Abstract
Objective information that can be passively obtained in an ambulatory setting could be potentially useful for determining appropriate care in blood pressure (BP) management. This study utilized digital medicine (DM) prototypes and telemetric data acquisition to directly confirm medication use and to assess habits of daily living in a hypertensive population. Thirty‐seven patients (23 men age 62±9 years) used the system for 6 weeks. DM prototypes consisted of valsartan 80 mg or 160 mg placed in a gelatin hemicapsule with an excipient tablet as a “stopper,” with a poppy seed–sized ingestible sensor (IS) made of foodstuff on its external surface and capable of creating a biogalvanic current on ingestion to alert a wearable sensor (WS) that was worn on the torso. Passive data collection included IS ingestion dates and times, daily step count, BP, and weight. Automatic short message service (SMS) reminders were sent whenever BP or weight values were not received. Passive detection of DM ingestion was 98% when compared with directly observed dosing. Mean taking and timing adherence rates were 90% and 83%, respectively, and the average step count at a pace of ≥60 steps per minute was 2.0±1.5 h/d. An automatic SMS was sent and 100% confirmed for 251 BP and 14 weight values that were not received. Mild and transient WS‐related skin irritation was the most common device‐related adverse event. There were no serious or unanticipated adverse events. Ninety percent of patients did not mind swallowing a DM capsule, and 75% had a positive overall experience with the system. Ambulatory evaluation of medication adherence and habits of daily living appear to be feasible and acceptable using DM and passive acquisition of telemetric data.
Currently, one third of patients in the United Kingdom have hypertension1 and only 10% of them of have controlled blood pressure (BP).2, 3 The National Institute for Health and Care Excellence (NICE) and the British Hypertension Society have issued joint guidelines for the management of hypertension,4 and the Steering Group on Improving the Use of Medicines (SGIUM) advocates the use of various means by which patients' medication adherence is assessed. Medicines Use Reviews were introduced in the community pharmacy contractual framework in 2005 and have been targeted for long‐term conditions. A New Medicines Service was introduced in 2011 for a number of conditions including hypertension.5 However, the only organized mechanism to assess adherence by these services is patient self‐reporting. Yet, patient questionnaires, pill counts, and prescription refill rates have been shown to be inaccurate. As a result, understanding outcomes in the context of medication adherence levels and daily health decisions is poorly understood, and there remains an unmet need for objective information in an ambulatory setting for determining whether daily health decisions, medication use, and/or pharmacologic unresponsiveness should be the focus of BP management.6
To address this need, digital dose forms of valsartan were integrated with passive telemetric data collection and bidirectional communication and piloted in a hypertensive population. The objectives included automatic recording, summarization, and communication of: (1) the regularity and pattern of individual medication‐taking, daily step count, and daily BP and weight; (2) safety; and (3) patient acceptability.
Methodology
The study was approved by an investigational review board (Ethical and Independent Review Services, Corte Madera, CA) and conducted per US Food and Drug Administration (FDA) guidance using an abbreviated investigational device exemption. Patients with essential hypertension and prescribed valsartan were recruited. Digital medicine (DM) prototypes consisted of valsartan 80 mg or 160 mg placed in a gelatin hemicapsule with an excipient tablet as a “stopper,” with a poppy seed–sized ingestible sensor (IS), which transmits a signal on ingestion (Figure 1). The excipient tablet consists of nonactive pharmaceutical materials that are listed as generally recognized as safe standards.7 The IS is made of foodstuff and creates a biogalvanic current upon coming into contact with gastric fluid which is used to transmit a signal that is detected by an adhesive wearable sensor (WS) patch worn on the lower torso. The WS stores ingestion dates and times, and measures and stores daily step count. Data are automatically encrypted and relayed to a secure server where they are automatically integrated with telemetered twice‐daily BP and once‐daily weight for display. The IS and WS were developed solely by Proteus Digital Health (Redwood City, CA). BP and weight values were obtained using a commercially available telemetric BP cuff and a commercially available telemetric weight scale. Clinical personnel and patients received instruction using the training materials provided by the manufacturers of the respective commercial products. Automatic reminders were sent via short message service (SMS) to participants whenever BP or weight values were not telemetered within a 24‐hour period (Figure 2).
