Abstract
Objective
The effect of prestroke aspirin use on initial severity, hemorrhagic transformation, and functional outcome of ischemic stroke is uncertain.
Methods
Using a multicenter stroke registry database, patients with acute ischemic stroke of three subtypes (large artery atherosclerosis [LAA], small vessel occlusion [SVO], or cardioembolism [CE]) were identified. NIH stroke scale (NIHSS) and hemorrhagic transformation at presentation and discharge modified Rankin Scale (mRS) were compared between prestroke aspirin users and nonusers.
Results
Among the 10,433 patients, 1,914 (18.3%) reported prestroke aspirin use. On crude analysis, initial NIHSS scores of aspirin users were higher than nonusers (mean difference: 0.35; 95% confidence interval [CI]: 0.04–0.66). However, a multivariable analysis with an application of inverse probability of treatment weighting based on a propensity score of prestroke aspirin, having an interaction effect of prestroke aspirin use and stroke subtype in the model, showed less stroke severity for aspirin users in LAA, but not in SVO and CE than for nonusers; mean difference in NIHSS scores in LAA was –0.97 (95% CI: –1.45 to –0.49). With respect to hemorrhagic transformation and mRS, no significant interaction effects were found. Prestroke aspirin use increased the risk of hemorrhagic transformation (adjusted odd ratio: 1.34; 95% CI: 1.05–1.73), but decreased the odds of the higher discharge mRS (0.86; 0.76–0.96).
Interpretation
Prestroke aspirin use may reduce initial stroke severity in atherothrombotic stroke and can improve functional outcome at discharge despite an increase of hemorrhagic transformation irrespective of stroke subtype. Ann Neurol 2016;79:560–568
Aspirin is well known to prevent stroke and cardiovascular disease in high‐risk persons; however, its value as a preventive in persons with low and medium cardiovascular risk profiles has not been proven.1, 2, 3 It has been hypothesized that aspirin, if administered before acute ischemic stroke onset, may be of value as an agent that may reduce initial stroke severity and improve overall clinical outcome.4, 5, 6, 7, 8, 9, 10 Some studies have shown an association between prestroke antiplatelet use and lesser stroke severity,4, 5, 6 whereas others have not.7, 8, 9 Furthermore, there is a paucity of clinical trial information about the possible aforementioned benefits of prestroke aspirin use balanced against the risk of hemorrhagic transformation, a most worrisome adverse event associated with aspirin administration.
We have previously reported that the effect of past antiplatelet use on initial stroke severity may differ by stroke subtype.11 The difference in baseline National Institutes of Health Stroke Scale (NIHSS) scores between the prestroke antiplatelet users and nonusers was significant only in patients with large artery atherosclerosis (LAA), but not in those with cardioembolic stroke (CE) and small vessel occlusion (SVO). This led us to conclude that stroke subtype was an important consideration when judging the possible benefits of prestroke aspirin administration. However, our previous report11 had several limitations: a small‐sized, single‐hospital‐based study; no analysis of bleeding risk or clinical outcome; and heterogeneity in types of antiplatelet agents included in the analysis.
In the absence of high‐level evidence from randomized, controlled clinical trials, observational data from a large clinical registry can be useful, if confounding, because of imbalances of baseline variables can be adequately controlled for in the analysis.12 Using a prospective multicenter stroke registry database,13 this study aimed to investigate the effect of prestroke aspirin use on stroke severity, hemorrhagic transformation at presentation, and functional outcome at discharge according to ischemic stroke subtype.
Materials and Methods
This study was a retrospective analysis based on the Clinical Research Center for Stroke‐5th division (CRCS‐5) registry database.13, 14 The CRCS‐5 registry is a prospective Web‐based registry of ischemic stroke patients who were admitted to participating university hospitals or regional stroke centers in South Korea since 2008. For the purpose of monitoring and improving the quality of stroke care, data on demographics, vascular risk factors, stroke characteristics, including stroke subtype, diagnostic studies, in‐hospital management, other laboratory findings, and functional outcomes, were prospectively collected using a standardized protocol. Details of the registry database are available elsewhere.13, 14
Using the registry, we identified consecutive patients with ischemic stroke who were admitted to the 12 participating centers within 7 days of symptom onset between April 2008 and September 2012 and had relevant cerebral lesions on diffusion‐weighted magnetic resonance imaging (DWI) consistent with acute ischemic stroke. Exclusion criteria were (1) stroke subtypes of other determined or undetermined etiology, (2) prestroke use of antithrombotics other than aspirin within 7 days of stroke onset, or (3) no information on study variables for the current analysis.
