Abstract
Background
There is an unmet need for general population‐based epidemiological data on rosacea based on contemporary diagnostic criteria and validated population survey methodology.
Objective
To evaluate the prevalence of rosacea in the general population of Germany and Russia.
Methods
General population screening was conducted in 9–10 cities per country to ensure adequate geographic representation. In Part I of this two‐phase study, screening of a representative sample of the general population (every fifth person or every fifth door using a fixed‐step procedure on a random route sample) was expedited with use of a questionnaire and algorithm based on current diagnostic criteria for rosacea. Of the subjects that screened positive in the initial phase, a randomly selected sample (every third subject) t`hen underwent diagnostic confirmation by a dermatologist in Part II.
Results
A total of 3052 and 3013 subjects (aged 18–65 years) were screened in Germany and Russia respectively. Rosacea prevalence was 12.3% [95%CI, 10.2–14.4] in Germany and 5.0% [95%CI, 2.8–7.2] in Russia. The profile of subjects with rosacea (75% women; mean age of 40 years; mainly skin phototype II or III, majority of subjects with sensitive facial skin) and subtype distribution were similar. Overall, 18% of subjects diagnosed with rosacea were aged 18–30 years. Over 80% were not previously diagnosed. Within the previous year, 47.5% of subjects had received no rosacea care and 23.7% had received topical and/or systemic drugs. Over one‐third (35% Germany, 43% Russia) of rosacea subjects reported a moderate to severe adverse impact on quality of life.
Conclusion
Rosacea is highly prevalent in Germany (12.3%) and Russia (5.0%). The demographic profile of rosacea subjects was similar between countries and the majority were previously undiagnosed.
Introduction
Rosacea is a chronic, inflammatory skin disorder comprising multiple signs and symptoms.1 Diagnostic criteria are clinical and have been defined by the National Rosacea Society Expert Committee (NRSEC), to comprise primary (flushing, non‐transient erythema, papules and pustules and telangiectasia) and secondary features (burning or stinging, plaques, dry appearance, oedema, ocular manifestations, peripheral location and phymatous changes).2 The presence of one or more of the primary features with a central distribution is indicative of rosacea.2 Rosacea can be divided into four subtypes of erythematotelangiectatic (ETR), papulopustular (PPR), phymatous (PR) and ocular rosacea. Patients can present with more than one subtype, but ETR and PPR are mutually exclusive.
Prior reports of rosacea prevalence range from <1% to 22%.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 This wide variance, however, may be due to shortcomings in case finding and study design.7 These studies, primarily conducted in Europe3, 5, 6, 8, 16, 17, 18, 19 and the United States,9, 10, 11 largely comprised phototypes I to III. Most studies collected data from dermatologist practices or outpatient hospital dermatology clinics and even the studies conducted in broader populations had limited generalizability due to the target population studied (e.g. working population)3, 5, 18 and/or geographical limitations (e.g. only one location).6, 13, 15, 16, 17 Furthermore, some studies did not include the ETR subtype5, 19, 20, which may be more prevalent than PPR.
This study aimed to determine the prevalence of rosacea based on general population screening followed by confirmatory diagnosis by dermatologists in two European countries.
Materials and methods
This was a multicenter, interventional, cross‐sectional study conducted in Germany and Russia. Cities were selected to ensure adequate geographic representation of the respective countries. The study was approved by the local ethics committees and all data were managed in accordance with the local data privacy regulations. All subjects randomized to attend the dermatologist provided informed consent.
In Part I, a target of 3000 consenting subjects aged 18–65 years old were interviewed in the street (Germany) or at their home (Russia) using a fixed‐step procedure on a random route sample (every 5th person or every 5th door). A quota sampling structure based on gender and age (census data from the German Federal Statistics Office and the Russian Federal Statistics State Service) was used to obtain a representative sample of the general population according to the region. For example, if a female subject was needed to match the quota, every fifth person would be contacted until a woman was encountered. Interviewers were non‐medical professionals trained to follow a neutral predefined script to prevent selection bias. Subjects were administered a rosacea screening instrument (Rosascreen) to assess signs and symptoms of rosacea (with representative photographs), skin phototype, socio‐demographic items, facial dermatoses and treatment history.
