Abstract
This project measured population salt intake in Samoa by integrating urinary sodium analysis into the World Health Organization's (WHO's) STEPwise approach to surveillance of noncommunicable disease risk factors (STEPS). A subsample of the Samoan Ministry of Health's 2013 STEPS Survey collected 24‐hour and spot urine samples and completed questions on salt‐related behaviors. Complete urine samples were available for 293 participants. Overall, weighted mean population 24‐hour urine excretion of salt was 7.09 g (standard error 0.19) to 7.63 g (standard error 0.27) for men and 6.39 g (standard error 0.14) for women (P=.0014). Salt intake increased with body mass index (P=.0004), and people who added salt at the table had 1.5 g higher salt intakes than those who did not add salt (P=.0422). A total of 70% of the population had urinary excretion values above the 5 g/d cutoff recommended by the WHO. A reduction of 30% (2 g) would reduce average population salt intake to 5 g/d, in line with WHO recommendations. While challenging, integration of salt monitoring into STEPS provides clear logistical and cost benefits and the lessons communicated here can help inform future programs.
Cardiovascular disease is the leading cause of mortality in Pacific Island countries (PICs).1 Increased blood pressure (BP) has been linked to increased risks of stroke and other adverse health outcomes.2 Reducing salt consumption lowers BP, is projected to deliver large health gains,3 and has been identified as a highly cost‐effective intervention for the prevention of noncommunicable diseases (NCDs).4, 5 Accordingly, the World Health Assembly has set targets for NCDs that include a 30% relative reduction in population salt intake by 2025.6, 7 For most PICs, including Samoa, a key step will be establishing baseline intake so that progress against the target can be measured.8 Measuring salt intake can be challenging and costly.9 A 2012 review of national approaches to monitoring salt intake highlighted the challenges, particularly for lower‐middle– and upper‐middle–income countries and the importance of identifying practical approaches without compromising accuracy.9 The review included three examples of lower‐middle–income countries (LMICs; China, Philippines, and Thailand) that had monitored salt intake and two examples of research studies from lower‐middle–income countries (India and Vietnam). However, only one LMIC (Vietnam) had collected urine samples. Other countries reported ongoing challenges relating to resources, practicalities, and obtaining reasonable response rates. Integrating salt monitoring into other planned surveys, such as the WHO's STEPwise approach to surveillance of noncommunicable disease risk factors (STEPS), should provide efficiencies to help overcome some of these issues.10
The objective of this study in Samoa was to establish current mean population salt intake and to test the feasibility of integrating urinary sodium analysis into WHO STEPs. The study is the first phase of a before‐and‐after intervention project investigating the cost‐effectiveness of strategies to reduce population salt intake through multisectorial interventions.11
Methods
Samoa is an LMIC located south of the equator, about halfway between Hawaii and New Zealand in the Polynesian region of the Pacific Ocean. It has a population of almost 200,000 and consists of four inhabited islands.12 The second Samoan STEPS survey took place between March and May 2013 as a partnership between the Samoan Ministry of Health, the WHO, and The George Institute for Global Health. The objectives were to assess and track changes in risk factors for chronic disease and measure baseline salt consumption. A detailed implementation plan outlining roles and responsibilities, budget, and timelines was agreed upon between the project partners. A Ministry of Agriculture agreement was also signed between the Ministry of Health, WHO and George Institute, and other partners to ensure protection of data. Ethics approval was obtained from the Health Research Committee (Samoa), the ethics review committee of the Western Pacific Regional Office of WHO, and the University of Sydney's human ethics research committee (protocol number: 15359).
Sampling Frame
The STEPs survey framework used a three‐stage (enumeration area [EA], household, and individual) cluster sampling procedure to select a representative sample of the adult population.13 Samoan permanent residents aged 18 to 64 years were included in the sampling frame based on a household list from 2011.12 The household list was stratified into four statistical regions, namely Apia Urban Area, North West Upolu, Rest of Upolu, and Savaii. Each region comprised a number of districts, villages, EAs, and the number of households. The Apia Urban Area region represented the urban population and the other three regions represented the rural population.
A survey sample size of 2880 was calculated according to the WHO standard formula for STEPS. EAs were selected from each stratum using probability proportional to size sampling. The 2011 Population Household List was then used to select households using equal probability systematic sampling, and an eligible individual from each household was selected using the Kish method.14 A systematic random sampling approach, taking every fifth household (starting at the fifth) in administrative order from the main STEPS sample with no replacement, was then used to invite 500 participants into the salt study.
Research Team and Logistics
The data collection framework for the STEPS survey was based on 52 field workers, divided into two teams of 26 people per team, then having two fixed points operating concurrently. Teams were constructed as follows: supervisors (1); recruitment field workers for the Kish selection method (4); field workers for the registration/check‐in point (2); interviewers for STEPS questionnaire (5); measurements (2); biochemistry testing (2); final check and feedback (1); salt data collectors (2); and drivers (4).
In addition, the survey manager (1), driver and three technical advisors (1 from WHO, 1 from the Ministry of Health's subsurvey, and 1 from the salt subsurvey) resulted in a total of 57 field workers including drivers.