Figure 1.
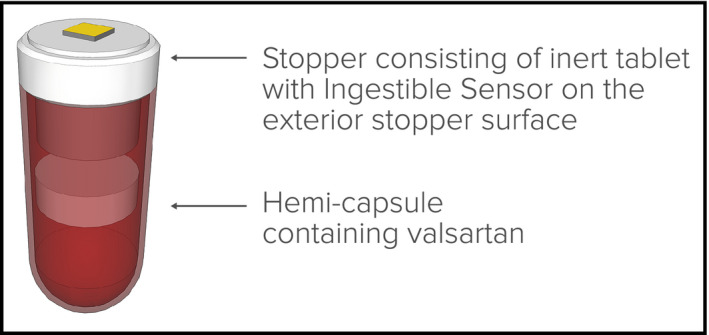
Prototype capsule and “stopper.”
Figure 2.
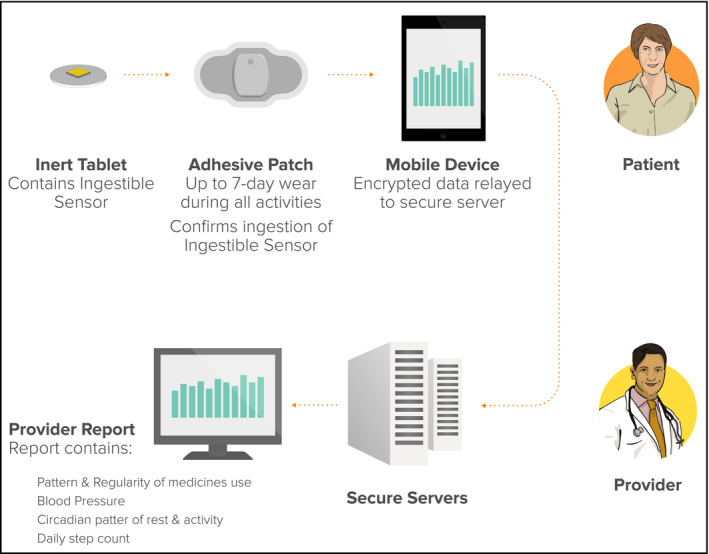
Service overview.
During weekly clinic visits, dosing was directly observed and compared with the accuracy of concurrent automated detection of ingestion (positive detection accuracy [PDA]). PDA was defined as the number of IS ingestions detected by the WS, divided by the number of IS ingestions that were confirmed by actual direct observation. A total of 278 observed ingestions was determined to be needed to achieve a statistical likelihood (P<.05) of demonstrating a PDA having a lower boundary of 95% within a 95% confidence interval (CI) having a point estimate of 97% for overall accuracy.
Taking adherence was defined as the number of digital valsartan tablets that were detected by the WS, divided by the number of digital doses that were prescribed. Timing (also known as “scheduling”) adherence was defined as the number of digital valsartan tablets that were detected within a ±1‐hour time window around the prescribed dosing time, divided by the number of digital valsartan tablets that were detected during that dosing interval.
None of the data that were acquired were used for diagnosis or treatment. On completion of the study, all patients completed a non‐validated, qualitative questionnaire concerning the prototype's utility, usability, and acceptability.
Results
Patient demographics and results are summarized in Table 1. Thirty‐seven patients (23 men; age 62±9 years) participated for 6 weeks. WS detection of the 510 digital valsartan doses that were directly observed in the clinic was 98% (PDA; 95% CI, 96.4–99.1; P<.05). Mean taking and timing adherence rates between clinic visits was 90% and 83% (Figure 3), respectively, with some tapering at weeks 5 and 6. Patients appeared to be more compliant with taking weight than BP measurements. An automatic SMS was sent and 100% confirmed for 250 BP and 17 weight values that were not telemetered within a 24‐hour period. Average step count was duration was 2.0±1.5 h/d at a pace of ≥60 steps per minute. The mean morning BP was 132/78 mm Hg and the mean evening BP was 127/73 mm Hg during system use.
