Abstract
Aims
To evaluate the safety and efficacy of once‐weekly dulaglutide 1.5 mg, a long‐acting glucagon‐like peptide‐1 receptor agonist, compared with placebo in patients with type 2 diabetes (T2D) on glimepiride monotherapy.
Methods
This phase III, randomized (4 : 1; dulaglutide:placebo), double‐blind, placebo‐controlled, 24‐week study compared the safety and efficacy of once‐weekly dulaglutide 1.5 mg with placebo in sulphonylurea‐treated (≥half‐maximal dose, stable ≥3 months) patients (N = 300) with T2D and inadequate glycaemic control [glycated haemoglobin (HbA1c) ≥7.5 and ≤9.5% (≥58 mmol/mol and ≤80 mmol/mol)]. Analysis was carried out according to intention‐to‐treat.
Results
At baseline, the mean participant age was 58 years; mean HbA1c was 8.4% (68 mmol/mol) and mean weight was 85.5 kg. Dulaglutide 1.5 mg was superior to placebo at 24 weeks for HbA1c reduction from baseline with a between‐group HbA1c difference of −1.3% [95% confidence interval (CI) −1.6, −1.0] or ‐14 mmol/mol (95% CI −17, −11); p < 0.001. A greater proportion of participants in the dulaglutide group reached an HbA1c level of <7.0% (53 mmol/mol) compared with placebo (55.3% vs 18.9%; p < 0.001). Dulaglutide significantly decreased fasting serum glucose from baseline compared with placebo (between‐group difference −1.86 mmol/l (95% CI −2.58, −1.14) or −33.54 mg/dl (95% CI −46.55, −20.53); p < 0.001. Weight was decreased significantly from baseline in the dulaglutide group (p < 0.001); the between‐group difference was not significant. The most common treatment‐emergent adverse events for dulaglutide 1.5 mg were gastrointestinal: nausea (10.5%), diarrhoea (8.4%) and eructation (5.9%). Total hypoglycaemia was higher with dulaglutide 1.5 mg vs placebo (2.37 and 0.07 events/participant/year, respectively; p = 0.025). No severe hypoglycaemia was reported.
Conclusions
Once‐weekly dulaglutide 1.5 mg had a favourable benefit/risk profile when added to glimepiride monotherapy.
Keywords: dulaglutide, glucagon‐like peptide‐1, type 2 diabetes
Introduction
Type 2 diabetes (T2D) is characterized by a gradual loss of β‐cell function that necessitates continued advancement of therapy to maintain glycaemic control 1. Along with lifestyle modifications, at diagnosis, patients with T2D are often initiated on metformin therapy as per current treatment guidelines 2; however, many patients experience significant gastrointestinal adverse events (AEs) with metformin, and up to 10% of patients are ultimately unable to tolerate it 3. Relative contraindications to metformin therapy, such as chronic kidney disease, symptomatic heart failure or liver disease, are very common in ambulatory patients 4, 5, 6, 7. For these patients, treatment with a sulphonylurea (SU) may be considered first‐line therapy. If the glycated haemoglobin (HbA1c) target is not achieved with an SU, recent treatment guidelines suggest adding a thiazolidinedione, a dipeptidyl peptidase‐4 (DPP‐4) inhibitor, a sodium glucose cotransporter‐2 inhibitor, a glucagon‐like peptide 1 (GLP‐1) receptor agonist or basal insulin, as a patient‐centred approach, individualized to concurrent medical history and patient preference 3. In general, combination therapy with drugs that use complementary mechanisms of action provide additive benefit, but occasionally they may unveil compensatory mechanisms that limit the effects of each individual drug 8. With increasing options for therapy, it is important to evaluate individual drug combinations in order to appropriately target therapies to individuals.
Dulaglutide, a once‐weekly GLP‐1 receptor agonist approved for the treatment of T2D, is a fusion protein that combines two identical human GLP‐1 receptor analogues, modified to resist DPP‐4 inactivation, with soluble human IgG4 Fc 9. The larger size of the molecule slows injection site absorption and minimizes renal clearance 9. Dulaglutide has a half‐life of ∼5 days and therefore is suitable for once‐weekly injection 10.
The Assessment of Weekly Administration of LY2189265 (Dulaglutide) in Diabetes‐8 (AWARD‐8) study was conducted to understand the safety and efficacy profile of dulaglutide in combination with an SU.
Materials and Methods
Study Participants
The present study included adult men and women [age ≥18 years, body mass index (BMI) ≤45 kg/m2] with T2D not optimally controlled [HbA1c ≥7.5 and ≤9.5% (≥58 and ≤80 mmol/mol)] with diet and exercise on a stable dose of SU that was at least 50% of the maximum dose per country‐specific label for at least 3 months before screening. Patients treated with any other antihyperglycaemic medication (including insulin) <3 months before screening were excluded from the study, as were patients with a history of pancreatitis, signs or symptoms of liver disease, impaired renal function (estimated glomerular filtration rate <30 ml/min/1.73 m2), elevated serum calcitonin concentration (20 ng/L), or recent history of severe hypoglycaemia. The study protocol was approved by local ethical review boards and patients provided written informed consent prior to any study procedures. The trial was conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonisation guideline on good clinical practice 11.