Collection of clinical information for the CRCS‐5 registry was approved by the local institutional review boards (IRBs) of all participating centers with a waiver of informed consent because of the provisions of study subject anonymity and determination of minimal study risk to participants. Also, use of the registry database and additional review of medical records for this study was approved by the local IRBs.
The following data were obtained from the registry database: (1) age, sex, and systolic and diastolic blood pressure at presentation; (2) fasting glucose and fasting low‐density lipoprotein (LDL) cholesterol; (3) history of hypertension, diabetes mellitus, hyperlipidemia, smoking, atrial fibrillation, coronary artery disease and past stroke; (4) stroke subtypes, classified as LAA, SVO, CE, stroke of undetermined etiology, and stroke of other determined etiology according to the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria15 with minor modifications16; (5) initial stroke impairment measured by NIHSS score, prestrike, and discharge functional disability measured by the modified Rankin Scale (mRS) score and use of thrombolytic therapies; (6) brain magnetic resonance imaging (MRI) findings, including DWI, and T2‐weighted gradient echo MRI (GE‐MRI); (7) hemorrhagic transformation at presentation (on initial MRI); and (8) use of antithrombotic drugs and statins preceding stroke. Prestroke aspirin (PA) users were defined as patients who had taken aspirin within 7 days before stroke onset to prevent vascular events. Nonusers (non‐PA users) were those who had not taken this antithrombotic drug during the time period. Temporary aspirin users taking aspirin for the purpose other than preventing vascular events were classified as nonaspirin users. Presence of hemorrhagic transformation was determined based on the formal readings of the initial brain MRI, including GE‐MRI. Local investigators routinely reviewed neuroimages in the course of diagnosis and treatment of individual patients.
Outcome measures were initial NIHSS scores, presence of hemorrhagic transformation at presentation, and discharge mRS scores.
Statistical Analysis
Comparisons of baseline characteristics for study subjects (Table 1) and outcomes by stroke subtypes were made between the PA and non‐PA users. For the comparisons, the Student t test and Pearson's chi‐squared test was used according to types of variables, as appropriate.
Table 1.
Characteristics of Patients With Acute Stroke According to Previous Aspirin Use
| Characteristics | PA Users (N = 1,914) | Non‐PA Users (N = 8519) | P a |
|---|---|---|---|
| Age, yr | 70.5 ± 10.6 | 66.8 ± 12.7 | <0.001 |
| Male | 1,064 (55.6) | 5,075 (59.6) | 0.001 |
| Hypertension | 1,613 (84.3) | 5,376 (63.1) | <0.001 |
| Diabetes mellitus | 758 (39.6) | 2,594 (30.4) | <0.001 |
| Hyperlipidemia | 682 (35.6) | 2,408 (28.3) | <0.001 |
| Smoking | 654 (34.2) | 3,629 (42.6) | <0.001 |
| Atrial fibrillation | 546 (28.5) | 1,270 (14.9) | <0.001 |
| History of TIA | 65 (3.4) | 184 (2.2) | 0.001 |
| History of stroke | 524 (27.4) | 1,005 (11.8) | <0.001 |
| History of CAD | 360 (18.8) | 412 (4.8) | <0.001 |
| SBP, mm Hg | 148.2 ± 26.8 | 149.2 ± 27.4 | 0.153 |
| DBP, mm Hg | 84.2 ± 15.6 | 87.1 ± 15.7 | <0.001 |
| WBCb, No./μl | 8,781 ± 17,352 | 8,697 ± 10,466 | 0.783 |
| Platelet, No./μl | 232,465 ± 74,704 | 240,196 ± 73,685 | <0.001 |
| Glucose, mg/dl | 122.0 ± 49.9 | 124.1 ± 53.9 | 0.119 |
| HbA1c, % | 6.53 ± 1.36 | 6.45 ± 1.48 | 0.035 |
| LDL cholesterol, mg/dl | 105 ± 35 | 115 ± 36 | <0.001 |
| Creatinine, mg/dl | 1.14 ± 1.17 | 0.97 ± 0.81 | <0.001 |
| INR | 1.02 ± 0.20 | 1.00 ± 0.14 | <0.001 |
| Prestroke mRS = 0 | 1,499 (78.3) | 7,456 (87.5) | <0.001 |
| Interval from onset to presentation, hr | 25.65 ± 35.67 | 25.37 ± 33.90 | 0.751 |
| TOAST classification | <0.001 | ||
| LAA | 784 (41.0) | 4,357 (51.1) | |
| SVO | 442 (23.1) | 2,409 (28.3) | |
| CE | 688 (35.9) | 1,753 (20.6) | |
| Previous statin use | 495 (25.9) | 411 (4.8) | <0.001 |
| Thrombolysis | 253 (13.2) | 1,001 (11.8) | 0.074 |
Values are number of patients (%) or mean ± standard deviation, unless otherwise indicated.
p values are calculated by chi‐squared test or Student t test, as appropriate.
