Abstract
Uncontrolled BK polyomavirus (BKPyV) replication in kidney transplant recipients (KTRs) causes polyomavirus‐associated nephropathy and allograft loss. Reducing immunosuppression is associated with clearing viremia and nephropathy and increasing BKPyV‐specific T cell responses in most patients; however, current immunoassays have limited sensitivity, target mostly CD4+ T cells, and largely fail to predict onset and clearance of BKPyV replication. To characterize BKPyV‐specific CD8+ T cells, bioinformatics were used to predict 9mer epitopes in the early viral gene region (EVGR) presented by 14 common HLAs in Europe and North America. Thirty‐nine EVGR epitopes were experimentally confirmed by interferon‐γ enzyme‐linked immunospot assays in at least 30% of BKPyV IgG–seropositive healthy participants. Most 9mers clustered in domains, and some were presented by more than one HLA class I, as typically seen for immunodominant epitopes. Specific T cell binding using MHC class I streptamers was demonstrated for 21 of 39 (54%) epitopes. In a prospective cohort of 118 pediatric KTRs, 19 patients protected or recovering from BKPyV viremia were experimentally tested, and 13 epitopes were validated. Single HLA mismatches were not associated with viremia, suggesting that failing immune control likely involves multiple factors including maintenance immunosuppression. Combining BKPyV load and T cell assays using immunodominant epitopes may help in evaluating risk and reducing immunosuppression and may lead to safe adoptive T cell transfer.
Short abstract
To better characterize BK polyomavirus–specific CD8+ T cells, the authors identify 39 mostly novel immunodominant 9mer epitopes in the early viral gene region by ELISpot, CD107a, killing assay, and streptamer binding, and find that most 9mers cluster in domains, and that some are presented by more than one HLA class I.
Abbreviations
- 9mP
9mer peptide pools
- 9msP
9mer peptide subpools
- 15mP
15mer peptide pools
- BKPyV
BK polyomavirus
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- ELISpot
enzyme‐linked immunospot
- EVGR
early viral gene region
- IFN‐γ
interferon γ
- HI
healthy individual participant
- IEDB
Immune Epitope Database and Analysis Resource
- IRB
institutional review board
- JCPyV
JC polyomavirus
- KT
kidney transplantation
- KTR
kidney transplant recipient
- LPP
long peptide pool
- LVGR
late viral gene region
- nOD
net optical density
- OD
optical density
- PBMCs
peripheral blood mononuclear cells
- PE
phycoerythrin
- PHA
phytohemagglutinin‐l
- PyVHC
polyomavirus‐associated hemorrhagic cystitis
- PyVAN
polyomavirus‐associated nephropathy
- SEB
Staphylococcus enterotoxin B
Introduction
BK polyomavirus (BKPyV) is a small nonenveloped double‐stranded DNA virus and one of, by now, at least 13 human polyomaviruses 1, 2. Specific antibody surveys indicate that BKPyV infects 80–95% of the human population, mostly during childhood and without specific symptoms or signs 3, 4, 5. BKPyV then persists in the renourinary tract, as demonstrated by asymptomatic shedding into the urine 4, 6, 7. Disease manifestations arise almost exclusively in persons with altered immune functions and appear to involve cofactors linked to specific clinical settings 8. Consequently, polyomavirus‐associated nephropathy (PyVAN) occurs in 1–15% of kidney transplant recipients (KTRs), whereas polyomavirus‐associated hemorrhagic cystitis (PyVHC) affects 5–20% of allogeneic hematopoietic stem cell transplant patients 2, 9, 10. PyVAN and PyVHC have a significant impact on morbidity and graft and patient survival 11, 12, 13, 14, 15, 16, 17, 18. Despite considerable virologic research 19, 20, 21, 22, 23, randomized clinical studies either are lacking or failed to demonstrate effective antiviral therapies 24. In kidney transplantation (KT), high‐level BKPyV viruria and viremia have been identified as markers of progression to PyVAN 25, thus current management strategies recommend screening KTRs for viremia followed by reducing immunosuppression 26, 27, 28. In prospective observational studies, this preemptive intervention has been successful, as shown by clearance of viremia and PyVAN in 80–100% of cases, with a low risk of subsequent acute rejection in 0–14% of patients 29, 30, 31, 32, 33. BKPyV viremia clearance has been paralleled by increasing BKPyV‐specific T cell responses in peripheral blood 30, 34, 35, 36. Because BKPyV‐specific T cell responses are ≈50‐ to 100‐fold lower than those to cytomegalovirus, these assays have not readily entered clinical practice. Moreover, the risk factors for BKPyV replication and nephropathy vary in different KT studies and include steroid pulses for acute rejection, maintenance immunosuppression such as tacrolimus–mycophenolate versus cyclosporine–mycophenolate, older age of recipients, male sex and a higher number of HLA mismatches 37, 38, 39, 40, 41, 42, 43, 44, 45. According to the recent Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients report, these risk factors are present in a substantial number of KT patients 46. Moreover, organs from BKPyV IgG–positive donors for recipients with low or undetectable antibodies may face an increased risk 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48.
We and others investigated cellular immune responses to overlapping peptide pools encoded in the early viral gene region (EVGR) or the late viral gene region (LVGR) of the BKPyV DNA genome 30, 35, 36, 49, 50. T cell responses to the LVGR‐encoded capsid viral protein VP1 were generally more pronounced than those to EVGR‐encoded viral proteins 30, 35, 49. Interferon γ (IFN‐γ) responses were largely derived from CD4+ T cells and, to a lesser extent, from CD8+ T cells 30, 35, 51, 52, 53. Because most of these studies used overlapping 15mer peptide pools (15mP), the contribution of individual CD8+ T cell–restricted epitopes to these responses is largely undefined. With few exceptions, HLA‐restricted T cell responses to BKPyV are mostly reported from HLA‐A*02 individuals 51, 54, 55, 56. To better characterize BKPyV‐specific CD8+ T cell epitopes, a bioinformatics approach was chosen to predict 9mer epitopes encoded in BKPyV EVGR and presented by 14 common HLA types in Europe and North America for experimental testing in healthy adult individuals and pediatric KTRs.
Materials and Methods
Healthy participants
Peripheral blood mononuclear cells (PBMCs) were prepared from 42 healthy individual (HIs), consisting of 34 blood donors from the Swiss Red Cross blood donation center in Basel, Switzerland, and from eight other healthy volunteers (Table S1). HLA types were determined with fee‐for‐service by the Transplantation Immunology Laboratory (Basel). Participants gave written informed consent for the protocol (IRB 267/06), which was approved by the local institutional review board (IRB).
Pediatric KTRs
A total of 118 consecutive pediatric KTRs were referred to the Genova Pediatric Kidney Transplant Program between March 2003 and November 2012. Three were aged >21 years but were still included in the cohort because they were initially received care as children for their end‐stage renal disease the Nephrology Unit, IRCCS, Genova, Italy. Cryopreserved PBMCs were analyzed from 19 KTRs protected (i.e. without BKPyV viremia) or recovering from BKPyV replication (Table S2). The study was approved by the local IRB (867/2014).
BKPyV IgG enzyme‐linked immunosorbent assay
BKPyV IgG serology was performed using BKPyV VP1‐derived virus‐like particles, as described previously 4, 57, 58.
In silico epitope prediction
The Syfpeithi database 59 provided information about HLA class I peptide binding affinity 60, whereas the Immune Epitope Database and Analysis Resource 61 provided a multiparametric prediction based on proteasomal cleavage, TAP transport and HLA class I peptide binding 62. The predictions were limited to HLA‐A and ‐B types present in >5% of the population within Europe or North America 63. For each HLA allele, the 20 epitopes within the BKPyV EVGR sequence displaying the best scores in both algorithms were considered.
BKPyV EVGR–derived peptides
A pool of 180 overlapping 15mP spanning BKPyV EVGR (Dunlop strain) or a pool of 11 longer LPm1‐11 peptides (long peptide pool [LPP]) covering immunodominant clusters of predicted BKPyV 9mer epitopes were used for in vitro T cell expansion. Cells were restimulated after expansion, as reported 35, using 15mP or a pool of 73 predicted 9mer peptides (9mP). The 9mer peptides were also resuspended in different subpools according to a checkerboard matrix approach, from A to H and from 1 to 9 (called 9msA–H and 9ms1–9). Each of the 73 peptides was present in two subpools. An additional set of 24 9mer peptides that initially were not predicted by computer algorithms and three longer peptides were later synthesized and used to assess “prediction gaps” in EVGR sequence. All peptides were >70% pure and resuspended in dimethyl sulfoxide (10 mg/mL; Eurogentec Deutschland GmbH, Köln, Germany).
In vitro expansion of T cells
Freshly isolated or thawed PBMCs were stimulated with LPP or 15mP (200 ng/mL) in 24‐well plates and incubated for 7–14 days at 37°C in 5% CO2 before performing phenotypic and functional assays. Recombinant human IL‐2 (20 U/mL; Peprotech, Rocky Hill, NJ) and recombinant IL‐7 (5 ng/mL; Peprotech) were added once a week.
