 2010, Han and colleagues ranked the stability of eight commonly used reference genes for qRT-PCR studies of the mouse mammary gland25. However, their study focussed on the pregnancy and lactation stages in the adult only. Moreover, a single algorithm was used to rank the genes, while several algorithms are currently available to provide a more comprehensive overview of reference gene stability and sensitivity12,26,27,28,29.
Here we describe the identification of novel candidate reference genes for the mouse mammary gland based on their stable expression in multiple microarray datasets. We validate Ctbp1, Phf7 and Prdx1 as tissue-specific reference genes. They show stable expression across multiple stages of postnatal mammary gland development and allow the detection of subtle changes in Wnt gene expression in whole mammary gland RNA preparations. As such, they outperform traditionally used reference genes and are exquisitely suited for comparative qRT-PCR analyses in this complex developing tissue.
Results
Selection of novel candidate reference genes from published microarray data
The mammary gland is composed of multiple different cell types, including basal and luminal epithelial cells, stromal fibroblasts and adipocytes, as well as cells contributing to other structures in the gland such as nerves and blood vessels. In addition, the gland shows dynamic growth and differentiation during puberty, pregnancy and lactation, as well as extensive tissue remodelling during involution (Fig. 1). To date, no dedicated attempt has been made to determine whether suitable reference genes exist for use in qRT-PCR studies of whole mammary gland preparations across all stages of postnatal development. Therefore, we set out to identify novel, tissue-specific candidate reference genes that are stably expressed from pre-puberty through puberty and adulthood by analyzing published expression datasets.
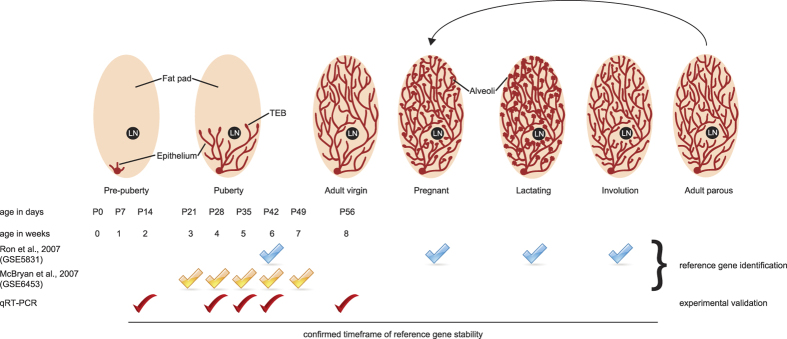
Figure 1. Postnatal development of the mouse mammary gland.
Top: scheme illustrating developmental dynamics of the mammary gland. Before puberty, the fat pad only contains a rudimentary epithelial tree that starts from the nipple. During puberty, the epithelial tree invades the surrounding fat pad. This process is driven by highly proliferative structures called terminal end buds (TEBs). At the start of adulthood, the epithelial tree has fully invaded the fat pad and the TEBs regress. During each pregnancy, the gland forms alveoli for milk production during lactation. After weaning of the pups, the epithelial tree reverts back to a virgin-like state in a process called involution. LN = lymph node. Bottom: overview of the experimental time points used to generate the microarray expression data sets GSE5831 and GSE6453, as well as the time points used for qRT-PCR validation experiments.
We identified two gene expression profiling studies in the NCBI gene expression omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) that compared different stages of mammary gland development (Fig. 1). Both datasets (GSE583130 and GSE645331) used microarrays to analyse the whole mammary gland transcriptome, but the studies were performed by different labs, on different mouse strains (C57BL6/J and CD-1, respectively) and using different array platforms. Furthermore, one experiment (GSE5831) compared adult stages (6 week old virgins, pregnancy day 14, lactation day 10 and involution day 4), whereas the other (GSE6453) compared pubertal time points (3, 4, 5, 6 and 7 week old females). We hypothesized that any gene that would be stably expressed across all of these arrays could qualify as a suitable reference gene. To identify these genes, we compared physiologically divergent stages using GEO2R, an interactive webtool that allows gene expression analysis of published microarray datasets using the GEOquery and limma R packages32.
