Abstract
We recently found an association between androgen deprivation therapy (ADT) and Alzheimer’s disease. As Alzheimer’s disease is a disease of advanced age, we hypothesize that older individuals on ADT may be at greatest risk. We conducted a retrospective multi-institutional analysis among 16,888 individuals with prostate cancer using an informatics approach. We tested the effect of ADT on Alzheimer’s disease using Kaplan–Meier age stratified analyses in a propensity score matched cohort. We found a lower cumulative probability of remaining Alzheimer’s disease-free between non-ADT users age ≥70 versus those age <70 years (p < 0.001) and between ADT versus non-ADT users ≥70 years (p = 0.034). The 5-year probability of developing Alzheimer’s disease was 2.9%, 1.9% and 0.5% among ADT users ≥70, non-ADT users ≥70 and individuals <70 years, respectively. Compared to younger individuals older men on ADT may have the greatest absolute Alzheimer’s disease risk. Future work should investigate the ADT Alzheimer’s disease association in advanced age populations given the greater potential clinical impact.
Prostate cancer is diagnosed in over 1 million patients each year1. Androgen deprivation therapy (ADT) is a mainstay of treatment for men with unfavorable and advanced prostate cancer2 with over 50% of prostate cancer patients in industrialized nations utilizing ADT3. Importantly, ADT has been associated with both improved overall survival and increased adverse health effects4,5 with emerging data indicating a detrimental impact on neurocognitive function6.
We previously demonstrated an association between ADT and Alzheimer’s disease7. In this study we found a large relative increased risk of Alzheimer’s disease among ADT users. Currently, it is unclear among which groups this association may have the greatest clinical significance8. Given that Alzheimer’s disease is a disease of older age, and that ADT is unlikely sufficient to cause Alzheimer’s disease in isolation, we hypothesize that ADT may have a larger absolute impact on Alzheimer’s disease risk among older patients. Here we utilize an informatics approach to analyze electronic medical data in over 5 million patients to examine the impact of age on the association of ADT with Alzheimer’s disease.
Materials and Methods
We used a validated text-processing pipeline to analyze electronic medical record data at Stanford University and Mount Sinai hospitals with study characteristics previously described7. Both data sources were accessed under approved institutional review board protocols. Access to Mt. Sinai data was obtained via an institutional research agreement. The institutional review board waived the requirement for patient consent as the data mining studies were deemed not to involve human participants.
Briefly, individuals with prostate cancer and follow-up ≥180 days after diagnosis were eligible. Prostate cancer was defined as (1) ICD-9 code (185), (2) billing code for radical prostatectomy (ICD-9 60.5 or CPT code 55810–55815, 55840–55845) plus either ADT use (in medication lists or clinical text) or clinical text evidence of prostate cancer diagnosis, or (3) clinical text evidence of prostate cancer diagnosis and ADT use (in medication lists or clinical text). The use of ADT was defined using data from clinical notes and medication lists including pharmacy orders.
Those with a history of dementia, stroke or chemotherapy use were excluded. Covariates were age at prostate cancer diagnosis, race, smoking status, use of anti-platelet, anti-coagulant, anti-hypertensive and statin medications, and a history of cardiovascular disease, diabetes or malignancy. Variables were defined using ICD-9 diagnostic codes, CPT codes, medications lists and clinical text7.
We examined the impact of ADT on Alzheimer’s disease stratified by age at diagnosis. Age 70 years was selected as the cut-off given existing management guidelines for cancer patients older than 70 regarding assessment for age appropriate intervention9. There was insufficient power to examine the association of ADT and Alzheimer’s disease in the <70 years subgroup given 9,112 non-ADT users with 35 Alzheimer’s disease cases and 1,105 ADT users with 3 Alzheimer’s disease cases, which allows a detectable hazard ratio (HR) ≥4.3.
Patient characteristics were compared using a t-test or chi-squared test. Hazard ratios were calculated using 1:5 propensity score matched and traditional multivariable adjusted Cox proportional hazards models to test the effect of use versus non-use of ADT, and ADT duration (no-ADT, <12 months, ≥12 months), on risk of Alzheimer’s disease. Proportional-hazards assumptions were evaluated by Schoenfeld’s residuals tests. Kaplan–Meier curves were compared among individuals <70 years, non-ADT users ≥70 years, and ADT users ≥70 years in the full and propensity score matched cohorts using log-rank and Cox tests for equality, respectively. A test for interaction was conducted between age and ADT using the Wald test. We additionally calculated the cumulative probability of developing Alzheimer’s disease at 5-years using the Kaplan-Meier method in the age-stratified propensity score matched cohort.