Enzyme‐linked immunospot assay
Enzyme‐linked immunospot (ELISpot) assay was performed, as described previously 35. Expanded T cells were cultured without (negative control) or with BKPyV‐specific peptides. Cells treated with Staphylococcus enterotoxin B (2 μg/mL; Sigma‐Aldrich, St. Louis, MO) or phytohemagglutinin‐l (PHA; 2 μg/mL; Roche Diagnostics GmbH, Mannheim, Germany) were used as positive control. ELISpot data are averaged in duplicate or triplicate wells with background wells subtracted. Responses greater than background plus 2 standard deviations were considered positive.
MHC streptamer staining
BKPyV‐specific T cells were stained with phycoerythrin (PE)‐ or allophycocyanin‐labeled streptamers (IBA GmbH, Göttingen, Germany) composed of ≈8–12 peptide‐loaded MHC molecules, allowing better sensitivity than tetramers or pentamers. The cells were then incubated with CD8‐PE‐Cy7 antibody (BD Biosciences, San Jose, CA) and analyzed on a flow cytometer (FACSCanto; BD Biosciences) using FACSDiva software.
CFSE proliferation assay
PBMCs were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE; eBioscience, Vienna, Austria) before being expanded with BKPyV‐derived peptides and analyzed by flow cytometry.
CD107a degranulation assay
Expanded T cells were rechallenged for 5 h with BKPyV 9mer peptides or phorbol 12‐myristate 13‐acetate (100 ng/mL; Sigma‐Aldrich) and ionomycin (1 µg/mL; Sigma‐Aldrich) as positive control in the presence of PE‐Cy7–labeled CD107a antibody (BD Biosciences) before being analyzed by flow cytometry.
Cytotoxicity assay
Expanded T cells were cocultured for 4 h with 51Cr‐labeled autologous PHA blasts pulsed with BKPyV 9mer peptide at different effector:target cell ratios. Counts per minute were taken with a β‐counter (TopCount; PerkinElmer, Waltham, MA).
Statistical analysis
Proportions of viremic or viruric KTRs, and proportions of matched or mismatched patient populations were compared using the Fisher exact test with GraphPad Prism version 4.00 (GraphPad Software, La Jolla, CA). Differences corresponding to p < 0.05 were considered statistically significant.
Additional material and methods can be found in the supporting information.
Results
Bioinformatic prediction of HLA‐A– and HLA‐B–binding BKPyV 9mer epitopes
Syfpeithi and IEBD programs were used to predict 20 top‐scoring 9mer epitopes encoded in the BKPyV EVGR for each of 14 HLA‐A and ‐B types prevalent in Europe and North America. These HLA types are common in many ethnics groups worldwide, as shown in Table S3. The predictions for each HLA type were visualized relative to the BKPyV EVGR sequence and arbitrarily numbered (Figure 1). Although each HLA type appeared to have its own unique 9mer pattern, there were clearly sequence stretches in which the predicted epitopes appeared to cluster locally as well as across several HLA types by both algorithms (Figure 1). To focus on immunodominant epitopes, a total of 73 predicted 9mer epitopes including epitopes identified in previous studies (Table S4) were selected from different prominent clusters present across most HLA‐A and ‐B types for chemical synthesis and experimental testing. Twenty‐four additional 9mer predictions outside of these clusters were synthesized, resulting in a total of 97 9mer‐epitope candidates. In addition, 11 longer peptide stretches were selected for domains in which several predicted 9mers overlapped.
Figure 1.
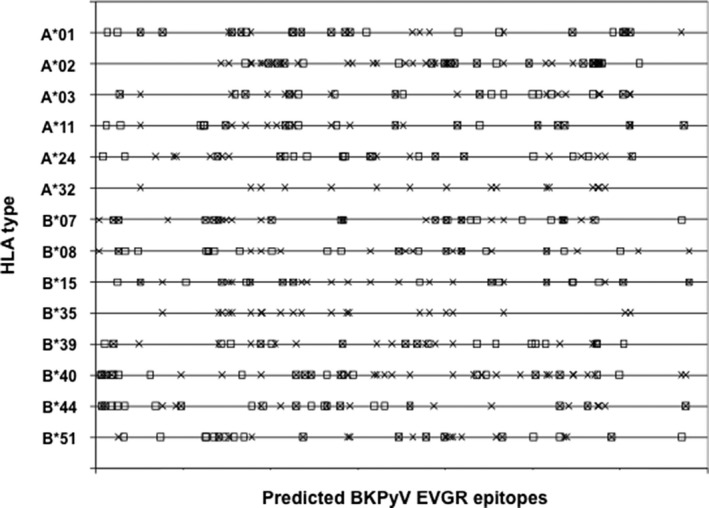
In silico prediction of BKPyV EVGR immunogenic epitopes. The graph depicts 20 top‐scoring 9mer epitopes predicted in BKPyV EVGR sequences for common HLA‐A and ‐B types in Europe and North America, according to the Immune Epitope Database (X) and Syfpeithi (□) algorithms. BKPyV, BK polyomavirus; EVGR, early viral gene region.
Experimental testing of predicted BKPyV 9mer epitopes in HIs
PBMCs were obtained from 42 HIs (median age 46 years) (Table S1) who were BKPyV‐IgG seropositive, as defined by the normalized OD492nm of >0.1 at 200‐fold dilution (Figure 2), which was previously shown to be very highly sensitive and specific 58. Because of the low BKPyV‐specific T cell frequency in PBMCs, an in vitro expansion protocol was adopted 35, and PBMCs were stimulated using BKPyV EVGR 15mP or LPP. IFN‐γ ELISpot assays were performed before and after expansion using 15mP, LPP, 9mP and 9msP for single‐epitope cross‐identification.
Figure 2.
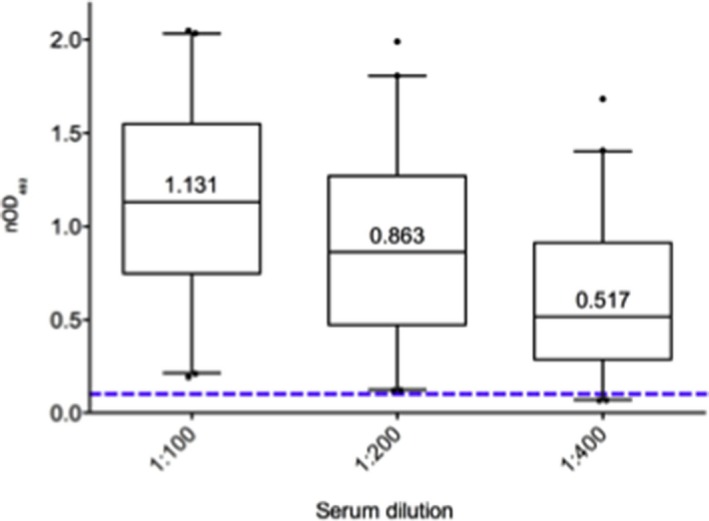
BKPyV IgG serology of 42 healthy individual participants. Normalized BKPyV IgG antibody levels are shown at 1:100, 1:200 and 1:400 dilutions (median, box shows 25th, 75th percentiles; whiskers 5% and 95%). Positive serological status was defined as OD492nm ≥0.100 (dotted line) at the 1:200 dilution. BKPyV, BK polyomavirus; nOD, net optical density.
The approach is illustrated in HI‐29: PBMCs were either stimulated directly or after expansion using the indicated peptide pools (Figure 3A). IFN‐γ ELISpot results showed that the in vitro T cell expansion protocol resulted in a significant increase of BKPyV‐specific T cells that responded to both 15mP and 9mP (Figure 3A). Rechallenge with 9mer subpools showed that the highest responses could be attributed to specific subpools, namely, 9msA and 9ms4 (Figure 3B). The single 9mer common to both of these subpools is 9m127. A slightly weaker response was also observed in 9ms5, suggesting some response to eight amino acid–overlapping 9m128 as well. Rechallenge of expanded cells with single 9mer‐peptides confirmed that the major response to 9msP was indeed attributable to 9m127, with a response similar to that observed with 9mP or 9msA and 9ms4 and, to a lesser extent, to 9m128 (Figure 3C). Based on the peptide length of nine amino acids, this functional IFN‐γ response should originate from CD8+ T cells. To address this directly, expanded T cells were stained with HLA‐B*07:02 9m127 streptamer and with CD8 surface marker and analyzed by flow cytometry (Figure 3D). A population of HLA‐B*0702–positive 9m127‐specific CD8+ T cells (right panel) could be detected, representing 3.9% of the total lymphocyte population.
Figure 3.
