Abstract
The bacterium Aeromonas salmonicida subsp. salmonicida is a common pathogen in fish farms worldwide. Since the antibiotic resistance of this bacterial species is on the increase, it is important to have a broader view on this issue. In the present study, we tested the presence of known plasmids conferring multi-drug resistance as well as antibiotic resistance genes by a PCR approach in 100 Canadian A. salmonicida subsp. salmonicida isolates. Our study highlighted the dominance of the conjugative pSN254b plasmid, which confers multi-drug resistance. We also identified a new multi-drug plasmid named pAsa8, which has been characterized by a combination of sequencing technologies (Illumina and Oxford nanopore). This new plasmid harbors a complex class 1 integron similar to the one of the Salmonella genomic island 1 (SGI1) found in Salmonella enterica and Proteus mirabilis. Consequently, in addition to providing an update on the A. salmonicida subsp. salmonicida isolates that are resistant to antibiotics, our data suggest that this bacterium is potentially an important reservoir of drug resistance genes and should consequently be monitored more extensively. In addition, we describe a screening method that has the potential to become a diagnostic tool that is complementary to other methods currently in use.
The Gram-negative bacterium Aeromonas salmonicida subsp. salmonicida is a fish pathogen that causes furunculosis worldwide1,2. In Quebec (Canada), furunculosis is the most common infection encountered in fish farms, especially brook trout farms, and causes between 30 and 60% of reported outbreaks every year3. Antibiotics are the most widely used treatment for furunculosis. In Canada, four antibiotics (oxytetracycline, florfenicol [a chloramphenicol analog], sulfadimethoxine/ormetoprim, and sulfadiazine/trimethoprim) are approved by the Veterinary Drugs Directorate (VDD) of Health Canada to treat infected fish. In Quebec, florfenicol is the antibiotic of choice because of its short withdrawal period (12 days) compared to antibiotics such as oxytetracycline and sulfadimethoxine/ormetoprim (42 days)3. However, intensive use of these four antibiotics has been correlated with growing number of antibiotic-resistant isolates3.
Antibiotic resistance genes in A. salmonicida subsp. salmonicida are mostly located on plasmids4. Among them, pSN254b and pAB5S9b plasmids, which bear multi-drug resistance genes, as well as variants of pRAS3 have been recently described in isolates from Canada5. To date, pSN254b has been identified in isolates from Quebec, New Brunswick (Canada) and Nova Scotia (Canada), while pAB5S9b and pRAS3 variants have been identified only in New Brunswick. pAB5S9b and pSN254b bear genes coding for resistance to the three antibiotics used in veterinary medicine in Quebec. There are currently no government-approved antibiotics that are effective against bacterial isolates that host either one of these two plasmids. For their part, pRAS3 variants contain a gene coding for tetracycline resistance5. In addition to these plasmids conferring drug resistance (R-plasmids), many others were found in A. salmonicida, some of them having a large-panel of genes causing resistance to antibiotics (Table 1).
Table 1. R-plasmids found in A. salmonicida.
| Plasmid | Length (kb) | R-genes | Found in Canada | Reference |
|---|---|---|---|---|
| pAsa7 | 5,276 | cat | No | 64 |
| pRAS3.2 | 11,823 | tetA | No | 65 |
| pRAS3.3 | 11,845 | tetA | Yes | 5 |
| pRAS3.1 | 11,851 | tetA | No | 65 |
| pAB5S9b | 25,540 | tet(H), floR, sul2, strA, strB | Yes | 5 |
| pASOT3 | ~39 | aadA2, tetA | No | 66,67 |
| pRAS1 | ~45 | dfrA16, tetA, sul1 | No | 17 |
| pAr-32 | ~47 | aadA2, sul1, catA2 | No | 17,21 |
| pASOT | ~47 | aadA2 or dfrIIc, tetA | No | 66,67 |
| pASOT2 | ~47 | aadA2, tetA | No | 66,67 |
| pRAS2 | ~48 | sul2, tet 31, strA, strB | No | 17,68 |
| pSN254b | 152,216 | tetA, floR, sul1, sul2, blaCMY, aadA, strA, strB, sugE2, qacEΔ1 | Yes | 5 |
| pAsa4 | 166,749 | tet(E), sul1, aadA, cat | Yes (pAsa4-like) | 5,43 |
Even if the plasmidome (i.e., the total plasmid content) of A. salmonicida subsp. salmonicida harbor multiple R-plasmids, the dispersion of these plasmids and the gravity of the situation are presently unknown. In order to determine the fraction of isolates bearing antibiotic-resistance genes and to infer the geographic distribution of the R-plasmids, we investigated a collection of 100 A. salmonicida subsp. salmonicida Canadian isolates, using, among other methods, optimized multiplex PCR assays. This approach was also done to assess the presence of new R-plasmids.
This screening showed that pSN254b is the most common R-plasmid in tested Canadian isolates of A. salmonicida subsp. salmonicida. A new R-plasmid, pAsa8, was also found, which had genetic features that provided additional evidence that A. salmonicida subsp. salmonicida is an important reservoir for mobile genetic elements as well as antibiotic resistance genes.
Results
Plasmid content determined by PCR genotyping
The presence of plasmids containing known antibiotic resistance genes (pAsa4, pAB5S9b, pRAS3.3, pSN254b) has been tested in 100 Canadian A. salmonicida subsp. salmonicida isolates5 using the primers listed in Supplementary Table S1. The results of the genotyping for the 100 isolates are given in Supplementary Table S2. The pSN254b plasmid was the most prevalent (25/100), while the other plasmids were detected in two to four isolates each.
Multiplex PCR to detect antibiotic resistance genes
We subsequently developed four multiplex PCR assays (see the Methods section) to detect the antibiotic resistance genes known to be present in A. salmonicida subsp. salmonicida isolates: floR, cat, sul1, sul2, tetA, tet(C), tet(E), and tet(H). The results of these tests for the 100 A. salmonicida subsp. salmonicida isolates are presented in Supplementary Table S2.
The floR gene was detected in 29 of the 100 isolates, while the cat gene was not detected in any of the isolates excepted in A449, which was the positive control. The floR gene, which has been detected in isolates with the pSN254b and pAB5S9b plasmids5, was also detected in the M15448-11 and M16474-11 isolates, despite the fact that they do not host either of the two plasmids. This result suggests that an unknown genomic entity might be responsible for the presence of the floR gene in these two isolates.
The sul1 and sul2 genes were detected in 30 and 28 isolates, respectively. Both genes were detected in isolates that contained pSN254b, while only sul2 was detected in isolates carrying pAB5S9b, as reported previously5. The sul1 gene was detected in four isolates (three from Quebec and one from Ontario (Canada)) with a pAsa4-like plasmid. This multiplex PCR experiment also revealed that the sul1 gene was present in M15448-11 and M16474-11.