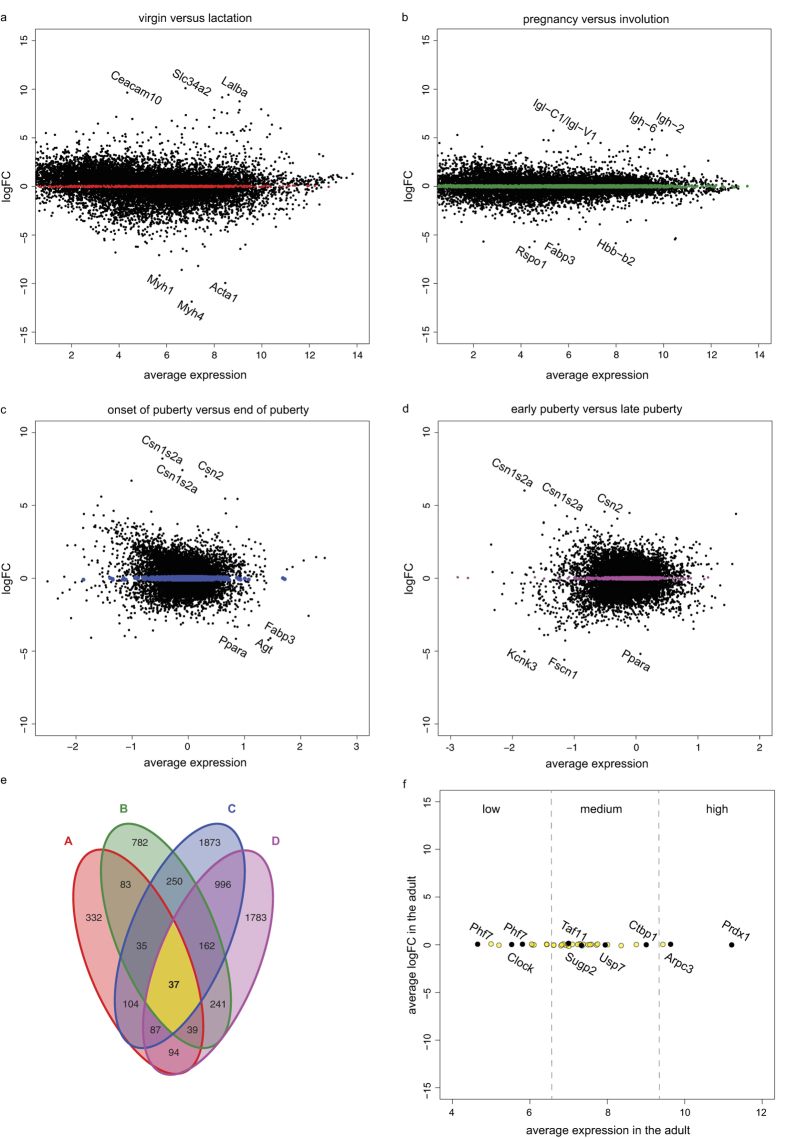
First, we performed differential gene expression analysis of GSE5831 for the virgin and lactating, terminally differentiated gland. We selected genes with a log fold change (logFC) between −0.1 and 0.1, thus reflecting stable expression between the two conditions (Fig. 2a). Next, we performed a similar analysis for the pregnant (actively growing) and involuting (regressing) gland (Fig. 2b). Of the 852 and 1754 genes that showed stable expression in the virgin/lactating and pregnant/involuting comparisons, respectively, only a subset (194 genes) approached a logFC of 0 in both.
Figure 2. Identification of novel candidate reference genes.
(a–d) MA plots for GSE5831 (a,b) and GSE6453 (c,d), comparing the virgin versus the lactating gland (a), the pregnant versus the involuting gland (b), the onset versus the end of puberty (3 versus 7 weeks, c) and early versus late puberty (4 weeks versus 6 weeks, d). The top 3 up- and downregulated genes for each of the four comparisons are indicated. Genes with a log fold change (logFC) between −0.1 and 0.1 are highlighted. (e) Venn diagram showing the overlap of genes with a logFC between −0.1 and 0.1 identified in a–d. Only 37 genes are shared between all four comparisons. (f) Ranking of the 37 genes with a logFC between −0.1 and 0.1 according to their average expression level in the GSE5831 dataset. The names of the candidate reference genes that were picked for further testing are highlighted.
To narrow down the number of potential candidate reference genes, we then performed differential gene expression analysis of GSE6453 for different pubertal stages, comparing the start and end of puberty (3-week versus 7-week old mice, Fig. 2c) as well as early and late puberty (4-week versus 6-week old mice, Fig. 2d). This resulted in a list of 37 genes that showed stable expression across all of the tested conditions (Fig. 2e and Supplementary Table S1). From this list, we selected a final set of 8 putative reference genes (Arpc3, Clock, Ctbp1, Phf7, Prdx1, Sugp2, Taf11 and Usp7, Fig. 2f). We made certain that the resulting candidates represented a variety of expression levels (Fig. 2f) and divergent functions as predicted by gene ontology analysis (Supplementary Table S1), and ensured that gene-specific qRT-PCR primers with unique, intron-spanning sequences and sufficient amplification efficiency could be designed (Supplementary Table S2).
Variation in expression levels of candidate reference genes
Previously, Han and colleagues investigated the stability of eight commonly used reference genes in the pregnant and lactating mouse mammary gland25. To determine whether any of these genes are also stably expressed over a larger developmental timeframe, we selected four of the most stable genes identified in this study (Rpl13a, Hprt, Gapdh and Actb) as well as four additional commonly used reference genes33,34,35 (18S, Hmbs, Tbp and Sdha) as a “traditional reference gene set”.
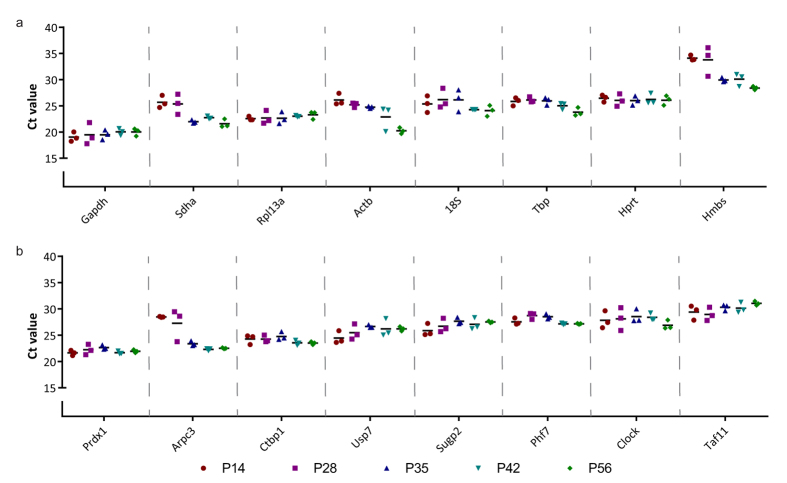
To test the variation in expression levels of the traditional and novel candidate reference genes over a large developmental time course, we performed qRT-PCR analyses on cDNA synthesized from whole mouse mammary gland RNA preparations ranging from pre-puberty (P14) to early, mid- and late puberty (P28, P35 and P42) and adult virgin FVB mice (P56). When the expression levels (Ct values) of all sixteen (candidate) reference genes are compared, variable expression patterns across the different stages of development emerge for both the traditional (Fig. 3a) and the newly identified (Fig. 3b) genes. Some genes show a relatively stable pattern throughout, with all Ct values clustering closely together irrespective of the developmental time point. For instance, across pre-puberty and adulthood the Ct values for Prdx1 and Ctbp1 show a total spread of only 2.0 and 1.9 cycles, respectively. Other genes show more variation and are therefore probably less suitable as reference genes for our purpose. As an example, the expression of Hmbs and Actb shows a spread of 8.0 and 7.7 cycles, respectively. The 3rd thoracic gland was analysed as a biological replicate and shows a similar pattern (Supplementary Fig. S1).
