Abstract
Aim
To investigate whether clinical inertia, the failure to intensify treatment regimens when required, exists in people with type 2 diabetes treated with basal insulin.
Methods
This was a retrospective cohort study involving patients with type 2 diabetes in the UK Clinical Practice Research Datalink database between January 2004 and December 2011, with follow‐up until December 2013.
Results
A total of 11 696 patients were included in the analysis. Among all patients, 36.5% had their treatment intensified during the study period; of these, the treatment of 50.0, 42.5 and 7.4% was intensified with bolus or premix insulin or glucagon‐like peptide‐1 receptor agonists, respectively. The median time from initiation of basal insulin to treatment intensification was 4.3 years [95% confidence interval (CI) 4.1, 4.6]. Among patients clinically eligible for treatment intensification [glycated haemoglobin (HbA1c) ≥7.5% (58 mmol/mol)], 30.9% had their treatment regimen intensified. The median time to intensification in this group was 3.7 years (95% CI 3.4, 4.0). Increasing age, duration of diabetes, oral antihyperglycaemic agent usage and Charlson comorbidity index score were associated with a significant delay in the time to intensification (p < 0.05). Among patients with HbA1c ≥7.5% (58 mmol/mol), 32.1% stopped basal insulin therapy.
Conclusions
Strategies should be developed to increase the number of patients undergoing therapy intensification and to reduce the delay in intensifying therapy for suitable patients on basal insulin. Initiatives to support patients continuing on insulin are also required.
Keywords: basal, glucagon‐like peptide‐1, glycaemic control, intensive insulin therapy, type 2 diabetes
Introduction
Type 2 diabetes is a progressive disease characterized by a decline in β‐cell function and loss of glycaemic control, with many patients ultimately requiring intensification of their treatment regimen 1. Guidelines for the treatment of patients with type 2 diabetes suggest that tight glycaemic control should be maintained [defined as glycated haemoglobin (HbA1c) <7.0% (53 mmol/mol)] through active titration of combinations of antihyperglycaemic medications and lifestyle modification, as appropriate 2, 3. Additional antihyperglycaemic drugs may be added if the HbA1c level continues to remain above the recommended target of 7.0% (53 mmol/mol). If HbA1c is ≥7.5% (58 mmol/mol), further intensification, including the use of insulin, is recommended 2, 3, 4. As people with diabetes move through the recommended treatment algorithm, those patients who are suboptimally controlled according to the guideline targets may be at greater risk of long‐term diabetes‐related complications 5, 6.
A major concern in the clinical community is the failure of a very high proportion of patients to reach the recommended glycaemic targets for a considerable period of time after the diagnosis of diabetes 7, 8, 9, 10, 11. Among those with poor glycaemic control, an overwhelmingly large proportion of patients experience a delay before their treatment is intensified 8, 11. This delay in treatment intensification, also termed ‘clinical inertia’, has been investigated in a number of studies 7, 8, 9, 10, 11. A recent study by Khunti et al. 8 reported that the average time to intensification with two oral antihyperglycaemic agents (OHAs) from one OHA, among patients with HbA1c >7.0% (53 mmol/mol), was ∼3 years. A major reason for clinical inertia is the failure to act by healthcare professionals in primary care 12.
A large proportion of patients with type 2 diabetes with poor glycaemic control receive insulin treatment, although studies have reported significant delay in initiation of insulin treatment after glycaemic failure with oral antidiabetes drugs 13, 14, 15. Failure to switch insulin regimens or to intensify treatment has been reported even when HbA1c remains well above glycaemic targets 16. Initiation of insulin treatment with basal insulin is often a preferred option for primary care physicians for its logistic ease and also for its relatively low risk of hypoglycaemia 15, 17, 18; however, there is no fixed standard for intensification of insulin treatment in patients who continue to have poor glycaemic control after insulin initiation, and it is often guided by individual patients' and their service providers' choices 3. Although several clinical trials have evaluated the efficacy of adding multiple insulin treatment regimens in patients with poorly controlled diabetes, studies evaluating the real‐world scenario in terms of robust and timely management of insulin treatment in patients with diabetes are scarce 15, 19, 20. Moreover, it is important that studies into clinical inertia are carried out regularly to keep pace with changes in patient demographics, therapy options and clinical guidelines.
In the present study, we investigated whether clinical inertia exists in a more progressed group of patients with type 2 diabetes; those who are treated with basal insulin ± OHAs. The specific objectives of our analysis were: (i) to estimate the likelihood of intensification and time from starting basal insulin to intensification, defined as adding bolus or premix insulin or glucagon‐like peptide‐1 (GLP‐1) receptor agonists (RAs); and (ii) to estimate the likelihood of intensification and time spent in poor glycaemic control [HbA1c >7.5% (58 mmol/mol)] before intensifying treatment.
Research Design and Methods
The data for this retrospective cohort study were extracted from the UK Clinical Practice Research Datalink (CPRD). The CPRD is one of the largest proprietary, anonymized, longitudinal, well validated databases derived from UK primary care and it contains patient‐level clinical information including diagnoses, mortality, laboratory results and prescription data 21. This database is representative of the UK general population, with age and sex distributions similar to those reported by the UK National Population Census 22. All information collected in the CPRD has been subjected to validation studies and has been proven to contain consistent and high‐quality data 23.