A 2-sided p-value < 0.05 was considered significant. Analyses were performed using Stata version 12.0 (StataCorp, College Station, TX) and R version 3.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline patient characteristics are shown in Table 1. No statistically significant differences existed between ADT and non-ADT users ≥70 years in the propensity score matched cohort. The median follow-up period was 2.7 years (interquartile range [IQR], 1.0–5.4 years). The median time to the diagnosis of Alzheimer’s disease was 4.0 years (IQR, 2.0 to 7.4 years).
Table 1. Baseline patient characteristics in the full and propensity score matched cohorts.
| Full cohort | Propensity score matched cohort | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All age-groups | Age ≥70 years subgroup | All age-groups | Age ≥70 years subgroup | |||||||||
| ADT (n = 2,397) | No ADT (n = 14,491) | p-value | ADT (n = 1,292) | No ADT (n = 5,379) | p-value | ADT (n = 2,397) | No ADT (n = 11,985) | p-value | ADT (n = 1,292) | No ADT (n = 6,339) | p-value | |
| Characteristic | ||||||||||||
| Age, mean years (SD) | 70.9 (10.8) | 66.7 (10.5) | <0.001 | 78.9 (6.9) | 77.5 (6.5) | <0.001 | 70.9 (10.8) | 70.9 (12.6) | 0.974 | 78.9 (6.9) | 78.9 (8.4) | 0.902 |
| Caucasian | 1243 (52) | 8426 (58) | <0.001 | 678 (52) | 3,249 (60) | <0.001 | 1243 (52) | 6,487 (54) | 0.115 | 678 (52) | 3,482 (55) | 0.213 |
| Ever smoker | 890 (37) | 3420 (24) | <0.001 | 461 (36) | 1,308 (24) | <0.001 | 890 (37) | 4,553 (38) | 0.539 | 461 (36) | 2,353 (37) | 0.450 |
| Anti-platelet use | 802 (33) | 3394 (23) | <0.001 | 515 (40) | 1,761 (33) | <0.001 | 802 (33) | 3,871 (32) | 0.393 | 515 (40) | 2,441 (39) | 0.483 |
| Anti-coagulant use | 420 (18) | 1885 (13) | <0.001 | 291 (23) | 1,085 (20) | 0.061 | 420 (18) | 1,950 (16) | 0.248 | 291 (23) | 1,388 (22) | 0.703 |
| Anti-hypertensive use | 1205 (50) | 5775 (40) | <0.001 | 738 (57) | 2,693 (50) | <0.001 | 1205 (50) | 6,015 (50) | 0.954 | 738 (57) | 3,644 (58) | 0.852 |
| Statin use | 559 (23) | 3135 (22) | 0.064 | 355 (28) | 1,512 (28) | 0.649 | 559 (23) | 2,651 (22) | 0.321 | 355 (28) | 1,690 (27) | 0.642 |
| Cardiovascular disease | 679 (28) | 3072 (21) | <0.001 | 483 (37) | 1,885 (35) | 0.114 | 679 (28) | 3,288 (27) | 0.491 | 483 (37) | 2,422 (38) | 0.667 |
| Diabetes | 514 (21) | 2295 (16) | <0.001 | 282 (22) | 1,045 (19) | 0.052 | 514 (21) | 2,499 (21) | 0.616 | 282 (22) | 1,408 (22) | 0.814 |
| Malignancy | 166 (7) | 1057 (7) | 0.519 | 111 (9) | 571 (11) | 0.031 | 166 (7) | 679 (6) | 0.073 | 111 (9) | 460 (7) | 0.212 |
ADT, Androgen deprivation therapy; SD, standard deviation.
All data reported as number (%) unless otherwise noted.