Although whote blood cell count was insignificant in baseline bivariate analysis, after performing imputations of missing values, it needed to be included in covariates for inverse probability of treatment weighting (PA 7,680 vs non‐PA 7,770; p = 0.117).
PA = prestroke aspirin; TIA = transient ischemic attack; CAD = coronary artery disease; SBP = systolic blood pressure; DBP = diastolic blood pressure; WBC = white blood cell count; HbA1c = glycated hemoglobin; LDL = low‐density lipoprotein; INR = international normalized ratio; TOAST = Trial of ORG 10172 in Acute Stroke Treatment15; mRS = modified Rankin Scale; LAA = large artery atherosclerosis; SVO = small vessel occlusion; CE = cardiac embolism.
To address an imbalance of baseline characteristics between the PA and non‐PA users, we used a propensity score (PS) analysis with an inverse probability of treatment weighting (IPTW) method. To obtain the PS, a probability of a subject receiving PA, a PS model was first developed using a multiple logistic regression model, in which all variables shown in Table 1 (except stroke subtype) were included.17 The discrimination ability of the model was examined using c‐statistics (c = 0.777). This score was used in the subsequent multivariable analyses for an outcome model with the IPTW method, where subjects who received PA were weighted by an inverse of their PS whereas those who received non‐PA were weighted by an inverse of 1 minus PS.18 When applying the IPTW method, a stabilized weight was used to maintain the number of subjects used in the weighted analysis as the number of original study subjects and to yield more precise interval estimates closer to 95% coverage probabilities..19, 20 To assess the likelihood of balancing baseline covariates between the two groups, a generalized estimating equation (GEE) analysis was performed by comparing absolute standardized differences of covariates before and after applying the PS analysis with stabilized IPTW. An absolute standardized difference <10% for a baseline covariate indicates a relatively small imbalance between groups.
In the outcome models, an interaction term between prestroke aspirin use and TOAST classification was included to examine whether the classification modified an effect of the aspirin use on outcomes. Effects of prestroke aspirin use were presented either as mean difference between the PA and non‐PA users and its 95% confidence intervals (CIs) for initial NIHSS scores, odds ratios (ORs) compared with no use and their 95% CIs for the presence of hemorrhagic transformation at presentation, or common ORs with 95% CIs for a shift in the direction of a higher mRS score at discharge (worse outcome). Specifically, binary logistic regression analysis was performed to examine the effect of prestroke aspirin use on the presence of hemorrhagic transformation at presentation, whereas ordinal logistic regression analysis with the proportional odds model was employed to evaluate its effect on the value of mRS scores at discharge. In the ordinal logistic regression analysis, we employed six levels of mRS by collapsing its value of 5 and 6 into a single level of extreme disability or death, and an assumption of proportional odds was satisfied. Therefore, the cumulative ORs can be interpreted as an OR of prestroke aspirin use for having a higher mRS compared to a lower mRS with any cut‐off point of mRS level after controlling for covariates.21, 22 p values reported were two‐tailed and a p value < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS (version 21; IBM Corporation, Armonk, NY) and SAS software (version 9.3; SAS Institute Inc., Cary, NC).
Results
Of 16,761 patients with acute ischemic stroke hospitalized within 7 days of onset with relevant ischemic lesions on DWI between April 2008 and September 2012 and enrolled into the CRCS‐5 registry, we excluded 6,328 for the following reasons: (1) 3,526 patients had other determined or undetermined stroke etiology according to TOAST classification; (2) 2,096 patients were on antithrombotics other than aspirin before stroke onset; and (4) 706 patients were missing glucose or LDL cholesterol values. Among the 10,433 patients enrolled in the study, a total of 1,914 (18.3%) reported aspirin use within 1 week before stroke onset. Among the initial 16,761 patients, 5,286 (31.5%) were on any antithrombotic drugs before stroke, and, as expected, aspirin was the most commonly used antithrombotic drug (Supplementary Table 1).