The cohort consisted of patients with type 2 diabetes who had started basal insulin therapy in the period between 1 January 2004 and 31 December 2011, with end of follow‐up on 31 December 2013 (minimum and maximum potential exposure times were 2 years and 10 years, respectively). The patients were classified as having type 2 diabetes according to Read/OXMIS codes, using algorithms based on age at diagnosis, type of treatment and age at treatment 24, 25. The patients were aged ≥18 years and had been registered in the CPRD for at least 180 days before the index date (starting basal insulin). The index date served as baseline for the study period. The duration of diabetes was the time from first diagnosis of diabetes in the CPRD until the index date. Basal insulin was defined according to British National Formulary chapter 6.1.2 (intermediate and long‐acting) 26. Poor glycaemic control was defined as a recording of HbA1c ≥7.5% (58 mmol/mol) taken >6 months after starting basal insulin: the 6‐month window was chosen to allow sufficient time to titrate patients to target HbA1c levels. In the second objective of our analysis – to estimate the likelihood of intensification and time spent in poor glycaemic control [HbA1c > 7.5% (58 mmol/mol)] before intensifying treatment – only those patients who were in poor glycaemic control contributed to ‘risk time’ (time in poor glycaemic control); all other patients were censored. To understand the influence of the HbA1c level on the decision of whether or not to intensify treatment, a sensitivity analysis was performed with an HbA1c threshold of 8.0% (64 mmol/mol). The influence of comorbidities on time to intensification was evaluated using the Charlson comorbidity index (CCI) score 27. CCI scores predict the 10‐year mortality for a patient who may have a range of comorbid conditions, such as renal failure, cancer or congestive heart failure. Each condition is assigned a score of 1, 2, 3 or 6, depending on the level of risk 27. In the present study, all patients were assigned a minimum value of one based on their diagnosis of type 2 diabetes. To assess whether the date of basal insulin initiation, and thus potential follow‐up time, affected the time to intensification, a sensitivity analysis of subcohorts starting basal insulin before 1 January 2006, 1 January 2009 and 1 July 2011 was performed.
The study protocol was approved by the Independent Scientific Advisory Committee of the CPRD (protocol number 14_027R).
Statistical Methods
Characteristics of the study population were presented by number (%), mean [standard deviation (s.d.)], or median [interquartile range (IQR)]), as appropriate. A Kaplan–Meier estimator was used to estimate the distribution of time from initiation of basal insulin to intensification, as well as time in poor glycaemic control until intensification. Time to event analysis (Cox regression) was used to investigate the effect of covariates on the distribution of time from basal insulin initiation until intensification, and the effect of covariates on time in poor glycaemic control until intensification, adjusting for background covariates. Index year was categorized into three subcohorts to assess potential changes in prescribing habits over time. The covariates included in both the analysis of the full cohort and the analysis of the subcohorts in poor glycaemic control [recording of HbA1c ≥ 7.5% (58 mmol/mol) after initiation of treatment with basal insulin] were: age; duration of diabetes diagnosis at the start of the cohort; body mass index (BMI); number of OHAs; CCI score; gender; and index‐year cohort for start of basal insulin. To investigate whether HbA1c level had an effect on the time to intensification, time‐varying covariates for HbA1c were included and served as a sensitivity analysis. Descriptive statistics were used to capture event/censoring patterns and to describe potential changes over time during follow‐up. Patients who did not intensify treatment were censored if HbA1c dropped below 7.5% (58 mmol/mol) or, in the case of death, transferring out of the practice or if there were missing data.
Results
Population Demographics and Baseline Characteristics
After exclusion criteria were applied to the primary dataset, 11 696 patients were available for inclusion in the full cohort (a detailed overview of the exclusion criteria is provided in Table S1). The baseline (start of basal insulin/index date) characteristics for the total, intensified and censored populations are shown in Table 1. Basal insulin was commenced in all patients at a mean ± standard deviation (s.d.) HbA1c of 9.7 ± 2.0% (82 mmol/mol), with 80.3% of patients being on ≥2 OHAs at commencement of insulin. Baseline characteristics for patients clinically eligible for intensification [HbA1c >7.5% (58 mmol/mol)] are provided in Table 2. The mean BMI ± s.d. at intensification, for patients who intensified was 31.3 ± 6.6 kg/m2 (n = 1627) with bolus insulin, 31.3 ± 6.2 kg/m2 (n = 1379) with premix insulin, and 38.6 ± 7.1 kg/m2 (n = 125) with GLP‐1 RAs.
Table 1.
Baseline (start of basal insulin) characteristics for the total, all intensified and all censored populations.