Data collection training for the survey teams took place from February 18 to February 22, 2013 (3 days), followed by pilot testing (2 days). The data collection took place from March to May (9 weeks), with data cleaning and analysis taking place in September.
Recruitment and Participant Consent
Participants were asked to provide written consent, were given instructions on completing the survey, and were invited to come to the survey site the next morning. Samoan communication protocols were followed to ensure community and individual participation. This was done through the usual collaboration with the Ministry of Women, Community, and Social Development. Participation in the survey was also promoted through the media. Selected participants who agreed to participate in the salt study were provided with additional participant information and consent forms. There was no exclusion based on current illness, use of medications, or any other aspect of demography or personal history.
Data Collection
Interviewer‐administered questionnaires were used to obtain demographic and risk factor information, including history of raised BP and current treatment or advice to reduce salt, and salt knowledge and behavior. All data were entered into handheld electronic devices. Physical measurements of body weight and height were taken to the nearest 0.1 kg and 0.1 cm, respectively, and used to calculate body mass index (BMI; kg/m2). Systolic BP (SBP) and diastolic BP (DBP) were measured in triplicate according to standard WHO procedures using Omron Premium HEM 7211 sphygmomanometers (Omron Healthcare, Kyoto, Japan).
Participants in the salt study were then provided with written and oral instructions on how to collect spot and 24‐hour urine samples based on established protocols.15 The urine collection was performed in the 24 hours following the STEPS survey to avoid bias introduced by the need for an overnight fast (required for assessing blood glucose). The spot urine samples were collected as part of the 24‐hour urine collection. Participants were provided with the two containers: a 5 L bottle for the specimen sample and a 1 L jug to collect the urine to put into the 5 L bottle. They were also provided with a cooler bag to store and carry the containers and were instructed to start urine collection at any time before noon that day, recording the time on the container provided.
A salt data collector then travelled around the next day (after 12 pm on day 3) to collect the urine samples and hand out the healthy compensation package (eg, Masima project T‐shirt, and healthy food vouchers) valued at 30 Samoan Tala ($11.62 US). A salt collection and tracking form was used by the salt data collectors to record all details. The urine samples were transported to a central laboratory where the volumes were measured and aliquots of both the spot and 24‐hour samples were analyzed for sodium, potassium, and creatinine.16
Data Entry and Analysis
Descriptive statistics for demographic and health status and consumer knowledge and behavior were recorded for both the salt and the non‐salt participants of the STEPS survey. Urine samples were excluded from the analysis if urine volume was <500 mL or creatinine was <4 mmol (women) or <6 mmol (men). Mean population intake estimates were obtained by sample weighting to the sex and age distribution of the population to provide overall and sex‐, age‐, and BMI‐specific estimates. Differences between subgroups were assessed using t tests. The proportion of the population above the WHO‐recommended guideline of 5 g/d of salt and sodium to potassium ratio were also calculated. Analysis of the consumer knowledge and behavior questions was undertaken and associations with salt intake levels were assessed.
The association between salt intake and participant characteristics were explored using regressions for region, age, sex, BMI, education, income, and employment.
Differences in the characteristics of the salt study participants and the non‐salt study STEPS participants were explored. Data analyses were undertaken using Proc Surveymeans and Proc Surveyfreq in SAS 9.3 (SAS Institute, Cary, NC) and incorporated the study design (strata, cluster, and weights) into the estimates. We included any variable with indications of association with salt consumption (P<.20) in adjusted models. Statistical significance was accepted at P<.05. All confidence interval estimations and tests were conducted using a two‐sided error rate of 5%. No missing data were imputed.
Lastly, measured 24‐hour sodium intake was compared with 24‐hour sodium intake estimated from spot urine samples using Bland‐Altman plots.
Results
Response Rate
From the 500 participants selected to participate in the salt study, 421 (84%) consented and completed the survey. Sixty‐three participants subsequently had to be excluded for lack of unique identifying numbers, 16 for missing urine volumes, and six for missing sex or age data, leaving 336 samples for urine analysis. A further 43 were then excluded for suspected incomplete 24‐hour urine collection, leaving a total of 293 participants (58.5% participation rate) for analysis of salt intake.
Descriptive Statistics
The majority (90%) of the participants from the salt subsample were from rural areas. The mean age was 36.4 years, mean BMI 31.92 kg/m2, and average SBP 126.5 mm Hg (Table 1). About 18.7% of the population were assessed as having hypertension (ie, SPB ≥140 mm Hg and/or DBP ≥90 mm Hg). Those included in the salt subsample were an average of 2 years younger (P=.0166) and less likely to be from urban areas (8.9% vs 22.1%, P<.0001) than the rest of the STEPS sample. Other characteristics were not different (all P>.05) (Annex S1).
Table 1.