Table 1 presents comparisons of baseline characteristics between the PA and non‐PA users. Meaningful imbalances (p values < 0.2) were detected for age, sex, hypertension, diabetes mellitus, hyperlipidemia, smoking, atrial fibrillation, history of transient ischemic attack (TIA), history of stroke, history of coronary artery disease, systolic blood pressure, diastolic blood pressure, platelet count, glucose, glycosylated hemoglobin, LDL cholesterol, creatinine, international normalized ratio (INR), pre‐stroke mRS of 0, stroke subtype (TOAST classification), previous statin use, and thrombolysis. After adjustments with IPTW using propensity scores, all the covariates were well balanced within a standardized difference of 0.1 except hypertension (Supplementary Table 2).
On crude analysis, the PA users had a higher initial NIHSS score than the non‐PA users (6.05 vs 5.69; mean difference [95% CI]:, 0.35 [0.04–0. 66]). However, the PA users had a lower initial NIHSS score than the non‐PA users (–0.61 [–0.89 to –0.34] in the multivariable analysis; –0.52 [–0.83 to –0.21] in the IPTW analysis with additional adjustments; Table 2). The application of IPTW revealed a statistically significant interaction between previous aspirin use and stroke subtype (p = 0.009). Specifically, the initial NIHSS scores were lower in the PA users than the non‐PA users with LAA subtype (6.91 vs 7.88; mean difference [95% CI]: –0.97; [–1.43 to –0.50]), whereas this was not the case in those with SVO and CE stroke subtypes (Table 2; Fig 1).
Table 2.
Comparison of Baseline NIHSS Scores Between PA and Non‐PA Users According to Stroke Mechanisms After Applying Stabilized IPTW
| PA Users | Non‐PA Users | LSM Difference | p a | P b | |
|---|---|---|---|---|---|
| Total | 6.74 (6.39–7.10) | 7.26 (6.97–7.56) | −0.52 (−0.83 to −0.21) | 0.001 | |
|
LAA SVO |
6.91 (6.35–7.47) 5.32 (4.84–5.81) |
7.88 (7.47–8.29) 5.39 (5.00–5.78) |
−0.97 (−1.43 to −0.50) −0.07 (−0.41–0.27) |
<0.001 0.680 |
0.009 |
| CE | 8.19 (7.47–8.92) | 8.45 (8.05–8.85) | −0.25 (−1.01–0.51) | 0.513 |
Values presented are mean (95% confidence interval [CI]) or least‐square mean (95% CI), as appropriate.
p values are calculated by analysis of covariance with the generalized estimating equation method adjusting for age, atrial fibrillation, hypertension, diabetes mellitus, stroke, glucose, glycated hemoglobin, low‐density lipoprotein cholesterol, and interval from onset to presentation.
An interaction effect p value between prestroke aspirin use and TOAST classification.
NIHSS = NIH stroke scale; PA = prestroke aspirin; IPTW = inverse probability of treatment weighting; LSM = least‐square mean; LAA = large artery atherosclerosis; SVO = small vessel occlusion; CE = cardiac embolism; TOAST = Trial of ORG 10172 in Acute Stroke Treatment.15
Figure 1.
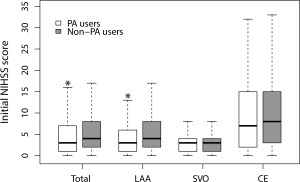
Comparison of initial NIHSS scores according to ischemic stroke subtypes after applying inverse probability of treatment weighting. *p < 0.01 by analysis of covariance with the generalized estimating equation method adjusting for age, atrial fibrillation, hypertension, diabetes mellitus, stroke, glucose, glycated hemoglobin, and low‐density lipoprotein cholesterol. CE = cardiac embolism; IPTW = inverse probability of treatment weighting; LAA = large artery atherosclerosis; NIHSS = NIH stroke scale; PA = prestroke aspirin; SVO = small vessel occlusion.
Hemorrhagic transformation at presentation was more frequently observed in the PA users compared to the non‐PA users (7.4% vs 4.3%; p < 0.001 for crude analysis), and the increased risk of hemorrhagic transformation with prestroke aspirin use was significant in the traditional multivariable analysis as well as the IPTW analysis with additional adjustments (Table 3). In contrast to baseline NIHSS, no significant interaction was found between prestroke aspirin use and stroke subtype (Supplementary Table 3).
Table 3.
Proportional OR for the Hemorrhagic Transformation and Discharge mRS Between PA and Non‐PA Users After Applying Stabilized IPTW
| Adjusted OR (95% CI) | p | |
|---|---|---|
| Hemorrhagic transformation | 1.35 (1.05–1.73) | 0.019a |
| Discharge mRSb | 0.86 (0.77–0.97) | 0.011c |
p value by binary logistic regression analysis with the GEE method adjusting for age, hypertension, diabetes mellitus, AF, stroke, glucose, HbA1c, low‐density lipoprotein, TOAST classification, and interval from onset to presentation.