The tet(C) gene was detected in four isolates with the pRAS3 plasmid while tet(H) was detected in two isolates harboring the pAB5S9b plasmid5. The tet(E) gene was present in four isolates with a pAsa4-like plasmid. The tetA gene was detected in isolates with the pSN254b plasmid5 as well as in the M15448-11 and M16474-11 isolates.
Characterization of pAsa8
The presence of multiple closely related antibiotic resistance genes in M15448-11 and M16474-11 isolates without the detection of any recognized plasmids in these isolates prompted us to sequence the complete DNA of M16474-11 by Illumina and Oxford nanopore (MinION) technologies. Only the DNA of the M16474-11 isolate was sequenced since both isolates (i.e., M15448-11 and M16474-11) exhibited the same profile.
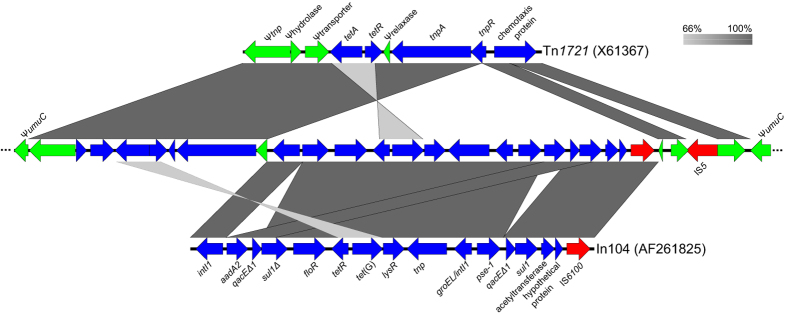
The sequencing revealed the presence of a new plasmid, that we named pAsa8, of 110 577 bp and which has a region containing many drug resistance genes. This region is the product of multiple integration events involving mobile genetic elements (Fig. 1). One of them is a transposon Tn17216, which is integrated in the gene umuC, encoding for a UV mutagenesis and repair protein, of the ancestral pAsa8. This transposon brings a tetA gene, causing resistance to tetracycline, which explains the positive result of the multiplex PCR.
Figure 1. Putative evolutionary scenario of the multidrug region of pAsa8.
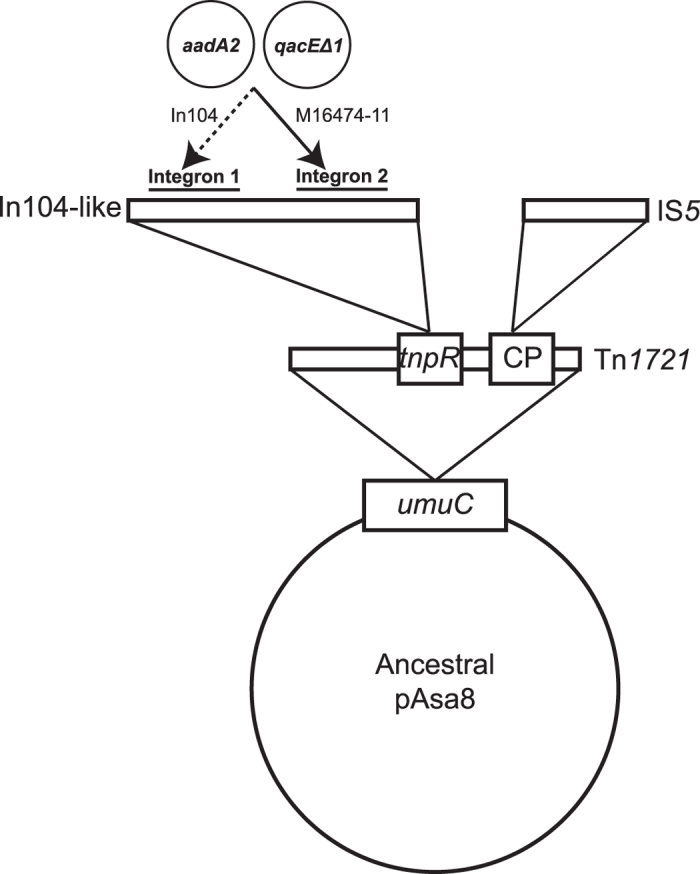
Only the elements involved are shown: the gene umuC of ancestral pAsa8, the genes tnpR and the one encoding for the chemotaxis protein (CP) located on the transposon Tn1721, the insertion sequence IS5 and finally the integron In104-like. Cassettes aadA2 and qacEΔ1 are located within integron 1 of In104 (dashed arrow), and also within integron 2 of In104-like found on pAsa8 (solid line arrow) (isolate M16474-11).
A second integration event involved a complex class 1 integron In104-like (In4-group) previously found in a 43-kb integrated element known as the Salmonella genomic island 1 (SGI1) found in Salmonella enterica7 and Proteus mirabilis8,9,10,11 and for which numerous variants were found12. The In104-like integron allows resistance to florfenicol/chloramphenicol (floR), tetracyclines (tet(G)), sulfonamides (sul1), ampicillin/carbenicillin (blaPSE-1) and streptomycin/spectinomycin (aadA2) (Fig. 2) in the previously described M16474-11 A. salmonicida subsp. salmonicida isolate. The complex In104 integron contains two functional IntI1 integron regions12,13 with many variants reported14. The integron regions found in the In104-like of A. salmonicida differs from those of the original In104 found in S. enterica serovar Typhimurium DT104 in the sense that the cassettes aadA2 and qacEΔ1 found in the first integron (IntI1) in S. enterica are in the second (groEL/IntI1) for A. salmonicida subsp. salmonicida (Fig. 2). The evolutionary scenario that would account for the cassette swap is not clear, but a BLASTn revealed that the second integron region (groEL/IntI1) is identical to one of the transposon Tn2610, which is formed by parts of Tn1721, Tn21 and SGI115. The third and last integration event involves the integration of an IS5 into the gene that encodes the chemotaxis protein of Tn1721.
Figure 2. Comparisons of the multi-drug region of pAsa8 with the transposon Tn1721 and the integron In104 found in SGI.
Interestingly, the IncU plasmid pRAS1, found in both typical and atypical A. salmonicida, was also reported to harbours a Tn1721 and an integron of the In4 family16,17. Unfortunately, this plasmid is not sequenced and consequently it would be perilous to postulate any evolutionary links between pRAS1 and pAsa8. As recently reviewed elsewhere, Tn1721 is present in several strains of the genus Aeromonas4.