Population Characteristics
| Baseline Characteristics | Total | Female | Male |
|---|---|---|---|
| Age, mean (SE), y | 36.4 (0.9) | 36.4 (0.9) | 36.4 (1.2) |
| Female, % | 58.4 | – | – |
| Rural, % | 90.0 | 85.8 | 93.2 |
| Education, % | |||
| Completed primary school or less | 19.7 | 16.1 | 22.2 |
| Completed secondary school | 61.6 | 68.9 | 56.5 |
| Completed tertiary school | 18.7 | 15.0 | 21.3 |
| Employed, % | 61.3 | 62.2 | 60.8 |
| Household size, mean (SE), No. | 8.6 (0.3) | 8.4 (0.5) | 8.7 (0.4) |
| Height, mean (SE), cm | 168.26 (0.86) | 161.67 (0.53) | 173.39 (0.74) |
| Weight, mean (SE), kg | 90.12 (1.21) | 88.87 (0.81) | 91.09 (1.85) |
| Body mass index, mean (SE), kg/m2 | 31.92 (0.39) | 34.04 (0.18) | 30.28 (0.57) |
| Waist circumference, mean (SE), cm | 100.26 (1.47) | 103.17 (1.55) | 98.48 (1.60) |
| Systolic BP, mean (SE), mm Hg | 126.5 (1.1) | 123.4 (2.3) | 128.6 (0.9) |
| Diastolic BP, mean (SE), mm Hg | 75.4 (1.0) | 75.4 (1.4) | 75.5 (1.2) |
| Hypertension, % | 18.7 | 15.6 | 20.8 |
| Uses BP‐lowering medications, % | 8.0 | 11.6 | 5.6 |
| History of stroke, % | 0.0 | 0.0 | 0.0 |
| History of heart attacks, % | 0.2 | 0.4 | 0.0 |
| History of hypertension, % | 7.2 | 7.7 | 6.8 |
| History of diabetes, % | 3.6 | 7.7 | 0.6 |
| Urinary volume, mean (SE), mL | 1260.7 (27.1) | 1167.0 (53.0) | 1332.0 (36.9) |
| Creatinine, mean (SE), mmol | 13.1 (0.4) | 9.9 (0.2) | 15.5 (0.4) |
Abbreviations: BP, blood pressure; SE, standard error.
Results of 24‐Hour Urinary Salt Intake
Weighted mean population urinary 24‐hour salt excretion for Samoan adults aged 18 to 64 years was 7.09 g (standard error [SE], 0.19) (Table 2). The mean salt excretion in men was 7.63 g/d (SE, 0.27) compared with 6.39 g/d (SE, 0.14) for women (P=.0014). This difference remained significant after adjusting for various confounders. A total of 69.3% of the population had a salt intake >5 g with 18.2% consuming more than 10 g of salt per day. The sodium/potassium ratio for the population was 2.48 (SE, 0.13).
Table 2.
Weighted Results for Salt Intake, Potassium Intake, and Sodium/Potassium Ratio
| Overall (N=293) | Female (n=171) | Male (n=122) | |
|---|---|---|---|
| Salt intake, mean (SE), g | 7.09 (0.19) | 6.39 (0.14) | 7.63 (0.27) |
| Salt intake above the 5 g WHO target, % | 69.3 | 65.2 | 72.4 |
| Salt intake above the 10 g WHO target, % | 18.2 | 11.7 | 23.2 |
| Potassium intake, mean (SE), mmol | 56.95 (2.47) | 45.17 (1.32) | 65.93 (3.63) |
| Sodium intake, mean (SE), mmol | 121.37 (3.31) | 109.31 (2.34) | 130.55 (4.69) |
| Sodium/potassium ratio, mean (SE) | 2.48 (0.13) | 2.74 (0.10) | 2.29 (0.16) |
Abbreviations: SE, standard error; WHO, World Health Organization.
Association Between Salt Intake and Other Key Variables
People with low BMI values ate on average 1.34 g less salt than those with higher BMI values (P=.004). People in urban areas ate significantly less salt than those in rural areas, although this difference became nonsignificant after adjusting for potential confounders (P=.0695). There were no significant associations between salt intake and age, education, employment, or hypertension status (Table 3).
Table 3.