Six levels were used after collapsing mRSs of 5 and 6 into a single level.
p value by ordinal logistic regression analysis with GEE method adjusting for age, hypertension, diabetes mellitus, stroke, glucose, HbA1c, low‐density lipoprotein, prestroke mRS, TOAST classification, in‐hospital antithrombotic drugs and interval from onset to presentation.
OR = odds ratio; mRS = modified Rankin Scale; PA = prestroke aspirin; IPTW = inverse probability of treatment weighting; CI = confidence interval; AF = atrial fibrillation; GEE = generalized estimating equation; HbA1c = glycated hemoglobin; TOAST = Trial of ORG 10172 in Acute Stroke Treatment.15
The median value in the discharge mRS scores was lower in the PA users than the non‐PA users, and, similar to hemorrhagic transformation, there was no significant interaction between prestroke aspirin use and stroke subtype (Supplementary Table 3; Fig 2). The ordinal logistic regression analysis with IPTW and additional adjustments for covariates and in‐hospital antithrombotic drug use provided the adjusted common OR of 0.86 in favor of prestroke aspirin use (Table 3). A shift in distribution of discharge mRS scores favoring prestroke aspirin use after applying IPTW is given in Figure 3.
Figure 2.
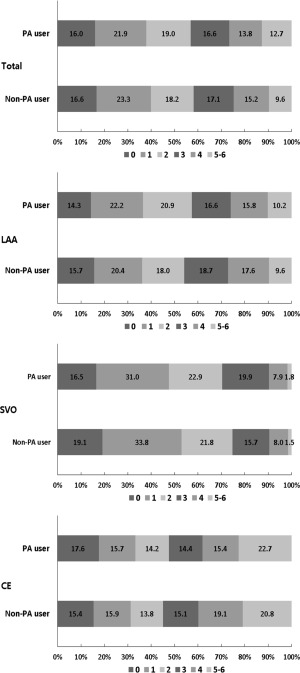
Distribution of modified Rankin Scale scores at discharge according to prestroke aspirin use before applying inverse probability of treatment weighting. CE = cardiac embolism; IPTW = inverse probability of treatment weighting; LAA = large artery atherosclerosis; PA = prestroke aspirin; SVO = small vessel occlusion.
Figure 3.
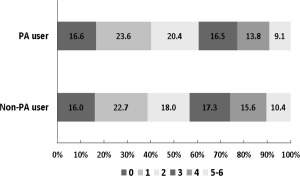
Distribution of modified Rankin Scale (mRS) scores at discharge after inverse probability of treatment weighting according prestroke aspirin use. A favorable shift in the mRS was observed in the prestroke aspirin users (odds ratio: 0.86; 95% confidence interval: 0.76–0.96; p = 0.008).
As post‐hoc sensitivity analysis, analyses for hemorrhagic transformation at presentation and discharge mRS were repeated after excluding 1,254 patients receiving thrombolysis. The results were similar to those of the original analysis; prestroke aspirin use decreased the odds of worse functional outcome (a higher mRS score) at discharge despite an increased risk of hemorrhagic transformation at presentation. There was no significant interaction between prestroke aspirin use and stroke subtype with respect to hemorrhagic transformation and discharge mRS (Supplementary Table 4).
Discussion
The main findings from our study can be summarized as follows: (1) There was a beneficial effect of prestroke aspirin use in relation to the reduction of initial stroke severity according to stroke subtype. The effect was evident in atherosclerotic stroke, but not in other subtypes, such as lacunar or cardioembolic stroke, and (2) although prestroke aspirin increased the risk of hemorrhagic transformation, it was associated with better short‐term functional outcome irrespective of stroke subtype.
Aspirin may reduce stroke severity by limiting clot size, extent of thrombosis, and subsequent embolism.23 In addition, aspirin may benefit patients with acute ischemic stroke by improving the microcirculation in the ischemic penumbra through inhibition of platelet‐derived vasoconstrictors, such as thromboxane A2.24, 25, 26 Anti‐inflammatory and neuroprotective effects can be expected as other beneficial mechanisms.27, 28, 29 Current guidelines recommend oral administration of aspirin within 24 to 48 hours of stroke onset in patients with acute cerebrovascular disease to reduce mortality and unfavorable outcomes.30 Our finding suggests that persons already taking aspirin may have better outcomes associated with acute ischemic stroke than those who are not already taking aspirin.