The pAsa8 backbone contains several genes encoding for hypothetical proteins and also has a region containing genes promoting conjugative transfers. However, many of these ORFs are likely pseudogenes or are highly derived relative to sequences in the GenBank nr/nt database (based on BLASTp). No type 2 toxin-antitoxin system was found by TAfinder. A BLASTn query of the nr/nt database with the pAsa8 sequence only gave partial alignments (the multidrug region). However, a BLASTn analysis against the Whole Genome Shotgun (WGS) database (Gammaproteobacteria, taxid:1236) resulted in significant homology to the draft genome of Aeromonas rivuli strain DSM 22539 (GenBank: CDBJ00000000.1). This sequence was a result of a recent large-scale study investigating Aeromonad taxonomy and was not extensively analyzed18. Interestingly, it was possible to map five contigs from the draft genome of A. rivuli on the sequence of pAsa8 using locally CONTIguator version 2.7.419. The five contigs covered almost all of the pAsa8 sequence, with the exception of the region containing the Tn1721 and the In104-like regions in pAsa8, thus indicating that a similar plasmid, without the integration of these mobile elements, could be present in the strain DSM 22539 of A. rivuli. A search for Tn1721 and In104-like in the complete draft genome of A. rivuli was negative.
Based on the discovery of pAsa8 and the presence of the tet(G) genes in this plasmid which was detected for the first time in A. salmonicida subsp salmonicida, primers targeting this gene were included in the fourth multiplex PCR assay (the one detecting tet(C) and tet(H)) (see the Methods section). An illustration of the results obtained with positive controls with all the four multiplex PCR assays designed in this study is shown in Fig. 3.
Figure 3.
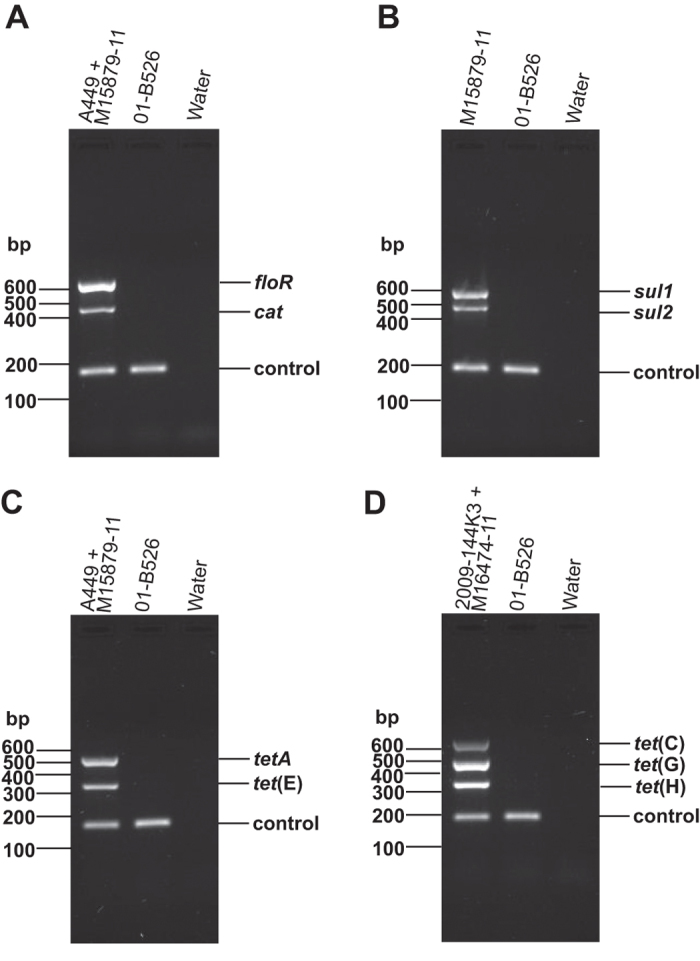
Multiplex PCR targeting genes coding for resistance to chloramphenicol/florfenicol (A), sulfonamides (B), and tetracyclines (C,D). Electrophoresis gels with amplicons generated using DNA isolated from positive controls (A449 + M15879-11 in (A,C), M15879-11 in B, 2009-144K3 + M16474-11 in D) and from a negative control (01-B526). A specific control for A. salmonicida subsp. salmonicida using a primer pair that amplified an element of the prophage 1 was included in each multiplex PCR reaction. The amplicon of this product can be seen at the bottom of each gel (control). (A) The target cat (448 bp) and floR genes (632 bp) and (B) the sul2 (449 bp) and sul1 (550 bp) genes were detected when present. (C) Amplicons for tet(E) (351 bp) and tetA (526 pb). (D) Amplicons for tet(H) (326 bp), tet(G) (460 bp), and tet(C) (629 bp). Water was used as a negative control.
In addition, other primers were designed to target the various parts of pAsa8 (Supplementary Table S1). Three targets were for the pAsa8 backbone and one for the Tn1721. The primers targeting tet(G) were used to detect the presence of the In104-like since this gene was actually only found to be In104-encoded in A. salmonicida subsp. salmonicida. Only two isolates, M15448-11 and M16474-11, gave PCR-positive results with all the primer pairs tested. No amplification occurred with any of the primer pairs in any other isolates.
Geographic distribution of isolates in Quebec harboring antibiotic resistance plasmids
Since a large number of isolates from Quebec were included in the present study, it was relevant to analyze the geographical distribution of the antibiotic-resistant isolates in Quebec. The province was divided into four regions: southeast (SE), southwest (SW), northeast (NE), and northwest (NW) (Fig. 4 and Table 2). The multidrug-resistant plasmid pSN254b was identified in many isolates from the SE, SW, and NW regions, while pAsa4 variants were only found in three isolates from the NW region. The two isolates containing pAsa8 were also from the NW region. The isolates from the SE, SW and NW regions displayed a high prevalence of antibiotic resistance while those from the NE region displayed no antibiotic resistance at all.
Figure 4. Map of the Quebec showing the regions to which the various isolates were assigned.
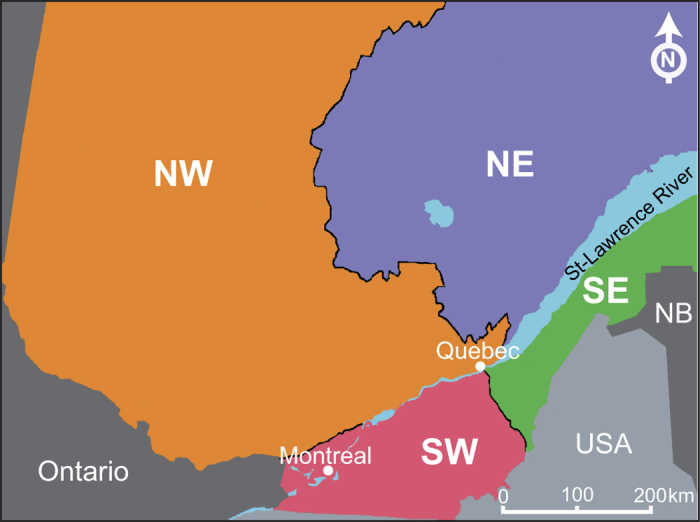
Northwest (NW, orange), northeast (NE, purple), southwest (SW, pink), and southeast (SE, green). Most of the fish farms analyzed were less than 200 km from the St. Lawrence River, which crosses the province from west to east. The map has been drawn using Adobe Photoshop CS4 version 11.0.2 (www.adobe.com).