Rosascreen is comprised of a subject questionnaire and an algorithm for rosacea case finding.21 Subjects were screen positive if they had at least one of the following: rhinophyma; central facial persistent erythema; facial warmth/stinging/burning for darker phototypes (i.e. IV, V and VI); previous rosacea diagnosis. Papules/pustules and ocular symptoms were not included in the algorithm due to their high prevalence in conditions not associated with rosacea (such as acne vulgaris and conjunctivitis respectively). The questions were developed by two dermatologists (JT and MB), using the NRSEC diagnostic criteria translated into layman's language, to detect primary and characteristic features of rosacea. It was pilot tested on subjects with and without rosacea for optimization of sensitivity, specificity and clarity. Prior to implementation in this study, it was tested on a small sample of people in Germany and Russia for comprehension and acceptability.
In Part II, a randomly selected population of screen‐positive subjects attended a dermatologist visit (every third subject was invited until the planned number of subjects had been recruited) for diagnostic confirmation of rosacea. Information about skin care, skin characteristics, comorbidities, features of rosacea and severity of symptoms were obtained to verify or reject the diagnosis of rosacea and identify subtypes. All randomly selected subjects completed the Dermatology Life Quality Index (DLQI) questionnaire.22 For those with a confirmed diagnosis, information on disease history was also collected.
Sample size calculations were based on the expected precision of the prevalence estimate accounting for variability from combining Parts I and II of the study. This calculation resulted from the product of a binomial probability law by a hypergeometric probability law. Assuming a prevalence of rosacea between 2% and 10%, a sample size of 3000 interviewed subjects in Part I and 150 subjects in Part II, the 95% confidence interval (CI) would range from 0.5–1.8%. Increasing the number of subjects visiting dermatologists would not have led to a meaningful improvement in precision.
Statistical analysis was carried out using SAS software version 9.2 (SAS Institute Inc., Cary, USA). Screen‐positive subjects who attended dermatologist consultations were compared to those who did not attend using a stepwise logistic regression procedure starting with all factors selected from univariate analyses. A raw estimate of prevalence was first obtained by multiplying the percentage of screen‐positive subjects in Part I by the percentage of subjects with confirmed rosacea in Part II in each subcategory, defined by the combination of factors found significant in the logistic model. The variance was estimated using the variance of the product of two variables. The adjusted prevalence of rosacea was obtained by the weighted sum of all raw estimates with 95%CI (to estimate precision) using the squared weighted sum of variances.
Results
Population surveys were conducted in 10 German cities from September 2013 to March 2014 and in nine Russian cities from November 2013 to February 2014 (Fig. 1). Of the 3052 subjects interviewed in Germany and 3013 in Russia, a total of 770 subjects (25.2%) in Germany and 423 (14.0%) in Russia were screen positive using the screening algorithm (Fig. 2 and Table 1). Screen‐positive subjects were representative of the general population for age and gender. Persistent central facial erythema was the dominant criterion for initial selection (see Table 1).
Figure 1.
Location of the screening sites in Germany (a) and Russia (b).
Figure 2.
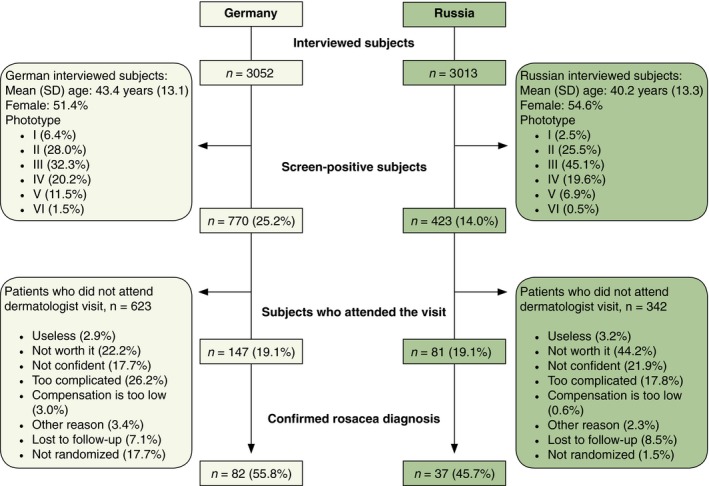
Disposition of subjects in Germany and in Russia.