Table 1.
Study Demographics
| Patients, No. | 37 |
| Men, No. (%) | 23 (62) |
| Race (Caucasian/black/Asian), % | 77/5/18 |
| Education (high school/college/graduate school/other), % | 23/54/21/2 |
| Height (cm)/weight (kg), ±standard deviation | 169±11/90±22 |
| Morning BP (mean) during system use, mm Hg | 132/78 |
| Evening BP (mean) during system use, mm Hg | 127/73 |
| Positive detection accuracy, % | 98 (95% CI, 96.4–99.1)a |
| Taking adherence (mean), % | 90 |
| Timing adherence (mean), % | 83 |
| Step count at pace ≥60 steps per min, h/d | 2.0±1.5 |
P<.05. Abbreviations: BP, blood pressure; CI, confidence interval.
Figure 3.
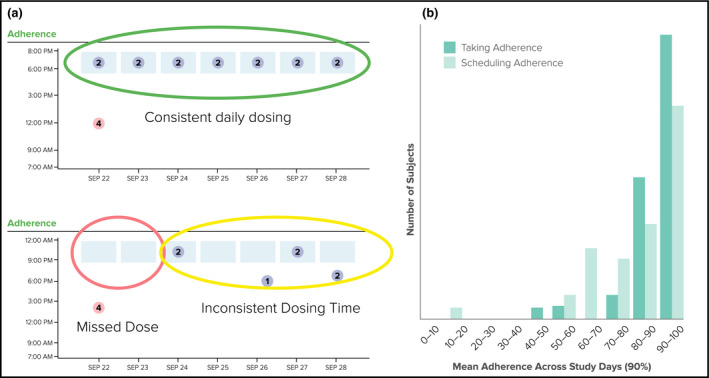
(a) Examples of consistent and inconsistent daily dosing while in a free‐living setting. (b) Relative mean taking and timing (“scheduling”) adherence over the study period.
Safety
Safety results are summarized in Table 2. No serious adverse events, unanticipated adverse events, or study procedure–related adverse events occurred during the study. Mild and transient WS‐related skin irritation occurred in 14 patients (40%), and there were no adverse events related to the IS.
Table 2.
Safety Summary
| Serious adverse events, No. | 0 |
| Unanticipated adverse events, No. | 0 |
| Study procedure‐related adverse events, No. | 0 |
| Nonserious adverse events (NSAEs), No. | 54 |
| Related/possibly related, No. (%) | 32 (59) |
| Mild, No. (%) | 27 (85) |
| Moderate, No. (%) | 5 (15) |
| Severe, No. (%) | 0 |
| Patients with related/possibly related, cutaneous NSAEs, No. | 14 |
| Patients with related/possibly related, cutaneous NSAEs, No. | 8 |
| Asthma attack | 1 |
| Abdominal cramping | 1 |
| Constipation | 2 |
| Nausea | 2 |
| Chest pain (noncardiac) | 1 |
| Bitter taste | 1 |
Acceptability
A summary of acceptability is provided in Figure 4. Ninety percent of patients did not mind swallowing the DM capsule, and 75% had a positive overall experience using the system. When asked more specifically, the majority of patients did not mind wearing the adhesive sensor for even longer periods, and expressed willingness to use such a product if it helped their physicians to take better care of them.
Figure 4.