Table 2. Geographical distribution of the antibiotic resistant isolates in Quebec.
| Regiona | NE | SE | SW | NW | Quebec as a whole | |
|---|---|---|---|---|---|---|
| Number of isolates | 16 | 11 | 14 | 45 | 86 | |
| Plasmid found in the isolates | pSN254b | 0 | 5 | 6 | 12 | 23 |
| pAsa4-like | 0 | 0 | 0 | 3 | 3 | |
| pAsa4 | 0 | 0 | 0 | 0 | 0 | |
| pAB5S9 | 0 | 0 | 0 | 0 | 0 | |
| pRAS3 | 0 | 0 | 0 | 0 | 0 | |
| pAsa8 | 0 | 0 | 0 | 2 | 2 | |
| Number of antibiotic-resistant isolates | 0 | 5 | 6 | 17 | 28 | |
| % of resistant isolatesb | 0 [0.00, 0.11] | 45 [0.20, 0.73] | 43 [0.20, 0.68] | 38 [0.25, 0.52] | 33 [0.23, 0.43] | |
asee Fig. 4.
bThe binomial 95% confidence interval (Jeffreys method) is indicated between brackets.
Discussion
One of the goals of this study was to evaluate the occurrence of genes in the fish pathogen A. salmonicida subsp. salmonicida that code for resistance to chloramphenicol/florfenicol, tetracycline, and sulfamethoxazole. Based on the multiplex PCR results, sulfonamide resistance was the most common antibiotic resistance detected in the Canadian isolates. The prevalence of florfenicol resistance in Quebec is most likely due to the intensive use of this antibiotic since 1999 to treat furunculosis3. The selective pressure caused by exposure to this antibiotic has promoted the spread of florfenicol-resistant isolates20. The multiplex PCR results showed that many isolates from Quebec contain resistance genes to the four antibiotics authorized for aquaculture use in the province. This is alarming since the Veterinary Drugs Directorate (VDD) of Health Canada has not authorized the use of any other antibiotics for treating fish infected by these multi-resistant isolates. However, given that the present study mainly investigated A. salmonicida subsp. salmonicida isolates from Quebec, more isolates from other Canadian provinces will have to be assessed to determine whether they also display the same trends.
The multiplex PCR-based method that we developed provide an accurate diagnostic tool allowing to identify genes on known plasmids in A. salmonicida subsp. salmonicida that code for resistance against the three major classes of antibiotics legally used in fish farming in Canada. While the present study focused only on A. salmonicida subsp. salmonicida isolates from Canada, this new diagnostic tool allowed us to uncover important information about the antibiotic resistance of this bacterium. This diagnostic method could also be used to study the diversity of antibiotic resistance plasmids in A. salmonicida subsp. salmonicida isolates from around the world and for conducting surveys to study temporal resistance evolution in general.
Since the discovery of transferable R-factors in A. salmonicida published in 1971 by Aoki21, many R-plasmids have been found in this bacterium (Table 1). However, actually only pSN254b (25/100), pRAS3 (4/100), pAsa4-like (4/100), pAB5S9b (2/100) and pAsa8 (2/100) were found in Canadian isolates. More specifically, the pSN254b multiple antibiotic resistance-encoding plasmid has been observed in many isolates in Quebec and is a major problem when it comes to the treatment of infected fish with antibiotics. The high prevalence of pSN254b can be mostly explained by (1) its capacity to be transferred by conjugation22 and (2) the presence of genes conferring resistance to various antibiotics, thus enhancing the selection pressure for isolates having this plasmid. In fact, up to 40% of the A. salmonicida subsp. salmonicida bacteria isolated from diseased fish in Quebec, except in the NE region, harbor antibiotic resistance genes (Table 2). pAsa4 and pAsa8 were found in the NW region while the pSN254b plasmid was present in the SW, SE, and NW regions.
The veterinarians who collected the A. salmonicida subsp. salmonicida isolates from diseased fish proposed three reasons explaining the geographic distribution of the antibiotic resistant isolates23. First, there is more infection outbreak in regions with higher aquaculture activity (mainly NW, SW and SE). Second, it is known that fish farmers exchange fish between regions which may contribute in part to the spread of the antibiotic resistant isolates. Finally, there is an increased likelihood of diagnosis for the fish farms in the surrounding regions of the veterinary service (e.g. NW and SW).
As mentioned in Vincent et al. (2014), plasmids conferring resistance to antibiotics may be transferable between A. salmonicida subsp. salmonicida and human pathogens such as Salmonella enterica5. This is exemplified by the IncA/C pSN254b plasmid, a variant of pSN254 found in S. enterica5. The plasmids of the IncA/C group are known to be conjugative and found, in addition to A. salmonicida and S. enterica, in a broad range of bacterium24 such as Yersinia pestis25, Klebsiella pneumonia26, Aeromonas hydrophila27,28, Photobacterium damselae subsp. piscicida29 and Escherichia coli30,31. The IncA/C plasmids are also known to regulate the excision of SGI132 and to serve as helpers for its mobilization in trans33. Interestingly, pAsa8 (a new R-plasmid described here) of the M16474-11 and M15448-11 A. salmonicida subsp. salmonicida isolates appeared to have a Tn1721 and a complex class 1 integron similar to the one found in SGI1. Even if we have a putative mechanism explaining the multiple integration events in the ancestral pAsa8 plasmid (Fig. 1), it is unclear if they occurred in A. salmonicida, in other bacteria such as S. enterica, or in both.