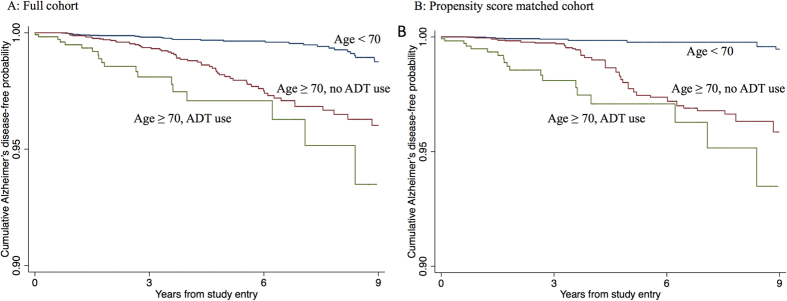
Kaplan-Meier curves (Fig. 1) demonstrated a lower cumulative probability of remaining Alzheimer’s disease-free between those age ≥70 years without ADT use versus those age <70 years (p < 0.001) and between ADT users ≥70 years versus non-ADT users ≥70 years (p = 0.034) in the propensity score matched cohort. The cumulative probability of developing Alzheimer’s disease at 5-years was 2.9%, 1.9% and 0.5% among ADT users ≥70 years, non-ADT users ≥70 years and individuals <70 years of age, respectively. There was a statistically significant association between ADT use and Alzheimer’s disease among those ≥70 years using propensity score matched (HR = 1.84; 95% confidence interval [CI], 1.07–3.17; p = 0.027) and traditional multivariable adjusted Cox regression analysis (HR = 2.04; 95% CI, 1.23–3.40; p = 0.006). Among individuals ≥70 years, a longer duration of ADT was associated with a greater risk of Alzheimer’s disease (HR = 1.41; 95% CI 1.01–1.96, p = 0.043). We did not find evidence of an interaction between ADT use and age (Wald 0.08, p = 0.782).
Figure 1.
Kaplan-Meier curves according to androgen deprivation therapy (ADT) status and age for the cumulative probability of remaining Alzheimer’s disease-free (y-axis) from the initiation of ADT, for ADT users, or from the time of prostate cancer diagnosis plus the median time to ADT use, for non-ADT users (x-axis) in the full (A) Age <70 versus Age ≥70, p < 0.001; Age < 70 versus Age ≥70 without ADT use, p < 0.001; Age ≥70 with ADT use versus Age ≥70 without ADT use, p = 0.008) and propensity score matched cohorts (B) Age <70 versus Age ≥70, p < 0.001; Age <70 versus Age ≥70 without ADT use, p < 0.001; Age ≥70 with ADT use versus Age ≥70 without ADT use, p = 0.034) AD, Alzheimer’s disease; ADT, androgen deprivation therapy.
Conclusions
Using an informatics approach we find that, compared to younger individuals, men aged 70 years or older on ADT have a clinically significant increase in absolute Alzheimer’s disease risk. We support this finding using both multivariable adjusted and propensity score matched models in a large cohort of individuals. This further supports the association between ADT and cognitive dysfunction and suggests that older men may be most susceptible and a particular high-risk subgroup deserving further investigation6,7.
Multiple studies now demonstrate an association between ADT and neurocognitive dysfunction6,7,10. The association of ADT and Alzheimer’s disease is supported by a number of plausible biologic mechanisms including through augmentation of β-amyloid protein levels11, interaction with the Apolipoprotein E gene12, a direct neuropathic effect13 and an increase in cardiometabolic disease5,14. If ADT is truly causally associated with Alzheimer’s disease it likely contributes within a multifactorial etiology. Given that age is the strongest risk factor for Alzheimer’s disease15 we therefore postulated that ADT use among older individuals might confer the greatest absolute risk of Alzheimer’s disease, as has previously been found regarding the impact of ADT on cardiovascular disease risk16. Our finding that older men on ADT have a greater absolute risk of Alzheimer’s disease compared to younger individuals is particularly relevant given concerns regarding aggressive treatment of prostate cancer among men with limited life expectancies17.
Limitations of this study include its retrospective design and the inability to conduct subgroup analysis according to ADT use versus non-use in the <70 years cohort due to low event rates. We were unable to account for prostate cancer specific characteristics, such as Gleason score. We were not powered to undertake subgroup analysis by type of ADT, which may be relevant given that some types of ADT might have a protective effect on Alzheimer’s disease18. Finally, we were unable to evaluate APOE ε4 allele status, which may interact with testosterone levels19.
In conclusion, we find that older men on ADT have the greatest absolute increased risk of Alzheimer’s disease. Future studies are required to determine the mechanism of this association and to develop preventative strategies and inform clinical practice. Prioritization of research and clinical intervention regarding adverse effects of ADT among older individuals may have the greatest clinical impact.
Additional Information
How to cite this article: Nead, K. T. et al. Influence of age on androgen deprivation therapy-associated Alzheimer’s disease. Sci. Rep. 6, 35695; doi: 10.1038/srep35695 (2016).
Acknowledgments
NHS acknowledges support from grant R01 LM011369 from National Library of Medicine (US) and grant R01 GM101430 from National Institute of General Medical Sciences (US).