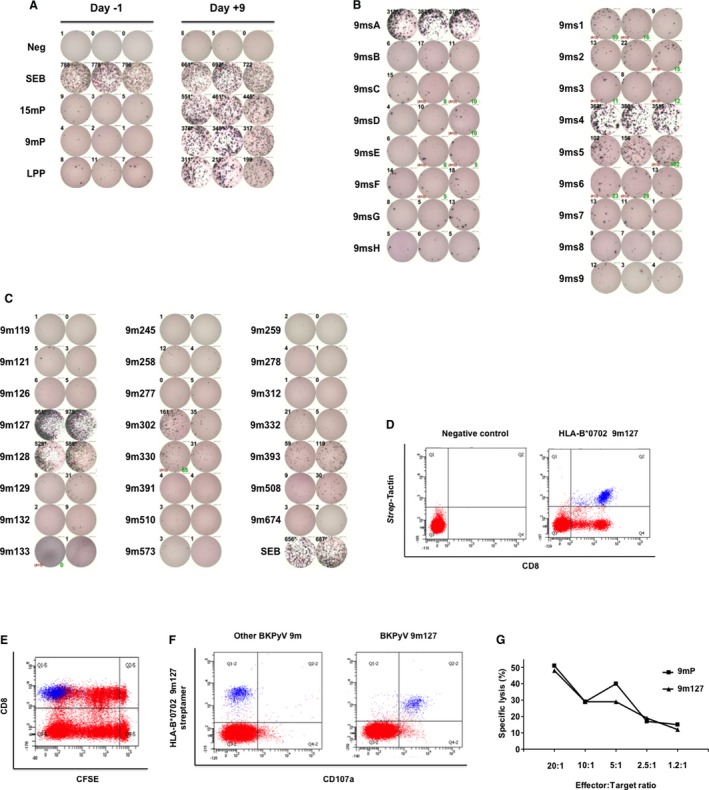
Characterization BKPyV EVGR 9mer‐specific immune responses. (A) Interferon γ enzyme‐linked immunospot assay using PBMCs directly after isolation from fresh blood (day −1; left panel) or after 9‐day expansion with BKPyV EVGR peptides (day +9; right panel). Cells were treated with medium (neg), SEB, 9mP, overlapping 15mP spanning the BKPyV EGFR sequence or with LPP. (B) Rechallenge of 9‐day expanded T cells with 9mer subpools 9msA to 9msH, and 9ms1 to 9ms9. Each peptide was present in two subpools for cross‐identification. (C) Identification of 9mer epitopes by restimulating expanded cell with single 9mer peptides contained in the subpools eliciting the highest responses (e.g. 9msA, 9ms4, 9ms5). (D) HLA streptamer staining using PE‐labeled Strep‐Tactin without (left panel) or with (right panel) HLA‐B*0702 molecules bearing 9m127 peptide. (E) CD8+ T cell proliferation during in vitro expansion. PBMCs were stained at day 0 with CFSE dye (red) that dilutes on cell division (x‐axis). HLA‐B*07–positive 9m127‐specific T cells are shown in blue. (F) Epitope‐specific degranulation of CD8+ T cells using PE‐Cy7‐labeled CD107a antibody (red) on 5‐h restimulation with 9m127 (right panel) or another BKPyV EVGR peptide (left panel). HLA‐B*07 9m127‐specific T cells are shown in blue. (G) The 9mer‐specific cytotoxic activity of expanded T cells. Autologous phytohemagglutinin‐L blasts stained with 51Cr and pulsed with 9mP (▪) or 9m127 (▴) were used as target cells and incubated for 4 h with expanded T cells (effector cells). Percentage of target cells lysis (y‐axis) at the different effector:target cells ratios (x‐axis) is shown. 9mP, 9mer‐peptide pool; 15mP, 15mer peptide pool; BKPyV, BK polyomavirus; CFSE, carboxyfluorescein diacetate succinimidyl ester; EVGR, early viral gene region; LPP, long peptide pool; Neg, negative; PBMCs, peripheral blood mononuclear cells; PE, phycoerythrin; SEB, Staphylococcus enterotoxin B.
To address proliferation following peptide stimulation, PBMCs were stained with CFSE before expansion and labeled for CD8 and HLA‐B*0702–positive 9m127 streptamer after expansion. CSFE dilution indicated the presence of at least nine divisions of the CD8+ T cell population (Figure 3E, red population). Gating on CCR7 and CD45RA revealed that a majority of CD8+ T cells undergoing seven, eight and nine division were of central or effector memory phenotype, in contrast to naïve CD8+ T cells that did not divide or that divided only once (Figure S1). HLA‐B*0702–positive 9m127‐specific CD8+ T cells showed the lowest CSFE signals, indicating that these cells had divided close to once per 1–2 days during the expansion period (Figure 3E, blue population).
To correlate HLA‐B*0702–positive 9m127‐specific CD8+ T cells and degranulation function, expanded T cells were stimulated with 9m127 or another BKPyV peptide (9m259) for 5 h in the presence of CD107a antibody and stained for HLA‐B*0702–positive 9m127 streptamers (Figure 3F). Only 9m127 induced degranulation of nearly the entire HLA‐B*0702–positive 9m127 CD8+ T cell population.
T cell functionality was also investigated in a killing assay in which lytic activity of expanded T cells against autologous 51Cr‐labeled PHA blasts pulsed with the single 9m127 or with 9mP was assessed (Figure 3G). The results showed that 9m127 mediates a mean specific lysis of 48% at an effector:target ratio of 20:1. This single 9m127 response was comparable to that mediated by 9mP, in line with an immunodominant BKPyV epitope.
This experimental approach permitted functional identification of candidate 9mer epitopes from BKPyV recognized by CD8+ T cells in strongly BKPyV‐seropositive HIs, even if cells were present at a low frequency among PBMCs. In some cases, the responses induced by 15mP and by 9mP did not correlate (data not shown), suggesting the presence of different epitope‐specific T cell populations among CD4+ or CD8+ subsets. Testing of expanded PBMCs from BKPyV‐ and JC polyomavirus (JCPyV)–seronegative HIs remained negative for 9mer responses but showed some residual 15mer responses (Figure S2, left panel). This indicated that this in vitro protocol of only 2 weeks expansion did not lead to significant priming of naïve CD8+ T cells, as detected by 9mer responses, but most likely induced proliferation of memory CD8+ T cells. Interestingly, HIs who were seronegative for BKPyV but seropositive for JCPyV showed a response to BKPyV EVGR 15mer and 9mer (Figure S2, right panel). This suggested the presence of LTag crossreactive responses from JCPyV‐specific T cells, as discussed previously 35.
In total, 42 HIs were analyzed, and the frequency of responses was summarized according HLA type in a heat map (Table 1). Only two 9mer responses had frequencies of <15% of HIs (9m316, 9m518). Many 9mer‐epitope responses could be detected in up to one‐third of HIs, but some epitopes had more frequent responses (40 epitopes elicited IFN‐γ production in 30–49% of donors; 17 epitopes induced IFN‐γ ELISpot responses in >50% of tested participants). The presence of response hotspots supports the notion that not all 9mer epitopes are equally potent, and certain clustering is observed. This is illustrated by the ELISpot results for selected epitopes (Figure 4). The overall results indicated that BKPyV EVGR‐specific 9mer T cell responses were heterogeneous in terms of frequency and strength. For some HLA types, positive responses were found more frequently and directed towards more epitopes compared to HLA types. For example, in participants positive for HLA‐A*03, ‐A*11, ‐B*07, ‐B*35, ‐B*39, ‐B*44 or ‐B*51, more than one epitope could be identified in ≥50% of HIs (Table 1). Single 9mer epitopes were associated with variable responses in HIs (Figure 4), whereby values of >69 spot‐forming units (SFU)/106 cells were regarded as strong responses in accordance with a previously defined threshold of protection from BKPyV viremia in KTRs 35.
Table 1.
BKPyV EVGR 9mer responses in IFN‐γ ELISpot assay
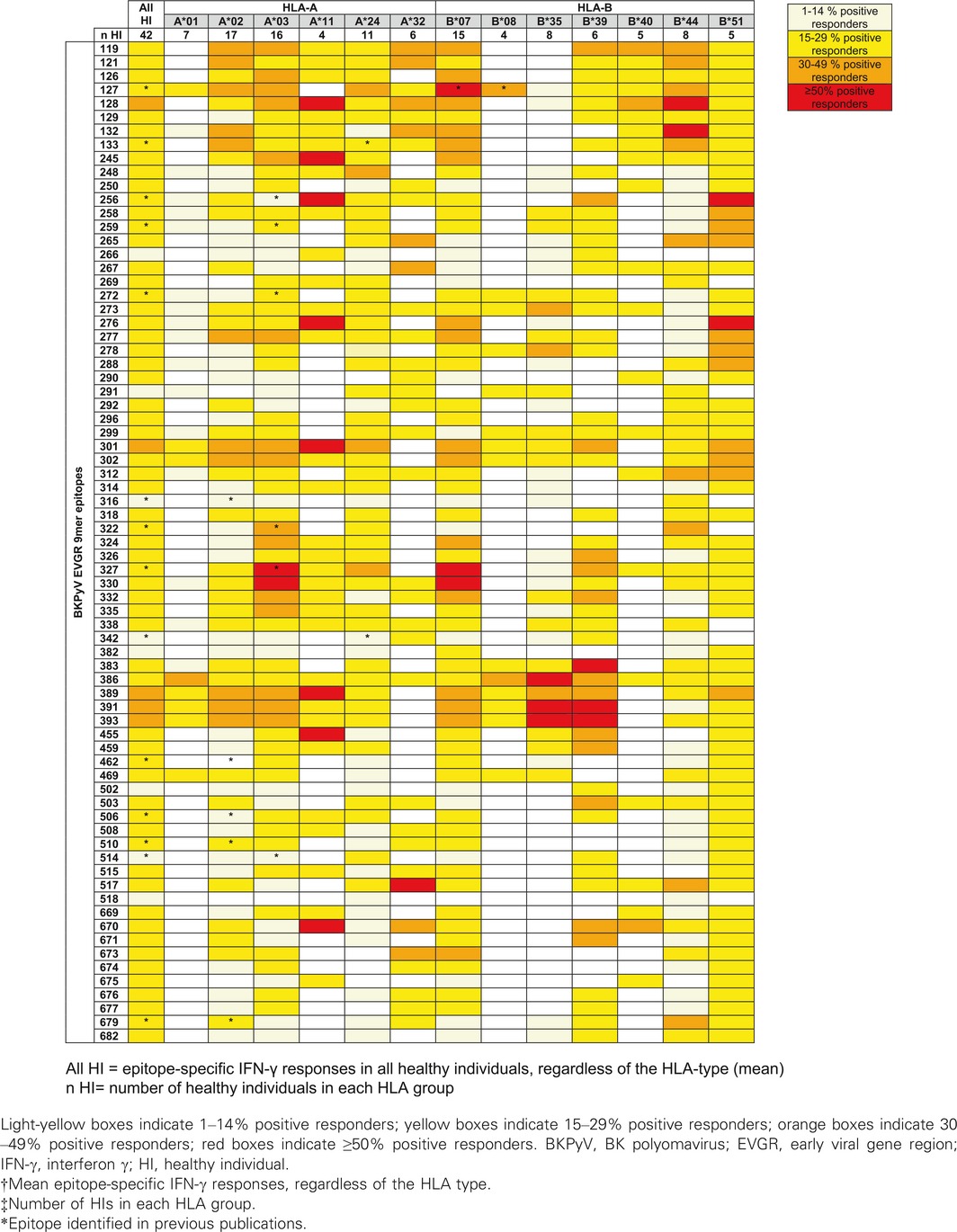
Figure 4.