In summary, we analyzed 100 A. salmonicida subsp. salmonicida Canadian isolates for their antibiotic resistance genes and R-plasmids repertoire to shed light on their occurrence and distribution. We found that the conjugative R-plasmid pSN254b is dominant with 25% of the isolates having it. In fact, 37% of the isolates have at least one R-plasmid. This situation is worrying considering that no government-approved antibiotics can be used against these isolates to prevent furunculosis. We suggest that A. salmonicida subsp. salmonicida should be monitored worldwide to verify the trend found in Canada revealed in this study. We also discovered and characterized a new R-plasmid named here pAsa8. This plasmid hosts multiple antibiotic resistance genes from mobile elements such as Tn1721 and a complex class 1 integron In104-like. Since In104 is usually found within the genomic island SGI1 of the human pathogen S. enterica, this study reinforces the idea that A. salmonicida subsp. salmonicida could be an important reservoir of mobile DNA conferring drug resistance and raises the possibility of exchange of this genetic material with other waterborne bacteria. If indeed this is the case, it is even more worrying from a One Health perspective (i.e. the idea that human, animal and environmental health are all interconnected)34. Considering antimicrobial resistance in a One Health context is a part of the fourth intervention plan of the last report produced by the Review on Antimicrobial Resistance35. It is now well known that environmental bacteria, such as Aeromonas36, may act as a reservoir of antibiotic resistance genes37,38,39. Therefore, our results add substantial evidence that there is an urgent need to develop alternative strategies to efficiently mitigate furunculosis prevalence without selecting for other resistance genes. Pre and probiotic strategies, phage therapy and vegetable extracts such as essential oils might be promising research avenues against A. salmonicida subsp. salmonicida as it is the case for other bacteria40,41,42.
Methods
Bacterial isolates and growth conditions
All 100 A. salmonicida subsp. salmonicida isolates used in this study were from Canada (Supplementary Table S2). The A449 reference strain from France43, for which the annotated chromosome and plasmid sequences are publicly available, was also included. All the isolates were grown on furunculosis agar for two or three days at 18 °C as previously described44.
PCR analyses
The DNA templates, PCR mixtures, and program cycles used in this study have been previously described45. The PCR assays were performed at least twice. Suitable positive and negative controls were included in each assay.
The PCR primers used to identify the isolates harboring antibiotic-resistance plasmids (pAsa4, pAB5S9, pSN254, pRAS3) are listed in Supplementary Table S1, as are the primers used to genotype pAsa8 of the M16474-11 isolate.
Multiplex PCR
The sequences of orthologous genes causing resistance to chloramphenicol/florfenicol (cat and floR), tetracycline (tetA, tet(C), tet(E), tet(H), tet(G) and sulfamethoxazole (sul1 and sul2) in A. salmonicida subsp. salmonicida were aligned to identify the conserved regions. The chloramphenicol resistance gene catA2 found on the pAr-32 plasmid identified in a Japanese isolate46 was not included in the analysis due to the lack of an appropriate positive control. The alignment for each resistance gene sequence was performed using MUSCLE47 and BLASTn48 through Geneious version 6.1.849. Primers were designed based on the conserved sequences using Primer350 also through Geneious version 6.1.8.
The DNA templates were prepared by resuspending an inoculum of bacterial culture in 1 mL of SWL buffer (50 mM KCl, 10 mM Tris, pH 8.3, 2.5 mM MgCl2, 0.45% NP-40, and 0.45% Tween 20). The lysates were heated for 15 min at 95 °C, and the DNA concentrations in the solutions were adjusted to 100 ng/μL. The PCR mixture contained 1X Go-Taq buffer (Promega, USA), 2 mM dNTP, 0.2 μM forward and reverse primers, 1 U of GoTaq (5 U; Promega), and 100 ng of DNA templates. Depending on the number of primers used for the multiplex PCR, various volumes of filtered water were added to adjust the final reaction volume to 20 μL. The PCR program was as follows: 2 min 30 s at 95 °C, 30 cycles of 30 s at 95 °C, 30 s at 60 °C, and 1 min 30 s at 68 °C, followed by a final 5-min extension step at 68 °C. The PCR products were separated on 1.3% agarose gels and were stained with 0.5 μg/mL of ethidium bromide. For all the multiplex PCR, water and 01-B526, an antibiotic-sensitive isolate51, were used as negative controls. The primers used for each multiplex PCR are given in Table 3.
Table 3. Primers used for the multiplex PCR.
| Primer | Sequence 5′−3′ | Tm (°C) | Amplicon size (bp) | Target |
|---|---|---|---|---|
| Multiplex PCR targeting genes coding for chloramphenicol/florfenicol resistance | ||||
| MT-ctrl-chloram-F1 | GCTTACCTCAGATAATGAGTCGTC | 54,8 | 172 | Prophage 1 (Control) |
| MT-ctrl-chloram-R1 | GCCAATAAGAGCCCTACTCTTC | 55 | ||
| MT-cat-F1 | CTATTTTGACAATACGCCCTGC | 54,3 | 448 | cat |
| MT-cat-R1 | CTTCCCAAACGTAAATATCGGC | 54 | ||
| MT-floR-F1 | TTGAGCCTCTATATGGTGATGC | 54,4 | 632 | floR |
| MT-floR-R1 | GTTGTCACGATCATTACAAGCG | 54,3 | ||
| Multiplex PCR targeting genes coding for sulfonamide resistance | ||||
| MT-ctrl_sul-F1 | TTCATTTCGTCTTGGGTCTAGC | 54,8 | 175 | Prophage 1 (Control) |
| MT-ctrl_sul-R1 | GGACTACAGATCTACCATAATCCG | 54 | ||
| MT-sul1-F1 | GGGCTACCTGAACGATATCC | 54,7 | 550 | sul1 |
| MT-sul1-R1 | CTAGGCATGATCTAACCCTCG | 54,4 | ||
| MT-sul2-F1 | ATCATCTGCCAAACTCGTCG | 55,2 | 449 | sul2 |
| MT-sul2-R1 | TTCTTGCGGTTTCTTTCAGC | 53,9 | ||
| Multiplex PCR targeting genes coding for tetracycline resistance | ||||
| First multiplex PCR targeting genes coding for tetracycline resistance | ||||
| MT-ctrltet1-F1 | CCAGAATGACGAATTGAATGTCG | 54,3 | 175 | Prophage 1 (Control) |
| MT-ctrltet1-R1 | GGACCTCTTTACTCCAGTCG | 54,4 | ||
| MT-tetA(E)pAsa4-F1 | GATGTCACACCTGAGGAATCC | 55,1 | 351 | tetA(E) |
| MT-tetA(E)pAsa4-R1 | TCCGAATAAAACCCATAATGTTGC | 53,9 | ||
| MT-tetApSN54b-F1 | CAAGCAGGATGTAGCCTGTG | 55,9 | 526 | tetA |
| MT-tetApSN54b-R1 | ATTGCCGATATCACTGATGG | 52,4 | ||
| Second multiplex PCR targeting genes coding for tetracycline resistance | ||||
| MT-ctrltet2-F1 | ATTCATTTCGTCTTGGGTCTAGC | 55 | 176 | Prophage 1 (Control) |
| MT-ctrltet2-R1 | GGACTACAGATCTACCATAATCCG | 54 | ||
| MT-tetHpAB5S9b-F1 | ACGACTGTCTGATAAATACGGC | 54,6 | 326 | tetH |
| MT-tetHpAB5S9b-R1 | ATATCGAGTGTGAAATAGCGGC | 54,9 | ||
| MT-tetA(C)pRAS3-F1 | CTGTAGGCATAGGCTTGGTTAT | 54,4 | 629 | tetA(C) |
| MT-tetA(C)pRAS3-R1 | CTGTCCTACGAGTTGCATGATA | 54,1 | ||
| MT-tetGs62-F1 | GGTTCGCATCAAACCATTCG | 54,8 | 460 | tetA(G) |
| MT-tetGs62-R1 | GCTTAGATTGGTGAGGCTCG | 55,6 | ||
In the first multiplex PCR, targeting floR and cat, a mix of the sequenced isolates M15879-11 and A44943,52 was used as a positive control. The floR gene gives resistance to both chloramphenicol and florfenicol while the cat gene provides resistance only to chloramphenicol53. Bioinformatics analyses confirmed that the M15879-11 isolate bears a pSN254b plasmid similar to that of the 2004-05MF26 isolate and had the same set of antibiotic resistance genes5,54. In the second multiplex PCR, developed to detect the sul1 and sul2 genes, the M15879-11 isolate was used as positive control. A subset of tetracycline resistance genes (tetA and tet(E)) was targeted by a third multiplex PCR. A mix of M15879-11 and A449 was used as a positive control for these genes. The last multiplex PCR assay aimed at detecting tet(C), tet(G) and tet(H). Two isolates were used as positive controls: 2009-144K3 (pAB5S9b and pRAS3.3) and M16474-11 (pAsa8).