This study shows that the effect of prestroke aspirin use on reducing stroke impairment differs by stroke mechanism. Different from myocardial infarction, artery‐to‐artery embolism is a dominant mechanism of atherosclerotic stroke.31 Aspirin may reduce not only the possibility of embolization from an unstable plaque, but also the size of emboli.32 Theoretically, antiplatelet therapy may be less beneficial in lacunar stroke than atherosclerotic stroke. In addition, potent antiplatelet therapy is known to increase the risk of bleeding in patients with lacunar stroke.33 With respect to atrial fibrillation, the most common cause of cardioembolic stroke, a meta‐analysis and a recent clinical guideline suggested no or minimal effect of aspirin, especially for primary prevention.34, 35 All of these results support our findings that prestroke aspirin use is more beneficial in patients with LAA than other stroke subtypes.
Risk of bleeding is increased by use of aspirin. It is well known that hemorrhagic transformation is expected to worsen clinical outcome in ischemic stroke patients. Therefore, it is anticipated that a good outcome in prestroke aspirin users may be attributed to a beneficial effect of prestroke aspirin use in those without hemorrhagic transformation. We estimated the effect of prestroke aspirin use on discharge mRS according to hemorrhagic transformation by introducing the presence of hemorrhagic transformation into the multivariable model with stabilized IPTW as a covariate and an interaction term between prestroke aspirin use and hemorrhagic transformation. As expected, the beneficial effect of prestroke aspirin use was only significant in patients without hemorrhagic transformation (yes [N = 509] vs no [N = 9,924], or for better mRS 0.83 [0.55–1.27] vs 0.86 [0.76–0.96]). However, interaction between hemorrhagic transformation and prestroke aspirin use was not significant (p = 0.912). Therefore, we could conclude that prestroke aspirin use improved outcome, but this was independent of hemorrhagic transformation.
In the analysis of the Safe Implementation of Treatments in Stroke International Stroke Thrombolysis Register (SITS‐ISTR), the risk of symptomatic intracerebral hemorrhage after intravenous thrombolysis was higher in the prestroke aspirin users compared with the no antiplatelet user group36; however, the risk was small compared with the benefit of thrombolysis. Because of the retrospective nature of this study, we could not analyze clinical impacts of hemorrhagic transformation at presentation on individual patients, and hence the relationship between hemorrhagic transformation and symptomatic deterioration could be considered uncertain in our study. However, potential residual confounding by thrombolysis on the association of prestroke aspirin use with hemorrhagic transformation and functional outcome was analyzed by sensitivity analysis excluding patients receiving thrombolysis, which showed no meaningful influence of thrombolysis on the study results.
Baseline characteristics were quite different between the PA and non‐PA users, as observed previously.7 An imbalance according to aspirin use was expected and inevitable, which may not be easily overcome by usual methods of multivariable analysis. It has been suggested that use of propensity scores eliminates a greater proportion of baseline imbalances between two groups than does covariate adjustment or stratification.37 It should be noted, however, that use of propensity scores cannot eliminate bias because of unmeasured confounders. Aspirin users may have higher socioeconomic status and therefore have better access to health care and better control of vascular risk factors than nonusers. In this context, aspirin would merely be a surrogate of better medical care. Education level is a well‐known variable that represents socioeconomic status. As a post‐hoc analysis, we included education level in the PS model to adjust for socioeconomic status and thus potential accessibility to health care. Education level was divided into uneducated or illiterate, 0 to 6 years, 7 to 12 years, and over 13 years of schooling. Education level was missing in 1,147 (11.0%) out of 10,433 patients. A subject's propensity score was then recalculated using the 23 variables listed in Table 1 and education level (Supplementary Table 5). After applying the stabilized IPTW, the impacts of prestroke aspirin use on initial NIHSS, hemorrhagic transformation at presentation, and discharge mRS were attenuated, but grossly unchanged (Supplementary Tables 6 and 7). Therefore, and based on our assumptions, we do not believe that accessibility to health care would substantially change the main results.