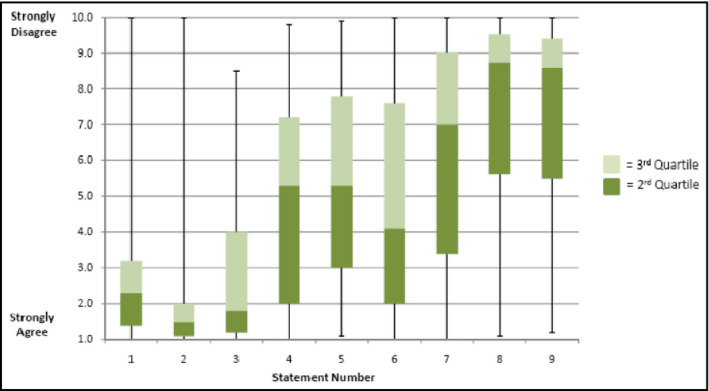
Post‐study questionnaire (statements 1–9) and patient responses. Statement 1: I had a good experience with the study. Statement 2: I did not mind seeing the sensor on the pills. Statement 3: I would be happy to use the system if it helped my doctor to take better care of me. Statement 4: I would be happy to use the system every day. Statement 5: I would be comfortable with using the system long term, more than 3 months. Statement 6: I would like getting reminders from my cell phone when I forgot to take my medicines. Statement 7: I would like a designated person (family member, friend, or caregiver) to be notified when I forgot to take my medicines. Statement 8: I worry about possible side effects from this new monitoring system. Statement 9: I worry about how this new method monitors me.
Discussion
Insufficient medical therapy is the most common reason for apparently resistant hypertension.8 At the same time, it is difficult to assess in clinical practice. Although clinicians can recognize good adherence, they are often unaware of how patients actually take their medicines.9 Thus, for patients with uncontrolled BP while prescribed antihypertensive therapy, there remains an unmet need for clinicians to objectively determine the medication‐taking behavior of their patients if they are to avoid underprescribing or overprescribing. Such information can be used to: (1) compare actual usage of the prescribed medicinal product by the patient in relation to the medicine's approved label; (2) determine whether maximum value is being obtained from prescribed medication(s); and (3) inform decisions that may avoid unnecessary dose escalation, unwanted polypharmacy, overprescribing and medication wastage. Key opinion leaders, regulators, and health systems have highlighted these as major unmet needs in current everyday practice.10, 11, 12, 13, 14, 15 Uncontrolled BP also takes a toll on healthcare resources. It has been reported that poor control of hypertension is associated with an increased number of office visits and greater medication expenditure: the mean number of office visits is 5.5 when diastolic BP is <85 mm Hg, whereas the mean number is 10.0 when diastolic BP is ≥100 mm Hg. Similarly, the mean number of office visits is 4.1 when systolic BP is <120 mm Hg, whereas the mean number is 9.7 when systolic BP is ≥180 mm Hg.16
This was a feasibility study and further evaluations are in progress to determine the potential role of digital health data in the management of hypertension. The questionnaires that were utilized were qualitative and not validated; therefore, it is possible that different results could occur using validated instruments. Nonetheless, the present study has demonstrated that DM in conjunction with remote, in‐home digital assessment of BP, weight, and habits of daily living has the potential to objectively assess patients for the management of BP. The means are available for digital health data that are passively acquired to be provided automatically to patients as part of ongoing feedback on their health behaviors. The same data could be of potential use to healthcare providers for determining whether lifestyle modification, medication use, and/or treatment escalation should be the focus for optimizing an individual's BP management.
The IS and WS are now FDA‐cleared1 and Commonwealth of Europe (CE)–marked class 2 medical devices2 that aid in the measurement of medication adherence. The WS has been improved with more tolerable adhesives and the approved devices have had favorable safety profiles: based on more than 6400 patch‐days of wear, approximately 12% of study patients have experienced self‐limited skin eruptions localized at the site of patch placement. There have now been more than 20,000 ingestions of the IS by more than 500 study patients, with an approximately 1% rate of nausea/vomiting being the adverse event having the highest rate related to the IS.17
The Medicines and Healthcare Products Review Agency has also approved the enclosure of United Kingdom–licensed human medicinal products with the IS within a whole capsule when prescribed by an appropriate healthcare practitioner for a specified patient under their care, where there is special need, in order to directly confirm and automatically log the dates and times of ingestion of the prescribed medicine to assess efficacy. WS with much better tolerated adhesives are now available, and the development of medicinals with an IS within each actual tablet is underway (Figure 5).
Figure 5.