DNA extraction and genomic sequencing
The total DNA of the M15879-11 and M16474-11 A. salmonicida subsp. salmonicida isolates was extracted using DNeasy Blood and Tissue kits (Qiagen, Canada) and was sequenced by next-generation sequencing (NGS) at the Plateforme d’Analyse Génomique of the Institut de Biologie Intégrative et des Systèmes (IBIS, Université Laval). The DNA of the M15879-11 isolate was fragmented at a 5-kb size and sequenced by pyrosequencing using a GS-FLX+ apparatus as previously described52. The resulting sequencing reads were de novo assembled with Newbler version 2.5.355. The total draft assembly of M15879-11 was annotated by the NCBI Prokaryotic Genome Annotation Pipeline (PGAP) and deposited in GenBank under accession number LAIS00000000.
The M16474-11 library was prepared using a KAPA Hyper Prep kit and was sequenced by a MiSeq (Illumina) sequencing system. The resulting reads were filtered with Trimmomatic version 0.3256 using the manual-recommended parameters for paired-end reads57. The DNA of M16474-11 was also extracted by phenol/chloroform by following the protocol Extracting DNA Using Phenol-Chloroform provided by Pacific Biosciences (http://www.pacb.com). The DNA was sheared at 8-kb using a standard Covaris g-TUBE protocol and was used to prepare an Oxford nanopore technologies sequencing library using the protocol GDE_1002_v1_revF_17Nov2015. The library was sequenced with a MinION sequencer. The basecalling was done by the Oxford nanopore technologies’s Metrichor cloud service with the 2D Basecalling for SQK-MAP006 version 1.69 application. All the reads (1D and 2D) ≥ 750 bp were converted from the native HDF5/FAST5 format to FASTQ by poretools version 0.5.158. Finally, a hybrid assembly of both Illumina and Oxford nanopore technologies reads was performed using SPAdes version 3.7.159 with kmer lengths of 21, 33, 55, 77, 99 and 127. The pAsa8 plasmid was recovered in a single contig. To assess the quality of the sequence, the Illumina reads were mapped on the pAsa8 sequence using BWA-MEM60 and evaluated with Pilon version 1.1761. No corrections occurred during step, reinforcing our confidence in the quality of the pAsa8 sequence. This sequence was annotated with the webserver RAST62 and manually curated. The sequence was analyzed using TAfinder (TADB) to find putative type 2 toxin-antitoxin systems63. The complete annotated sequence of pAsa8 was deposited in GenBank under the accession number KX364409.
Additional Information
How to cite this article: Trudel, M. V. et al. Diversity of antibiotic-resistance genes in Canadian isolates of Aeromonas salmonicida subsp. salmonicida: dominance of pSN254b and discovery of pAsa8. Sci. Rep. 6, 35617; doi: 10.1038/srep35617 (2016).
Supplementary Material
Acknowledgments
We thank Valérie E. Paquet, Jean-Guillaume Emond-Rheault, Brian Boyle, and Mathilde Goldschmitt for their technical support. We also thank Katherine H. Tanaka (Université Laval) for her critical reading of the manuscript. MVT received scholarships under the CREATE program of Ressources Aquatiques Québec (RAQ). ATV received a scholarship from Fonds de la recherche québécois sur la nature et les technologies (FRQNT) and also an Alexander Graham Bell Canada Graduate Scholarship from the Natural Sciences and Engineering Research Council of Canada (NSERC). SAA received a Canada Graduate Scholarship from the NSERC. ML is supported by scholarships from NSERC and FRQNT. The MinION was acquired as part of the Oxford Nanopore MinION access program. This project was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC), the Société de recherche et de développement en aquaculture continentale (SORDAC), Resources Aquatiques Québec (RAQ), and the Innovamer program of the Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec.
Footnotes
Author Contributions Conceived and designed the experiments: M.V.T., A.T.V., N.D. and S.J.C. Performed the experiments: M.V.T., A.T.V., S.A.A. and M.L. Analyzed the data: M.V.T., A.T.V., S.A.A. and S.J.C. Contributed reagents/materials/analysis tools: N.D., A.I.C. and S.J.C. Wrote the paper: M.V.T., A.T.V., N.D., A.I.C. and S.J.C. All authors reviewed the manuscript.