| Total population | All intensified | All censored | ||||
|---|---|---|---|---|---|---|
| n | Mean* ± s.d. | n | Mean* ± s.d. | n | Mean* ± s.d. | |
| Age, years | 11 696 | 65.5 ± 13.2 | 4269 | 61.3 ± 12.6 | 7427 | 67.9 ± 12.9 |
| Age at diagnosis of diabetes, years† | 11 696 | 57.4 ± 12.9 | 4269 | 53.7 ± 12.1 | 7427 | 59.5 ± 12.8 |
| Male, % | 6513 | 55.7 | 2472 | 57.9 | 4041 | 54.4 |
| Female, % | 5183 | 44.3 | 1797 | 42.1 | 3386 | 45.6 |
| Duration of diabetes, years† | 11 696 | 8.2 ± 6.3 | 4269 | 7.7 ± 5.9 | 7427 | 8.5 ± 6.5 |
| BMI, kg/m2 | 8513 | 30.6 ± 6.5 | 3296 | 31.3 ± 6.6 | 5217 | 30.1 ± 6.4 |
| Body weight, kg | 8579 | 86.8 ± 20.6 | 3322 | 89.6 ± 20.7 | 5594 | 85.0 ± 20.3 |
| HbA1c | ||||||
| % | 9811 | 9.7 ± 2.0 | 3698 | 9.8 ± 1.9 | 6496 | 9.7 ± 2.0 |
| mmol/mol | 82.5 ± 22 | 83.6 ± 20.8 | 82.5 ± 22 | |||
| Number of OADs | ||||||
| 0 | 557 | 4.8 | 165 | 3.9 | 392 | 5.3 |
| 1 | 1752 | 15.0 | 458 | 10.7 | 1294 | 17.4 |
| ≥2 | 9387 | 80.3 | 3646 | 85.4 | 5741 | 77.3 |
| CCI score | ||||||
| 1 | 3910 | 33.4 | 1642 | 38.5 | 2268 | 30.5 |
| 2 | 2007 | 17.2 | 780 | 18.3 | 1227 | 16.5 |
| 3 | 2576 | 22.0 | 906 | 21.2 | 1670 | 22.5 |
| ≥4 | 3203 | 27.4 | 941 | 22.0 | 2262 | 30.5 |
BMI, body mass index; CCI, Charlson comorbidity index; HbA1c, glycated haemoglobin; OAD, oral antidiabetic drug; s.d., standard deviation.
Values for male/female, number of OADs and CCI scores are per cent.
If before regimen start.
Table 2.
Baseline (start of basal insulin) characteristics of the population clinically eligible for intensification [glycated haemoglobin ≥7.5% (58 mmol/mol)].
| Total population | All intensified | All censored | ||||
|---|---|---|---|---|---|---|
| n | Mean* ± s.d. | n | Mean* ± s.d. | n | Mean* ± s.d. | |
| Age, years | 6072 | 66.1 ± 12.7 | 1879 | 61.9 ± 11.7 | 4193 | 67.9 ± 12.1 |
| Age at diagnosis of diabetes, years† | 6072 | 56.7 ± 12.0 | 1879 | 53.3 ± 11.3 | 4193 | 58.2 ± 11.9 |
| Male, % | 3397 | 55.9 | 1094 | 58.2 | 2303 | 54.9 |
| Female, % | 2675 | 44.1 | 785 | 41.8 | 1890 | 45.1 |
| Time since diagnosis of diabetes, years† | 6072 | 9.5 ± 6.0 | 1879 | 8.7 ± 5.5 | 4193 | 9.8 ± 6.2 |
| BMI, kg/m2 | 3828 | 31.3 ± 6.3 | 1210 | 32.1 ± 6.3 | 2618 | 31.0 ± 6.2 |
| Body weight, kg | 3846 | 88.4 ± 19.8 | 1215 | 91.0 ± 19.9 | 2631 | 87.2 ± 19.6 |
| HbA1c, at baseline/initiation of insulin | ||||||
| % | 5425 | 9.8 ± 1.9 | 1708 | 9.9 ± 1.7 | 3717 | 9.8 ± 1.9 |
| mmol/mol | 83.6 ± 20.8 | 84.7 ± 18.6 | 83.6 ± 20.8 | |||
| HbA1c, at first recording ≥7.5% (58 mmol/mol)‡, % | 6072 | 73.8 ± 14.2 | 1879 | 77.0 ± 15.3 | 4193 | 71.6 ± 14.2 |
| Number of OADs | ||||||
| 0 | 583 | 9.6 | 165 | 8.8 | 418 | 10.0 |
| 1 | 2780 | 45.8 | 864 | 46.0 | 1916 | 45.7 |
| ≥2 | 2709 | 44.6 | 850 | 45.2 | 1859 | 44.3 |
| CCI score | ||||||
| 1 | 1533 | 25.3 | 577 | 30.7 | 956 | 22.8 |
| 2 | 767 | 13.1 | 273 | 14.5 | 524 | 12.5 |
| 3 | 1472 | 24.2 | 462 | 24.6 | 1010 | 24.1 |
| ≥4 | 2270 | 37.4 | 567 | 30.2 | 1703 | 40.6 |
BMI, body mass index; CCI, Charlson comorbidity index; HbA1c, glycated haemoglobin; OAD, oral antidiabetic drug; s.d, standard deviation.
Values for male/female, number of OADs and CCI scores are per cent.
If before regimen start.
n is greater than HbA1c at baseline because not all patients had a recording at baseline.