Study Design
This 24‐week, phase IIIb, multicentre, randomized, parallel‐arm, double‐blind, superiority trial compared dulaglutide 1.5 mg (initiated at the full 1.5 mg dose), administered once weekly as a subcutaneous injection, versus placebo in patients with T2D who had inadequate glycaemic control with SU monotherapy. Participants were randomized in a 4 : 1 ratio to dulaglutide or matching placebo stratified by country and baseline HbA1c. During the lead‐in period, eligible participants either continued their prestudy dose of glimepiride or replaced their previous SU with an approximately equivalent dose of glimepiride. The lead‐in period was 2 weeks for all participants regardless of prestudy SU. Participants maintained their lead‐in glimepiride dose throughout the study, but the dose could be reduced, followed by discontinuation, in the case of hypoglycaemia or for an AE. All participants were taught injection techniques and glucose monitoring before randomization. Patients with severe, persistent hyperglycaemia based on mean fasting self‐monitored plasma glucose (SMPG) measurements and prespecified criteria (Table S1, Supporting Information) could either increase the glimepiride dose or initiate additional glycaemic rescue therapy.
Efficacy Measurements
The primary objective of the present study was to show that dulaglutide was superior to placebo, as measured by HbA1c change from baseline at 24 weeks. To control for type I error, a sequential gatekeeping strategy 12 was used to compare treatments regarding selected secondary objectives at 24 weeks once the primary objective was achieved, in the following order: (i) the percentage of patients achieving an HbA1c <7.0% (<53 mmol/mol); (ii) change from baseline in fasting serum glucose (FSG; central laboratory measurement); and (iii) change from baseline in body weight. In addition, the percentage of patients achieving HbA1c ≤6.5% (48 mmol/mol), and seven‐point SMPG profiles were evaluated for efficacy. All HbA1c measurements were determined by a central laboratory.
Safety Measurements
Safety assessments included AE and serious AEs (SAEs). Hypoglycaemia was defined as plasma glucose ≤3.9 mmol/l (≤70 mg/dl) and/or signs and/or symptoms associated with hypoglycaemia 13. Hypoglycaemia was also analysed at the <3.0 mmol/l (<54 mg/dl) threshold. Severe hypoglycaemia was defined as an episode requiring the assistance of another person to actively administer carbohydrate, glucagon or other resuscitative actions 13. The rate and incidence of hypoglycaemia and severe hypoglycaemia were evaluated, as were vital signs (seated heart rate and blood pressure), ECGs, dulaglutide antidrug antibodies (measured by immunoassay), and the occurrence of severe, persistent hyperglycaemia requiring rescue therapy. Suspected cardiovascular events and pancreatic events were adjudicated; C‐cell hyperplasia, C‐cell neoplasms, serum calcitonin, development of antidrug antibodies and allergic/hypersensitivity reactions were monitored as AEs of special interest.
Statistical Analysis
It was estimated that 285 randomized (4 : 1; dulaglutide:placebo) patients (228 completers) would provide 90% power to show superiority of dulaglutide 1.5 mg versus placebo for change in HbA1c from baseline to 24 weeks at the two‐sided significance level of 0.05, assuming that dulaglutide reduced HbA1c by 0.7% (8 mmol/mol) more than placebo, with a standard deviation (s.d.) of 1.3% (14 mmol/mol). Efficacy and safety analyses were performed using the intention‐to‐treat population, defined as all randomized patients who took ≥1 dose of study medication. Efficacy (e.g. HbA1c, FSG, weight) and hypoglycaemia measurements were censored after therapeutic intervention for persistent hyperglycaemia (post‐rescue). A mixed‐model for repeated measures (MMRM) was used as the primary analysis model, with treatment, country, visit and treatment‐by‐visit as fixed effects, baseline as a covariate, and patient as a random effect. The secondary analysis for the primary endpoint was analysis of covariance (ancova) for change in HbA1c from baseline to endpoint, with country and treatment as fixed effects and baseline as a covariate. Body weight was analysed using MMRM and ancova and adjusted for baseline values. MMRM was used for analyses of other continuous measures (vital signs, seven‐point SMPG, etc.). The chi‐squared test was used for categorical measures. The percentages of patients achieving HbA1c targets [last observed carried forward (LOCF)] were analysed using a logistic regression model for repeated measures with factors of treatment, country, baseline HbA1c, visit and visit‐by‐treatment interaction. Hypoglycaemia rate was analysed using a generalized linear model with negative binomial distribution. The study was registered with ClinicalTrials.gov with the number: NCT01769378.
Results
Participant Baseline Demographics and Disposition
A total of 549 participants were screened and 300 were randomized to treatment with once‐weekly dulaglutide (N = 240) or placebo (N = 60). Overall, 29 participants [dulaglutide, n = 25 (10.4%); placebo, n = 4 (6.7%)] discontinued treatment (study and/or study drug) before week 24, with 215 participants (89.6%) in the dulaglutide group and 56 participants (93.3%) in the placebo group completing treatment (Figure 1). Twelve participants [dulaglutide, n = 5 (2.1%); placebo, n = 7 (11.7%)] received rescue therapy for severe, persistent hyperglycaemia.
Figure 1.