Association Between Levels of Salt Intake, Patient Characteristics, and BP
| Sociodemographic Characteristics | Crude | Adjusted | |||
|---|---|---|---|---|---|
| No. | Mean (95% CI) | P Value | Mean (95% CI) | P Value | |
| Region | |||||
| Urban | 26 | 5.97 (4.73–7.20) | .0293 | 5.83 (4.29–7.37) | .0695 |
| Rural | 267 | 7.22 (6.82–7.62) | 7.12 (6.73–7.52) | ||
| Sex | |||||
| Men | 122 | 7.63 (6.96–8.30) | .0014 | 7.02 (5.93–8.11) | .0122 |
| Women | 171 | 6.39 (6.05–6.72) | 5.93 (5.17–6.70) | ||
| Age, y | |||||
| 18–44 | 203 | 7.38 (6.59–8.18) | .0986 | 6.43 (5.28–7.57) | .0654 |
| 45–64 | 90 | 6.33 (5.67–6.99) | 5.21 (3.98–6.43) | ||
| Age continuous (/5 y) | −0.04 (−0.10 to 0.01) | .1012 | −0.05 (−0.10 to 0.00) | .0567 | |
| Education | |||||
| Primary school or less completed | 51 | 7.46 (5.26–9.66) | .9385 | ||
| Secondary school completed | 151 | 7.07 (5.90–8.24) | |||
| Post‐secondary/university | 37 | 7.40 (5.18–9.63) | |||
| Employment status | |||||
| Employed/domestic duties | 133 | 7.09 (6.22–7.96) | .3016 | ||
| Unemployed/student | 104 | 7.37 (6.68–8.06) | |||
| BMI, kg/m2 | |||||
| Underweight/normal | 34 | 6.40 (5.36–7.44) | .0222 | 5.15 (4.00–6.30) | .0004 |
| Overweight/obese | 251 | 7.25 (6.82–7.68) | 6.49 (5.64–7.33) | ||
| BMI continuous | −0.06 (−0.10 to −0.02) | .0108 | −0.02 (−0.05 to 0.01) | .1663 | |
| BP, mm Hg | |||||
| Systolic BP <140 and diastolic BP <90 | 205 | 7.35 (6.46–8.23) | .2080 | ||
| Systolic BP ≥140 or diastolic BP ≥90 | 50 | 6.40 (5.56–7.24) | |||
| Systolic BP continuous (/10 mm) | −0.02 (−0.06 to 0.02) | .2057 | |||
Abbreviation: CI, confidence interval. The multivariate (adjusted) regression includes variables with a P value <.2 in the unadjusted analysis. This version includes the continuous forms for age, body mass index (BMI), and blood pressure (BP). The coefficients for these variables represent a change in salt excretion according to the units on the table.
Knowledge and Behaviors Related to Salt
More than 80% of the participants (82.8%) knew that salt could cause serious health problems (Table 4) but about half reported behaviors expected to lead to higher salt intake. People who added salt to food or at the table had about 1.5 g higher salt intake than those who did not (P=.0422) (Table 5). There were no significant associations between any other aspects of behavior to control salt intake and actual salt intake (all P>.05).
Table 4.
Salt Knowledge and Behavior
| Knowledge and Behavior Toward Salt | Percentage |
|---|---|
| Always/often add salt to food | 46.4 |
| Always/often add salt while cooking | 50.1 |
| Always/often consume processed food high in salt | 54.8 |
| Agreed that too much salt could pose serious health problems | 82.8 |
| Perceived salt consumption | |
| Too much | 18.9 |
| Just the right amount | 52.2 |
| Too little | 21.9 |
| Perceived healthy salt consumption | |
| <10 g or 2 teaspoons | 3.1 |
| <5 g or 1 teaspoon | 24.5 |
| <2 g or 1/2 teaspoon | 52.0 |
| Salt intake control | |
| Avoid processed food | 58.7 |
| Look at sodium labels on food | 42.6 |
| Do not add salt on the table | 43.8 |
| Buy low‐salt alternatives | 54.9 |
| Do not add salt when cooking | 49.0 |
| Use spices other than salt when cooking | 43.9 |
| Avoid eating out | 60.9 |
Table 5.
Association Between Levels of Salt Intake and Salt Knowledge and Behavior
| Salt Knowledge, Attitudes, and Behavior Variables | Crude | Adjusted | ||
|---|---|---|---|---|
| Mean (95% CI) | P Value | Mean (95% CI) | P Value | |
| Add salt to food | ||||
| Always/often/sometimes | 7.56 (6.70–8.41) | .0240 | 7.54 (6.36–8.72) | .0422 |
| Rarely/never | 6.05 (5.04–7.05) | 6.05 (5.11–7.00) | ||
| Add salt while cooking | ||||
| Always/often/sometimes | 7.39 (6.56–8.22) | .1537 | 6.81 (5.97–7.66) | .9696 |
| Rarely/never | 6.29 (4.90–7.68) | 6.78 (5.28–8.29) | ||
| Consume processed food high in salt | ||||
| Always/often/sometimes | 7.33 (6.42–8.24) | .4584 | ||
| Rarely/never | 6.39 (4.02–8.76) | |||
| Perceived salt consumption | ||||
| Too/far too much | 6.08 (4.60–7.55) | .2607 | ||
| Just the right amount | 7.68 (6.12–9.23) | |||
| Too/far too little | 7.09 (5.20–8.98) | |||
| Don't know | 6.65 (5.58–7.72) | |||
| Could using too much salt pose serious health problems? | ||||
| Yes | 7.34 (6.34–8.34) | .4209 | ||
| No | 6.85 (5.35–8.36) | |||
| Don't know | 6.39 (5.56–7.23) | |||
| Perceived healthy salt consumption | ||||
| <10/5 g (2/1 teaspoons) | 6.73 (6.28–7.19) | .3651 | ||
| <2 g (1/2 teaspoon) | 7.77 (6.14–9.40) | |||
| Don't know | 6.40 (5.45–7.35) | |||
| Take action to control salt intake | ||||
| No | 6.96 (6.09–7.83) | .6669 | ||
| Yes | 7.18 (6.49–7.87) | |||
Abbreviation: CI, confidence interval. The multivariate (adjusted) regression includes variables with a P value <.2 in the unadjusted analysis.