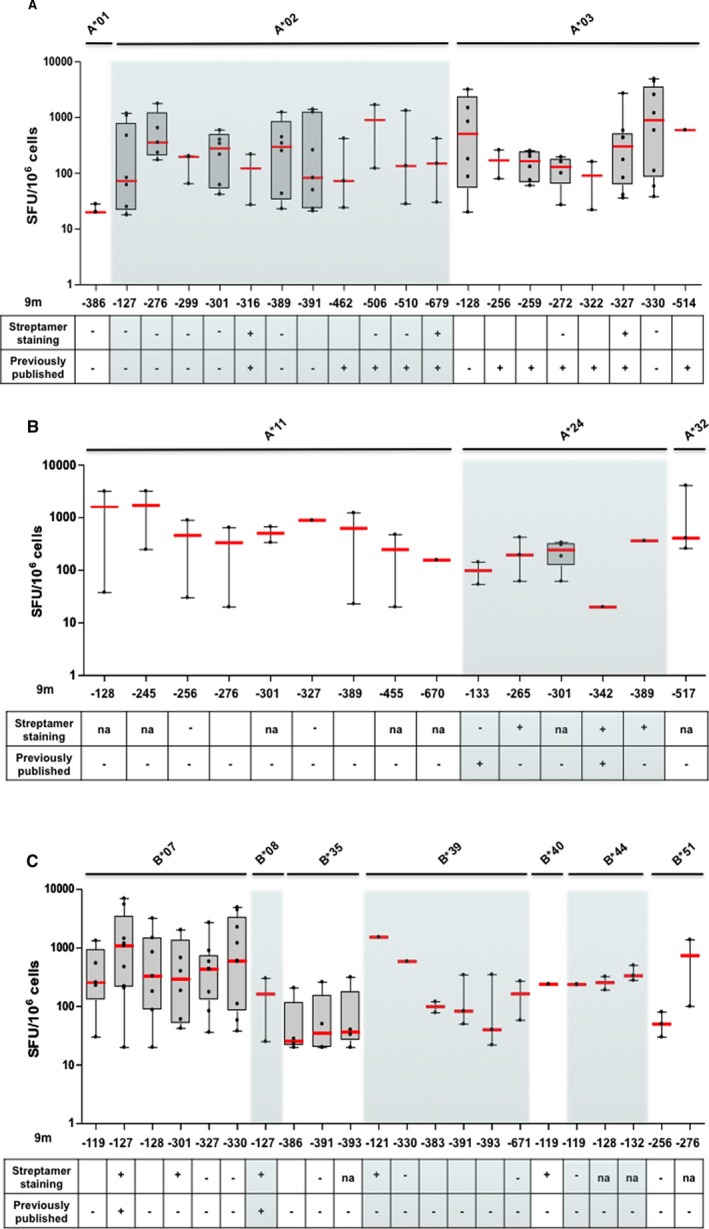
Breadth and strength of BKPyV EVGR epitope‐specific immune responses in HIs. The 9mer‐epitope responses in interferon γ enzyme‐linked immunospot assay (dots) found in at least 40% of HIs, listed in Table 1, with positive streptamer staining or previous publication (median in red, box 25th and 75th percentiles, whiskers 5th and 95th). BKPyV, BK polyomavirus; HI, healthy individual; na, not available; SFU, spot‐forming unit.
In summary, 9m301 elicited specific responses in 47% of participants with HLA‐B*07 with a median of 292 SFU/106 cells. The same 9mer epitope was found to be immunogenic in 35% of donors with HLA‐A*02 and 45% with HLA‐A*24. In addition, 9m327 induced a median response of 304 SFU/106 cells in eight HIs who were positive for HLA‐A*03. Overall, 9m330 induced fairly strong responses in 53% of HIs who were positive for HLA‐A*03, with a median of 593 SFU/106 cells. Moreover, 9m330‐specific IFN‐γ production could also be detected in some HIs with HLA‐B*07 and ‐B*39. Finally, 9m389 elicited a median response of 299 SFU/106 cells in six participants with HLA‐A*02 (35%) and elicited IFN‐γ production in 50% of participants with HLA‐A*11 and 18% with HLA‐A*24 (Table 1).
In line with the predicted clusters, some areas in the EVGR appeared to be more immunogenic than others (Table 1). The domain spanning from 9m383 to 9m393 could induce frequent responses in participants with HLA‐A*02, ‐A*11, ‐B*35 and ‐B*39. The domain from 9m119 to 9m133 appeared to be highly immunogenic across different HLA types. Additional epitope clusters in the 221–240 and 536–645 EVGR areas also appeared to be more immunogenic (Table S5); however, they were tested in only few participants and thus deserve further investigation.
HLA restriction of BKPyV EVGR‐specific T cell responses in HIs
To address HLA specificity of IFN‐γ–inducing 9mer epitopes, cells were stained with MHC streptamers (Table 2). Some MHC streptamers showed strong staining such as HLA‐B*07– and ‐B*08–positive 9m127 streptamers that were identified in 89% and 67% HIs with mean values of 0.86% and 0.27%, respectively. T cells presenting 9m127 via the HLA‐A*02 molecule could not be detected, despite high 9m127‐specific IFN‐γ T cell responses among HLA‐A*02–positive HIs (Figure 4A), suggesting that those responses were not HLA‐A*02 restricted. Conversely, HLA‐A*02–positive T cells specific for 9m679 could be detected in 50% of tested participants with HLA‐A*02, despite a low number of responsive donors in the IFN‐γ ELISpot assay (18%).
Table 2.
HLA‐A and ‐B specificity of BKPyV EVGR CD8+ T cell responses in HIs
| HLA type | Streptamer | Tested HIs (n) | HIs with positive response (%) | Epitope‐specific CD8+ cells T cells (mean %) |
|---|---|---|---|---|
| A*02 | 316 | 6 | 33 | 0.04 |
| 679 | 6 | 50 | 0.04 | |
| A*03 | 327 | 5 | 40 | 0.01 |
| A*24 | 265 | 4 | 25 | 0.02 |
| 312 | 4 | 25 | 0.02 | |
| 342 | 7 | 14 | 0.04 | |
| 389 | 7 | 14 | 0.02 | |
| B*07 | 127 | 9 | 89 | 0.86 |
| 301 | 1 | 100 | 0.23 | |
| B*08 | 127 | 3 | 67 | 0.27 |
| B*35 | 121 | 1 | 100 | 0.03 |
| 240 | 3 | 67 | 0.16 | |
| 391 | 4 | 50 | 0.03 | |
| B*39 | 121 | 2 | 50 | 0.19 |
| 240 | 2 | 50 | 0.04 | |
| B*40 | 119 | 2 | 50 | 0.3 |
| 330 | 1 | 100 | 0.25 | |
| 631 | 1 | 100 | 0.17 |
BKPyV, BK polyomavirus; EVGR, early viral gene region; HI, healthy individual.
Of note, HLA‐A*03–positive 9m327‐specific T cells could be detected in 40% of HIs with HLA‐A*03, but 9m327 elicited IFN‐γ responses in donors with HLA‐A*03, ‐A*11 and ‐B*07. HLA‐A*24–positive 9m389‐specific T cells could be detected in 14% of HIs, but 9m389 elicited IFN‐γ responses in donors with HLA‐A*02, ‐A*11 and ‐A*24. HLA‐B*07–positive 9m301‐specific T cells could be detected in the only tested participants with HLA‐B*07, but 9m301 elicited IFN‐γ responses in donors with HLA‐A*02, ‐*A24 and ‐B*07. Finally, HLA‐B*40–positive 9m119‐specific T cells could be detected in 50% of HIs with HLA‐B*40 but 9m119 elicited IFN‐γ responses in donors wit HLA‐B*07, ‐B*40 and ‐B*44.