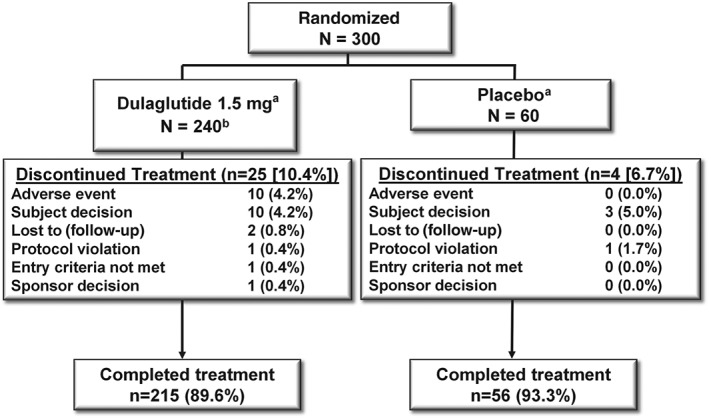
Participant disposition. aRequired glycaemic rescue: dulaglutide 1.5 mg, n = 5 (2.1%); placebo, n = 7 (11.7%). bOne patient was randomized to dulaglutide but not treated (investigator decision, entry criteria not met).
Baseline characteristics were generally similar between the groups (Table 1). The overall mean (s.d.) participant age was 58 (9.7) years, 56% were women and 83% were white. The baseline mean (s.d.) duration of diabetes was 7.6 (5.1) years and the baseline mean (s.d.) HbA1c was 8.4 (0.7)% [68 (8) mmol/mol]. Baseline body weight was significantly lower in the dulaglutide group (84.5 kg) compared with the placebo group (89.5 kg; p = 0.038). Mean (s.d.) glimepiride doses were similar at baseline [dulaglutide, 4.8 (1.6) mg/day; placebo, 4.7 (1.6) mg/day] and after 24 weeks [dulaglutide = 4.7 (1.6) mg/day; placebo = 4.9 (1.9) mg/day]. A total of 22 participants [dulaglutide, n = 16 (6.7%); placebo, n = 6 (10.0%)] decreased or stopped glimepiride therapy (p = 0.407).
Table 1.
Baseline demographics.
| Dulaglutide 1.5 mg | Placebo | |
|---|---|---|
| n = 239 | n = 60 | |
| Sex, n (%) | ||
| Male | 104 (43.5) | 28 (46.7) |
| Female | 135 (56.5) | 32 (53.3) |
| Age, years | 57.7 (10.2) | 58.2 (7.4) |
| Race, n (%) | ||
| American Indian or Alaskan Native | 21 (8.8) | 5 (8.3) |
| Asian | 3 (1.3) | 2 (3.3) |
| Black or African American | 7 (2.9) | 4 (6.7) |
| Multiple | 6 (2.5) | 2 (3.3) |
| White | 202 (84.5) | 47 (78.3) |
| Ethnic origin, n (%) | ||
| Hispanic or Latino | 112 (46.9) | 27 (45.0) |
| Not Hispanic or Latino | 127 (53.1) | 33 (55.0) |
| Weight, kg | 84.5 (16.4)* | 89.5 (18.6) |
| BMI, kg/m2 | 30.9 (5.2) | 32.4 (5.9) |
| Diabetes duration | 7.8 (5.3) | 6.8 (3.8) |
| HbA1c, % | 8.4 (0.7) | 8.4 (0.7) |
| HbA1c, mmol/mol | 68 (8) | 68 (8) |
| FSG, mmol/l | 9.9 (2.9) | 9.7 (2.5) |
| FSG, mg/dl | 177.6 (52.9) | 175.1 (44.9) |
| Glimepiride dose, mg/day | 4.8 (1.6) | 4.7 (1.6) |
| Seated systolic blood pressure, mm Hg | 132 (13) | 130 (12) |
| Seated diastolic blood pressure, mm Hg | 78 (9) | 78 (8) |
| Seated heart rate, beats/min | 75 (10) | 74 (10) |
BMI, body mass index; FSG, fasting serum glucose; s.d., standard deviation.
p = 0.038 versus placebo; intention‐to‐treat population, all values are mean (s.d.) unless otherwise noted.
Efficacy
Glycaemic Control
Dulaglutide reduced HbA1c by −1.4% (−15 mmol/mol) from baseline compared with −0.1% (−1 mmol/mol) for placebo, with a between‐group difference of −1.3% [95% confidence interval (CI) −1.6, −1.0) or −14 mmol/mol (95% CI: −17, −11); p < 0.001]. This significant difference met the primary endpoint of superiority versus placebo for this study (Figure 2A). Dulaglutide significantly improved HbA1c versus placebo at all post‐baseline time points, beginning at 4 weeks (Figure 2B). At 24 weeks, 55.3% (dulaglutide) and 18.9% (placebo) of participants achieved an HbA1c target of <7.0% (<53 mmol/mol; p < 0.001 dulaglutide vs placebo), while 40% (dulaglutide) and 9.4% (placebo) of participants achieved an HbA1c target of ≤6.5% (≤48 mmol/mol; p < 0.001 dulaglutide vs placebo; Figure 2C). Dulaglutide reduced FSG from baseline to 24 weeks [dulaglutide −1.70 and placebo 0.16 mmol/l (−30.60 and 2.93 mg/dl, respectively)]; the between‐group least‐squares (LS) mean difference of −1.86 mmol/l (95% CI −2.58, −1.14) was statistically significant [−33.54 mg/dl (95% CI −46.55, −20.53); p < 0.001; Figure 2D]. At all time points, the LS mean values for seven‐point SMPG were significantly reduced in the dulaglutide‐treated group (all p < 0.001; Figure 2E). Postprandial (after breakfast, lunch and dinner) and bedtime (03:00 h) SMPG values in the placebo group were significantly reduced compared with baseline at 24 weeks (all p < 0.050). Additionally, at 24 weeks, for each of the seven SMPG measurements, mean values in the dulaglutide group were significantly decreased compared with placebo (all p < 0.001).