Estimation of 24‐Hour Sodium Intake From Spot Samples
From the four different equations used to estimate 24‐hour sodium excretion from spot urine samples at the population level, the INTERSALT method had the closest match in population mean to the 24‐hour collection and showed much better agreement in the Bland‐Altman plots (Figure) than any of the other existing methods. However, it still overestimated salt intake by more than a gram and a half (8.56 g compared with 6.91 g from 24‐hour, Table 6). The methods based on sodium/creatinine ratio (Tanaka, Kawasaki and Mage) overestimated the population levels of salt excretion.
Figure 1.
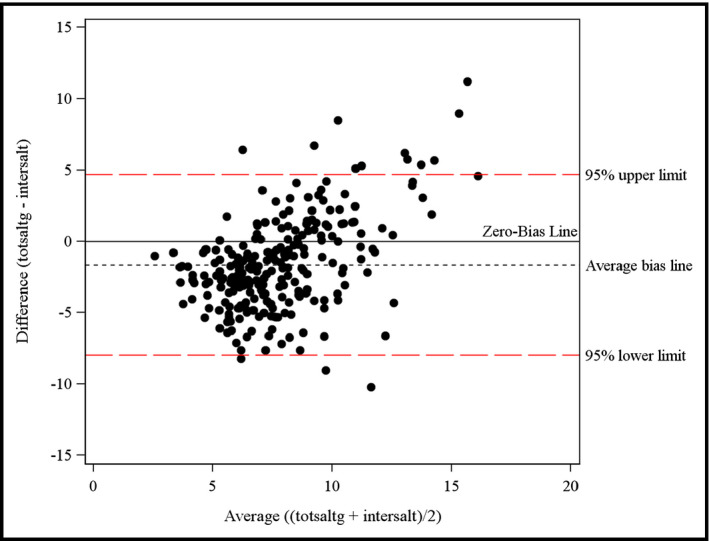
Bland‐Altman plot for 24‐hour sodium excretion estimated from spot urine using the INTERSALT method.
Table 6.
Mean and Intraclass Correlation Coefficients (ICCs) of 24‐Hour Salt Excretion Estimated From Spot Urine Samples Using Different Equations
| Equation | Mean | ICC |
|---|---|---|
| Measured 24‐hour | 6.91 | – |
| INTERSALT | 8.56 | 0.36 |
| Tanaka | 8.99 | 0.40 |
| Kawasaki | 11.86 | 0.26 |
| Mage | 12.09 | 0.09 |
Discussion
In 2013, WHO incorporated the monitoring of salt intake through urine collection into its STEPS protocol. This project was the first time this was done in Samoa and the documenting of the process and the lessons learned will help to inform both national implementation of STEPS surveys to incorporate salt measurements and global policy on monitoring salt intake. Mean population salt intake in Samoa was low compared with average intake levels in many other low‐ and middle‐income countries,17 although still higher than recommended by WHO and with clear scope to reduce intake to the 5 g/d guideline.18 The fact that salt levels were higher among men than women is consistent with most populations.19 About half of the participants said they were trying to reduce their salt intake and people who said that they sometimes or always added salt to food ate on average 1.5 g more salt than those who rarely or never added salt to foods, which suggests that targeting this behavior could make a difference.
The observation that there was no association between salt intake and most other aspects of consumer behavior is in line with recent studies in Australia20 and confirms current thinking that any approach to reduce population salt intake should have a strong emphasis on changing the food environment, rather than focusing solely on consumer education.21 In Samoa, this could include the adoption of existing regional targets for salt levels in foods22, 23 and working with importers and retailers to ensure that the products that are imported meet these targets. In addition, since a substudy performed as part of this project showed that iodine consumption in women aged 18 to 45 years was insufficient (median urinary iodine excretion, 88 μg/L; interquartile range, 54–121 μg/L) and 62% of the women had urinary iodine excretion <100 μg/L,24 efforts to reduce salt intake in Samoa should include efforts to achieve universal salt iodization.25
Study Limitations and Strengths
Several study limitations should be noted when interpreting these results. First, low response rates and the relatively high proportion of nonusable urine samples meant the participants in the final salt subsample were nonrepresentative (mean age differed by 2 years) and more likely to be from rural areas. The participation rate of 58.5% was, however, higher than for many other salt surveys,26, 27, 28 and most characteristics of the salt participants were comparable to those of the non‐salt STEPs participants. Volume and creatinine cutoff values were used to ensure completeness and mean volume and creatinine levels of the participants were in line with other studies.29, 30, 31 Moreover, inclusion of the participants who could not be matched or had urine samples that did not meet the volume or creatinine levels for completeness in the analysis of salt intake also had no impact on calculated intake levels. Second, while it is always possible that the behavior of participants was affected through participation in the survey, ie, that people knew that their salt consumption was being measured and therefore reduced their consumption of salt during the time of the survey, resulting in a lower estimate of population salt intake,32, 33 this is unlikely to have affected the results in view of the challenges associated with changing behavior around salt usage.34 While lower than expected, we believe the salt intake levels should be reasonably accurate.