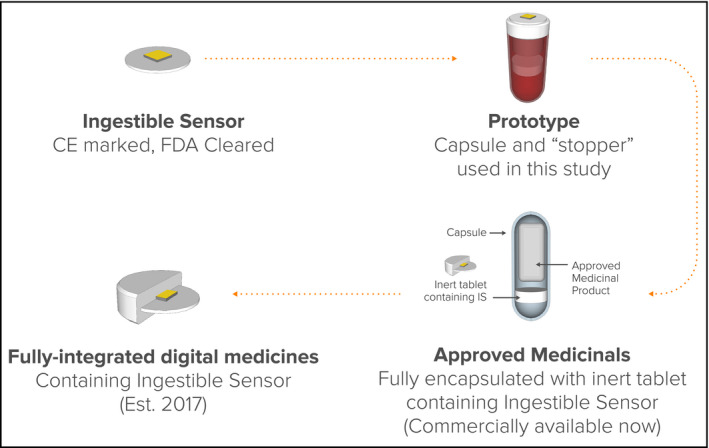
Development pipeline for digital medicines. CE indicates Commonwealth of Europe; FDA, US Food and Drug Administration.
Two other recent clinical studies have utilized the IS in patients with uncontrolled hypertension. In the recently completed UK Hypertension Registry, the IS was prescribed for coingestion at the time of each dosing period of prescribed medications to assess hypertension treatment efficacy in 151 patients with uncontrolled BP while prescribed two or more antihypertensives. The average age was 69 years, 30% had diabetes as a comorbidity, and 38% were chronically prescribed three or more antihypertensive drugs before use of digital health service (DHS). A root cause for uncontrolled BP was identified in 100% of patients: 57% had pharmaceutical resistance and 43% had inadequate medication use. Overall, 38% were found to be capable of achieving BP control on their currently prescribed medications, 57% were found to have a need for additional pharmacologic treatment, and 6% needed additional adherence support. Primary practices found the offering easy to use, and the majority of patients expressed a positive experience in using it.18
In a second study, coingestion of the IS was used by pharmacists participating in the New Medicines Service in 15 rural community pharmacies. The service provided community pharmacists confirmation of medication‐taking and objective measures of lifestyle activities for evidence‐based prescribing and making lifestyle recommendations for achieving BP control. The majority of pharmacists found that the DHS helped to create a collaborative experience with their patients; 91% of those surveyed noted that having objective longitudinal trends in actual medication use and lifestyle behavior aided them in counseling and/or making specific recommendations. Approximately 30% of patients with uncontrolled baseline BP met BP goals (<140/90 mm Hg) after 2 weeks with no changes in their prescribed medicinal therapy. More than 85% of the patients surveyed found the DHS to be usable and useful, and the majority of pharmacists found that the DHS helped to create a collaborative experience with their patients.19
Among individuals who are capable of achieving BP control with no changes in their existing therapy, the controlled BP can also be used subsequently as a legitimate surrogate for medication‐taking during subsequent months. BP elevation during this period of time can be addressed through medication reviews and adherence counseling in lieu of additional clinic visits, prescribing, or specialty referrals for hypertension management.
Conclusions
Digital health monitoring based on, passive acquisition of objective data regarding medication use, and integrating it with reliable and pertinent physiologic and behavioral information, may provide an opportunity for patients and their healthcare providers, caregivers, and family members to engage in better informed dialogues regarding health status, habits of daily living, and response to treatment. Such information may also serve to support patient education and to promote patient engagement in self‐care, while providing an important step towards addressing national mandates to improve on the prescribing of medicines.
J Clin Hypertens (Greenwich). 2016;18:901–906. DOI: 10.1111/jch.12787. © 2016 The Authors. The Journal of Clinical Hypertension Published by Wiley Periodicals, Inc.
Notes
FDA has determined these products to be “substantially equivalent” in safety and effectiveness to another lawfully marketed device, or to a standard recognized by the FDA when used for the same intended purposes.
Medium risk medical devices–hearing aids, syringes are examples that are also in this category—approved for use in humans.