Figure 2.
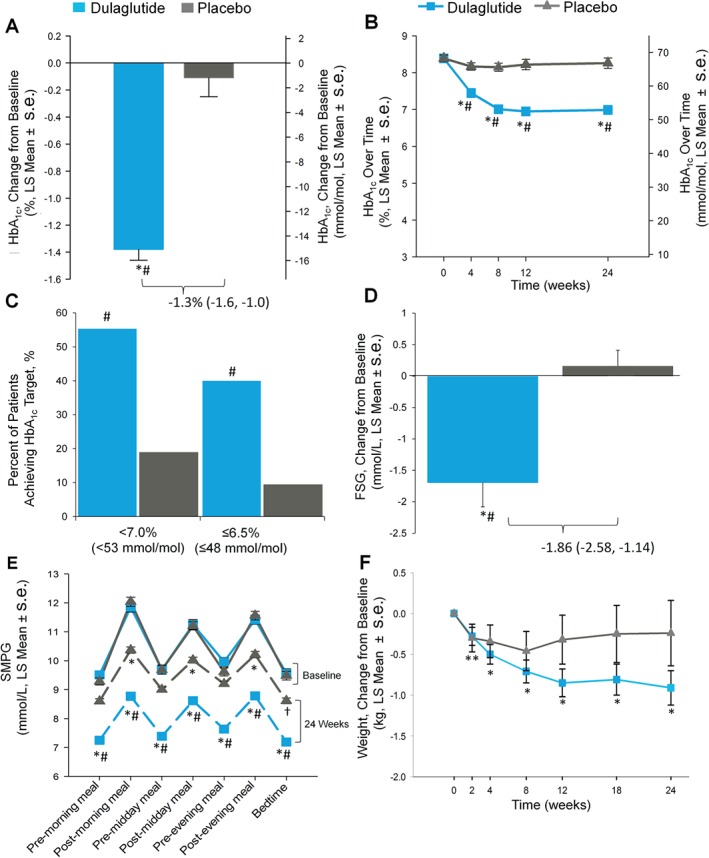
Trial outcome measures: (A) Change in glycated haemoglobin (HbA1c) from baseline at week 24, intention‐to‐treat (ITT) without post‐rescue values [mixed model for repeated measures (MMRM)], *p < 0.001, change from baseline; #p < 0.001, dulaglutide versus placebo. (B) HbA1c values change from baseline over time to week 24 (MMRM), *p < 0.001, change from baseline; #p < 0.001, dulaglutide versus placebo. (C) Percentage of patients achieving HbA1c targets, ITT, without post‐rescue values, Logistic regression, #p < 0.001, dulaglutide versus placebo. (D) Change in fasting serum glucose concentrations from baseline to week 24 (central laboratory) ITT without post‐rescue values analysis of covariance (LOCF) *p < 0.001, change from baseline; #p < 0.001, dulaglutide vs placebo. (E) Seven‐point self‐monitored plasma glucose (SMPG) by time of day, ITT without post‐rescue values (MMRM), *p < 0.001, †p < 0.050, change from baseline; # p < 0.001, dulaglutide vs placebo. Solid lines indicate baseline, dashed lines indicate endpoint data. (F) Body weight change over time from baseline to 24 weeks, ITT without post‐rescue values (MMRM), dulaglutide change from baseline **p < 0.050, *p < 0.001. LS, least‐squares; s.e., standard error.
Body Weight
Weight change over time is shown in Figure 2F. At study endpoint (24 weeks) the LSM [standard error (s.e.)] change in weight from baseline was −0.91 (0.21) kg for dulaglutide (p < 0.001) and −0.24 (0.40) kg for placebo (p = 0.553). The between‐group difference was not significant with an LS mean (s.e.) of −0.68 (0.43) kg (p = 0.120, 95% CI −1.53, 0.18).
Safety
Overall, nine participants (3.8%) in the dulaglutide group and no participant in the placebo group experienced an SAE (Table S2, Supporting Information). One death was reported in a participant randomized to the dulaglutide treatment group who discontinued the study as a result of enterocolitis (5 weeks after randomization). The participant was subsequently hospitalized 7 weeks after discontinuing the study for community‐acquired pneumonia and died 2 days later (adjudicated as an infectious disease death; sepsis). One participant was admitted to the hospital with an episode of hypoglycaemia [blood glucose 62 mg/dl (3.4 mmol/l)]; the participant was discharged and considered recovered by the investigator. The investigator judged the hypoglycaemic event to be mild, not severe. As a result of the hospitalization, however, the event was reported, as per protocol, as an SAE.