Table 1.
Proportion of subjects presenting with the screening criteria for rosacea (several screening criteria could be found in the same subject)
| Screening criteria (n,%) | Interviewed subjects | Screen‐positive subjects | ||
|---|---|---|---|---|
| Germany (N = 3052) | Russia (N = 3013) | Germany (N = 770) | Russia (N = 423) | |
| Central facial persistent erythema | 704 (23.1) | 343 (11.4) | 704 (91.4) | 343 (81.1) |
| Thickened skin on the nose | 63 (2.1) | 53 (2.5) | 63 (8.2) | 53 (12.5) |
| Prior diagnosis of rosacea or acne rosacea | 61 (2.0) | 19 (0.6) | 61 (7.9) | 19 (4.5) |
| Dark phototype (IV–VI) associated with facial warmth, stinging and/or burning | 82 (2.7) | 53 (1.8) | 82 (10.6) | 74 (17.5) |
Following randomization of the screen‐positive subjects, 147 (19.1%) in Germany and 81 (14.0%) in Russia attended confirmatory dermatologist visits. The planned sample size of 150 subjects was not reached in Russia as many screen‐positive subjects refused to participate in Part II. The most common reason given for non‐participation was that it was not worth it as their skin problem was not serious/disturbing (22.2% in Germany and 44.2% in Russia) (see Fig. 2).
Of subjects attending the dermatologist visit, rosacea was confirmed for 82 subjects (55.8%) in Germany and 37 subjects (45.7%) in Russia (Fig. 2). For the remaining subjects, alternate diagnoses comprised: no evident facial skin disease (39.1% Germany, 22.7% Russia); acne vulgaris (26.6% Germany, 56.8% Russia); seborrheic dermatitis (12.5% Germany, 38.6% Russia); perioral dermatitis (12.5% Germany); and facial dermatitis (11.4% Russia). Strict application of NRSEC criteria of the signs symptoms recorded by the dermatologist would have increased the number of subjects with rosacea to 90 (61.2%) in Germany and 46 (56.8%) in Russia.
Adjusted (for significant differences between screen‐positive subjects in Parts I and II) rosacea prevalence was 12.3% [95%CI, 10.2–14.4] for Germany and 5.0% [95%CI, 2.8–7.2] for Russia (Table 2). Adjusted prevalence for the ETR subtype was 9.2% for Germany and 4.0% for Russia (see Table 2).
Table 2.
Adjusted prevalence of rosacea and rosacea subtypes in the general population of Germany and Russia
| Germany | Russia | |
|---|---|---|
| Adjusted prevalence (%, [95%CI]) | 12.3% [10.2–14.4] | 5.0% [2.8–7.2] |
| Rosacea subtypes (%, [95%CI]) | ||
| Erythematotelangiectatic | 9.2% [7.2–11.2] | 4.0% [1.8–6.2] |
| Papulopustular | 3.1% [2.0–4.2] | 0.9% [0.4–1.3] |
| Phymatous | 0.5% [0.0–0.9] | 0.2% [0.0–0.5] |
| Ocular | 0.8% [0.2–1.5] | 0.4% [0.1–0.7] |
Subjects could have more than one subtype but erythematotelangiectatic and papulopustular subtypes were mutually exclusive.