This study has several limitations. First, although we attempted to control confounding using IPTW, the lack of randomization still leads to the possibility of existing unknown sources of biases that may have influenced the findings of this observational study. In particular, use of aspirin for prevention of thrombotic events could be a clinical indicator for a greater severity of illness. This unmeasured confounder, however, would have led to bias toward the null.38 That is, the bias would suppress a positive effect, if any, of prestroke aspirin use, which supports the robustness of our findings. Second, because did not consider initial infarct size and occurrence of stroke recurrence or progression during hospitalization in the analysis, the mechanism to explain the reduction of initial severity and the improvement of discharge outcome by prestroke aspirin use could not be explored in this study. Third, we assessed functional outcome of stroke with mRS at discharge instead of at 3 months. A 3‐month mRS is a more generally accepted outcome measure in clinical studies.39 However, mRS at 7 to 10 days has been reported to strongly correlate with 3‐month mRS.40 Fourth, we had no information on duration of prestroke aspirin use. However, based on the predetermined definition of prestroke aspirin use in our stroke registry, most aspirin administration was for the purpose of primary or secondary prevention of vascular events and may be assumed as chronic use. The effect of temporary aspirin use for other indications was not evaluated. Last, the data were prospectively collected, but retrospectively extracted from a pre‐existent registry database and thereby might not be as accurate as those data from clinical trials. However, to ensure the quality of data, the data were collected by trained registrars using standardized protocols and the overall process of case registration, monitoring of the data quality, and inquiry and correction of erroneous data were managed and supervised through monthly meetings by a steering committee; the details of these processes are published elsewhere.13, 14
In conclusion, this study suggests that aspirin may reduce stroke severity, at least for atherosclerotic stroke, and improve functional outcome even when it fails to prevent stroke. The effect of prestroke aspirin may act differently according to stroke mechanism on reducing stroke severity. A further examination of its clinical implication should therefore be necessary.
Author Contributions
J.‐M.P., J.S.L., J.L., and H.‐J.B. contributed to conception and design of the study. J.‐M.P., K.K., Y.‐J.C., K.‐S.H., K.B.L., T.H.P., S.J.L., Y.K., M.‐K.H., J.L., J.‐K.C., D.‐H.K., D.‐E.K., J.‐T.K., J.C.C., K.‐H.Y., B.‐C.L., J.S.L., J.L., P.B.G., and H.‐J.B. contributed to acquisition and analysis of data. J.‐M.P., J.S.L., J.L., P.B.G., and H.‐J.B. contributed to drafting the manuscript and subsequent revisions of the manuscript.
Potential Conflicts of Interest
Dr. Gorelick serves on the Steering Committee of the Bayer‐sponsored ARRIVE trial of aspirin administration in primary stroke prevention and is a consultant to New Haven Pharmaceuticals for a long‐acting aspirin preparation, Durlaza.
Supporting information
Additional supporting information can be found in the online version of this article
Supporting Information
Acknowledgment
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C2020) and by a grant of Bayer Korea, Ltd.
References
- 1. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–2236. [DOI] [PubMed] [Google Scholar]
- 2. Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:517–584. [DOI] [PubMed] [Google Scholar]
- 3. Antithrombotic Trialists C, Baigent C, Blackwell L, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta‐analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sanossian N, Saver JL, Rajajee V, et al. Premorbid antiplatelet use and ischemic stroke outcomes. Neurology 2006;66(3):319–323. [DOI] [PubMed] [Google Scholar]
- 5. Ovbiagele B, Buck BH, Liebeskind DS, et al. Prior antiplatelet use and infarct volume in ischemic stroke. J Neurol Sci 2008;264:140–144. [DOI] [PubMed] [Google Scholar]
- 6. O'Donnell M, Oczkowski W, Fang J, et al. Preadmission antithrombotic treatment and stroke severity in patients with atrial fibrillation and acute ischaemic stroke: an observational study. Lancet Neurol. 2006;5:749–754. [DOI] [PubMed] [Google Scholar]
- 7. Paciaroni M, Agnelli G, Caso V, et al. Prior use of antithrombotic agents and neurological functional outcome at discharge in patients with ischemic stroke. J Thromb Haemost 2006;4:1957–1961. [DOI] [PubMed] [Google Scholar]
- 8. Rist PM, Buring JE, Kase CS, et al. Effect of low‐dose aspirin on functional outcome from cerebral vascular events in women. Stroke 2013;44:432–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ricci S, Lewis S, Sandercock P, et al. Previous use of aspirin and baseline stroke severity: an analysis of 17 850 patients in the International Stroke Trial. Stroke 2006;37:1737–1740. [DOI] [PubMed] [Google Scholar]