Figure 3. Ct values of reference genes for biological replicas of #4 mammary gland.
Scatter plot depicting Ct values for all sixteen reference genes in the 4th inguinal mammary gland over a developmental time series from pre-puberty (P14) to puberty (P28, P35 and P42) and adult (P56). Each data point represents a single biological replicate. (a) Traditionally used reference genes. (b) Novel candidate reference genes. Genes were plotted in order of their absolute expression levels, from high expression (lowest Ct value) to low expression (n = 3 biological replicates for each developmental time point).
GeNorm stability analysis of candidate reference genes
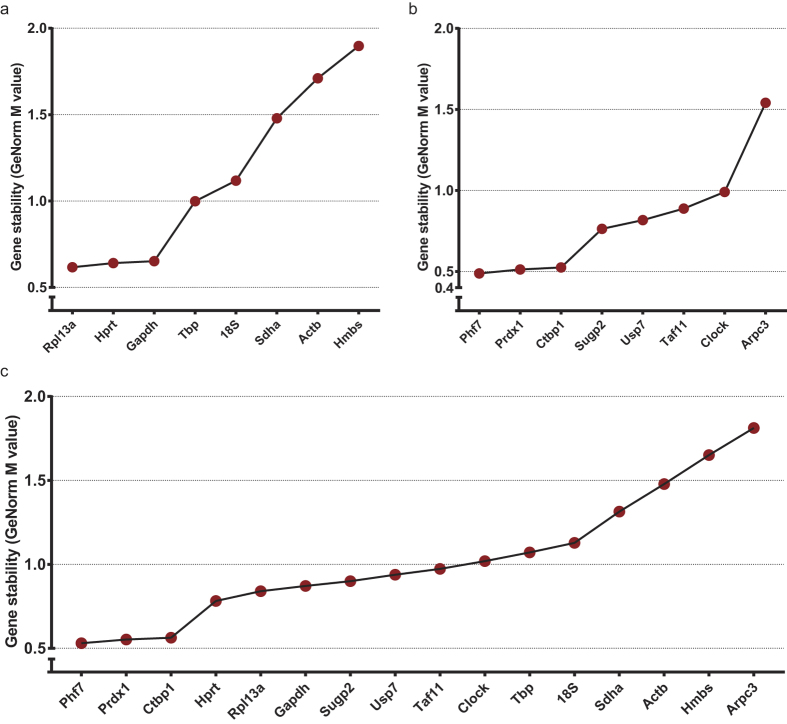
Reference genes can be ranked based on their expression stability over all samples using the GeNorm algorithm12. The underlying principle of this algorithm is that ideal reference genes show stable expression patterns regardless of the experimental parameters (in our case: different developmental time points). When multiple bona-fide reference genes are analysed, their average expression should best represent the “true” normalisation factor. Therefore, GeNorm not only scores for stable expression per candidate, but also for gene expression variation of each individual reference compared to the average reference gene expression. By performing a pairwise variation of any candidate reference gene compared to the average expression of the entire pool, a so-called M-value is calculated for each gene, which serves as a measure for gene stability: the lower the value, the more stably a gene is expressed. Next, the genes are ranked based on M-value, by performing stepwise exclusion of the gene with the highest M-value (i.e. poorest stability). M-value calculation and stepwise exclusion are repeated until only two genes remain. These final two genes are ranked based on their individual stability value.
M-values should be as low as possible. However, they strongly depend on the complexity of the experimental samples and fixed criteria or cut offs do not exist36. For homologous samples, such as cell lines, the M-value can drop below 0.2. In contrast, for complex tissues M-values of 0.5 or even >1.0 have been reported6,7,8,23,37. When the traditional gene set was analysed, Rpl13a, Hprt and Gapdh came out as the best references, with an M-value of 0.62 for Rpl13a (Fig. 4a). GeNorm analysis for the novel candidates revealed Phf7, Prdx1 and Ctbp1 as best performing references in this set (Fig. 4b). The M-values for these genes (0.49, 0.51 and 0.53, respectively) are lower than the best M-value for the traditional gene set. Combined analysis of both novel (Arpc3, Clock, Ctbp1, Phf7, Prdx1, Sugp2, Taf11 and Usp7) and traditional (18S, Actb, Gapdh, Hbms, Hprt, Rpl13a, Sdha and Tbp) reference genes confirms that Phf7, Prdx1 and Ctbp1 rank as the most stable genes across all developmental time points analysed, outperforming Rpl13a, Hprt and Gapdh (Fig. 4c).
Figure 4. Reference gene ranking according to GeNorm.
Graph showing candidate reference gene ranking according to the M-value for gene expression stability in the developing mammary gland. (a) Ranking of eight traditional reference genes. (b) Ranking of eight novel candidate reference genes. (c) Comprehensive ranking of the traditional and candidate reference gene sets. Ctbp1, Phf7 and Prdx1 have a lower M-value, indicating higher stability.