Demographics of rosacea subjects with a confirmed diagnosis were similar in Germany and Russia (Table 3). Overall, the majority (74.8%) were female, mean age was 41.8 years, skin phototype was mainly II or III (88.2%) and most (78.2%) had facial skin sensitivity. Less than one‐fifth (16.8%) were previously diagnosed, 45% of whom reported onset of symptoms before the age of 20 years (Table 3). Overall, 23.7% of subjects had received topical and/or systemic medications within the previous year (see Fig. 3).
Table 3.
Characteristics of subjects with confirmed rosacea per country and overall
| Characteristics | Germany (N = 82) | Russia (N = 37) | Overall (N = 119) |
|---|---|---|---|
| Age | |||
| Mean (SD), years | 42.8 (11.8) | 39.5 (11.4) | 41.8 (11.8) |
| Age category n (%) | |||
| <30 | 14 (17.1) | 7 (18.9) | 21 (17.6) |
| 30–39 | 21 (25.6) | 13 (35.1) | 34 (28.6) |
| 40–49 | 18 (22.0) | 9 (24.3) | 27 (22.7) |
| 50–59 | 26 (31.7) | 8 (21.6) | 34 (28.6) |
| > 60 | 3 (3.7) | 0 (0.0) | 3 (2.5) |
| Gender n (%) | |||
| Females | 61 (74.4) | 28 (75.7) | 89 (74.8) |
| Skin phototype (Fitzpatrick) n (%) | |||
| I | 5 (6.1) | 5 (13.5) | 10 (8.4) |
| II | 44 (53.7) | 14 (37.8) | 58 (48.7) |
| III | 32 (39.0) | 15 (40.5) | 47 (39.5) |
| IV | 1 (1.2) | 3 (8.1) | 4 (3.4) |
| V | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| VI | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sensitive facial skin n (%) | |||
| Yes | 62 (75.6) | 31 (83.8) | 93 (78.2) |
| Previous diagnosis of rosacea n (%) | |||
| No | 66 (80.5) | 33 (89.2) | 99 (83.2) |
| Yes | 16 (19.5) | 4 (10.8) | 20 (16.8) |
| If yes, age of onset | |||
| 0–9 years | 5 (31.3%) | 1 (25.0%) | 6 (30.0%) |
| 10–19 years | 2 (12.5%) | 1 (25.0%) | 3 (15.0%) |
| 20–29 years | 5 (31.3%) | 2 (50.0%) | 7 (35.0%) |
| 30–39 years | 1 (6.3%) | 0 (0.0%) | 1 (5.0%) |
| 40–49 years | 2 (12.5%) | 0 (0.0%) | 2 (10.0%) |
| ≥ 50 years | 1 (6.3%) | 0 (0.0%) | 1 (5.0%) |
| Family member(s) diagnosed with rosacea n (%) | |||
| Missing data | 17 NA | 6 NA | 23 NA |
| Yes | 33 (50.8) | 17 (54.8) | 50 (52.1) |
| Subtype of rosacea n (%) | |||
| Erythematotelangiectatic | 57 (69.5) | 23 (62.2) | 80 (67.2) |
| Papulopustular | 25 (30.5) | 11 (29.7) | 36 (30.3) |
| Phymatous | 4 (4.9) | 2 (5.4) | 6 (5.0) |
| Ocular | 6 (7.3) | 7 (18.9) | 13 (10.9) |
Figure 3.
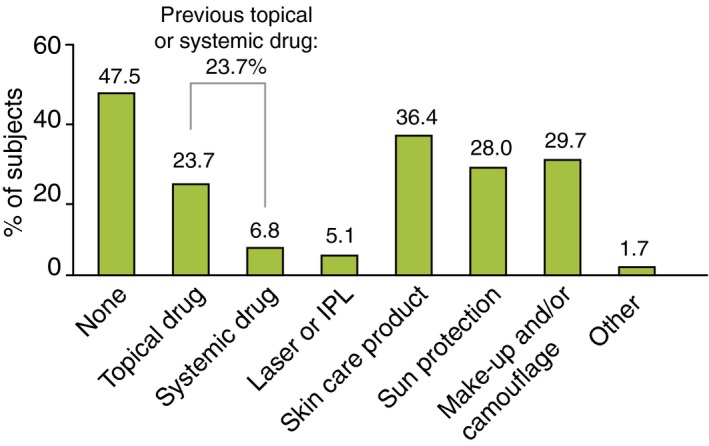
Rosacea care in the binational sample of 119 subjects with a confirmed diagnosis of rosacea IPL= Intense Pulsed Light.