Potential barriers to the effective implementation of the survey included the difficulty in communicating the importance of obtaining complete 24‐hour urine samples to the participants and the logistics of transporting urine samples from different parts of the Islands to the laboratory. Specialist training and close supervision of the salt data collectors ensured that data were properly recorded and the inclusion of laboratory technicians and data analysts in planning ensured a good understanding of what is required and when. It should be noted that some of the challenges faced were in terms of salt monitoring in general and not specific to integration into the STEPS process. Collection of 24‐hour urine samples requires a high level of resources and supervision, which is sometimes difficult to provide as part of large‐scale national population surveys. However, survey integration offers clear benefits, particularly in terms of costs associated with the sampling, logistics, and travel.
The relatively high potassium intake levels align with the findings of lower‐than‐anticipated sodium intake, suggesting that the transition to processed food consumption35 in Samoa is limited, with the diet still including substantial intakes of fresh vegetables.36 Preliminary analysis of the Food and Agriculture's Household Income and Expenditure Survey confirms that around 55% of total energy intake is derived from vegetables (primarily root vegetables). This is in contrast to Fiji where salt intake is much higher, partly because of the influence of the Indian diet but likely also because of the greater transition toward high‐salt processed foods.37
While other individual studies of salt intake in LMICs or low‐income countries have been documented,29, 31, 38, 39, 40 few studies focus on the methods used or how they can be applied to other populations. This study has demonstrated that while it is possible to measure salt intake through the integration of 24‐hour urinary sodium analysis into STEPS, it is not without its challenges. Undertaking large‐scale multifactorial surveys such as STEPS requires large capacity in terms of training, recruitment, and supervision of fieldworkers; use of equipment for biochemical measurement; and transport of people and equipment to sites. The addition of salt monitoring added further logistical complexity in relation to instructions and materials for urine collection, laboratory analysis of urine, transport/storage of urine, and data analysis.
WHO is now considering using spot urine samples to assess population salt intake as part of STEPS in order to monitor progress toward the global targets. Several equations have been developed and tested and proven potentially useful for estimating mean population 24‐hour salt intake from spot urine samples.41, 42, 43, 44 However, they have yet to be tested on a wide range of population groups. The analysis of the spot samples from this survey showed a moderate correlation and the estimations from spot samples were overestimated by about 1.5 g (Annex 3). While the estimation from spot samples effectively demonstrates that salt intakes are well above the WHO recommendations and may provide a basis from which to monitor change, more research will be needed before we are able to confidently use spot urine samples to estimate 24‐hour urine excretion for this region.
Conclusions
This is the first time salt intake has been reported in Samoa and that urinary sodium analysis has been undertaken as part of the WHO STEPS process. As such, it represents a huge step forward in terms of scalability and sustainability of salt reduction programs. The data have already been used to inform a national salt reduction intervention in Samoa. Population‐based salt reduction strategies are cost‐effective and cost‐saving in most settings.45 Accurate measurement of salt intake is a fundamental step in the development and monitoring of national programs to reduce population salt intake. Such programs are required to ensure that countries are on track to achieve the global target of a 30% reduction in population salt intake by 2025, which is projected to save around 2 million lives a year as well as millions of dollars in healthcare spending.46 These new data and experiences further support the widespread rollout of national monitoring of salt intake to support the increased salt reduction efforts around the world.47
Sources of Funding
Funding support STEPs was provided by the Ministry of Health Samoa and WHO. Funding support for the salt study was provided by the Ministry of Health, Samoa, and a National Health and Medical Research Council of Australia project grant through the Global Alliance for Chronic Diseases hypertension program (1040178). Jacqui Webster was supported from 2012 to 2014 by a National Heart Foundation and Stroke Foundation postdoctoral research fellowship. She is currently supported by a joint National Health and Medical Research Council and Heart Foundation Career Development Fellowship (1082924). Bruce Neal is supported by a National Health and Medical Research Council of Australia Fellowship.
Supporting information
Annex S1. The characteristics of the salt subsample compared with the non‐salt participants in the STEPS survey.
Annex S2. Sensitivity analysis comparing knowledge and attitudes related to salt for the salt subsample compared with the non‐salt sample of STEPS participants.
Annex S3. Estimation of 24‐hour salt consumption from spot samples.
Table S1. Mean and median of estimated 24‐hour salt excretion.
Table S2. The intraclass correlation coefficient between 24‐hour salt collection and different SPOT methods.
Figure S1. Distribution and summary information on estimated 24‐hour salt excretion.
Figure S2. Bland‐Altman plot for 24 hour sodium excretion estimated from spot urine using different estimating equations.
Acknowledgments
We acknowledge the participants in the 2013 WHO STEPs survey and particularly those who collected urine samples. The authors thank the Ministry of Health, The NHS Laboratory Services, the Samoa Bureau of Statistics, the NCD Committee, the STEPS team, and the nurses and all of the participants for their interest in and support for the study in Samoa. We also acknowledge the support of the WHO staff in Samoa and Geneva for their contributions to this project. We acknowledge the support of the George Institute statistics team including Laurent Billot and Kris Rogers for their advice and support on the data analysis for this report.
J Clin Hypertens (Greenwich). 2016;18:884–891. DOI: 10.1111/jch.12778. © 2016 The Authors. The Journal of Clinical Hypertension Published by Wiley Periodicals, Inc.