A similar proportion of participants experienced treatment‐emergent AEs (TEAEs) in the dulaglutide group (n = 111, 46.4%) compared with the placebo group (n = 23, 38.3%; p = 0.259). The most frequent TEAEs in the dulaglutide group were gastrointestinal: nausea (n = 25, 10.5%), diarrhoea (n = 20, 8.4%), eructation (n = 14, 5.9%) and vomiting (n = 10, 4.2%; Table 2). The majority of reported events were mild to moderate in severity and were transient. The incidence of gastrointestinal AEs peaked by 2 weeks and then declined to an incidence similar to that in the placebo group by 6 weeks (data not shown).
Table 2.
Safety assessments up to 24weeks.
| Dulaglutide 1.5 mg | Placebo | |
|---|---|---|
| Safety Assessment | n = 239 | n = 60 |
| Patients with ≥1 SAE† | 9 (3.8) | 0 (0.0) |
| Patients with ≥1 AE | 111 (46.4) | 23 (38.3) |
| AEs (occurring in ≥5% in either group) | ||
| Nausea | 25 (10.5)* | 0 (0.0) |
| Diarrhoea | 20 (8.4)* | 0 (0.0) |
| Eructation | 14 (5.9) | 0 (0.0) |
| Upper respiratory tract infections | 13 (5.4) | 2 (3.3) |
| Hyperglycaemia | 1 (0.4)* | 3 (5.0) |
| Vital signs, LS mean change from baseline (s.e.) | ||
| Systolic blood pressure, mm Hg | −0.52 (0.96) | 0.00 (1.54) |
| Diastolic blood pressure, mm Hg | −0.03 (0.61) | −0.76 (0.98) |
| Heart rate, bpm | 2.92 (0.67)* | 0.30 (1.09) |
| LS mean (s.e.) change from baseline in ECG PR interval, ms | 3.9 (1.25)* | −1.0 (1.82) |
| Median change from baseline in pancreatic enzymes, LOCF, (Q1,Q3), U/l | ||
| Total amylase | 8.0 (1, 18)* | 2.0 (−5, 11) |
| Lipase | 8.0 (1, 18)* | 4.5 (−3, 16) |
| Patients with treatment‐emergent pancreatic enzymes >1 × ULN | ||
| Total amylase | 28 (12.0) | 7 (11.7) |
| Lipase | 70 (29.9) | 19 (31.7) |
| Patients with treatment‐emergent pancreatic enzymes ≥3 × ULN | ||
| Total amylase | 0 (0.0) | 0 (0.0) |
| Lipase | 7 (3.0) | 0 (0.0) |
| Treatment‐emergent dulaglutide antidrug antibodies, n (%) | ||
| Patients with ≥1 treatment‐emergent dulaglutide antidrug antibodies | 2 (0.8) | NA |
| Dulaglutide‐neutralizing antibodies | 1 (0.4) | NA |
| Native‐sequence GLP‐1 cross‐reactive antibodies | 1 (0.4) | NA |
| Native‐sequence GLP‐1 neutralizing antibodies | 0 (0.0) | NA |
Intention‐to‐treat population. incidence n (%); AE, adverse event; GLP‐1, glucagon like peptide‐1, LS, least squares; ULN, upper limit of normal; SAE, serious adverse event; s.e., standard error.
p < 0.05 versus placebo.
Reported SAEs are listed in the supplement.
Total hypoglycaemia (plasma glucose ≤3.9 mmol/l) occurred in 50 participants (20.9%) in the dulaglutide and in two participants (3.3%) in the placebo group (p = 0.001), with a mean (sd) rate of 2.37 (7.22) events/participant/year for dulaglutide compared with 0.07 (0.39) for placebo (p = 0.001). A greater proportion of participants reported an episode of documented symptomatic hypoglycaemia (p < 0.05) in the dulaglutide group compared with the placebo group (Table 3). There were no cases of severe hypoglycaemia. No differences were observed when hypoglycaemia was evaluated at the <3.0 mmol/l threshold (Table S3, Supporting Information).
Table 3.
Hypoglycaemia through 24 weeks.
| Dulaglutide 1.5 mg | Placebo | |
|---|---|---|
| Hypoglycaemia | n = 239 | n = 60 |
| Total hypoglycaemia(†) | ||
| Incidence, n (%) | 50 (20.9)* | 2 (3.3) |
| Rate (events/patient/year), mean (sd) | 2.37 (7.2)* | 0.07 (0.39) |
| Documented symptomatic‡ | ||
| Incidence, n (%) | 27 (11.3)* | 1 (1.7) |
| Rate (events/patient/year), mean (s.d.) | 0.90 (3.97) | 0.04 (0.28) |
| Nocturnal‡ | ||
| Incidence n (%) | 16 (6.7) | 1 (1.7) |
| Rate (events/patient/year), mean (s.d) | 0.29 (1.89) | 0.04 (0.28) |
| Severe hypoglycaemia | 0 (0.0) | 0 (0.0) |
Intention‐to‐treat population. s.d., standard deviation.
p < 0.05 versus placebo.
Plasma glucose ≤3.9 mmol/l (70 mg/dl) ± symptoms.