Relative distribution of rosacea subtypes was similar between countries except for the ocular subtype, which was twice as frequent in the Russian as the German population (18.9% vs. 7.3%) (Table 3). Overall, 67.2% of subjects had ETR subtype, 30.3% PPR subtype and 5.0% phymatous subtype. Severity of symptoms were similar in German and Russian subjects, except for flushing, which was mostly mild in German subjects (68.4%) whereas it was moderate (50.0%) or mild (47.2%) in Russian subjects (Fig. 4).
Figure 4.
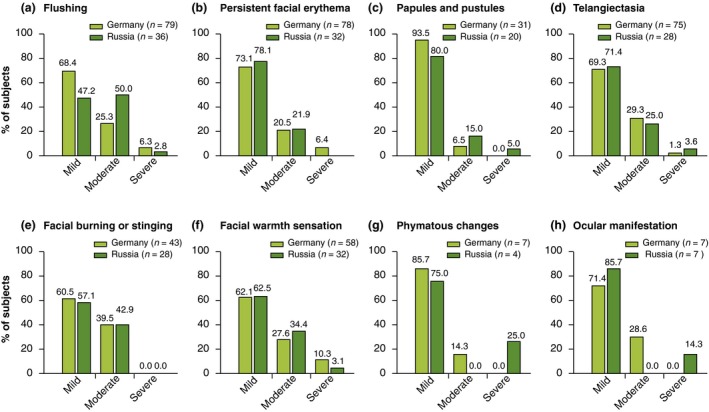
Severity of primary (a–d) and secondary (e–h) features of rosacea in subjects with a confirmed diagnosis of rosacea. Percentages were calculated on subjects presenting the feature and several symptoms could be found in the same patient.
The order of onset of clinical features was flushing first (77.0%), erythema second (61.9%), and papules and pustules appeared third (50.0%).
Mean DLQI scores in subjects with confirmed rosacea was 5.4 in Germany and 6.8 in Russia. The proportion of subjects in Germany and Russia reporting an adverse impact (DLQI score ≥ 6) on skin‐related quality of life (QoL) were 35.4% and 43.2% respectively (Fig. 5).
Figure 5.
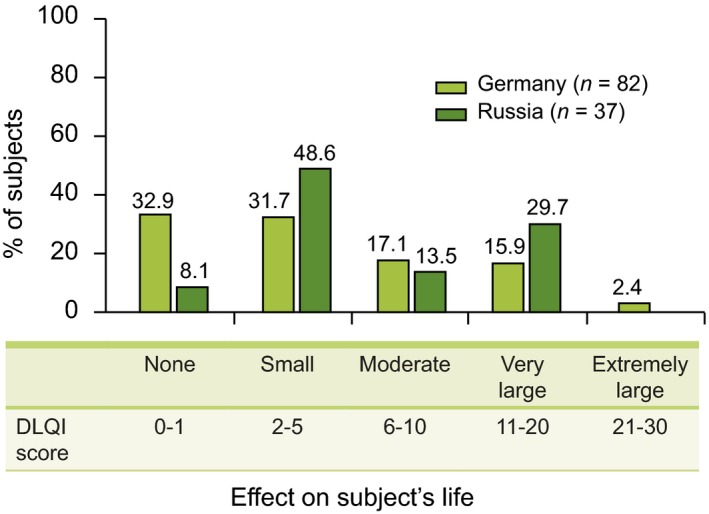
Effect of rosacea on quality of life (QoL) in German and Russian subjects – DLQI questionnaire.