References
- 1. Institute of Health Metrics and Evaluation. The Global Burden of Disease: generating evidence, guiding policy. East Asia and Pacific Regional Edition. Washington, DC: Human Development Network, The World Bank; 2014. [Google Scholar]
- 2. Polonia J, Martins L. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens. 2009;23:771–772. [DOI] [PubMed] [Google Scholar]
- 3. He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta‐analysis of randomised trials. BMJ. 2013;346:f1325. [DOI] [PubMed] [Google Scholar]
- 4. Smith‐Spangler CM, Juusola JL, Enns EA, et al. Population strategies to decrease sodium intake and the burden of cardiovascular disease. Ann Intern Med. 2010;152:481–487. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization and World Economic Forum . From burden to “best buys”: reducing the economic impact of non‐communicable diseases in low‐ and middle‐income countries. http://apps.who.int/medicinedocs/en/d/Js18804en/. Accessed January 4, 2016.
- 6. UN General Assembly 66th Session . A/66/L.1. Sept 16, N.Y.U.N., 2011. Political declaration of the High‐level Meeting of the General Assembly on the Prevention and Control of Non‐communicable Diseases.
- 7. World Health Organization . Global Action Plan for the Prevention and Control of NCDs 2013–2020. http://www.who.int/nmh/events/ncd_action_plan/en/. Accessed May 1, 2014.
- 8. Christoforou A, Snowdon W, Laesango N, et al. Progress on salt reduction in the pacific islands: from strategies to action. Heart Lung Circ. 2015;24:503–509. [DOI] [PubMed] [Google Scholar]
- 9. Hawkes C, Webster J. National approaches to monitoring population salt intake: a trade‐off between accuracy and practicality? PLoS One. 2012;7:e46727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The World Health Organisation . European Regional Technical Consultation on Noncommunicable Disease Surveillance, Monitoring and Evaluation. http://www.euro.who.int/en/health-topics/noncommunicable-diseases/pages/resolutions-and-meeting-reports/european-regional-technical-consultation-on-noncommunicable-disease-surveillance,-monitoring-and-evaluation.-report-of-consultation. Accessed January 4, 2016.
- 11. Webster J, Snowdon W, Moodie M, et al. Cost‐effectiveness of reducing salt intake in the Pacific Islands: protocol for a before and after intervention study. BMC Public Health. 2014;14:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Samoan Bureau of Statistics . Samoan Census 2011. http://www.sbs.gov.ws/. Accessed May 28, 2014.
- 13. World Health Organization . STEPS Sample Size Calculator and Sampling Spreadsheet. World Health Organization web site. http://www.who.int/chp/steps/resources/sampling/en/. Accessed May 28, 2014.
- 14. Kish L. Sampling organizations and groups of unequal sizes. Am Sociol Rev. 1965;30:564–572. [PubMed] [Google Scholar]
- 15. WHO/PAHO Regional Expert Group for Cardiovasular Disease Prevention through Population‐Wide Dietary Salt Reduction . Protocol for Population Level Sodium Determination in 24‐Hour Urine Samples. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 16. Dyer A, Elliott P, Shipley M. Urinary electrolyte excretion in 24 hours and blood pressure in the INTERSALT study. II. Estimate of electrolyte‐blood pressure associations corrected for regression dilution bias. The INTERSALT Cooperative Research Group. Am J Epidemiol. 1994;139:940–951. [DOI] [PubMed] [Google Scholar]
- 17. Powles J, Fahimi S, Micha R, et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013;3:e003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The World Health Organization . A comprehensive global monitoring framework including indicators and a set of voluntary global targets for the prevention and control of noncommunicable diseases. Second WHO discussion paper 23. Geneva, 2012.
- 19. Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol. 2009;38:791–813. [DOI] [PubMed] [Google Scholar]
- 20. Land MA, Webster J, Chalmers J, et al. Correlation of salt intake with knowledge, attitudes and behaviours in Australian Adults. 24th Scientific Meeting of the International Society of Hypertension. Sydney, Australia; Journal of Hypertension; 2012;30 suppl B:1119. [Google Scholar]
- 21. Webster JL, Dunford EK, Hawkes C, Neal BC. Salt reduction initiatives around the world. J Hypertens. 2011;29:1043–1050. [DOI] [PubMed] [Google Scholar]
- 22. Webster J. Getting Serious about Salt in the Pacific: proposals for regional actions to support the development and monitoring of national salt reduction strategies in the Pacific Islands. http://www.georgeinstitute.org/publications/getting-serious-about-salt-in-the-pacific-islands-proposals-for-regional-actions-to. Accessed January 4, 2016.