Finally, some T cell–activating 9mers were presented by more than one HLA molecule, namely, 9m121 (HLA‐B*35 and ‐B*39), 9m127 (HLA‐B*07 and ‐B*08), and 9m240 (HLA‐B*35 and ‐B*39) (Table 2).
BKPyV EVGR‐specific epitope CD8+ T cell responses in pediatric KTRs
To extend the results from HIs to the clinical setting, BKPyV‐specific T cell responses were investigated in 19 pediatric KTRs who were protected or recovered from BKPyV viremia (Table S2). Several epitopes identified in HIs could be confirmed in these 19 KTRs (Table 3).
Table 3.
BKPyV EVGR‐specific T cell responses in kidney transplant recipients
| IFN‐γ ELISpot | MHC‐streptamers | ||||||
|---|---|---|---|---|---|---|---|
| HLA type | BKPyV epitope | Tested KTRs (n) | KTRs with positive response (%) | SFU/106 cells (mean) | Tested KTRs (n) | KTRs with positive response (%) | Epitope‐specific CD8+ T cells (mean %) |
| A*01 | 256 | 2 | 50 | 100 | – | – | – |
| 327 | 2 | 100 | 40 | 3 | 67 | 0.10 | |
| 386 | – | – | – | 3 | 33 | 0.02 | |
| A*02 | 316 | – | – | – | 5 | 20 | 0.14 |
| 302 | 8 | 12 | 245 | – | – | – | |
| 389 | 9 | 67 | 312 | – | – | – | |
| 391 | 4 | 25 | 840 | – | – | – | |
| 393 | – | – | – | 3 | 33 | 0.06 | |
| 510 | 10 | 50 | 95 | – | – | – | |
| 536 | – | – | – | 4 | 25 | 0.14 | |
| 679 | 9 | 89 | 324 | 11 | 36 | 0.09 | |
| A*03 | 256 | 3 | 30 | 120 | – | – | – |
| 272 | – | – | – | 3 | 67 | 0.12 | |
| 327 | 3 | 30 | 270 | 1 | 100 | 0.05 | |
| A*11 | 256 | 3 | 30 | 120 | – | – | – |
| 276 | 3 | 30 | 465 | – | – | – | |
| 327 | 3 | 67 | 270 | 3 | 33 | 0.08 | |
| 389 | 3 | 33 | 70 | – | – | – | |
| A*24 | 127 | 3 | 33 | 200 | – | – | – |
| 342 | – | – | – | 1 | 100 | 0.08 | |
| 389 | 4 | 50 | 370 | 2 | 50 | 0.02 | |
| B*07 | 119 | 2 | 50 | 130 | 2 | 50 | 0.07 |
| 127 | 2 | 100 | 280 | 2 | 50 | 0.08 | |
| 240 | – | – | – | 1 | 100 | 0.03 | |
| 301 | – | – | – | 1 | 100 | 0.09 | |
| 389 | 2 | 50 | 285 | – | – | – | |
| B*08 | 127 | 1 | 100 | 150 | – | – | – |
| 462 | – | – | – | 1 | 100 | 0.08 | |
| 679 | – | – | – | 1 | 100 | 0.07 | |
| B*35 | 240 | 5 | 80 | 410 | – | – | – |
| 391 | 5 | 40 | 590 | – | – | – | |
| 633 | 5 | 40 | 145 | – | – | – | |
| B*51 | 256 | 4 | 25 | 180 | – | – | – |
| 389 | 5 | 40 | 135 | 2 | 50 | 0.10 | |
| 506 | 2 | 100 | 175 | – | – | – | |
BKPyV, BK polyomavirus; EVGR, early viral gene region; IFN‐γ, interferon γ; KTR, kidney transplant recipient; SFU, spot‐forming unit.
Overall, 9m389 was recognized in 67% of patients with HLA‐A*02, with a mean value of 312 SFU/106 cells, but HLA‐A*02 restriction could not be confirmed. This epitope induced T cell responses in 33%, 50%, 50%, and 40% of patients with HLA‐A*11, ‐A*24, ‐B*07, and ‐B*51, respectively, with HLA specificity confirmed for HLA‐A*24 and ‐B*51 molecules. In addition, 9m679 was found to be immunogenic in eight of nine tested patients with HLA‐A*02, and specific CD8+ T cells were detectable in 36% of patients with HLA‐A*02. ELISpot assays could confirm 9m327 and MHC streptamer staining in KTRs who were positive for HLA‐A*01, ‐A*03, and ‐A*11. Furthermore, 9m127 elicited T cell responses in 100% of patients with HLA‐B*07 and ‐B*08, and MHC streptamer staining identified CD8+ T cells for one patient with HLA‐B*07.
Four 9mer epitopes elicited functional IFN‐γ responses in HIs and KTRs and were HLA‐specific in both cohorts: 9m127 (HLA‐B*07 specific), 9m327 (HLA‐A*03 specific), 9m389 (HLA‐A*24 specific) and 9m679 (HLA‐A*02 specific). Three 9mer responses were found in KTRs but not in HIs (9m302, 9m536, and 9m633).
BKPyV replication prevalence in pediatric KTRs
To evaluate the potential association of BKPyV replication with specific HLA‐A and ‐B types, a prospective cohort of 118 consecutive pediatric KTRs was analyzed, of whom 38 (32%) experienced BKPyV viremia. The rate of BKPyV viremia was not equally distributed across HLA types or mismatches (Figure 5). Although the overall sample size was too small for statistically supported conclusions, patients with HLA‐A*01 seemed to have lower rates of viremia compared with the overall population (p < 0.05), and mismatching for this allele was present in about half of the KTRs. Similarly, a trend for a lower rate of BKPyV viremia was seen among patients positive for HLA‐A*26, ‐A*28 and ‐B*57, without a clear association with matching. Interestingly, 60% of nonviremic patients with HLA‐B*07 were matched for this particular allele, whereas all viremic patients with HLA‐B*07 were mismatched (Figure 5). This observation would be in line with the hypothesis that the absence of HLA‐B*07 might increase the risk of viremia if confirmed by an independent study of larger sample size. In contrast, there was a higher proportion of HLA‐B*35–matched patients among viremic KTRs than among the nonviremic KTRs (p < 0.05). Finally, viremia seemed to be independent of matching for HLA‐A*01, ‐A*24, ‐A*29, ‐B*08, and ‐B*51 types, for which the proportions of matched patients among viremic and nonviremic patients were similar (Figure 5). A higher rate of high‐level viruria was found in patients with HLA‐A*31 (p < 0.05), whereas a trend toward a lower rate was found in patients with HLA‐B*57 (p < 0.05) and HLA‐A*01 (p = 0.08) (data not shown). Consequently, although immunogenic properties of some 9mer epitopes could be confirmed in KTRs, a simple association of single mismatching with risk or matching with protection could not be derived from this pediatric cohort.
Figure 5.
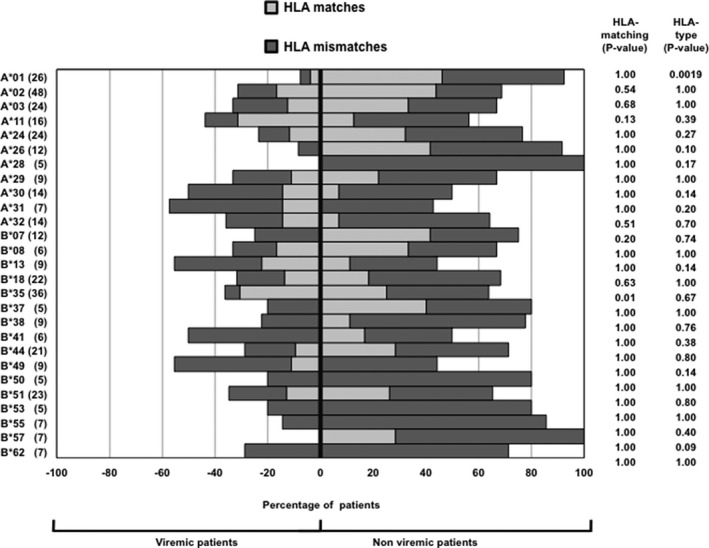
BKPyV viremia and HLA‐matching in 118 pediatric KTRs. The percentages of viremic (left side) and nonviremic (right side) KTRs according to HLA type are shown. For each HLA type, the percentage of patients displaying matched (light gray) or mismatched (dark gray) allele with their kidney donor is shown. The number of KTRs with the most common HLA types is indicated at left. BKPyV viremia was analyzed for single HLA types versus the whole population (p‐value for HLA type) or by comparing viremia occurrence in matched and mismatched patients (p‐value for HLA matching; Fisher exact test). BKPyV, BK polyomavirus; KTR, kidney transplant recipient.