Discussion
This study estimated the prevalence of rosacea in adult populations with a comprehensive approach based on screening a representative sample of the general population using a rosacea screening instrument, followed by diagnostic verification by a dermatologist. The screening instrument (Rosascreen), comprising a questionnaire and algorithm, was developed by dermatologists expert in rosacea using NRSEC diagnostic criteria.21 In pilot testing, it had 100% sensitivity (i.e. true positive cases) and 63–71% specificity. The initial screening phase, with use of Rosacreen, should therefore have detected the vast majority of rosacea cases. Indeed, as the prevalence estimates in our study exceeded those from most previous general population studies, it appears unlikely that the screening process excluded many subjects with rosacea. Furthermore, the subsequent step of confirmation by dermatologists would have excluded false‐positive subjects.
Our prevalence estimates of 5% and 12% for Russia and Germany, respectively, are consistent with previous studies. The prevalence of rosacea in previous European studies generally ranged from 2% to 10%5, 8, 18 with one study reporting 22%.3 The reported prevalence in Germany of 2.2–2.3% may have been underestimated as rosacea was one of multiple potential cutaneous diagnoses based on a general skin examination, criteria for case finding were not explicitly defined and rosacea subtyping was not performed.5, 18 Furthermore, previous studies often did not consider the ETR subtype when assessing prevalence as it was considered only of cosmetic relevance.5, 19, 20 Excluding the ETR subtype, the prevalence of rosacea in our study would be more in accord with previous results at 3.1%. Indeed, the ETR subtype was the most frequent in both countries involving 69.5 and 62.2% of German and Russian confirmed subjects respectively. These results are similar to the 64% previously reported in Germany16, but lower than the 78% reported in Estonia.3
Rosacea prevalence in this study was higher in Germany than Russia despite no substantive differences in skin phototype, age or gender distribution. The difference was apparent at screening with a higher screen‐positive rate in Germany (25.2% vs. 14.0%), largely due to the twofold higher rate of self‐reported persistent erythema in Germany (23.1% vs. 11.4%). Furthermore, there was a higher rate of diagnostic confirmation of rosacea in Germany (55.8% vs. 45.7%). Dermatologists participating in the study established a diagnosis using their clinical judgement (they were provided with diagnostic criteria based on NRSEC guidelines) and recorded the signs and symptoms observed. It is noteworthy that a secondary analysis of these records and application of NRSEC criteria gave a higher rate (56.8% vs. 45.7%) of confirmed cases of rosacea in Russia, mainly due to slightly more cases of phymatous rosacea.
Limitations of this study include lack of representation of rural regions as the surveys were performed in cities to facilitate recruitment of subjects and dermatologists. Nevertheless, general population representation was addressed by using quotas based on demographic census data for gender and age characteristics in the selected cities. Randomization of screen‐positive subjects to attend dermatology visits was undertaken to minimize selection bias during the confirmatory phase. Although a high number of subjects refused to attend the dermatologist visit, the representativeness of those attending was evaluated and the prevalence of rosacea was adjusted to compensate for any differences observed.
Our results on temporal development of clinical features support previous studies at patient23 and molecular level1, 24 demonstrating sequential progression with episodic flushing appearing first followed by persistent erythema and then papules/pustules (insufficient data was available on the order of appearance of phymatous changes).
The demographic profile of subjects with rosacea in this study was similar in both countries and the female predominance, mean age and light skin phototype correspond to the previously established profile of subjects with rosacea.3, 5, 6, 25 Overall, 18% of rosacea subjects were younger than 30 years, which suggests that rosacea may be more common in younger subjects than previously recognized.3, 8, 26 Clinical severity of rosacea associated signs and symptoms were predominantly mild.