Comprehensive ranking of Prdx1, Phf7 and Ctbp1 as the most stable reference genes in the postnatal mammary gland
For a more comprehensive overview of reference gene suitability, we used the web-based tool RefFinder26 to rank the candidate reference genes according to three additional algorithms: DeltaCt29, BestKeeper27 and NormFinder28. DeltaCt ranks genes based only on the spread in Ct values (similar as depicted in Fig. 3), considering the gene with the least variation to be the best ranking reference. BestKeeper performs pairwise variations for all possible pairs of reference genes and calculates a geometric mean to determine which genes have highly similar expression profiles, creating a ranking based on the fit of the geometric means. NormFinder analyses the variance in expression data of all given candidate reference genes, with the assumption that an ideal reference gene should only differ mildly from the overall estimate.
The different approaches from the above mentioned algorithms lead to small differences in their respective reference gene rankings, but ideally the overall trend should be the same. Table 1 shows the comprehensive ranking of all novel candidates and traditional reference genes for the different algorithms. Indeed, the results confirm the GeNorm analysis: Arpc3, Hmbs, Actb and Sdha rank poorly for all algorithms and are thus considered to be less suitable reference genes in all tests. In contrast, Prdx1, Phf7 and Ctbp1 are ranked in the top four in all tests, complemented with either Tbp, Hprt or Rpl13a depending on the algorithm. A comprehensive ranking of the results from all four algorithms thus confirms Prdx1, Phf7 and Ctbp1 as the top three reference genes (Table 1 and Supplementary Fig. S2).
Table 1. Reference gene ranking according to four algorithms.
| Ranking | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta CT | Prdx1 | Ctbp1 | Phf 7 | Tbp | Hprt | Rpl13a | Sugp2 | Gapdh | Clock | 18S | Usp7 | Taf11 | Sdha | Actb | Hmbs | Arpc3 |
| BestKeeper | Prdx1 | Ctbp1 | Rpl13a | Phf 7 | Tbp | Hprt | Sugp2 | Gapdh | Taf11 | Usp7 | Clock | 18S | Sdha | Actb | Hmbs | Arpc3 |
| NormFinder | Phf 7 | Tbp | Prdx1 | Ctbp1 | Hprt | Rpl13a | Clock | Sugp2 | Gapdh | 18S | Usp7 | Taf11 | Sdha | Actb | Hmbs | Arpc3 |
| GeNorm | Phf 7 | Prdx1 | Ctbp1 | Hprt | Rpl13a | Gapdh | Sugp2 | Usp7 | Taf11 | Clock | Tbp | 18S | Sdha | Actb | Hmbs | Arpc3 |
| Overall ranking | Prdx1 | Phf 7 | Ctbp1 | Tbp | Rpl13a | Hprt | Sugp2 | Gapdh | Clock | Usp7 | Taf11 | 18S | Sdha | Actb | Hmbs | Arpc3 |
DeltaCt, BestKeeper, NormFinder and GeNorm were used to rank reference genes for suitability. Ranking was performed in RefFinder (DeltaCt, BestKeeper and NormFinder) or qBase + (GeNorm). Individual rankings are shown in the top four rows. The overall comprehensive ranking is shown in the bottom row. A similar analysis performed separately on the traditional and the novel reference gene sets is presented in Supplementary Fig. S2.
Validation of novel reference genes by qRT-PCR analysis of Wnt gene expression
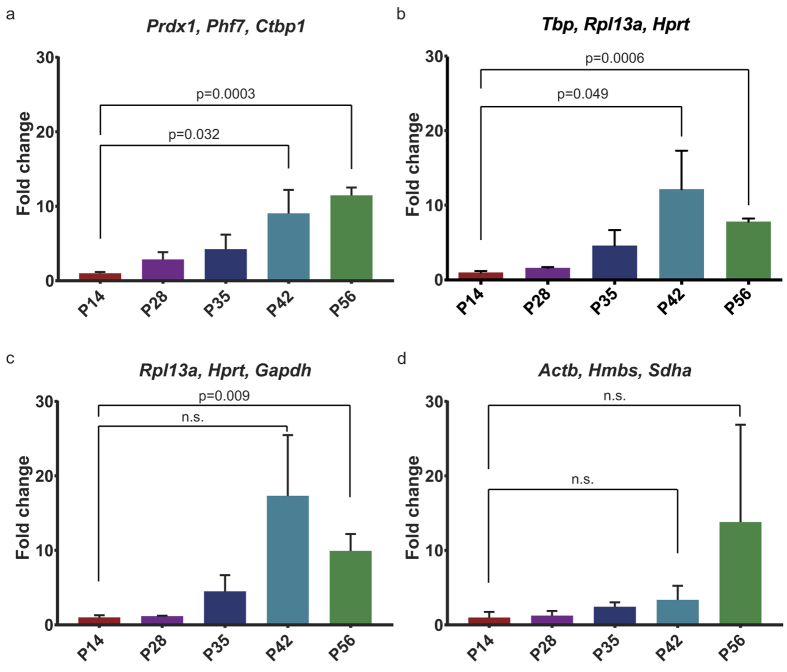
To validate that Prdx1, Phf7 and Ctbp1 can be used as reliable reference genes for the postnatal mouse mammary gland, we tested their ability to reveal subtle changes in gene expression across mammary gland development. For this purpose, we focused on Wnt gene expression. Wnt signalling is crucial for mammary gland development and function, and Wnt-responsive stem cells reside in the mammary gland from an early time point on refs 38, 39, 40. Multiple Wnt genes have previously been shown to be expressed in the mammary gland, but so far, Wnt gene expression has mainly been studied using semi-quantitative methods34,41. Furthermore, Wnt genes are typically expressed at relatively low levels, making it challenging to detect expression level changes in RNA preparations from the whole mammary gland. Wnt4 is known to be expressed in the adult mammary gland, where it plays a role in the estrus cycle42 and in side-branching during pregnancy43. A recent study also revealed a role for Wnt4 in branching morphogenesis during puberty44, but if and how Wnt4 expression levels change during postnatal mammary gland development remains incompletely understood. To this end, we quantified Wnt4 expression across pre-puberty, puberty and adulthood. Only when Prdx1, Phf7 and Ctbp1 (our set of novel and preferred reference genes) are used for normalization, a steady increase in Wnt4 levels is revealed between P14 and P56 (Fig. 5a). Subtle changes in Wnt4 expression are also apparent when Tbp, Rpl13a and Hprt (the most reliable traditional reference genes) are used as a reference geneset (Fig. 5b). Using a set of even slightly less stable reference genes (Rpl13a, Hprt and Gapdh) already obscures this trend (Fig. 5c). Finally, when we normalized Wnt4 expression to the three commonly used reference genes that were least stable in our analysis (Actb, Hmbs and Sdha), no statistically significant changes can be detected between the different developmental stages at all (Fig. 5d). This underscores the importance of using properly validated reference genes for the tissue of choice and confirms that the new reference gene set comprised of Prdx1, Phf7 and Ctbp1 allows small changes in developmental gene expression to be detected with high precision in a complex mammary tissue preparation.