References
- Hiney M. & Olivier G. In Fish diseases and disorders, vol III: viral, bacterial and fungal infections (eds Woo P. T. K. & Bruno D. W.) 341–425 (CAB publishing Oxford, 1999). [Google Scholar]
- Dallaire-Dufresne S., Tanaka K. H., Trudel M. V., Lafaille A. & Charette S. J. Virulence, genomic features, and plasticity of Aeromonas salmonicida subsp. salmonicida, the causative agent of fish furunculosis. Vet Microbiol 169, 1–7, 10.1016/j.vetmic.2013.06.025 (2014). [DOI] [PubMed] [Google Scholar]
- Morin R. L’utilisation des antibiotiques pour combattre la furonculose chez l’omble de fontaine génère de l’antibiorésistance chez Aeromonas salmonicida. L’aquicole 15, 2–6 (2010). [Google Scholar]
- Piotrowska M. & Popowska M. Insight into the mobilome of Aeromonas strains. Front Microbiol 6, 494, 10.3389/fmicb.2015.00494 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A. T. et al. Detection of variants of the pRAS3, pAB5S9, and pSN254 plasmids in Aeromonas salmonicida subsp. salmonicida: multidrug resistance, interspecies exchanges, and plasmid reshaping. Antimicrob Agents Chemother 58, 7367–7374, 10.1128/AAC.03730-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allmeier H., Cresnar B., Greck M. & Schmitt R. Complete nucleotide sequence of Tn1721: gene organization and a novel gene product with features of a chemotaxis protein. Gene 111, 11–20 (1992). [DOI] [PubMed] [Google Scholar]
- Boyd D. A., Peters G. A., Ng L. & Mulvey M. R. Partial characterization of a genomic island associated with the multidrug resistance region of Salmonella enterica Typhymurium DT104. FEMS Microbiol Lett 189, 285–291 (2000). [DOI] [PubMed] [Google Scholar]
- Bi S. et al. New variant Salmonella genomic island 1-U in Proteus mirabilis clinical and food isolates from South China. J Antimicrob Chemother 66, 1178–1179, 10.1093/jac/dkr030 (2011). [DOI] [PubMed] [Google Scholar]
- Lei C. W. et al. Molecular characteristics of Salmonella genomic island 1 in Proteus mirabilis isolates from poultry farms in China. Antimicrob Agents Chemother 58, 7570–7572, 10.1128/AAC.03992-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebor E. & Neuwirth C. Emergence of Salmonella genomic island 1 (SGI1) among Proteus mirabilis clinical isolates in Dijon, France. J Antimicrob Chemother 68, 1750–1756, 10.1093/jac/dkt100 (2013). [DOI] [PubMed] [Google Scholar]
- Qin S. et al. Emergence of Extensively Drug-Resistant Proteus mirabilis Harboring a Conjugative NDM-1 Plasmid and a Novel Salmonella Genomic Island 1 Variant, SGI1-Z. Antimicrob Agents Chemother 59, 6601–6604, 10.1128/AAC.00292-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss J., Nagy B. & Olasz F. Stability, entrapment and variant formation of Salmonella genomic island 1. PLoS One 7, e32497, 10.1371/journal.pone.0032497 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael G. B., Hall R., Fanning S. & Schwarz S. In Salmonella in domestic animals (eds Barrow P. A. & Methner U.) 120–135 (2013). [Google Scholar]
- Mulvey M. R., Boyd D. A., Olson A. B., Doublet B. & Cloeckaert A. The genetics of Salmonella genomic island 1. Microbes Infect 8, 1915–1922, 10.1016/j.micinf.2005.12.028 (2006). [DOI] [PubMed] [Google Scholar]
- Takaya A., Watanabe M. & Yamamoto T. Organization of tn2610 containing two transposition modules. Antimicrob Agents Chemother 50, 1143–1147, 10.1128/AAC.50.4.1143-1147.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandaa R. A. & Enger O. Transfer in Marine Sediments of the Naturally Occurring Plasmid pRAS1 Encoding Multiple Antibiotic Resistance. Appl Environ Microbiol 60, 4234–4238 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorum H., L’Abee-Lund T. M., Solberg A. & Wold A. Integron-containing IncU R plasmids pRAS1 and pAr-32 from the fish pathogen Aeromonas salmonicida. Antimicrob Agents Chemother 47, 1285–1290 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colston S. M. et al. Bioinformatic genome comparisons for taxonomic and phylogenetic assignments using Aeromonas as a test case. MBio 5, e02136, 10.1128/mBio.02136-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galardini M., Biondi E. G., Bazzicalupo M. & Mengoni A. CONTIGuator: a bacterial genomes finishing tool for structural insights on draft genomes. Source Code Biol Med 6, 11, 10.1186/1751-0473-6-11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon B. A. The biology of antibiotic resistance. World Aquac 32, 63–65 (2001). [Google Scholar]
- Aoki T., Egusa S., Kimura T. & Watanabe T. Detection of R factors in naturally occurring Aeromonas salmonicida strains. Appl Microbiol 22, 716–717 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh D. et al. Transferable, multiple antibiotic and mercury resistance in Atlantic Canadian isolates of Aeromonas salmonicida subsp. salmonicida is associated with carriage of an IncA/C plasmid similar to the Salmonella enterica plasmid pSN254. J Antimicrob Chemother 61, 1221–1228, 10.1093/jac/dkn123 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhland C. F. Rapport des activités du Laboratoire d’Ichtyopathologie (1 avril 2004 – 31 mars 2005). 16 pages (Saint-Hyacinthe (Québec), 2005).
- Johnson T. J. & Lang K. S. IncA/C plasmids: An emerging threat to human and animal health? Mob Genet Elements 2, 55–58, 10.4161/mge.19626 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch T. J. et al. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS One 2, e309, 10.1371/journal.pone.0000309 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doublet B. et al. Complete nucleotide sequence of the multidrug resistance IncA/C plasmid pR55 from Klebsiella pneumoniae isolated in 1969. J Antimicrob Chemother 67, 2354–2360, 10.1093/jac/dks251 (2012). [DOI] [PubMed] [Google Scholar]
- Aoki T., Egusa S., Ogata Y. & Watanabe T. Detection of resistance factors in fish pathogen Aeromonas liquefaciens. J Gen Microbiol 65, 343–349, 10.1099/00221287-65-3-343 (1971). [DOI] [PubMed] [Google Scholar]
- Fricke W. F. et al. Comparative genomics of the IncA/C multidrug resistance plasmid family. J Bacteriol 191, 4750–4757, 10.1128/JB.00189-09 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. J. et al. Complete DNA sequence and analysis of the transferable multiple-drug resistance plasmids (R Plasmids) from Photobacterium damselae subsp. piscicida isolates collected in Japan and the United States. Antimicrob Agents Chemother 52, 606–611, 10.1128/AAC.01216-07 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call D. R. et al. blaCMY-2-positive IncA/C plasmids from Escherichia coli and Salmonella enterica are a distinct component of a larger lineage of plasmids. Antimicrob Agents Chemother 54, 590–596, 10.1128/AAC.00055-09 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Alarcon C., Singer R. S. & Johnson T. J. Comparative genomics of multidrug resistance-encoding IncA/C plasmids from commensal and pathogenic Escherichia coli from multiple animal sources. PLoS One 6, e23415, 10.1371/journal.pone.0023415 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss J. et al. The master regulator of IncA/C plasmids is recognized by the Salmonella Genomic island SGI1 as a signal for excision and conjugal transfer. Nucleic Acids Res 43, 8735–8745, 10.1093/nar/gkv758 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douard G., Praud K., Cloeckaert A. & Doublet B. The Salmonella genomic island 1 is specifically mobilized in trans by the IncA/C multidrug resistance plasmid family. PLoS One 5, e15302, 10.1371/journal.pone.0015302 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T. P. et al. Antibiotic resistance is the quintessential One Health issue. Trans R Soc Trop Med Hyg 110, 377–380, 10.1093/trstmh/trw048 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J. Tackling Drug-Resistant Infections Globally: final report and recommendations. (2016).