Although only 16.8% of subjects with confirmed rosacea had previously been diagnosed, 25% had received drug treatments and/or 5% laser or intense pulsed light therapy. Hence, some subjects received treatment without a diagnosis and may have been misdiagnosed. This highlights that rosacea is a poorly understood disease and most subjects had received suboptimal care for their disease with about half having received no care for their rosacea within the previous year.
The mean DLQI scores reflect small to moderate effect on QoL.27 However, over one‐third of subjects had scores indicative of moderate to severe impact. Rosacea can significantly affect QoL manifested as embarrassment, anxiety and depression.28 These findings imply that appropriate diagnosis and management may alleviate the impact of this condition and improve the QoL of this not inconsiderable proportion of the population.
In conclusion, rosacea prevalence was 12.3% in Germany and 5.0% in Russia and the majority of subjects were previously undiagnosed. We highlight the use of this methodology in surveying large populations and anticipate that future studies will provide more information on rosacea prevalence, demographic features and clinical and psychosocial impact in diverse global populations.
Acknowledgements
The RISE study acknowledge the dermatologists who participated in the study: Helmut Schöfer, Stafen Golsch, Dirk Debus, Phillipp‐Marcel Buck, Maja Hofmann, Reinhard Müller, Bernard Homey, Ralf Denfeld, Stefan Beissert and Rainer Maria Kopner in Germany, and Alexey Kubanov, Elena Araviiskaia, Olga Sidorenko, Evgeniy Orlov, Oksana Bitkina, Tatyana Pisklakova, Nina Filimonkova, Tatyana Reshetnikova and Rasim Abdrakhmanov in Russia. The authors thank Will Maier for assistance in study design and Céline Faure for medical writing support.
Conflicts of interest
J. Tan, M. Berg, H. Schöfer, and E. Araviiskaia have been investigators, advisors and speakers for Galderma; J. Tan and M. Berg have been consultants for Galderma; H. Schöfer has been an advisor and speaker for Medigene, speaker for Meda, GSK/Stiefel, and Abbott; E. Araviiskaia has been an advisor and speaker for L'Oreal, La Roche Posay, Vichy, Bioderma, Pierre Fabre, Uriage, Glenmark, GSK/Stiefel and speaker for Merck Sharp & Dohme, Bayer Health Care, Mertz. F. Audibert and N. Kerrouche are full‐time employees of Galderma R&D SNC.
Funding sources
The study was funded by Galderma R&D SNC.
References
- 1. Steinhoff M, Buddenkotte J, Aubert J et al Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc 2011; 15: 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilkin J, Dahl M, Detmar M et al Standard classification of rosacea: Report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol 2002; 46: 584–587. [DOI] [PubMed] [Google Scholar]
- 3. Abram K, Silm H, Oona M. Prevalence of rosacea in an Estonian working population using a standard classification. Acta Derm Venereol 2010; 90: 269–273. [DOI] [PubMed] [Google Scholar]
- 4. Khaled A, Hammami H, Zeglaoui F et al Rosacea: 244 Tunisian cases. Tunis Med 2010; 88: 597–601. [PubMed] [Google Scholar]
- 5. Schaefer I, Rustenbach SJ, Zimmer L, Augustin M. Prevalence of skin diseases in a cohort of 48,665 employees in Germany. Dermatol 2008; 217: 169–172. [DOI] [PubMed] [Google Scholar]
- 6. Kyriakis KP, Palamaras I, Terzoudi S, Emmanuelides S, Michailides C, Pagana G. Epidemiologic aspects of rosacea. J Am Acad Dermatol 2005; 53: 918–919. [DOI] [PubMed] [Google Scholar]
- 7. Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol 2013; 69(6 Suppl 1): S27–S35. [DOI] [PubMed] [Google Scholar]