Plasma glucose ≤3.9 mmol/l (70 mg/dl).
Mean serum calcitonin levels were unchanged during the study, and there were no reports of C‐cell hyperplasia or medullary thyroid carcinoma. Small median increases in serum lipase and total amylase that remained within normal range were observed for dulaglutide compared with placebo (p < 0.05 both). The percentage of participants with treatment‐emergent lipase or amylase levels above the upper limit of normal (ULN) or ≥3 × ULN was similar between groups, although a numerically greater proportion of participants on dulaglutide (3.0%) had elevations ≥3 × ULN in lipase compared with placebo (0%; p = 0.351; Table 2). There were no cases of adjudicated acute or chronic pancreatitis.
Dulaglutide significantly increased heart rate from baseline compared with placebo (p = 0.024; 2.92 vs 0.30 beats/min). There were no significant changes in LS mean systolic or diastolic blood pressure in either treatment group at 24 weeks (Table 2). Statistically significant increases in PR interval were also observed for dulaglutide. Two participants in the dulaglutide group had adjudicated (confirmed) cardiovascular events: one experienced a cerebrovascular accident during treatment and another was hospitalized with cardiac failure during the safety follow‐up period.
One participant reported a mild systemic hypersensitivity reaction of urticaria, but did not develop dulaglutide antidrug antibodies. Two participants (0.8%) randomized to dulaglutide developed treatment‐emergent dulaglutide antidrug antibodies. One participant developed antidrug antibodies that were both dulaglutide‐neutralizing and cross‐reactive with native‐sequence GLP‐1. Neither participant reported a systemic hypersensitivity reaction and both experienced significant reductions in HbA1c from baseline [−1.1% (−12 mmol/mol) and −1.4% (−15 mmol/mol)] that were sustained up to 24 weeks. No injection site reactions were reported.
Discussion
Once‐weekly dulaglutide was well tolerated and effective when used in combination with SU therapy. Dulaglutide 1.5 mg treatment resulted in greater HbA1c reductions, more participants reaching HbA1c targets, and greater fasting glucose reductions compared with placebo. No between‐group differences in weight were observed. These results suggest that when patients are no longer achieving glycaemic control with an SU, dulaglutide could be an effective treatment to consider. When advancing therapy for patients with T2D, choices should be individualized and include considerations of cost, risk of hypoglycaemia, weight, side effects and quality of life 2.
The efficacy profile observed with dulaglutide 1.5 mg in AWARD‐8 is consistent with previous studies. Specifically, the observed HbA1c reduction of −1.4% (−15 mmol/mol) was similar to reductions reported for the dulaglutide 1.5 mg dose at 26 weeks in the six completed phase III studies [ranging from −0.8 to −1.6% (−9 to −17 mmol/mol)], as was the percentage of participants achieving the HbA1c target of <7.0% (<53 mmol/mol ) with 55% of participants achieving this target in the present study compared with 58–78% in previous studies 14, 15, 16, 17, 18, 19, 20. Previous studies of dulaglutide 1.5 mg have shown weight reductions of −0.9 to −3.2 kg at 26 weeks with an attenuation of weight loss when used in combination with therapies typically associated with weight gain. In AWARD‐8, the concomitant SU probably offset further weight reduction with dulaglutide 1.5 mg. One other study, AWARD‐2, has been completed where dulaglutide was used in combination with maximally tolerated doses of both an SU (glimepiride mean dose 6.3 mg) and metformin. In AWARD‐2, participants treated with dulaglutide 1.5 mg had a mean weight reduction of −1.82 kg at 26 weeks. This finding is somewhat different from the present study and could be related to the concomitant metformin used in the AWARD‐2 study which may have partially mitigated the SU effect on weight. In looking at hypoglyceamia risk between these two studies of dulaglutide in combination with SU, in AWARD‐2, 55% of participants experienced hypoglycaemia over 52 weeks which is higher than the rate observed in the present study (21% over 24 weeks); differences in hypoglycaemia may be related to higher glimepiride dosing in AWARD‐2 (6.3 vs 4.8 mg in AWARD‐8), differing time points for reporting, and differing patient populations with respect to baseline HbA1c and duration of diabetes.
Within the GLP‐1 receptor agonist class, two other agents have specifically evaluated add‐on to SU monotherapy: liraglutide and exenatide twice daily. With respect to the HbA1c reduction, in AWARD‐8, from a baseline HbA1c of 8.4% (68 mmol/mol) dulaglutide 1.5 mg resulted in a HbA1c reduction of −1.4% (−15 mmol/mol). In the Liraglutide Effect and Action in Diabetes (LEAD‐1) study, from a baseline HbA1c of 8.5% (69 mmol/mol), liraglutide 1.8 mg demonstrated an HbA1c reduction of −1.1% (−12 mmol/mol), while in the AMIGO‐2 study (AC2993: Diabetes Management for Improving Glucose Outcomes), exenatide 10 µg twice daily resulted in a HbA1c reduction of −0.86% (−9 mmol/mol), from a baseline of 8.6% (70 mmol/mol) 21, 22, 23. For weight changes, similarly to the present study, liraglutide 1.8 mg did not result in significant reductions in weight compared with placebo 21. In contrast, patients on exenatide twice daily in combination with SU had a small but statistically significant placebo‐adjusted weight change of −0.9 kg (95% CI −1.7, −0.0) 22, 23. Direct comparison of hypoglycaemia across these studies is confounded by the different definitions of hypoglycaemia and differences in the respective SU dosages at baseline and subsequent allowable adjustments for hypoglycaemia in each study protocol. The initial dose of glimepiride in this study was determined based on the prestudy (at least half‐maximum) dose in this study, while 2–4 mg glimepiride was used in LEAD‐1 and half‐maximum dosing of SU was used in AMIGO‐2 23, 24.