Discussion
BKPyV‐associated nephropathy is now widely recognized as an emerging complication in KT 64, 65, 66, 67. Insufficient BKPyV‐specific T cell control of the recipient over viral replication in donor allograft is suspected as the common denominator and key mechanism 67. Independent single‐center studies indicated that reducing immunosuppression was associated with increasing BKPyV‐specific T cell responses and coincided with clearance of viremia and nephropathy 30, 34, 35, 36. Alternatively, the decline of cellular immunity at 1 month after transplantation has been proposed to identify patients at increased risk of BKPyV viremia, but no or low IFN‐γ responses in at least half of patients impeded the predictive value for individual patients 53. Given these experiences and the fact that mostly CD4+ T cell responses were measured by overlapping 15mP, better characterization of BKPyV epitope‐specific CD8+ T cell response seems needed to improve the current understanding and clinical utility of BKPyV‐specific cellular immunity. Because we observed that BKPyV‐specific CD8+ T cell responses are more frequently directed to LTag than VP1 30, 35, we focused on the BKPyV EVGR–encoded epitopes rather than the capsid antigens 68, 69. The following aspects emerged from the present study.
First, systematic in silico analysis of immunogenic epitopes predicts both immunogenic hotspot clusters and gaps in BKPyV EVGR for each of the 14 major HLA class I types. Predicted areas were similarly clustered across different HLA class I types, and this observation argues for potential immunodominant domains, in which the virus would be particularly susceptible to immune control and selection pressure. Because these domains are present in EVGR‐encoding crucial viral regulatory proteins early in the viral replication cycle, at least transient escape from cellular immune control would be particularly important for BKPyV replication 70, 71.
Second, the expansion protocol used in the present study has been devised to overcome the low frequency of BKPyV‐specific T cell responses to overlapping 15mP 35 and was used successfully in the prospective study of pediatric KTRs 30. Our results demonstrate that functional CD8+ T cell responses can be amplified which selectively target few 9mer epitopes located in predicted immunodominant clusters. CFSE dye dilution and streptamer staining supported this central observation, indicating that these cells were among the most active, dividing approximately once every 1–2 days. The 9mer responses were functionally defined by IFN‐γ secretion but could be linked in principle to 9mer‐specific cell surface expression of CD107a, a marker of granzyme and perforin degranulation, and to cytotoxic activity in 51Cr release assays.
Third, although certain 9mer epitopes were shown to be selective for specific HLA types by streptamer staining of CD8+ T cells, presentation by different HLA class I types could be observed. This has been demonstrated for HLA types that belong to crossreacting group 1C (e.g. HLA‐A*01, ‐A*03 and ‐A*11) or 7C (e.g. HLA‐B*07 and ‐B*08) 72.
Fourth, 10 of the 9mer epitopes identified in HIs could be confirmed in an unrelated cohort of 19 pediatric KTRs who were protected or recovering from BKPyV replication. Consequently, predicted and tested immunodominant responses could be linked to the clinically relevant situation of immunosuppressed KTRs.
Interestingly, three additional 9mer responses found in KTRs had been predicted but were not detected in HIs. This might indicate that responses in HIs may be lower and hence less detectable. Conversely, the additional responses in pediatric KTRs might result from extensive exposure to BKPyV replication and the more vigorous immune response described for children 5.
The fact that the major site of BKPyV replication is in donor cells of the renal allograft deserves consideration because this might affect HLA presentation and modify T cell receptor recognition of BKPyV epitopes. Interestingly, in our prospective cohort of 118 pediatric KTRs, single HLA mismatching could not be associated with BKPyV viremia. Although sample size might be an important aspect 73, it could also reflect the fact that other risk factors besides HLA‐mismatching including maintenance immunosuppression can promote progression to viremia and nephropathy equally well and thereby contribute to the failing balance between BKPyV replication in the graft and BKPyV‐specific T cell control in an individual patient.
Previous studies focused mainly on persons with HLA‐A*02 41, 51, 55, 71 and more rarely on epitopes restricted for HLA‐A*01, ‐A*03, ‐A*11, ‐B*07 and ‐B*08 54, 56, 74. Most published epitopes could be confirmed in our study, such as the HLA‐A*02–restricted epitopes 9m679 and 9m316 or the HLA‐B*07– and HLA‐B*08–restricted 9m127, validating our approach and underscoring their potential immunodominance. Our approach expands this list to at least 39 mostly new 9mer epitopes, of which 21 were linked by streptamer staining to a range of HLA types. These findings may be of high interest, especially because BKPyV epitope‐specific T cells could be sorted using specific MHC streptamers, further expanded in vitro after removal of the MHC‐streptamer complex and used for adoptive T cell transfer. In addition, some of the epitopes identified in the present study have common sequences with the closely related JCPyV, suggesting that BKPyV‐specific T cells might induce cross‐protection to JCPyV, which is also associated with disease in some immunocompromised patients.
Our study has several limitations that should be considered for a balanced interpretation of the results. First, not all IFN‐γ responses could be linked unambiguously to HLA types present in functionally responding HIs or KTRs by streptamers. There may be several reasons for this, including low T cell frequencies, technical properties of certain streptamers that reduce sensitivity, or the fact that some 9mer epitopes eliciting IFN‐γ responses were presented by HLA molecules not predicted in this study, especially in the transplantation context. These aspects might be better addressed as prediction and technology improves. Second, some aspects of BKPyV‐specific CD8+ T cell characterization (proliferation during expansion, cytotoxicity, degranulation) could not be performed for all epitopes but could be addressed at least in principle in experimental animal models 75, 76. Third, the use of cryopreserved samples instead of fresh PBMCs might have reduced detection of some rare BKPyV‐specific T cells, even if immunodominant responses were still detectable. Fourth, the investigation of cellular immune responses in KTRs was done in a relatively small cohort; therefore, the number of identified epitopes was limited. Overall, the results are encouraging to take this approach to larger clinical cohorts and relevant prospective study settings.
We conclude that in vitro cellular expansion using 15mP EVGR peptides or longer peptides allowed us to experimentally test the immunogenicity of 9mer epitopes predicted by in silico algorithms. We could identify immunogenic 9mer epitopes in both HIs and KTRs and extended the current list to at least 39. Several epitopes were located in clusters and induced specific responses in different common HLA class I types, as expected for immunodominant epitopes. Further studies will have to prove how this information can be best harnessed for clinically relevant immune monitoring and possibly foster adoptive T cell transfer for prophylaxis and therapy in kidney and hematopoietic stem cell transplant patients.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
Figure S1: Phenotype of dividing cells during in vitro expansion. Peripheral blood mononuclear cells from a healthy donor were labeled with CFSE before being expanded in vitro and stained with CD45RA and CCR7 markers. (A) CFSE content of CD8+ T cells after in vitro expansion. Gates P11–P20 allow discrimination of different CD8+ T cell divisions (P11 = no division; P12 = one division; P13 = two divisions, and so forth). (B) CD45RA and CCR7 expression by CD8+ T cells at different division stages. Each panel is gated in one of the 10 gates (P11–P20). CFSE, carboxyfluorescein diacetate succinimidyl ester.
Figure S2: Immune responses of BKPyV‐seronegative HIs. PBMCs from HIs who were seronegative for both BKPyV and JCPyV (HI‐43, HI‐44, HI‐45 and HI‐46; left panel) or seropositive for JCPyV and seronegative for BKPyV (HI‐47, HI‐48; normalized enzyme‐linked immunosorbent assay net OD (58)) (right panel) were expanded for 14 days in the presence of BKPyV 15mP and restimulated with BKPyV 15mP, LPP or 9mP in interferon γ enzyme‐linked immunospot assay. 9mP, 9mer‐peptide pool; 15mP, 15mer peptide pool; BKPyV, BK polyomavirus; HI, healthy individual participant; JCPyV, JC polyomavirus; LPP, long peptide pool; OD, optical density; SFU, spot‐forming unit.
Table S1: Characteristics of 42 healthy individuals.
Table S2: Characteristics of 19 pediatric kidney transplant recipients.
Table S3: HLA allele frequencies in worldwide populations.
Table S4: Previously published BKPyV EVGR 9mer epitopes predicted in our study.
Table S5: BKPyV 9mer responses in IFN‐γ ELISpot assay ‐ additional 24 epitopes.
Acknowledgments
We would like to thank Dr. Adrian Egli, Clinical Microbiology, University Hospital Basel, for helpful discussions and critical reading of the manuscript; Marion Wernli for excellent technical assistance and performing IgG serology assays; and Sabrina Wilk, Flavio Lombardo and the members of the transplantation and clinical virology research group for support and helpful discussions. M. Cioni is currently located at the Nephrology, Dialysis and Transplantation Unit, IRCCS G. Gaslini Institute, Genova, Italy.