- 23. Downs SM, Christoforou A, Snowdon W, et al. Setting targets for salt levels in foods: a five‐step approach for low‐ and middle‐income countries. Food Policy. 2015;55:101–108. [Google Scholar]
- 24. Land MA, Webster J, Ma G, et al. Salt intake and iodine status of women in Samoa. Asia Pac J Clin Nutr. 2016;25(1). DOI: 10.6133/apjcn.2016.25.1.09 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25. Webster J, Land MA, Christoforou A, et al. Reducing dietary salt intake and preventing iodine deficiency: towards a common public health agenda. Med J Aust. 2014;201:507–508. [DOI] [PubMed] [Google Scholar]
- 26. Ortega RM, López‐Sobaler AM, Ballesteros JM, et al. Estimation of salt intake by 24 h urinary sodium excretion in a representative sample of Spanish adults. Br J Nutr. 2011;105:787–794. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Z, Cogswell ME, Gillespie C, et al. Association between usual sodium and potassium intake and blood pressure and hypertension among U.S. adults: NHANES 2005–2010. PLoS One. 2013;8:e75289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wyness LA, Butriss JL, Stanner SA. Reducing the population's sodium intake: the UK Food Standards Agency's salt reduction programme. Public Health Nutr. 2012;15:254–261. [DOI] [PubMed] [Google Scholar]
- 29. Enkhtungalag B, Batjargal J, Chimedsuren O, et al. Developing a national salt reduction strategy for Mongolia. Cardiovasc Diagn Ther. 2015;5:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Land MA, Jeffery P, Webster J, et al. Protocol for the implementation and evaluation of a community‐based intervention seeking to reduce dietary salt intake in Lithgow, Australia. BMC Public Health. 2014;14:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cappuccio F, Kerry S, Micah F, et al. A community programme to reduce salt intake and blood pressure in Ghana. BMC Public Health. 2006;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jürgens G. Method of estimating sodium intake and its possible influence on NHANES III outcome. Arch Intern Med. 2011;171:2063–2064. [DOI] [PubMed] [Google Scholar]
- 33. Kim HJ, Oh K. Methodological issues in estimating sodium intake in the Korea National Health and Nutrition Examination Survey. Epidemiol Health. 2014;36:e2014033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Webster JL, Li N, Dunford EK, et al. Consumer awareness and self‐reported behaviours related to salt consumption in Australia. Asia Pac J Clin Nutr. 2010;19:550–554. [PubMed] [Google Scholar]
- 35. Fa'alili‐Fidow J, McCool J, Percival T. Trade and health in Samoa: views from the insiders. BMC Public Health 2014;14:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seiden A, Hawley NL, Schulz D, et al. Long‐term trends in food availability, food prices, and obesity in Samoa. Am J Hum Biol. 2012;24:286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Snowdon W, Raj A, Reeve E, et al. Processed foods available in the Pacific Islands. Global Health. 2013;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. He FJ, Wu Y, Feng XX, et al. School based education programme to reduce salt intake in children and their families (School‐EduSalt): cluster randomised controlled trial. BMJ 2015;350:h770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang S, Hu X, Chen H, et al. The positive effect of an intervention program on the hypertension knowledge and lifestyles of rural residents over the age of 35 years in an area of China. Hypertens Res. 2011;34:503–508. [DOI] [PubMed] [Google Scholar]
- 40. Tian H, Nan Y, Shao RC, et al. Associations between blood pressure and dietary intake and urinary excretion of electrolytes in a Chinese population. Hypertension. 1995;13:49–56. [PubMed] [Google Scholar]
- 41. Brown IJ, Dyer AR, Chan Q, et al. Estimating 24‐hour urinary sodium excretion from casual urinary sodium concentrations in Western populations: the INTERSALT study. Am J Epidemiol. 2013;177:1180–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ji C, Miller MA, Venezia A, et al. Comparisons of spot vs 24‐h urine samples for estimating population salt intake: validation study in two independent samples of adults in Britain and Italy. Nutr Metab Cardiovasc Dis. 2014;24:140–147. [DOI] [PubMed] [Google Scholar]
- 43. Cogswell ME, Wang CY, Chen TC, et al. Validity of predictive equations for 24‐h urinary sodium excretion in adults aged 18–39 y. Am J Clin Nutr. 2013;98:1502–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang CY, Cogswell ME, Loria CM, et al. Urinary excretion of sodium, potassium, and chloride, but not iodine, varies by timing of collection in a 24‐hour calibration study. J Nutr. 2013;143:1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Asaria P, Chisholm D, Mathers C, et al. Chronic disease prevention: health effects and financial costs of strategies to reduce salt intake and control tobacco use. Lancet. 2007;370:2044–2053. [DOI] [PubMed] [Google Scholar]
- 46. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Trieu K, Neal B, Hawkes C, et al. Salt reduction initiatives around the world––a systematic review of progress towards the global target. PLoS One. 2015;10:e0130247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Annex S1. The characteristics of the salt subsample compared with the non‐salt participants in the STEPS survey.
Annex S2. Sensitivity analysis comparing knowledge and attitudes related to salt for the salt subsample compared with the non‐salt sample of STEPS participants.
Annex S3. Estimation of 24‐hour salt consumption from spot samples.
Table S1. Mean and median of estimated 24‐hour salt excretion.
Table S2. The intraclass correlation coefficient between 24‐hour salt collection and different SPOT methods.
Figure S1. Distribution and summary information on estimated 24‐hour salt excretion.
Figure S2. Bland‐Altman plot for 24 hour sodium excretion estimated from spot urine using different estimating equations.