The AEs reported with dulaglutide in the present trial are consistent with the known effects of the GLP‐1 receptor agonist class. Dulaglutide was associated with a higher incidence of gastrointestinal AEs, most commonly nausea, diarrhoea and eructation, consistent with prior dulaglutide studies and the GLP‐1 receptor agonist class 17, 18, 25. The events were mostly mild to moderate, occurred early and were transient in nature. Consistent with the GLP‐1 receptor agonist class and previous dulaglutide reports, small increases in heart rate and PR interval were observed 18, 26, 27, 28. While higher than placebo, the incidence of hypoglycaemic events was relatively low (21%), despite a robust effect on glycaemic control with concomitant SU therapy; no severe events were reported. The incidence of dulaglutide antidrug antibodies was very low (0.8%) and there were no associated systemic or injection site hypersensitivity reactions. Consistent with the class, measurable increases in pancreatic enzymes were observed, but there were no adjudicated cases of pancreatitis and no cases of pancreatic carcinoma 18, 27, 29, 30. In addition, there were no reports of medullary thyroid carcinoma.
Limitations of the present study include the relatively short duration of the trial, the use of non‐uniform doses of glimepiride and the lack of an HbA1c stabilization period for patients switching from an approximately equivalent dose of another SU to glimepiride during the lead‐in period. In addition, while the 4 : 1 treatment allocation limited placebo exposure and still allowed assessment of dulaglutide in combination with SU, it may have limited our ability to identify uncommon AEs in the placebo group. While the tolerability of dulaglutide in combination with glimepiride was acceptable in this study, further investigation of the utility of GLP‐1 receptor agonists in broader populations or using alternative approaches are worthwhile, including the combination with other SUs and other emerging glucose‐lowering agents.
Overall, the AWARD‐8 study results suggest a favourable benefit/risk profile, including a clinically significant reduction in HbA1c without weight gain or severe hypoglycaemia, for once‐weekly dulaglutide 1.5 mg as an add‐on intervention in patients with T2D treated with glimepiride monotherapy.
Conflict of Interest
K. M. D. declares consulting with Lilly and GSK and research with GSK, Merck, Astra Zeneca, Mylan, Regeneron and Grifols. R. W. reports personal fees from Member Advisory Board Astra Zeneca, Boehringer Ingelheim, Eli Lilly, Janssen, MSD, Novo Nordisk, Sanofi and Takeda, outside the submitted work. J. L. F., K. E. R., H. H. J. and J. A. S. are employees of and own stock in Eli Lilly and Company. F. P. M. and E. P. have no conflicts of interest to declare.
K. M. D., J. L. F., H. H. J. and J. S. contributed to the study design. J. L. F., H. H. J., J. S. and K. E. R. contributed to the conduct of the study. R.W., F. P. M. and E. P. enrolled patients and contributed to the collection of data in the study. K. M. D., R. W., F. P. M., E. P., J. L. F., H. H. J., J. S. and K. E. R. contributed to the data analysis and interpretation of data and made intellectual contributions to the content, writing and editing of the final manuscript.
Supporting information
Table S1. Fasting plasma glucose threshold values for patients with severe, persistent hyperglycaemia.
Table S2. Serious adverse events.
Table S3. Hypoglycaemia up to 24 weeks [plasma glucose <3.0 mmol/l (54 mg/dl)].
Acknowledgements
The authors acknowledge, with gratitude, Brian F. Teske, PhD (Eli Lilly and Company) for preparing the first and subsequent drafts of the manuscript, and Faithe Hamer (Eli Lilly and Company) for data collection and management. This work was funded by Eli Lilly and Company.