Cioni M, Leboeuf C, Comoli P, Ginevri F & Hirsch HH. Characterization of Immunodominant BK Polyomavirus 9mer Epitope T Cell Responses. Am J Transplant 2016; 16: 1193–1206
References
- 1. Rinaldo CH, Hirsch HH. The human polyomaviruses: From orphans and mutants to patchwork family. APMIS 2013; 121: 681–684. [DOI] [PubMed] [Google Scholar]
- 2. Pergam SA, Hirsch HH. Human adenovirus, polyomavirus, and parvovirus infections in patients undergoing hematopoietic stem‐cell transplantation In: Forman SJ, Antin H, Appelbaum FR, editors. Thomas' Hematopoietic Cell Transplantation. 5th ed Honoken, NJ: John Wiley & Sons Ltd; 2016: 1090–1104. [Google Scholar]
- 3. Knowles WA, Pipkin P, Andrews N, et al. Population‐based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol 2003; 71: 115–123. [DOI] [PubMed] [Google Scholar]
- 4. Egli A, Infanti L, Dumoulin A, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis 2009; 199: 837–846. [DOI] [PubMed] [Google Scholar]
- 5. Schmidt T, Adam C, Hirsch HH, et al. BK polyomavirus‐specific cellular immune responses are age‐dependent and strongly correlate with phases of virus replication. Am J Transplant 2014; 14: 1334–1345. [DOI] [PubMed] [Google Scholar]
- 6. Chesters PM, Heritage J, McCance DJ. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J Infect Dis 1983; 147: 676–684. [DOI] [PubMed] [Google Scholar]
- 7. Polo C, Perez JL, Mielnichuck A, Fedele CG, Niubo J, Tenorio A. Prevalence and patterns of polyomavirus urinary excretion in immunocompetent adults and children. Clin Microbiol Infect 2004; 10: 640–644. [DOI] [PubMed] [Google Scholar]
- 8. Hirsch HH. BK virus: Opportunity makes a pathogen. Clin Infect Dis 2005; 41: 354–360. [DOI] [PubMed] [Google Scholar]
- 9. Rinaldo CH, Tylden GD, Sharma BN. The human polyomavirus BK (BKPyV): Virological background and clinical implications. APMIS 2013; 121: 728–745. [DOI] [PubMed] [Google Scholar]
- 10. Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis 2003; 3: 611–623. [DOI] [PubMed] [Google Scholar]
- 11. Binet I, Nickeleit V, Hirsch HH, et al. Polyomavirus disease under new immunosuppressive drugs: A cause of renal graft dysfunction and graft loss. Transplantation 1999; 67: 918–922. [DOI] [PubMed] [Google Scholar]
- 12. Randhawa PS, Finkelstein S, Scantlebury V, et al. Human polyoma virus‐associated interstitial nephritis in the allograft kidney. Transplantation 1999; 67: 103–109. [DOI] [PubMed] [Google Scholar]
- 13. Drachenberg CB, Beskow CO, Cangro CB, et al. Human polyoma virus in renal allograft biopsies: Morphological findings and correlation with urine cytology. Hum Pathol 1999; 30: 970–977. [DOI] [PubMed] [Google Scholar]
- 14. Cesaro S, Brugiolo A, Faraci M, et al. Incidence and treatment of hemorrhagic cystitis in children given hematopoietic stem cell transplantation: A survey from the Italian association of pediatric hematology oncology‐bone marrow transplantation group. Bone Marrow Transplant 2003; 32: 925–931. [DOI] [PubMed] [Google Scholar]
- 15. Erard V, Kim HW, Corey L, et al. BK DNA viral load in plasma: Evidence for an association with hemorrhagic cystitis in allogeneic hematopoietic cell transplant recipients. Blood 2005; 106: 1130–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Giraud G, Bogdanovic G, Priftakis P, et al. The incidence of hemorrhagic cystitis and BK‐viruria in allogeneic hematopoietic stem cell recipients according to intensity of the conditioning regimen. Haematologica 2006; 91: 401–404. [PubMed] [Google Scholar]
- 17. Svahn BM, Alvin O, Ringden O, Gardulf A, Remberger M. Costs of allogeneic hematopoietic stem cell transplantation. Transplantation 2006; 82: 147–153. [DOI] [PubMed] [Google Scholar]
- 18. Zaia J, Baden L, Boeckh MJ, et al. Viral disease prevention after hematopoietic cell transplantation. Bone Marrow Transplant 2009; 44: 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Farasati NA, Shapiro R, Vats A, Randhawa P. Effect of leflunomide and cidofovir on replication of BK virus in an in vitro culture system. Transplantation 2005; 79: 116–118. [DOI] [PubMed] [Google Scholar]
- 20. Bernhoff E, Gutteberg TJ, Sandvik K, Hirsch HH, Rinaldo CH. Cidofovir inhibits polyomavirus BK replication in human renal tubular cells downstream of viral early gene expression. Am J Transplant 2008; 8: 1413–1422. [DOI] [PubMed] [Google Scholar]
- 21. Sharma BN, Li R, Bernhoff E, Gutteberg TJ, Rinaldo CH. Fluoroquinolones inhibit human polyomavirus BK (BKV) replication in primary human kidney cells. Antiviral Res 2011; 92: 115–123. [DOI] [PubMed] [Google Scholar]
- 22. Bernhoff E, Tylden GD, Kjerpeseth LJ, Gutteberg TJ, Hirsch HH, Rinaldo CH. Leflunomide inhibition of BK virus replication in renal tubular epithelial cells. J Virol 2010; 84: 2150–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rinaldo CH, Gosert R, Bernhoff E, Finstad S, Hirsch HH. 1‐O‐hexadecyloxypropyl cidofovir (CMX001) effectively inhibits polyomavirus BK replication in primary human renal tubular epithelial cells. Antimicrob Agents Chemother 2010; 54: 4714–4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knoll GA, Humar A, Fergusson D, et al. Levofloxacin for BK virus prophylaxis following kidney transplantation: A randomized clinical trial. JAMA 2014; 312: 2106–2114. [DOI] [PubMed] [Google Scholar]
- 25. Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal‐transplant recipients. N Engl J Med 2002; 347: 488–496. [DOI] [PubMed] [Google Scholar]
- 26. Hirsch HH, Brennan DC, Drachenberg CB, et al. Polyomavirus‐associated nephropathy in renal transplantation: Interdisciplinary analyses and recommendations. Transplantation 2005; 79: 1277–1286. [DOI] [PubMed] [Google Scholar]
- 27. Hirsch HH, Randhawa P. BK polyomavirus in solid organ transplantation. Am J Transplant 2013; 13(Suppl 4): 179–188. [DOI] [PubMed] [Google Scholar]
- 28. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(Suppl 3): S1–155. [DOI] [PubMed] [Google Scholar]
- 29. Brennan DC, Agha I, Bohl DL, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant 2005; 5: 582–594. [DOI] [PubMed] [Google Scholar]
- 30. Ginevri F, Azzi A, Hirsch HH, et al. Prospective monitoring of polyomavirus BK replication and impact of pre‐emptive intervention in pediatric kidney recipients. Am J Transplant 2007; 7: 2727–2735. [DOI] [PubMed] [Google Scholar]
- 31. Schaub S, Hirsch HH, Dickenmann M, et al. Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus‐associated nephropathy. Am J Transplant 2010; 10: 2615–2623. [DOI] [PubMed] [Google Scholar]
- 32. Sood P, Senanayake S, Sujeet K, et al. Management and outcome of BK viremia in renal transplant recipients: A prospective single‐center study. Transplantation 2012; 94: 814–821. [DOI] [PubMed] [Google Scholar]
- 33. Menter T, Mayr M, Schaub S, Mihatsch MJ, Hirsch HH, Hopfer H. Pathology of resolving polyomavirus‐associated nephropathy. Am J Transplant 2013; 13: 1474–1483. [DOI] [PubMed] [Google Scholar]
- 34. Comoli P, Azzi A, Maccario R, et al. Polyomavirus BK‐specific immunity after kidney transplantation. Transplantation 2004; 78: 1229–1232. [DOI] [PubMed] [Google Scholar]
- 35. Binggeli S, Egli A, Schaub S, et al. Polyomavirus BK‐specific cellular immune response to VP1 and large T‐antigen in kidney transplant recipients. Am J Transplant 2007; 7: 1131–1139. [DOI] [PubMed] [Google Scholar]
- 36. Schachtner T, Muller K, Stein M, Diezemann C, Sefrin A, Babel N, et al. BK virus‐specific immunity kinetics: a predictor of recovery from polyomavirus BK‐associated nephropathy. Am J Transplant. 2011; 11(11): 2443–2452. [DOI] [PubMed] [Google Scholar]
- 37. Hirsch HH, Vincenti F, Friman S, et al. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: A prospective, randomized, multicenter study. Am J Transplant 2013; 13: 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schold JD, Rehman S, Kayler LK, Magliocca J, Srinivas TR, Meier‐Kriesche HU. Treatment for BK virus: Incidence, risk factors and outcomes for kidney transplant recipients in the United States. Transpl Int 2009; 22: 626–634. [DOI] [PubMed] [Google Scholar]
- 39. Borni‐Duval C, Caillard S, Olagne J, et al. Risk factors for BK virus infection in the era of therapeutic drug monitoring. Transplantation 2013; 95: 1498–1505. [DOI] [PubMed] [Google Scholar]
- 40. Dharnidharka VR, Cherikh WS, Abbott KC. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation 2009; 87: 1019–1026. [DOI] [PubMed] [Google Scholar]
- 41. Manitpisitkul W, Drachenberg C, Ramos E, et al. Maintenance immunosuppressive agents as risk factors for BK virus nephropathy: A case‐control study. Transplantation 2009; 88: 83–88. [DOI] [PubMed] [Google Scholar]
- 42. Geddes CC, Gunson R, Mazonakis E, et al. BK viremia surveillance after kidney transplant: Single‐center experience during a change from cyclosporine‐to lower‐dose tacrolimus‐based primary immunosuppression regimen. Transpl Infect Dis 2011; 13: 109–116. [DOI] [PubMed] [Google Scholar]