Figure 5. Reference gene validation.
Bar graphs showing qRT-PCR analysis of Wnt4 expression in the developing mouse mammary gland between P14 and P56. The lowest value (P14) was set to 1. (a) Relative Wnt4 expression normalised to Prdx1, Phf7 and Ctbp1, which were identified as novel reference genes in this study. (b) Relative Wnt4 expression normalised to Rpl13a, Hprt and Tbp, which were identified as the best traditional reference genes in this study. (c) Relative Wnt4 expression normalised to Rpl13a, Hprt and Gapdh, which were previously identified as the best reference genes for the mouse mammary gland25. (c) Relative Wnt4 expression normalised to Actb, Hmbs and Sdha. Although frequently used as references, these genes rank poorly in our stability analyses. Statistically significant differences are indicated with their respective p-values. Error bars represent SEM (n = 3 biological replicates for each developmental time point). n.s. = not statistically significant.
Discussion
Traditionally, reference genes used for normalization purposes in qRT-PCR experiments have been chosen based on their supposed role in cellular housekeeping processes. However, these genes do not perform well as reference genes in all settings, especially when complex tissues or larger developmental time series are concerned33. It is therefore important to critically select and evaluate candidate reference genes for each specific experimental condition.
In this study we report the identification and validation of novel reference genes for qRT-PCR analysis of a wide range of postnatal stages of mouse mammary gland development based on published mammary gland microarray expression data.
Efforts to detect novel reference genes for a diverse set of human samples by comparing large published datasets have been made before14,15,16,17,18. A study by de Jonge et al.16 analysed an astonishing number of 13.629 human microarray sets to determine which genes are stably expressed. The resulting genes, mostly encoding ribosomal proteins, showed improved stability values over traditionally used reference genes such as GAPDH, ACTB and HPRT. The top ranking genes for human samples also showed high stability values in mouse microarray data16. However, the resulting genes were not analysed for tissue-specificity and were not experimentally validated for the mouse mammary gland. Moreover, since almost all of the identified candidate reference genes were involved in very similar biological processes, they may be co-regulated, making it more difficult to test their suitability as true reference genes12.
Since reference gene stability can be particularly variable depending on the biological data set, reference genes should be validated on any organism- and tissue-specific experimental setting. We employed two separate microarray expression sets to obtain gene expression data for the mouse mammary gland at multiple developmental time points (puberty, pregnancy, lactation and involution) and for two different mouse strains30,31. We hypothesized that any gene showing stable expression across all these different conditions, would serve as a suitable reference gene for the postnatal mouse mammary gland. A total of 37 genes met our selection criteria (i.e. a logFC between −0.1 and 0.1 in four different cross comparisons). Of note, our approach allows the identification of both low, medium and highly expressed genes (Fig. 2f), unlike traditional “housekeeping” genes, which are often expressed at significantly higher levels than the experimental genes of interest. A final set of eight novel candidate reference genes was selected based on average expression levels, amplification efficiency and gene ontology analysis (Supplementary Table S1). The latter was performed to reduce the chance of selecting co-regulated genes with similar functions.
The resulting eight candidate reference genes were Arpc3, Clock, Ctbp1, Phf7, Prdx1, Sugp2, Taf11 and Usp7. We compared the performance of these genes to eight traditional reference genes (Actb, Gapdh, Hprt, Rpl13a, 18S, Hmbs, Sdha and Tbp) using the GeNorm, DeltaCt, BestKeeper and NormFinder algorithms to determine the most stably expressed reference genes (Fig. 4 and Table 1)12,27,28,29. Out of the traditional reference gene set, Tbp, Rpl13a and Hprt perform better than the previously suggested combination of Gapdh, Rpl13a and Hprt25. However, our newly identified genes Prdx1, Phf7 and Ctbp1 rank as most stable overall. In addition, they are also in the top four of most stable reference genes in terms of their individual rankings (Table 1 and Supplementary Figure S2) and show a small spread in Ct values (Fig. 3), further supporting their suitability as reference genes in the mouse mammary gland. Interestingly, CTBP1 and PRDX1 have previously been found to be stably expressed in a variety of human samples, suggesting they may be more widely applicable17,18,19. In fact, PRDX1 was recently shown to be the only reference gene commonly identified in fifteen published human reference gene studies19.