References
- 1. Stratton IM, Adler AI, Neil HA et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 3. Jabbour S, Ziring B. Advantages of extended‐release metformin in patients with type 2 diabetes mellitus. Postgrad Med 2011; 123: 15–23. [DOI] [PubMed] [Google Scholar]
- 4. Pongwecharak J, Tengmeesri N, Malanusorn N, Panthong M, Pawangkapin N. Prescribing metformin in type 2 diabetes with a contraindication: prevalence and outcome. Pharm World Sci 2009; 31: 481–486. [DOI] [PubMed] [Google Scholar]
- 5. AHA . Heart Disease and Stroke Statistics: 2005 Update. Dallas: American Heart Association, 2005. [Google Scholar]
- 6. Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003; 289: 3273–3277. [DOI] [PubMed] [Google Scholar]
- 7. Davis TM, Peters KE, Bruce DG, Davis WA. Prevalence, incidence, and prognosis of hepatobiliary disease in community‐based patients with type 2 diabetes: the Fremantle Diabetes Study. J Clin Endocrinol Metab 2012; 97: 1581–1588. [DOI] [PubMed] [Google Scholar]
- 8. Abdul‐Ghani M. Where does combination therapy with an SGLT2 inhibitor plus a DPP‐4 inhibitor fit in the management of type 2 diabetes? Diabetes Care 2015; 38: 373–375. [DOI] [PubMed] [Google Scholar]
- 9. Glaesner W, Vick AM, Millican R et al. Engineering and characterization of the long‐acting glucagon‐like peptide‐1 analogue LY2189265, an Fc fusion protein. Diabetes Metab Res Rev 2010; 26: 287–296. [DOI] [PubMed] [Google Scholar]
- 10. De la Pena A, Loghin C, Cui X et al. Pharmacokinetics of once weekly dulaglutide in patients with type 2 diabetes mellitus. Diabetes 2014; 63(Suppl. 1): A251. [Google Scholar]
- 11. World Medical Association declaration of Helsinki . Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997; 277: 925–926. [PubMed] [Google Scholar]
- 12. Dmitrienko A, Tamhane AC. Gatekeeping procedures in clinical trials In: Dmitrienko A, Tamhane AC, Bretz F, eds. Multiple Testing Problems in Pharmaceutical Statistics. New York: Chapman and Hall/CRC Press, 2009: 171–176. [Google Scholar]
- 13. Seaquist ER, Anderson J, Childs B et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. J Clin Endocrinol Metab 2013; 98: 1845–1859. [DOI] [PubMed] [Google Scholar]
- 14. Blonde L, Jendle J, Gross J et al. Once‐weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD‐4): a randomised, open‐label, phase 3, non‐inferiority study. Lancet 2015; 385: 2057–2066. [DOI] [PubMed] [Google Scholar]
- 15. Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD‐5). Diabetes Care 2014; 37: 2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Umpierrez G, Tofe Povedano S, Perez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD‐3). Diabetes Care 2014; 37: 2168–2176. [DOI] [PubMed] [Google Scholar]
- 17. Wysham C, Blevins T, Arakaki R et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD‐1). Diabetes Care 2014; 37: 2159–2167. [DOI] [PubMed] [Google Scholar]
- 18. Dungan KM, Povedano ST, Forst T et al. Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet 2014; 384: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 19. Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and safety of once‐weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD‐2). Diabetes Care 2015; 38: 2241–2249. [DOI] [PubMed] [Google Scholar]
- 20. Dungan KM, Raz I, Skrivanek Z, Sealls W, Fahrbach JL. Achieving the composite endpoint of glycated haemoglobin <7.0%, no weight gain and no hypoglycaemia in the once‐weekly dulaglutide AWARD programme. Diabetes Obes Metab 2016; 18: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. VICTOZA [prescribing information] . Novo Nordisk Inc., Plainsboro, 2013. Available from URL: http://www.novo‐pi.com/victoza.pdf. Accessed 12 August 2015. [Google Scholar]
- 22. BYETTA [prescribing information] AstraZeneca Pharmaceuticals, Wilmington: Available from URL: http://www.azpicentral.com/byetta/pi_byetta.pdf#page=1. Accessed 12 August 2015. [Google Scholar]
- 23. Buse JB, Henry RR, Han J et al. Effects of exenatide (exendin‐4) on glycemic control over 30 weeks in sulfonylurea‐treated patients with type 2 diabetes. Diabetes Care 2004; 27: 2628–2635. [DOI] [PubMed] [Google Scholar]
- 24. Marre M, Shaw J, Brandle M et al. Liraglutide, a once‐daily human GLP‐1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD‐1 SU). Diabet Med 2009; 26: 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buse JB, Rosenstock J, Sesti G et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet 2009; 374: 39–47. [DOI] [PubMed] [Google Scholar]
- 26. Ferdinand KC, White WB, Calhoun DA et al. Effects of the once‐weekly glucagon‐like peptide‐1 receptor agonist dulaglutide on ambulatory blood pressure and heart rate in patients with type 2 diabetes mellitus. Hypertension 2014; 64: 731–737. [DOI] [PubMed] [Google Scholar]
- 27. Blevins T, Pullman J, Malloy J et al. DURATION‐5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab 2011; 96: 1301–1310. [DOI] [PubMed] [Google Scholar]
- 28. Pratley RE, Nauck M, Bailey T et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26‐week, randomised, parallel‐group, open‐label trial. Lancet 2010; 375: 1447–1456. [DOI] [PubMed] [Google Scholar]
- 29. DeVries JH, Bain SC, Rodbard HW et al. Sequential intensification of metformin treatment in type 2 diabetes with liraglutide followed by randomized addition of basal insulin prompted by A1C targets. Diabetes Care 2012; 35: 1446–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jensen TM, Saha K, Steinberg WM. Is there a link between liraglutide and pancreatitis? A post hoc review of pooled and patient‐level data from completed liraglutide type 2 diabetes clinical trials. Diabetes Care 2015; 38: 1058–1066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Fasting plasma glucose threshold values for patients with severe, persistent hyperglycaemia.
Table S2. Serious adverse events.
Table S3. Hypoglycaemia up to 24 weeks [plasma glucose <3.0 mmol/l (54 mg/dl)].