- 10. Kwok CS, Skinner J, Metcalf AK, et al. Prior antiplatelet or anticoagulant therapy and mortality in stroke. Heart 2012;98:712–717. [DOI] [PubMed] [Google Scholar]
- 11. Kim WJ, Ko Y, Yang MH, et al. Differential effect of previous antiplatelet use on stroke severity according to stroke mechanism. Stroke 2010;41:1200–1204. [DOI] [PubMed] [Google Scholar]
- 12. Bufalino VJ, Masoudi FA, Stranne SK, et al. The American Heart Association's recommendations for expanding the applications of existing and future clinical registries: a policy statement from the American Heart Association. Circulation 2011;123:2167–2179. [DOI] [PubMed] [Google Scholar]
- 13. Kim BJ, Han MK, Park TH, et al. Current status of acute stroke management in Korea: a report on a multicenter, comprehensive acute stroke registry. Int J Stroke 2013;9:514–518. [DOI] [PubMed] [Google Scholar]
- 14. Kim BJ, Park JM, Kang K, et al. Case characteristics, hyperacute treatment, and outcome information from the Clinical Research Center for Stroke–fifth division registry in South Korea. J Stroke 2015;17:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adams H, Jr , Bendixen B, Kappelle L, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 16. Ko Y, Lee S, Chung JW, et al. MRI‐based algorithm for acute ischemic stroke subtype classification. J Stroke 2014;16:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. [Google Scholar]
- 18. Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–560. [DOI] [PubMed] [Google Scholar]
- 19. Brookhart MA, Wyss R, Layton JB, et al. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes 2013;6:604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV‐positive men. Epidemiology 2000;11:561–570. [DOI] [PubMed] [Google Scholar]
- 21. Scott SC, Goldberg MS, Mayo NE. Statistical assessment of ordinal outcomes in comparative studies. J Clin Epidemiol 1997;50:45–55. [DOI] [PubMed] [Google Scholar]
- 22. Shuaib A, Lees KR, Lyden P, et al. NXY‐059 for the treatment of acute ischemic stroke. N Engl J Med 2007;357:562–571. [DOI] [PubMed] [Google Scholar]
- 23. Joseph R, Han E, Tsering C, et al. Platelet activity and stroke severity. J Neurol Sci 1992;108:1–6. [DOI] [PubMed] [Google Scholar]
- 24. Hallenbeck JM, Furlow TW. Prostaglandin I2 and indomethacin prevent impairment of post‐ischemic brain reperfusion in the dog. Stroke 1979;10:629–637. [DOI] [PubMed] [Google Scholar]
- 25. Rosenblum WI, El‐Sabban F. Platelet aggregation in the cerebral microcirculation: effect of aspirin and other agents. Circ Res 1977;40:320–328. [DOI] [PubMed] [Google Scholar]
- 26. Joseph R, D'Andrea G, Oster SB, et al. Whole blood platelet function in acute ischemic stroke. Importance of dense body secretion and effects of antithrombotic agents. Stroke 1989;20:38–44. [DOI] [PubMed] [Google Scholar]
- 27. Riepe MW, Kasischke K, Raupach A. Acetylsalicylic acid increases tolerance against hypoxic and chemical hypoxia. Stroke 1997;28:2006–2011. [DOI] [PubMed] [Google Scholar]
- 28. Grilli M, Pizzi M, Memo M, et al. Neuroprotection by aspirin and sodium salicylate through blockade of NF‐κB activation. Science 1996;274:1383–1385. [DOI] [PubMed] [Google Scholar]
- 29. Kuhn W, Muller T, Bttner T, et al. Aspirin as a free radical scavenger: consequences for therapy of cerebrovascular ischemia. Stroke 1995;26:1959–1960. [PubMed] [Google Scholar]
- 30. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 31. Panel, Mohr JP, Albers GW, et al. Etiology of stroke. Stroke 1997;28:1501–1506. [DOI] [PubMed] [Google Scholar]
- 32. Grotta JC, Lemak NA, Gary H, et al. Does platelet antiaggregant therapy lessen the severity of stroke? Neurology 1985;35:632–636. [DOI] [PubMed] [Google Scholar]
- 33. The SPS3 Investigators . Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med 2012;367:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 35. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Diedler J, Ahmed N, Sykora M, et al. Safety of intravenous thrombolysis for acute ischemic stroke in patients receiving antiplatelet therapy at stroke onset. Stroke 2010;41:288–294. [DOI] [PubMed] [Google Scholar]
- 37. Austin PC, Mamdani MM. A comparison of propensity score methods: a case‐study estimating the effectiveness of post‐AMI statin use. Stat Med 2006;25:2084–2106. [DOI] [PubMed] [Google Scholar]
- 38. Katz MH. Multivariable Analysis: A Practical Guide for Clinicians, 2nd ed. Cambridge, UK: Cambridge University Press; 2006. [Google Scholar]
- 39. Lees KR, Bath PMW, Schellinger PD, et al. Contemporary outcome measures in acute stroke research: choice of primary outcome measure. Stroke 2012;43:1163–1170. [DOI] [PubMed] [Google Scholar]
- 40. Ovbiagele B, Saver JL. Day‐90 acute ischemic stroke outcomes can be derived from early functional activity level. Cerebrovasc Dis 2010;29:50–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information can be found in the online version of this article
Supporting Information