Time to Intensification: All Patients
The total time of exposure for all 11 696 patients was 24 561 patient‐years and the totals for intensified and censored patients were 6493 and 18 122 patient‐years, respectively. Among all patients, 36.5% had their treatment intensified during the observational period and 63.5% were censored. Censoring was attributable to stopping basal insulin therapy (63.4%) and reaching the end of follow‐up (36.6%). The median time from initiation of basal insulin to intensification with bolus or premix insulin, or GLP‐1, was 4.3 years (95% CI 4.1, 4.6) for all patients, regardless of HbA1c level. The estimated probability of having treatment intensified at end of follow‐up was 67.8% (data not shown). Age, duration of diabetes, number of OHAs, CCI score, cohort year, baseline HbA1c and BMI all had a significant association with the time to intensification. Among the covariates included in the Cox proportional hazards analysis, only gender had no effect. Hazard ratios >1 indicated a shorter time to intensification, while values <1 indicated a longer time (delay) in intensification. Increasing age and duration of diabetes were associated with a delay in intensification, whereas increasing BMI was associated with a shorter time to intensification. For the CCI score there was no difference across the categories. The results for the number of OHAs were mixed, with any OHA use being associated with a delay; however, those individuals on ≥2 OHAs had a slightly reduced delay compared with those on one OHA. The index date subcohorts showed a successive decrease in the time to intensification, with the delay being shortest in the most recent cohort (1 January 2009 to 30 June 2011; Figure 1). Date of basal initiation did not affect time to intensification (Table 3). Of all patients whose treatment was intensified, 50.0 and 42.5% had treatment intensified with bolus or premix insulin, respectively, and the treatment of 7.4% of individuals was intensified with GLP‐1 RAs (data not shown).
Figure 1.
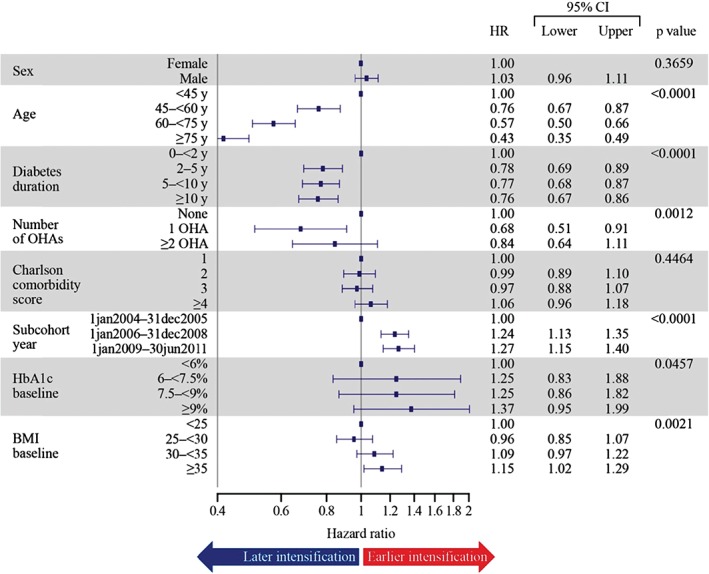
Effect of covariates on time to intensification (all patients). Multivariate Cox proportional hazard model. Hazard ratios (HRs) >1 indicate a shorter time to intensification (sooner), whereas values <1 indicate a longer time (delay/later) in intensification. BMI, body mass index; CI, confidence interval; HbA1c, glycated haemoglobin; OHA, oral hypoglycaemic agent; y, years.
Table 3.
Time to intensification in total population and patients clinically eligible for intensification [glycated haemoglobin ≥7.5% (58 mmol/mol)] by basal initiation date.
| Subcohort by date of basal initiation | Median time from basal insulin initiation to intensification, total population, years | n | Median time from first HbA1c ≥7.5% to intensification, years | n |
|---|---|---|---|---|
| Initiated basal before 1 January 2006 | 4.7 | 3 194 | 3.7 | 1 830 |
| Initiated basal before 1 January 2009 | 4.3 | 7 919 | 3.4 | 4 262 |
| Initiated basal before 1 July 2011 | 4.3 | 11 696 | 3.7 | 6 072 |
| All (for verification) | 4.3 | 11 696 | 3.7 | 6 072 |
HbA1c, glycated haemoglobin.
Time to Intensification for Clinically Eligible Patients: HbA1c ≥7.5%
The proportion of patients with HbA1c ≥7.5% (58 mmol/mol) in the main cohort was 51.9% (n = 6072). Total years of exposure were 9229 years. Among patients with HbA1c ≥7.5% (58 mmol/mol), 30.9% of patients had their treatment intensified. In this cohort, 69.1% of patients (n = 4193) were censored because: (i) HbA1c dropped below 7.5% (58 mmol/mol) (30.2%) and remained there after the 6‐month titration phase; (ii) patients ended treatment with basal insulin (46.5%); or (iii) end of follow‐up was reached (23.3%). In patients who underwent therapy intensification, the percentages of those with HbA1c levels ≥7.5, 8.0 and 9.0% when intensifying were 96.1, 88.9 and 60.5%, respectively. The median time to intensification with bolus or premix insulin or GLP‐1 after the first recording of HbA1c ≥7.5% (58 mmol/mol) was 3.7 years (95% CI 3.4; 4.0). The probability of having treatment intensified at end of follow‐up was 75.8% (Figure 2A). A sensitivity analysis using an HbA1c of ≥8.0% (64 mmol/mol) showed a median time to intensification of 3.2 years (95% CI 2.8, 3.5), showing only a small reduction in the time to intensification between these two thresholds (data not shown).
Figure 2.