By using bona-fide and highly stable reference genes, even subtle changes in developmental gene expression can be picked up by qRT-PCR on a complex tissue sample (Fig. 5). Furthermore, in concordance with published guidelines for performing qRT-PCR experiments36,45 the use of three reference genes minimizes errors. Most qRT-PCR software programs accept the use of multiple reference genes for normalization. Alternatively, the geometric mean of three (or more) reference genes can be used for normalization, making this a feasible approach for most researchers today46. However, our validation experiment stresses the importance of selecting the most stable reference gene set (Fig. 5). In this respect, Prdx1, Phf7 and Ctbp1 perform better over a large developmental time series than previously identified reference genes for the mouse mammary gland25.
The fact that Prdx1, Phf7 and Ctbp1 outperform traditionally used reference genes, demonstrates the feasibility of our strategy to select tissue-specific, stably expressed genes based on existing microarray data. However, it should be noted that not all candidates showed stable expression upon further testing. In fact, Arpc3 is one of the less well performing reference genes. One explanation is that our experimental samples contain an earlier developmental time point than the microarray datasets used to identify candidate genes. Arpc3 expression in 2-week old mice (P14) deviates from the expression values at the other time points (Fig. 3b). Another explanation is that strain-specific differences could play a role. Candidates were identified based on microarray experiments on C57BL/6 and CD-1 tissues, but our experimental validation was performed on FVB tissues. This further underscores the importance of testing and validating candidate reference genes, even if they are tissue-specific.
In conclusion, large datasets such as microarray or RNAseq analyses allow the selection of tissue-specific reference genes, although these should always be tested experimentally for stability in the biological samples of interest. Using this approach, we have identified Prdx1, Phf7 and Ctbp1 as novel, tissue-specific reference genes for qRT-PCR studies of the postnatal mouse mammary gland, and validated that this reference gene set is suitable for detecting subtle gene expression changes that occur during the pre-pubertal, pubertal and adult stages.
Materials and Methods
Animals
FVB/N mice were purchased from Envigo. Breeding for the appropriate developmental time points (P14, P28, P35, P42 and P56) was performed in-house. Mice were housed in open conventional cages on a 12 h light/dark cycle and received food and water ad libitum. All experiments were performed in accordance with institutional and national guidelines and regulations. All experimental protocols were approved by the Animal Welfare Committee of the University of Amsterdam. As biological replicates, n = 3 animals were used for each developmental time point.
Selection of candidate reference genes
A total of eight reference genes commonly used in qRT-PCR analyses for mouse tissues for which primers were already available were selected as “traditional” reference genes. Another set of eight novel candidate reference genes was selected from published mammary gland microarray datasets (GEO accession numbers GSE583130 and GSE645331) as described in the text. Briefly, GEO2R analysis was performed in RStudio using the GEOquery32 and limma47 R packages from the Bioconductor project to determine the log fold change (logFC) between virgin and lactating mice (GSE5831), pregnant and involuting mice (GSE5831), 3 versus 7 week old mice (GSE6453) and 4 versus 6 week old mice (GSE6453).
Genes with a logFC > −0.1 | <0.1 in each of these comparisons were selected as putative tissue-specific reference genes. These gene lists are provided in Supplementary Table S1. Gene ontology term analysis was performed using the “biological process” and “molecular function” GO terms from the GEO2R analysis to ensure genes with different cellular functions were used to build the final set of eight candidate reference genes to prevent the selection of co-regulated genes.
Primer design and validation
Primer sequences for the eight traditional reference genes were already available in the lab. Novel primers were selected from Primerbank (https://pga.mgh.harvard.edu/primerbank/) when available or designed with the Roche Universal Probe Library Assay Design Centre (https://lifescience.roche.com/). Only intron-spanning primer pairs were used. Specific amplification was confirmed by a single peak after melting curve analysis, a single band of appropriate size on an agarose gel and sequence verification. The amplification efficiency of each primer pair was determined by generating standard curves, using different dilutions of mixed mammary gland cDNA samples as input (see below). The amplification efficiency of the primers was calculated from the slope of the standard curve using Biogazelle qBase + software. All primer sequences, amplicon lengths and amplification efficiencies are available in Supplementary Table S2.
RNA isolation and cDNA synthesis
Total RNA was isolated from the pooled left and right #4 (inguinal) mammary glands of each biological replicate (n = 3 for each time point) using TRIzol reagent (Fisher Scientific) according to the manufacturer’s guidelines. RNA from the pooled left and right #3 mammary glands was isolated separately. Residual genomic DNA was digested by RQ1 RNAse-free DNAse treatment (Promega) according to the manufacturer’s instructions. The RNA concentration was determined using a Nanodrop spectrophotometer. The purity of all samples was assessed by the absorbance ratios of OD260/280 and OD260/230. cDNA was synthesized from 2 μg RNA using SuperScript® II Reverse Transcriptase (Invitrogen) and Random Hexamers (Fisher Scientific), according to the manufacturer’s instructions. The cDNA was diluted 10-fold for subsequent qRT-PCR analysis. Standard curves were generated by diluting the cDNA 2-, 5-, 10-, 100- and 1000-fold.