- Gordon L. et al. Complete sequence of the floR-carrying multiresistance plasmid pAB5S9 from freshwater Aeromonas bestiarum. J Antimicrob Chemother 62, 65–71, 10.1093/jac/dkn166 (2008). [DOI] [PubMed] [Google Scholar]
- Biyela P. T., Lin J. & Bezuidenhout C. C. The role of aquatic ecosystems as reservoirs of antibiotic resistant bacteria and antibiotic resistance genes. Water Sci Technol 50, 45–50 (2004). [PubMed] [Google Scholar]
- Hatosy S. M. & Martiny A. C. The ocean as a global reservoir of antibiotic resistance genes. Appl Environ Microbiol 81, 7593–7599, 10.1128/AEM.00736-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington E. M. et al. The role of the natural environment in the emergence of antibiotic resistance in gram-negative bacteria. Lancet Infect Dis 13, 155–165, 10.1016/S1473-3099(12)70317-1 (2013). [DOI] [PubMed] [Google Scholar]
- Haba E. et al. Rhamnolipids as emulsifying agents for essential oil formulations: antimicrobial effect against Candida albicans and methicillin-resistant Staphylococcus aureus. Int J Pharm 476, 134–141, 10.1016/j.ijpharm.2014.09.039 (2014). [DOI] [PubMed] [Google Scholar]
- Krylov V. N. Bacteriophages of Pseudomonas aeruginosa: long-term prospects for use in phage therapy. Adv Virus Res 88, 227–278, 10.1016/B978-0-12-800098-4.00005-2 (2014). [DOI] [PubMed] [Google Scholar]
- Llewellyn M. S., Boutin S., Hoseinifar S. H. & Derome N. Teleost microbiomes: the state of the art in their characterization, manipulation and importance in aquaculture and fisheries. Front Microbiol 5, 207, 10.3389/fmicb.2014.00207 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith M. E. et al. The genome of Aeromonas salmonicida subsp. salmonicida A449: insights into the evolution of a fish pathogen. BMC Genomics 9, 427, 10.1186/1471-2164-9-427 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher R. K. et al. Alteration of virulence factors and rearrangement of pAsa5 plasmid caused by the growth of Aeromonas salmonicida in stressful conditions. Vet Microbiol 152, 353–360, 10.1016/j.vetmic.2011.04.034 (2011). [DOI] [PubMed] [Google Scholar]
- Trudel M. V. et al. Insertion sequence AS5 (ISAS5) is involved in the genomic plasticity of Aeromonas salmonicida. Mob Genet Elements 3, e25640, 10.4161/mge.25640 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T., Mitoma Y. & Crosa J. H. The characterization of a conjugative R-plasmid isolated from Aeromonas salmonicida. Plasmid 16, 213–218 (1986). [DOI] [PubMed] [Google Scholar]
- Edgar R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32, 1792–1797, 10.1093/nar/gkh340 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25, 3389–3402 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M. et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649, 10.1093/bioinformatics/bts199 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untergasser A. et al. Primer3–new capabilities and interfaces. Nucleic Acids Res 40, e115, 10.1093/nar/gks596 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charette S. J. et al. Draft genome sequence of the virulent strain 01-B526 of the fish pathogen Aeromonas salmonicida. J Bacteriol 194, 722–723, 194/3/722 [pii] 10.1128/JB.06276-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A. T., Boyle B., Derome N. & Charette S. J. Improvement in the DNA sequencing of genomes bearing long repeated elements. J Microbiol Methods 107, 186–188, 10.1016/j.mimet.2014.10.016 (2014). [DOI] [PubMed] [Google Scholar]
- Mascaretti O. A. Bacteria versus antibacterial agents: an integrated approach. (ASM Press, 2003). [Google Scholar]
- Vincent A. T. et al. Draft genome sequences of two Aeromonas salmonicida subsp. salmonicida isolates harboring plasmids conferring antibiotic resistance. FEMS Microbiol Lett 362, 10.1093/femsle/fnv002 (2015). [DOI] [PubMed] [Google Scholar]
- Margulies M. et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature 437, 376–380, 10.1038/nature03959 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A. M., Lohse M. & Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120, 10.1093/bioinformatics/btu170 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coil D., Jospin G. & Darling A. E. A5-miseq: an updated pipeline to assemble microbial genomes from Illumina MiSeq data. Bioinformatics 31, 587–589, 10.1093/bioinformatics/btu661 (2015). [DOI] [PubMed] [Google Scholar]
- Loman N. J. & Quinlan A. R. Poretools: a toolkit for analyzing nanopore sequence data. Bioinformatics 30, 3399–3401, 10.1093/bioinformatics/btu555 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankevich A. et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19, 455–477, 10.1089/cmb.2012.0021 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. & Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760, 10.1093/bioinformatics/btp324 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B. J. et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One 9, e112963, 10.1371/journal.pone.0112963 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek R. et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res 42, D206–D214, 10.1093/nar/gkt1226 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y. et al. TADB: a web-based resource for Type 2 toxin-antitoxin loci in bacteria and archaea. Nucleic Acids Res 39, D606–D611, 10.1093/nar/gkq908 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A. T. et al. Antibiotic resistance due to an unusual ColE1-type replicon plasmid in Aeromonas salmonicida. Microbiology, 10.1099/mic.0.000286 (2016). [DOI] [PubMed] [Google Scholar]
- L’Abee-Lund T. M. & Sorum H. A global non-conjugative Tet C plasmid, pRAS3, from Aeromonas salmonicida. Plasmid 47, 172–181 (2002). [DOI] [PubMed] [Google Scholar]
- Adams C. A., Austin B., Meaden P. G. & McIntosh D. Molecular characterization of plasmid-mediated oxytetracycline resistance in Aeromonas salmonicida. Appl Environ Microbiol 64, 4194–4201 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Abee-Lund T. M. & Sorum H. Class 1 integrons mediate antibiotic resistance in the fish pathogen Aeromonas salmonicida worldwide. Microb Drug Resist 7, 263–272, 10.1089/10766290152652819 (2001). [DOI] [PubMed] [Google Scholar]
- L’Abee-Lund T. M. & Sorum H. Functional Tn5393-like transposon in the R plasmid pRAS2 from the fish pathogen Aeromonas salmonicida subspecies salmonicida isolated in Norway. Appl Environ Microbiol 66, 5533–5535 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.