- 43. Dadhania D, Snopkowski C, Ding R, et al. Epidemiology of BK virus in renal allograft recipients: Independent risk factors for BK virus replication. Transplantation 2008; 86: 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Awadallah Y, Duquesnoy R, Randhawa P, Shapiro R. HLA susceptibility to BKV infection. Am J Transplant 2006; 6: 640; author reply 641. [DOI] [PubMed] [Google Scholar]
- 45. Ramos E, Drachenberg CB, Papadimitriou JC, et al. Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J Am Soc Nephrol 2002; 13: 2145–2151. [DOI] [PubMed] [Google Scholar]
- 46. Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2013 annual data report: Kidney. Am J Transplant 2015; 15(Suppl 2): 1–34. [DOI] [PubMed] [Google Scholar]
- 47. Bohl DL, Storch GA, Ryschkewitsch C, et al. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am J Transplant 2005; 5: 2213–2221. [DOI] [PubMed] [Google Scholar]
- 48. Sood P, Senanayake S, Sujeet K, et al. Donor and recipient BKV‐specific IgG antibody and posttransplantation BKV infection: A prospective single‐center study. Transplantation 2013; 95: 896–902. [DOI] [PubMed] [Google Scholar]
- 49. Binggeli S, Egli A, Dickenmann M, Binet I, Steiger J, Hirsch HH. BKV replication and cellular immune responses in renal transplant recipients. Am J Transplant 2006: 2218–2219. [DOI] [PubMed] [Google Scholar]
- 50. Weist BJ, Schmueck M, Fuehrer H, Sattler A, Reinke P, Babel N. The role of CD4(+) T cells in BKV‐specific T cell immunity. Med Microbiol Immunol. 2014; 203: 395–408. [DOI] [PubMed] [Google Scholar]
- 51. Provenzano M, Bracci L, Wyler S, et al. Characterization of highly frequent epitope‐specific CD45RA+/CCR7+/− T lymphocyte responses against p53‐binding domains of the human polyomavirus BK large tumor antigen in HLA‐A*0201+ BKV‐seropositive donors. J Transl Med 2006; 4: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Krymskaya L, Sharma MC, Martinez J, et al. Cross‐reactivity of T lymphocytes recognizing a human cytotoxic T‐lymphocyte epitope within BK and JC virus VP1 polypeptides. J Virol 2005; 79: 11170–11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schachtner T, Stein M, Babel N, Reinke P. The loss of BKV‐specific immunity from pretransplantation to posttransplantation identifies kidney transplant recipients at increased risk of BKV replication. Am J Transplant 2015; 15: 2159–2169. [DOI] [PubMed] [Google Scholar]
- 54. Li J, Melenhorst J, Hensel N, et al. T‐cell responses to peptide fragments of the BK virus T antigen: Implications for cross‐reactivity of immune response to JC virus. J Gen Virol 2006; 87: 2951–2960. [DOI] [PubMed] [Google Scholar]
- 55. Randhawa PS, Popescu I, Macedo C, et al. Detection of CD8+ T cells sensitized to BK virus large T antigen in healthy volunteers and kidney transplant recipients. Hum Immunol 2006; 67: 298–302. [DOI] [PubMed] [Google Scholar]
- 56. Ramaswami B, Popescu I, Macedo C, et al. HLA‐A01‐, ‐A03‐, and ‐A024‐binding nanomeric epitopes in polyomavirus BK large T antigen. Hum Immunol 2009; 70: 722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kardas P, Sadeghi M, Weissbach FH, et al. Inter‐ and intralaboratory comparison of JC polyomavirus antibody testing using two different virus‐like particle‐based assays. Clin Vaccine Immunol 2014; 21: 1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kardas P, Leboeuf C, Hirsch HH. Optimizing JC and BK polyomavirus IgG testing for seroepidemiology and patient counseling. J Clin Virol 2015; 71: 28–33. [DOI] [PubMed] [Google Scholar]
- 59. Welcome to SYFPEITHI . [cited 2016 Jan 1]. Available from: http://www.syfpeithi.de/0-Home.htm.
- 60. Schuler MM, Nastke MD, Stevanovikć S. SYFPEITHI: Database for searching and T‐cell epitope prediction. Methods Mol Biol 2007; 409: 75–93. [DOI] [PubMed] [Google Scholar]
- 61. Immune Epitope Database and Analysis Resource . [cited 2016 Jan 1]. Available from: http://www.iedb.org/home_v3.php.
- 62. Vita R, Zarebski L, Greenbaum JA, et al. The immune epitope database 2.0. Nucleic Acids Res 2010; 38: D854–D862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Allele*Frequencies in Worldwide Populations . [cited 2016 Jan 1]. Available from: http://www.allelefrequencies.net/
- 64. Fishman JA. BK virus nephropathy–polyomavirus adding insult to injury. N Engl J Med. 2002; 347(7): 527–530. [DOI] [PubMed] [Google Scholar]
- 65. Ramos E, Drachenberg CB, Wali R, Hirsch HH. The decade of polyomavirus BK‐associated nephropathy: State of affairs. Transplantation 2009; 87: 621–630. [DOI] [PubMed] [Google Scholar]
- 66. Kuypers DR. Management of polyomavirus‐associated nephropathy in renal transplant recipients. Nat Rev Nephrol 2012; 8: 390–402. [DOI] [PubMed] [Google Scholar]
- 67. Comoli P, Hirsch HH, Ginevri F. Cellular immune responses to BK virus. Curr Opin Organ Transplant 2008; 13: 569–574. [DOI] [PubMed] [Google Scholar]
- 68. Mueller K, Schachtner T, Sattler A, et al. BK‐VP3 as a new target of cellular immunity in BK virus infection. Transplantation 2011; 91: 100–107. [DOI] [PubMed] [Google Scholar]
- 69. Trydzenskaya H, Sattler A, Muller K, et al. Novel approach for improved assessment of phenotypic and functional characteristics of BKV‐specific T‐cell immunity. Transplantation 2011; 92: 1269–1277. [DOI] [PubMed] [Google Scholar]
- 70. Gosert R, Rinaldo CH, Funk GA, et al. Polyomavirus BK with rearranged noncoding control region emerge in vivo in renal transplant patients and increase viral replication and cytopathology. J Exp Med 2008; 205: 841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sester M, Leboeuf C, Schmidt T, Hirsch HH. The ABC of virus‐specific T‐cell immunity in solid organ transplantation. Am J Transplant. 2015 Dec 23. doi: 10.1111/ajt.13684. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 72. Wade JA, Hurley CK, Takemoto SK, et al. HLA mismatching within or outside of cross‐reactive groups (CREGs) is associated with similar outcomes after unrelated hematopoietic stem cell transplantation. Blood 2007; 109: 4064–4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Masutani K, Ninomiya T, Randhawa P. HLA‐A2, HLA‐B44 and HLA‐DR15 are associated with lower risk of BK viremia. Nephrol Dial Transplant 2013; 28: 3119–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chen Y, Trofe J, Gordon J, Autissier P, Woodle ES, Koralnik IJ. BKV and JCV large T antigen‐specific CD8(+) T cell response in HLA A*0201(+) kidney transplant recipients with polyomavirus nephropathy and patients with progressive multifocal leukoencephalopathy. J Clin Virol 2008; 42: 198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wilson JJ, Pack CD, Lin E, et al. CD8 T cells recruited early in mouse polyomavirus infection undergo exhaustion. J Immunol 2012; 188: 4340–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hofstetter AR, Ford ML, Sullivan LC, et al. MHC class Ib‐restricted CD8 T cells differ in dependence on CD4 T cell help and CD28 costimulation over the course of mouse polyomavirus infection. J Immunol 2012; 188: 3071–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Phenotype of dividing cells during in vitro expansion. Peripheral blood mononuclear cells from a healthy donor were labeled with CFSE before being expanded in vitro and stained with CD45RA and CCR7 markers. (A) CFSE content of CD8+ T cells after in vitro expansion. Gates P11–P20 allow discrimination of different CD8+ T cell divisions (P11 = no division; P12 = one division; P13 = two divisions, and so forth). (B) CD45RA and CCR7 expression by CD8+ T cells at different division stages. Each panel is gated in one of the 10 gates (P11–P20). CFSE, carboxyfluorescein diacetate succinimidyl ester.
Figure S2: Immune responses of BKPyV‐seronegative HIs. PBMCs from HIs who were seronegative for both BKPyV and JCPyV (HI‐43, HI‐44, HI‐45 and HI‐46; left panel) or seropositive for JCPyV and seronegative for BKPyV (HI‐47, HI‐48; normalized enzyme‐linked immunosorbent assay net OD (58)) (right panel) were expanded for 14 days in the presence of BKPyV 15mP and restimulated with BKPyV 15mP, LPP or 9mP in interferon γ enzyme‐linked immunospot assay. 9mP, 9mer‐peptide pool; 15mP, 15mer peptide pool; BKPyV, BK polyomavirus; HI, healthy individual participant; JCPyV, JC polyomavirus; LPP, long peptide pool; OD, optical density; SFU, spot‐forming unit.
Table S1: Characteristics of 42 healthy individuals.
Table S2: Characteristics of 19 pediatric kidney transplant recipients.
Table S3: HLA allele frequencies in worldwide populations.
Table S4: Previously published BKPyV EVGR 9mer epitopes predicted in our study.
Table S5: BKPyV 9mer responses in IFN‐γ ELISpot assay ‐ additional 24 epitopes.