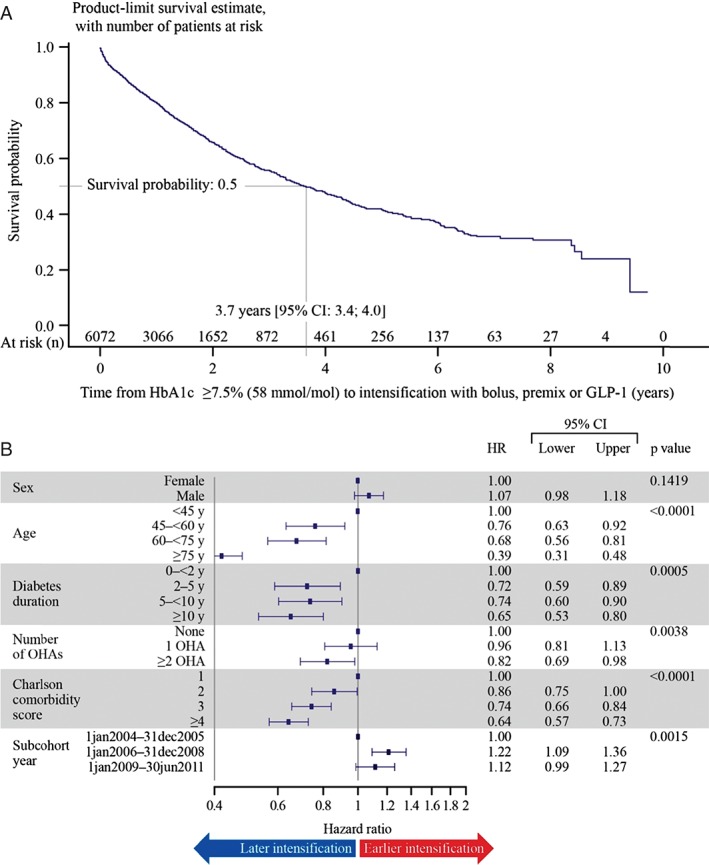
(A) Time (in years) from initiation of basal insulin therapy to intensification with bolus or premix insulin, or glucagon‐like peptide‐1(GLP‐1) in clinically eligible patients [glycated haemoglobin (HbA1c) ≥7.5% (58 mmol/mol)]. (B) Effect of covariates on time to intensification in clinically eligible patients [HbA1c ≥7.5% (58 mmol/mol), occurring at least 6 months after initiating basal insulin]. BMI, body mass index; CI, confidence interval; HR, hazard ratio; OHA, oral hypoglycaemic agent; y, years.
The time to intensification after the first recording of HbA1c ≥7.5% (58 mmol/mol) was influenced by age, duration of diabetes, number of OHAs, CCI score and cohort index date. Increasing age, duration of diabetes, OHA usage and CCI score were associated with a longer delay in intensification. Although the number of years' duration of diabetes had a significant effect on time to intensification, there was no difference between the 2‐ to <5‐year duration group and the 5‐ to <10‐year duration group. The longest delay was observed in the group with >10 years' diabetes duration. The most recent subcohort index dates showed a trend for decreasing time to intensification, although there was a slight increase between the cohorts 1 January 2006 to 31 December 2008 and 1 January 2009 to 30 June 2011 (Figure 2B); i.e. 46.9 and 43.1% of patients whose treatment was intensified were intensified with bolus or premix insulin, respectively, and 10.0% of individuals were intensified with GLP‐1 RAs (data not shown). The mean ± s.d. HbA1c at the time of intensification was 9.3 ± 1.44% (n = 569), 9.6 ± 1.09% (n = 24) and 9.3 ± 1.78% (n = 75) for patients whose treatment was intensified with bolus insulin, premix insulin or GLP‐1 RAs, respectively. The date of basal initiation did not affect time to intensification (Table 3). The mean ± s.d. first HbA1c measurement taken 6–12 months after treatment intensification, for patients continuing on treatment, was 8.4 ± 1.46%, 8.9 ± 1.35% and 8.2 ± 2.05% for bolus insulin, premix insulin or GLP‐1, representing respective decreases from baseline of −0.9 ± 1.52%, −0.7 ± 1.79% and −1.2 ± 2.16% (data not shown).
Discussion
Using data from a nationally representative database, the results of our analysis show for the first time that clinical inertia exists among patients with type 2 diabetes treated with basal insulin. For patients clinically eligible for intensification of treatment who were intensified, there was a median time of 3.7 years from starting basal insulin until intensification, despite having an HbA1c >7.5% (58 mmol/mol). Just over one‐third of patients had their treatment intensified. It should be noted that many patients with HbA1c ≥7.5% (58 mmol/mol) did not have their treatment intensified at all and many stopped basal insulin completely.
This delay in treatment intensification was more pronounced among older people and those with a longer time since diagnosis. Those patients with poor glycaemic control with a higher CCI score (more comorbidities) were also likely to wait longer before having their treatment intensified. Multi‐morbidity has been shown to be a barrier to prescribing 28. The higher threshold of ≥8.0% (64 mmol/mol) showed a slight reduction in the time to intensification (3.2 years) compared with ≥7.5% (58 mmol/mol), and the covariate analyses showed that there was a significant reduction in time to intensification with increasing HbA1c level. One possible explanation for this is that higher baseline HbA1c is responsible for the reduction in time to intensification; this may be part of a planned, stepwise intensification process by the treating physician.
Rather than treating to target, these intensification delays suggest that many patients only have their treatment regimen intensified once they have exceeded target HbA1c levels for an extended duration, and some patients do not appear to have their treatment intensified at all. The causes of these delays are complex and include concerns among healthcare providers regarding hypoglycaemia, weight gain and patient acceptance 12, 29, 30. These may, in part, be prudent decisions made by physicians who wish to avoid the risk of hypoglycaemia among people with diabetes who are perceived as frail, but equally they may represent hesitation to reduce HbA1c in people with diabetes who would benefit greatly from improved glycaemic control 15.