- 8. Berg M, Lidén S. An epidemiological study of rosacea. Acta Derm Venereol 1989; 69: 419–423. [PubMed] [Google Scholar]
- 9. Moustafa F, Hopkinson D, Huang KE, Feldman S. Prevalence of rosacea in community settings. J Cutan Med Surg 2015; 19: 149–152. [DOI] [PubMed] [Google Scholar]
- 10. Bamford JT, Gessert CE, Renier CM et al Childhood stye and adult rosacea. J Am Acad Dermatol 2006; 55: 951–955. [DOI] [PubMed] [Google Scholar]
- 11. Engel A, Johnson ML, Haynes SG. Health effects of sunlight exposure in the United States: results from the first national health and nutrition examination survey 1971‐1974. Arch Dermatol 1988; 124: 72–79. [PubMed] [Google Scholar]
- 12. Lomholt G. Prevalence of skin diseases in a population; a census study from the Faroe Islands. Dan Med Bull 1964; 11: 1–7. [PubMed] [Google Scholar]
- 13. Breton AL, Truchetet F, Véran Y et al Prevalence analysis of smoking in rosacea. J Eur Acad Dermatol Venereol 2011; 25: 1112–1113. [DOI] [PubMed] [Google Scholar]
- 14. Shin JB, Kim IH. A Clinical Study of 90 Patients with Rosacea Korean. J Dermatol 2007; 45: 1161–1169. [Google Scholar]
- 15. Bae YI, Yun SJ, Lee JB, Kim SJ, Won YH, Lee SC. Clinical evaluation of 168 korean patients with rosacea: the sun exposure correlates with the erythematotelangiectatic subtype. Ann Dermatol 2009; 21: 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guyomar M, Petit L, Ekanayake‐Bohlig S et al A Clinical Picture of Rosacea: description of a panel of 130 Rosacea patients recruited in Hamburg. Germany. Poster presented at: 22nd World Congress of Dermatology, 2011; Seoul, Korea. [Google Scholar]
- 17. Lazaridou E, Fotiadou C, Ziakas N, Giannopoulou C, Apalla Z, Ioannides D. Clinical and laboratory study of ocular rosacea in northern Greece. J Eur Acad Dermatol Venereol 2011; 25: 1428–1431. [DOI] [PubMed] [Google Scholar]
- 18. Augustin M, Herberger K, Hintzen S, Heigel H, Franzke N, Schäfer I. Prevalence of skin lesions and need for treatment in a cohort of 90 880 workers. Br J Dermatol 2011; 165: 865–873. [DOI] [PubMed] [Google Scholar]
- 19. McAleer MA1, Fitzpatrick P, Powell FC. Papulopustular rosacea: prevalence and relationship to photodamage. J Am Acad Dermatol 2010; 63: 33–39. [DOI] [PubMed] [Google Scholar]
- 20. Doe PT, Asiedu A, Acheampong JW. Rowland Payne CM. Skin diseases in Ghana and the UK. Int J Dermatol 2001; 40: 323–326. [DOI] [PubMed] [Google Scholar]
- 21. Czernielewski J, Berg M, Tan J, Audibert F, Kerrouche N. Development of a rosacea screening questionnaire. Poster presented at: 23rd World Congress of Dermatology 2015; Vancouver, Canada. [Google Scholar]
- 22. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 23. Tan J, Blume‐Peytavi U, Ortonne JP et al An observational cross‐sectional survey of rosacea: clinical associations and progression between subtypes. Br J Dermatol 2013; 169: 555–562. [DOI] [PubMed] [Google Scholar]
- 24. Buhl T, Sulk M, Nowak P et al Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of Th1/Th17 Pathways. J Invest Dermatol 2015; 135: 2198–2208. [DOI] [PubMed] [Google Scholar]
- 25. Powell FC. Clinical practice. Rosacea. N Engl J Med 2005; 352: 793–803. [DOI] [PubMed] [Google Scholar]
- 26. Jansen T, Plewig G. Rosacea: classification and treatment. J R Soc Med 1997; 90: 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994‐2007: a comprehensive review of validation data and clinical results. Br J Dermatol 2008; 159: 997–1035. [DOI] [PubMed] [Google Scholar]
- 28. Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol 2014; 71: 973–980. [DOI] [PubMed] [Google Scholar]