Quantitative Real-time PCR (qRT-PCR)
qRT-PCR was performed using an Applied Biosystems 7500 Real-Time PCR System. PCR reactions (total 20 μl) were set up containing 13 μl RNAse-free H2O, 4 μl 5× HOT FIREPol® EvaGreen® qRT-PCR Mix Plus ROX (Solis Biodyne), 0.5 μl of each specific forward and reverse primer (10 μM stock) and 2 μl of diluted cDNA template. The reactions were set up in technical triplicates in 96-well PCR plates. One negative control (no-RT) reaction was included for each sample/primer combination. Thermal cycling was performed, starting with an initial step at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s and annealing at 60 °C for 60 s. Each run was completed with a melting curve analysis.
Testing expression stability of candidate reference genes
Raw Ct values were used for GeNorm analysis using the Biogazelle qBase + software12. All technical triplicates were included to calculate a single Ct value for each gene and biological sample. To minimize the effect of spread within technical replicates, Ct values were calculated based on the median48. Expression stability values (M) were calculated as described in the text.
Median Ct values of the technical triplicates were also used as import for the web-based tool RefFinder26 (http://fulxie.0fees.us). The resulting rankings for the DeltaCt, BestKeeper and NormFinder algorithms were used to assign an appropriate weight to each of the individual reference genes. To this end, all reference genes were given a value (1–16) for their position. The same was done for the GeNorm ranking derived from the qBase + software. An overall final ranking was calculated based on the geometric mean of their four individual positions.
Wnt4 expression analysis
Relative expression levels of Wnt4 were calculated by the comparative Delta-Ct method49,50, taking the different amplification efficiencies into account, and were normalized to three reference genes (either Prdx1, Phf7 and Ctbp1; or Rpl13a, Hprt and Tbp; or Rpl13a, Hprt and Gapdh; or Actb, Hmbs and Sdha) using the Biogazelle qBase + software. Statistical analyses were done using qBase + Statistics Wizard and the Analysis Toolpack Plugin for Microsoft Excel 2016. An F-test for variances was performed to test whether the data distribution was equal. Subsequently, a t-test was performed to test whether there was a difference between respective samples.
Additional Information
How to cite this article: van de Moosdijk, A. A. A. and van Amerongen, R. Identification of reliable reference genes for qRT-PCR studies of the developing mouse mammary gland. Sci. Rep. 6, 35595; doi: 10.1038/srep35595 (2016).
Supplementary Material
Acknowledgments
We thank Eva Naninck for providing primer sequences for some of the traditional reference genes and for initial help with the qBase + software, and Amber Zeeman for help with the animal breeding. We thank all lab members for fruitful discussions and Joachim Goedhart and Katrin Wiese for critical feedback on the manuscript. This research was funded by the University of Amsterdam (MacGillavry fellowship to R.v.A.), the Netherlands Organization for Scientific Research (NWO ALW VIDI 864.13.002 to R.v.A.) and the Dutch Cancer Society (KWF grants ANW 2013-6057 and 2015-8014 to R.v.A.).
Footnotes
Author Contributions A.A.A.v.d.M. and R.v.A. designed the study. A.A.A.v.d.M. performed the laboratory experiments. A.A.A.v.d.M. and R.v.A. analysed the data. A.A.A.v.d.M. and R.v.A. wrote the manuscript.
References
- Richert M. M., Schwertfeger K. L., Ryder J. W. & Anderson S. M. An atlas of mouse mammary gland development. J. Mammary Gland Biol. Neoplasia 5, 227–241 (2000). [DOI] [PubMed] [Google Scholar]
- Watson C. J. & Khaled W. T. Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development 135, 995–1003 (2008). [DOI] [PubMed] [Google Scholar]
- Visvader J. E. & Stingl J. Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes Dev. 28, 1143–1158 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias H. & Hinck L. Mammary gland development. Wiley Interdisciplinary Reviews: Developmental Biology 1, 533–557 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhorne-Pollet S., Thélie A. & Pollet N. Validation of novel reference genes for RT-qPCR studies of gene expression in Xenopus tropicalis during embryonic and post-embryonic development. Dev. Dyn. 242, 709–717 (2013). [DOI] [PubMed] [Google Scholar]
- Liu C. et al. Reference gene selection for quantitative real-time RT-PCR normalization in the half-smooth tongue sole (Cynoglossus semilaevis) at different developmental stages, in various tissue types and on exposure to chemicals. PLoS One 9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. et al. Selection of reference genes for RT-qPCR analysis in a predatory biological control agent, Coleomegilla maculata (Coleoptera: Coccinellidae). Sci. Rep. 5, 18201 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X., Zhang F., Tao Y., Song S. & Fang J. Reference gene selection for quantitative real-time PCR normalization in different cherry genotypes, developmental stages and organs. Sci. Hortic. (Amsterdam). 181, 182–188 (2015). [Google Scholar]
- Nakamura A. M. et al. Reference genes for accessing differential expression among developmental stages and analysis of differential expression of OBP genes in Anastrepha obliqua. Sci. Rep. 6, 17480 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z.-J., Tian C., Jiang Q., Li X.-H. & Zhuang J. Selection of suitable reference genes for qRT-PCR normalization during leaf development and hormonal stimuli in tea plant (Camellia sinensis). Sci. Rep. 6, 19748 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.-Y. et al. Validation and Comparison of Reference Genes for qPCR Normalization of Celery (Apium graveolens) at Different Development Stages. Front. Plant Sci. 7, 1–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen T. D. & Zakrajsek B. A. Effect of experimental treatment on housekeeping gene expression: Validation by real-time, quantitative RT-PCR. J. Biochem. Biophys. Methods 46, 69–81 (2000). [DOI] [PubMed] [Google Scholar]