Physicians adopting a patient‐centred approach may also treat patients differently based on their history of glycaemic control. For example, a patient with HbA1c of 7.2% which, over 2 years, crept above 7.5%, may be considered less in need of treatment intensification than a patient who has never achieved the target of <7.5%. Another reason for the delay in intensification of insulin therapy could be that the delay in moving people with diabetes from OHAs onto basal insulin has a ripple effect, with patients being more progressed in their disease, more frail, experiencing more complications, and therefore being less able to tolerate further intensification 8. There are also delays in intensification with basal insulin once they have been initiated 31.
The fact that more patients had their treatment intensified with bolus insulin or premix instead of GLP‐1 RAs in the present study probably reflects the recent introduction of GLP‐1 RAs during the study period. Secondly, initiation of GLP‐1 RAs may precede basal insulin. Although GLP‐1 RAs were not licensed for combination with basal insulin during a significant proportion of the study period, results from the Association of British Clinical Diabetologists (ABCD) exenatide audit indicate that the combination was being used off‐label 32. It is conceivable, and indeed likely, that a larger percentage of patients are currently having their treatment intensified with GLP‐1 RAs as these become more widely adopted.
A large proportion of patients clinically eligible for intensification stopped basal insulin altogether. The reasons for stopping basal insulin are not reported in the CPRD but there are several possible explanations. It could be that patient non‐adherence to the treatment regimen is a factor in both the initial elevation of blood glucose concentrations and subsequently stopping basal insulin. For example, the fear of hypoglycaemia, inconvenience of self‐injection/blood glucose monitoring, concerns over weight gain, or simply the patient not accepting the need for insulin could all lead to omission of injections and non‐adherence. This ‘psychological insulin resistance’ is normally associated with the initiation of insulin but may persist in patients who have commenced basal insulin therapy 33. Alternatively, the physician may deem the patient to be too elderly or frail to derive sufficient benefit from continued administration of basal insulin, and that the risk of hypoglycaemia outweighs the benefit of a lower risk of diabetes‐related complications. It would be difficult to confirm the reasons for stopping basal insulin using routine data. Patient questionnaires or qualitative studies, similar to those used to investigate the barriers to insulin initiation 34, 35, are urgently needed to uncover the causes.
The Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) and Action to Control Cardiovascular Risk in Diabetes (ACCORD) trials raised questions over the benefits of tight glucose control in patients with type 2 diabetes 36. In the ADVANCE study, intensive glucose control did not change the incidence of retinopathy or macrovascular outcomes, whilst in ACCORD, intensive therapy produced mixed results 36. By contrast, results from the 10‐year follow‐up to the UK Prospective Diabetes Study (UKPDS) have shown that there are long‐term benefits from improving glycaemic control that extend to a reduction in cardiovascular diseases 6. The disparity between the benefit of intensive blood glucose control in the ADVANCE, ACCORD and UKPDS trials may be partly explained by the enrolment of older patient populations in ADVANCE and ACCORD, where patients also had a longer duration of diagnosis. Furthermore, the studies were of shorter duration. This supports the need to intensify blood glucose‐lowering therapy in a timely manner to ensure that the benefits of reduced blood glucose are realized 6, 8. The duration of the present study was not sufficient to assess whether failure to intensify treatment leads to poorer clinical outcomes; however, the findings of the UKPDS suggest that, particularly in younger patients, this is likely to be the case 6.
The present study has several limitations. Notably, not all people with diabetes had regular HbA1c measurements, and BMI recordings were not available for some people with diabetes. Insulin dose was not taken into account as this is not recorded in the CPRD, yet an increase in basal insulin dose should legitimately be considered an intensification step. It may be that uptitration occurred very slowly in some patients – ‘titration inertia’ – and exceeded the 6‐month window allocated in the present study before recording time with HbA1c ≥7.5% (58 mmol/mol) 37. Treatment adherence also affected our analysis. Those people with diabetes who did not adhere to treatment regimens were censored shortly after the index date. The Quality and Outcomes Framework (QOF) points (the annual reward and incentive programme detailing general practice achievement results) for individual practices were not accounted for in our analysis 38. This would have provided deeper insight into the standard of care for people with diabetes in the UK, and may have consequences for the delay in intensification among those practices that score high or low for diabetes care, but it is unlikely to have affected the mean or median time to intensification because practices participating in the CPRD are representative and most practices already meet QOF targets. Additionally, QOF was introduced in 2004, which makes it likely that most practices would have been following QOF procedures during the study period. Unfortunately, it was not possible to determine why such a large proportion of patients clinically eligible for intensification discontinued insulin. We speculate that these patients may have been on very low doses of insulin; however, the focus of our investigation is on the time to intensification – the reasons for discontinuation should be the focus of a separate study.
The strengths of the present analysis include the large cohort size, despite ineligibility as a result of selection criteria, and its nationally representative make‐up. This provides a clearer picture of clinical inertia in the UK than smaller studies would be capable of providing. This study has also taken into account many covariates, which improves the robustness of the analysis. Our sensitivity analysis showed that duration of follow‐up did not affect the time to intensification in either the total or clinically eligible populations.