Footnotes
N.H.S. is an inventor on patents owned by Stanford University that enable the use of clinical text for data-mining: Methods for Ontology based Analytics and numbers: US13/273,038, US13/420,402, US13/424,375, and US13/424,376.
Author Contributions Conception and design: K.T.N., G.G., C.C., N.J.L and N.H.S. Financial and Administrative support: N.H.S. Provision of study materials or patients: J.T.D. and N.H.S. Data analysis and interpretation: K.T.N., S.S., N.J.L. and N.H.S. Manuscript writing and final approval writing: All authors.
References
- Ferlay J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. Journal international du cancer 136, E359–386, doi: 10.1002/ijc.29210 (2015). [DOI] [PubMed] [Google Scholar]
- Bolla M. et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 360, 103–106 (2002). [DOI] [PubMed] [Google Scholar]
- Meng M. V. et al. Contemporary patterns of androgen deprivation therapy use for newly diagnosed prostate cancer. Urology 60, 7–11; discussion 11–12 (2002). [DOI] [PubMed] [Google Scholar]
- D’Amico A. V., Chen M. H., Renshaw A., Loffredo M. & Kantoff P. W. Long-term Follow-up of a Randomized Trial of Radiation With or Without Androgen Deprivation Therapy for Localized Prostate Cancer. Jama 314, 1291–1293, 10.1001/jama.2015.8577 (2015). [DOI] [PubMed] [Google Scholar]
- Keating N. L., O’Malley A. J., Freedland S. J. & Smith M. R. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. Journal of the National Cancer Institute 102, 39–46, 10.1093/jnci/djp404 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez B. D. et al. Course and Predictors of Cognitive Function in Patients With Prostate Cancer Receiving Androgen-Deprivation Therapy: A Controlled Comparison. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 33, 2021–2027, 10.1200/JCO.2014.60.1963 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nead K. T. et al. Androgen Deprivation Therapy and Future Alzheimer’s Disease Risk. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 34, 566–571, 10.1200/JCO.2015.63.6266 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P. L. Rethinking the Balance of Risk and Benefit of Androgen Deprivation Therapy for Intermediate-Risk Prostate Cancer. International journal of radiation oncology, biology, physics 94, 975–977, 10.1016/j.ijrobp.2016.02.003 (2016). [DOI] [PubMed] [Google Scholar]
- Droz J. P. et al. Management of prostate cancer in older patients: updated recommendations of a working group of the International Society of Geriatric Oncology. The Lancet. Oncology 15, e404–414, 10.1016/S1470-2045(14)70018-X (2014). [DOI] [PubMed] [Google Scholar]
- McGinty H. L. et al. Cognitive functioning in men receiving androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer 22, 2271–2280, 10.1007/s00520-014-2285-1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandy S. et al. Chemical andropause and amyloid-beta peptide. Jama 285, 2195–2196 (2001). [DOI] [PubMed] [Google Scholar]
- Hofman A. et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet 349, 151–154, 10.1016/S0140-6736(96)09328-2 (1997). [DOI] [PubMed] [Google Scholar]
- Vest R. S. & Pike C. J. Gender, sex steroid hormones, and Alzheimer’s disease. Hormones and behavior 63, 301–307, 10.1016/j.yhbeh.2012.04.006 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaitin C. et al. Metabolic syndrome and risk for incident Alzheimer’s disease or vascular dementia: the Three-City Study. Diabetes care 32, 169–174, 10.2337/dc08-0272 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay J. et al. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. American journal of epidemiology 156, 445–453 (2002). [DOI] [PubMed] [Google Scholar]
- D’Amico A. V. et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 25, 2420–2425, 10.1200/JCO.2006.09.3369 (2007). [DOI] [PubMed] [Google Scholar]
- Daskivich T. J. et al. Variation in treatment associated with life expectancy in a population-based cohort of men with early-stage prostate cancer. Cancer 120, 3642–3650, 10.1002/cncr.28926 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico A. V., Braccioforte M. H., Moran B. J. & Chen M. H. Luteinizing-hormone releasing hormone therapy and the risk of death from Alzheimer disease. Alzheimer disease and associated disorders 24, 85–89 (2010). [DOI] [PubMed] [Google Scholar]
- Panizzon M. S. et al. Testosterone modifies the effect of APOE genotype on hippocampal volume in middle-aged men. Neurology 75, 874–880, 10.1212/WNL.0b013e3181f11deb (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]