- Jin P. et al. Selection and validation of endogenous reference genes using a high throughput approach. BMC Genomics 5, 55 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo A. et al. Statistical modeling for selecting housekeeper genes. Genome Biol. 5, R59 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge H. J. M. et al. Evidence based selection of housekeeping genes. PLoS One 2, 1–5 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Jo M., Lee J., Koh S. S. & Kim S. Identification of novel universal housekeeping genes by statistical analysis of microarray data. J. Biochem. Mol. Biol. 40, 226–231 (2007). [DOI] [PubMed] [Google Scholar]
- Kwon M. J. et al. Identification of novel reference genes using multiplatform expression data and their validation for quantitative gene expression analysis. PLoS One 4 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Li D. & Sun B. Do housekeeping genes exist? PLoS One 10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslak J. et al. Screening for the Most Suitable Reference Genes for Gene Expression Studies in Equine Milk Somatic Cells. PLoS One 10, e0139688 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzellitti S., Kiwan A., Valbonesi P. & Fabbri E. Selection of best-performing reference gene products for investigating transcriptional regulation across silvering in the European eel (Anguilla anguilla). Sci. Rep. 5, 16966 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petriccione M., Mastrobuoni F., Zampella L. & Scortichini M. Reference gene selection for normalization of RT-qPCR gene expression data from Actinidia deliciosa leaves infected with Pseudomonas syringae pv. actinidiae. Sci. Rep. 5, 16961 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliep M. et al. Evaluation of Reference Genes for RT-qPCR Studies in the Seagrass Zostera muelleri Exposed to Light Limitation. Sci. Rep. 5, 17051 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. C. et al. Evidence based selection of commonly used RT-qPCR reference genes for the analysis of mouse skeletal muscle. PLoS One 9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L. Q. et al. Selection and use of reference genes in mouse mammary glands. Genet. Mol. Res. 9, 449–456 (2010). [DOI] [PubMed] [Google Scholar]
- Xie F., Xiao P., Chen D., Xu L. & Zhang B. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 80, 75–84 (2012). [DOI] [PubMed] [Google Scholar]
- Pfaffl M. W., Tichopad A., Prgomet C. & Neuvians T. P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol. Lett. 26, 509–515 (2004). [DOI] [PubMed] [Google Scholar]
- Andersen C. L., Jensen J. L. & Ørntoft T. F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 64, 5245–5250 (2004). [DOI] [PubMed] [Google Scholar]
- Silver N., Best S., Jiang J. & Thein S. L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 7, 33 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron M. et al. Combining mouse mammary gland gene expression and comparative mapping for the identification of candidate genes for QTL of milk production traits in cattle. BMC Genomics 8, 183 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBryan J., Howlin J., Kenny P. a., Shioda T. & Martin F. ERalpha-CITED1 co-regulated genes expressed during pubertal mammary gland development: implications for breast cancer prognosis. Oncogene 26, 6406–6419 (2007). [DOI] [PubMed] [Google Scholar]
- Sean D. & Meltzer P. S. GEOquery: A bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics 23, 1846–1847 (2007). [DOI] [PubMed] [Google Scholar]
- Thellin O. et al. Housekeeping genes as internal standards: Use and limits. J. Biotechnol. 75, 291–295 (1999). [DOI] [PubMed] [Google Scholar]
- Silberstein G. B., Van Horn K., Hrabeta-Robinson E. & Compton J. Estrogen-triggered delays in mammary gland gene expression during the estrous cycle: Evidence for a novel timing system. J. Endocrinol. 190, 225–239 (2006). [DOI] [PubMed] [Google Scholar]
- Kosir R. et al. Determination of reference genes for circadian studies in different tissues and mouse strains. BMC Mol. Biol. 11, 60 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans J., Mortier G., De Paepe A., Speleman F. & Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8, R19 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins P. K. et al. Selection of reliable reference genes for RT-qPCR analysis during developmental stages and abiotic stress in Setaria viridis. Sci. Rep. 6, 28348 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen R., Bowman A. N. & Nusse R. Developmental Stage and Time Dictate the Fate of Wnt/β-Catenin-Responsive Stem Cells in the Mammary Gland. Cell Stem Cell 11, 387–400 (2012). [DOI] [PubMed] [Google Scholar]
- De Visser K. E. et al. Developmental stage-specific contribution of LGR5+ cells to basal and luminal epithelial lineages in the postnatal mammary gland. J. Pathol. 228, 300–309 (2012). [DOI] [PubMed] [Google Scholar]
- Wang D. et al. Identification of multipotent mammary stem cells by protein C receptor expression. Nature 517, 81–84 (2015). [DOI] [PubMed] [Google Scholar]
- Weber-Hall S. J., Phippard D. J., Niemeyer C. C. & Dale T. C. Developmental and hormonal regulation of Wnt gene expression in the mouse mammary gland. Differentiation 57, 205–214 (1994). [DOI] [PubMed] [Google Scholar]
- Joshi P. a. et al. Progesterone induces adult mammary stem cell expansion. Nature 465, 803–807 (2010). [DOI] [PubMed] [Google Scholar]
- Brisken C. et al. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 14, 650–654 (2000). [PMC free article] [PubMed] [Google Scholar]
- Rajaram R. D. et al. Progesterone and Wnt4 control mammary stem cells via myoepithelial crosstalk. EMBO J. 34, 641–652 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin S. A. et al. The MIQE guidelines:Minimum Information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622 (2009). [DOI] [PubMed] [Google Scholar]
- Dean B., Udawela M. & Scarr E. Validating reference genes using minimally transformed qpcr data: findings in human cortex and outcomes in schizophrenia. BMC Psychiatry 16, 154 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J., Preter K. De, Pattyn F. & Poppe B. GeNorm software manual. Last Updat. March 2014, 1–16 (2007).
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2^− ΔΔCT Method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- Schmittgen T. D. & Livak K. J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.