In conclusion, the present study shows that there is a significant delay in the intensification of treatment in people with type 2 diabetes with poor glycaemic control, and that many patients do not have their treatment intensified at all. Clinical inertia appears to exist at both the initiation and intensification of insulin therapy 8. This may have a negative impact on the long‐term outcomes for patients. More detailed studies, perhaps using patient and physician questionnaires, should attempt to establish the reasons for a delay in intensification, particularly among elderly people with diabetes and those with comorbidities.
Conflict of Interest
K. K. has acted as a consultant and speaker for Novartis, Novo Nordisk, Sanofi‐Aventis, Lilly, Merck Sharp & Dohme, Janssen, Astra Zeneca and Boehringer Ingelheim. He has received grants in support of investigator and investigator‐initiated trials from Novartis, Novo Nordisk, Sanofi‐Aventis, Lilly, Pfizer, Boehringer Ingelheim, Merck Sharp & Dohme, Janssen and Roche. K. K. has received funds for research, honoraria for speaking at meetings and has served on advisory boards for Lilly, Sanofi‐Aventis, Merck Sharp & Dohme and Novo Nordisk, Boehringer Ingelheim, Janssen and Astra Zeneca. M. J. D. has acted as consultant, advisory board member and speaker for Novo Nordisk, Sanofi‐Aventis, Eli Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Astra Zeneca and Janssen, and as a speaker for Mitsubishi Tanabe Pharma Corporation. She has received grants in support of investigator and investigator‐initiated trials from Novo Nordisk, Sanofi‐Aventis and Eli Lilly. M. A. has acted as a consultant for Novo Nordisk. A. N. and B. L. T. are employees and shareholders of Novo Nordisk A/S. S. K. P. has acted as a consultant and/or speaker for Novartis, GI Dynamics, Roche, AstraZeneca, Guangzhou Zhongyi Pharmaceutical and Amylin Pharmaceuticals LLC. He has received grants in support of investigator and investigator‐initiated clinical studies from Merck, Novo Nordisk, Astra Zeneca, Hospira, Amylin Pharmaceuticals, Sanofi‐Aventis and Pfizer.
K. K. and S. K. P. contributed equally to the study design, statistical analysis plan, data interpretation and writing of this manuscript. A. N. and B. L. T. contributed to the study design, analysis and interpretation of data, and review of the manuscript. M. J. D. contributed to the research idea, and the interpretation of the data and results, and commented on the manuscript. M. A. contributed to the study design, statistical analysis and review of the manuscript.
K. K. is the guarantor of this work and, as such, had full access to the study data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
All authors were involved in the decision to submit the article for publication. Novo Nordisk sponsored data analysis, medical writing and editorial/submission support, contributed to study design, analysis and interpretation of data, and was also responsible for obtaining data from the CPRD.
Supporting information
Table S1. Subject disposition for analysis of subjects initiating basal insulin therapy.
Acknowledgements
The authors acknowledge medical writing support from Paul Tisdale, PhD and editorial/submission support from Daria Renshaw, both of Watermeadow Medical Limited, UK (an Ashfield company). This support was funded by Novo Nordisk. K. K. and M. J. D. would like to acknowledge support for this work from the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care East Midlands, and the NIHR Leicester‐Loughborough Diet, Lifestyle and Physical Activity Biomedical Research Unit, University of Leicester. S. K. P. acknowledges infrastructure research support from the Australian Government's National Collaborative Research Infrastructure Strategy initiative through Therapeutic Innovation Australia.
References
- 1. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care 2009; 32(Suppl. 2): S151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association . Standards of medical care in diabetes—2014. Diabetes Care 2014; 37(Suppl. 1): S14–80. [DOI] [PubMed] [Google Scholar]
- 3. Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 4. Chatterton H, Younger T, Fischer A, Khunti K; Programme development group. Risk identification and interventions to prevent type 2 diabetes in adults at high risk: summary of NICE guidance. BMJ 2012; 345: e4624. [DOI] [PubMed] [Google Scholar]
- 5. Stratton IM, Adler AI, Neil HAW et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 7. Paul S, Thorsted BL, Wolden M, Klein K, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Diabetologia 2013; 56: S534–S535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care 2013; 36: 3411–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yam FK, Adams AG, Divine H, Steinke D, Jones MD. Clinical inertia in type 2 diabetes: a retrospective analysis of pharmacist‐managed diabetes care vs. usual medical care. Pharmacol Pract 2013; 11: 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coan KE, Schlinkert AB, Beck BR et al. Clinical inertia during postoperative management of diabetes mellitus: relationship between hyperglycemia and insulin therapy intensification. J Diabetes Sci Technol 2013; 7: 880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care 2004; 27: 1535–1540. [DOI] [PubMed] [Google Scholar]
- 12. Zafar A, Davies M, Azhar A, Khunti K. Clinical inertia in management of T2DM. Prim Care Diabetes 2010; 4: 203–207. [DOI] [PubMed] [Google Scholar]
- 13. Rubino A, McQuay LJ, Gough SC, Kvasz M, Tennis P. Delayed initiation of subcutaneous insulin therapy after failure of oral glucose‐lowering agents in patients with type 2 diabetes: a population‐based analysis in the UK. Diabet Med 2007; 24: 1412–1418. [DOI] [PubMed] [Google Scholar]