References
- 1. National Institute for Care and Excellence (NICE) Clinical Guideline CG 68 Stroke.
- 2. Caro JJ, Speckman JL, Salas M, et al. Effect of initial drug choice on persistence with antihypertensive therapy: the importance of actual practice data. CMAJ./1999; 1999;160:41–46. [PMC free article] [PubMed] [Google Scholar]
- 3. Health Survey for England , 2014: Trend Tables. 16 December 2015. http://www.hscic.gov.uk/catalogue/PUB19297. Accessed January 4, 2016.
- 4. McCormack T, Arden C, Begg A, et al. Optimizing hypertension treatment: NICE/BHS guideline implementation and audit for best practice. Br J Cardiol. 2013;20(suppl 1):S1–S16. [Google Scholar]
- 5. Improving the use of medicines for better outcomes and reduced waste. Report and Action Plan of the Steering Group on Improving the Use of Medicines. 2012. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/126846/Improving-the-use-of-medicines-for-better-outcomes-and-reduced-waste-An-action-plan.pdf.pdf. Accessed September 9, 2015.
- 6. Insull W Jr. Statement of the problem and pharmacological and clinical requirements for the ideal marker. Control Clin Trials. 1984;5(4 suppl):459–462. [DOI] [PubMed] [Google Scholar]
- 7. United States Food and Drug Administration/Center for Drug Evaluation and Research . Inactive Ingredient Database. http://www.accessdata.fda.gov/scripts/cder/iig/index.cfm. Accessed September 9, 2015.
- 8. Tobe SW, Lewanczuk R. Resistant hypertension. Can J Cardiol. 2009;25:315–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burnier M, Wuerzner G, Struijker‐Boudier H, Urquhart J. Measuring, analysing, and managing drug adherence in resistant hypertension. Hypertension. 2013;62:218–225. [DOI] [PubMed] [Google Scholar]
- 10. Horne R. 2005. Concepts and terminology. In: Horne R, Weinman J, Barber N, et al. Concordance, Adherence and Compliance in Medicine Taking—Report for the National Coordinating Centre for NHS Service Delivery and Organisation Research and Development.
- 11. NICE clinical guideline 76, Medicines Adherence: Involving patients in decisions about prescribed medicines and supporting adherence 2009. [PubMed]
- 12. NICE Guide to resources Medicines Adherence: Implementing NICE Guidance 2009. www.nice.org.uk/nicemedia/live/11766/43741/43741.doc. Accessed September 9, 2015.
- 13. Department of Health: Improving the use of medicines for better outcomes and reduced waste: An Action Plan October 2011.
- 14. NHS England: making medicines‐taking a better experience. https://www.england.nhs.uk/wp-content/uploads/2014/04/mo-ws-report-02-14.pdf. Accessed January 12, 2016.
- 15. Improving medications adherence. SBIR Healthcare and NHS England competition for development contracts. May. 2014. https://www.innovateuk.org/documents/1524978/1866952/SBRI%20Healthcare%20Spring%202014%20-%20Improving%20medicines%20adherence%20-%20Competition%20brief. Accessed September 9, 2015.
- 16. Paramore LC, Halpern MT, Lapuerta P, et al. Impact of poorly controlled hypertension on health care resource utilization and cost. Am J Manag Care. 2001;7:389–398. [PubMed] [Google Scholar]
- 17. Proteus Personal Monitor [package insert]. Proteus Digital Health, Inc, Redwood City, CA. [Google Scholar]
- 18. Naik R, Macey N, West RJ, et al. An ingestible sensor and wearable patch tracking adherence and activity patterns identified underlying factors leading to persistent hypertension: a real‐world registry study. Presented at: the Annual Meeting of the European Society of Cardiology. London, England; 2015 (Abstract).
- 19. Nobel K, Xiang P, Kim YA, et al. Medication Adherence and Activity Patterns Measured by Sensor‐Technologies Guided Hypertension Management in the Community Pharmacy. Presented at: the 4th World Congress of Clinical Pharmacy. London, England; 2015 (Abstract).