- 14. Vaag A, Lund SS. Insulin initiation in patients with type 2 diabetes mellitus: treatment guidelines, clinical evidence and patterns of use of basal vs premixed insulin analogues. Eur J Endocrinol 2012; 166: 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mosenzon O, Raz I. Intensification of insulin therapy for type 2 diabetic patients in primary care: basal‐bolus regimen versus premix insulin analogs: when and for whom? Diabetes Care 2013; 36(Suppl. 2): S212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blak BT, Smith HT, Hards M, Curtis BH, Ivanyi T. Optimization of insulin therapy in patients with type 2 diabetes mellitus: beyond basal insulin. Diabet Med 2012; 29: e13–20. [DOI] [PubMed] [Google Scholar]
- 17. Holman RR, Thorne KI, Farmer AJ et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 2007; 357: 1716–1730. [DOI] [PubMed] [Google Scholar]
- 18. Holman RR, Farmer AJ, Davies MJ et al. Three‐year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 2009; 361: 1736–1747. [DOI] [PubMed] [Google Scholar]
- 19. Sharplin P, Gordon J, Peters JR, Tetlow AP, Longman AJ, McEwan P. Switching from premixed insulin to glargine‐based insulin regimen improves glycaemic control in patients with type 1 or type 2 diabetes: a retrospective primary‐care‐based analysis. Cardiovasc Diabetol 2009; 8: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. LaSalle JR, Berria R. Insulin therapy in type 2 diabetes mellitus: a practical approach for primary care physicians and other health care professionals. J Am Osteopath Assoc 2013; 113: 152–162. [PubMed] [Google Scholar]
- 21. Khan NF, Harrison S, Rose P. Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract 2010; 60: e128–e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Williams T, Van Staa T, Puri S, Eaton S. Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther Adv Drug Saf 2012; 3: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tate AR, Beloff N, Al‐Radwan B et al. Exploiting the potential of large databases of electronic health records for research using rapid search algorithms and an intuitive query interface. J Am Med Inform Assoc 2014; 21: 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Lusignan S, Sadek N, Mulnier H, Tahir A, Russell‐Jones D, Khunti K. Miscoding, misclassification and misdiagnosis of diabetes in primary care. Diabet Med 2012; 29: 181–189. [DOI] [PubMed] [Google Scholar]
- 25. Mulnier HE, Seaman HE, Raleigh VS et al. Risk of stroke in people with type 2 diabetes in the UK: a study using the General Practice Research Database. Diabetologia 2006; 49: 2859–2865. [DOI] [PubMed] [Google Scholar]
- 26. National Institute for Health and Care Excellence (NICE) . 6.1 drugs used in diabetes 2015 (January). Available from URL: http://www.evidence.nhs.uk/formulary/bnf/current/6‐endocrine‐system/61‐drugs‐used‐in‐diabetes. Accessed 1 October 2015.
- 27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 28. Schuling J, Gebben H, Veehof LJ, Haaijer‐Ruskamp FM. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Fam Pract 2012; 13: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patel N, Stone MA, Chauhan A, Davies MJ, Khunti K. Insulin initiation and management in people with type 2 diabetes in an ethnically diverse population: the healthcare provider perspective. Diabet Med 2012; 29: 1311–1316. [DOI] [PubMed] [Google Scholar]
- 30. Zafar A, Stone MA, Davies MJ, Khunti K. Acknowledging and allocating responsibility for clinical inertia in the management of type 2 diabetes in primary care: a qualitative study. Diabet Med 2015; 32: 407–413. [DOI] [PubMed] [Google Scholar]
- 31. Khunti K, Davies MJ, Kalra S. Self‐titration of insulin in the management of people with type 2 diabetes: a practical solution to improve management in primary care. Diabetes Obes Metab 2013; 15: 690–700. [DOI] [PubMed] [Google Scholar]
- 32. Thong KY, Jose B, Sukumar N et al. Safety, efficacy and tolerability of exenatide in combination with insulin in the Association of British Clinical Diabetologists nationwide exenatide audit*. Diabetes Obes Metab 2011; 13: 703–710. [DOI] [PubMed] [Google Scholar]
- 33. Polonsky WH, Fisher L, Guzman S, Villa‐Caballero L, Edelman SV. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care 2005; 28: 2543–2545. [DOI] [PubMed] [Google Scholar]
- 34. Lee YK, Ng CJ, Lee PY et al. What are the barriers faced by patients using insulin? A qualitative study of Malaysian health care professionals' views. Patient Prefer Adherence 2013; 7: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brod M, Alolga SL, Meneghini L. Barriers to initiating insulin in type 2 diabetes patients: development of a new patient education tool to address myths, misconceptions and clinical realities. Patient 2014; 7: 437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bloomgarden ZT. Glycemic control in diabetes: a tale of three studies. Diabetes Care 2008; 31: 1913–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khunti K, Damci T, Meneghini L, Pan CY, Yale JF; SOLVE Study Group . Study of once daily levemir (SOLVE™): insights into the timing of insulin initiation in people with poorly controlled type 2 diabetes in routine clinical practice. Diabetes Obes Metab 2012; 14: 654–661. [DOI] [PubMed] [Google Scholar]
- 38. Health and Social Care Information Centre (HSCIC) . Quality and outcomes framework. Available from URL: http://www.hscic.gov.uk/qof. Accessed 1 October 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Subject disposition for analysis of subjects initiating basal insulin therapy.


