ABSTRACT
Long‐term synaptic plasticity, represented by long‐term depression (LTD) and long‐term potentiation (LTP) comprise cellular processes that enable memory. Neuromodulators such as serotonin regulate hippocampal function, and the 5‐HT4‐receptor contributes to processes underlying cognition. It was previously shown that in the CA1‐region, 5‐HT4‐receptors regulate the frequency‐response relationship of synaptic plasticity: patterned afferent stimulation that has no effect on synaptic strength (i.e., a θm‐frequency), will result in LTP or LTD, when given in the presence of a 5‐HT4‐agonist, or antagonist, respectively. Here, we show that in the dentate gyrus (DG) and CA3 regions of freely behaving rats, pharmacological manipulations of 5‐HT4‐receptors do not influence responses generated at θm‐frequencies, but activation of 5‐HT4‐receptors prevents persistent LTD in mossy fiber (mf)‐CA3, or perforant path‐DG synapses. Furthermore, the regulation by 5‐HT4‐receptors of LTP is subfield‐specific: 5‐HT4‐receptor‐activation prevents mf‐CA3‐LTP, but does not strongly affect DG‐potentiation. These data suggest that 5‐HT4‐receptor activation prioritises information encoding by means of LTP in the DG and CA1 regions, and suppresses persistent information storage in mf‐CA3 synapses. Thus, 5‐HT4‐receptors serve to shape information storage across the hippocampal circuitry and specify the nature of experience‐dependent encoding. © 2016 The Authors Hippocampus Published by Wiley Periodicals, Inc.
Keywords: 5‐HT4, LTD, LTP, mossy fibers, dentate gyrus
Abbreviations
- ANOVA
analysis of variance
- DG
dentate gyrus
- fEPSP
field excitatory post‐synaptic potential
- HFS
high‐frequency stimulation
- HT
5‐hydroxytryptamine
- LFS
Low‐frequency stimulation
- LTD
long‐term depression
- LTP
long‐term potentiation
- NS
not significant
- PS
population spike
- SSRI
selective serotonin reuptake inhibitor
- STP
Short‐term potentiation
- wHFS
weak HFS
- wLFS
weak LFS.
INTRODUCTION
Serotonin (5‐hydroxytryptamine, 5‐HT) is involved in a broad spectrum of physiological (Buhot, 1997) and pathological (Reynolds et al., 1995) processes. Fourteen 5‐HT‐receptor subtypes have been identified (Barnes and Sharp, 1999). The 5‐HT4‐receptor is particularly interesting, as it has been implicated in the enhancement of cognition (Fontana et al., 1997; Kennett et al., 1997; Marchetti‐Gauthier et al., 1997; Meneses and Hong, 1997; Meneses, 1998; Hille et al., 2008). Strikingly, patients suffering from Alzheimer's disease undergo a substantial loss of hippocampal 5‐HT4‐receptors (Reynolds et al., 1995), and inhibition of 5‐HT4‐receptors leads to impairments of olfactory associative memory (Marchetti et al., 2000), an ability that is markedly impaired in Alzheimer's disease (Nordin and Murphy, 1998; Conti et al., 2013). Moreover, in healthy humans low selective serotonin reuptake inhibitor (SSRI) doses improve memory, whereas high doses impair it (Dumont et al., 2005). Notably, recent studies suggest a role for 5‐HT4‐receptor expression as a neural marker for learning and memory (Manuel‐Apolinar et al., 2005; Madsen et al., 2011; Meneses, 2015), and revealed an inverse correlation of 5‐HT4‐receptor expression with memory performance in humans (Haahr et al., 2013). Conversely, activation of 5‐HT4‐results in dendritic spine growth after a learning‐event, that is not evident in naïve rodents (Marchetti et al., 2004; Restivo et al., 2008) and augments learning and memory in rodents (Lamirault and Simon, 2001; Lelong et al., 2001; Bockaert et al., 2004, 2011; King et al., 2008) and primates (Terry et al., 1998). The physiological mechanisms underlying the regulation by 5‐HT4‐receptor of learning and memory processes are currently unclear, however.
Long‐term hippocampus‐dependent memory is tightly linked to persistent forms of synaptic plasticity such as long‐term potentiation (LTP) (Kemp and Manahan‐Vaughan, 2004; Malenka and Bear, 2004; Whitlock et al., 2006; Lynch et al., 2007; Nabavi et al., 2014; Lynch and Baudry, 2015) and long‐term depression (LTD) (Etkin et al., 2006; Kemp and Manahan‐Vaughan, 2004, 2007; Goh and Manahan‐Vaughan, 2013a). Strikingly, the degree of activation of the 5‐HT4‐receptor has a profound influence on the ability of CA1 synapses to express synaptic plasticity (Kemp and Manahan‐Vaughan, 2005). Low, or blocked activity results in the strengthening of weak synaptic depression into persistent LTD, whereas activation of the receptor results in strengthening of weak synaptic potentiation into persistent LTP (Kemp and Manahan‐Vaughan, 2005). Moreover, afferent stimulation in frequency ranges that correspond to θm (Bienenstock et al., 1982; Dudek and Bear, 1993), that can be considered to be ambiguous, as they are too fast to induce lasting LTD and too slow to induce lasting LTP, result in LTD if 5‐HT4‐receptors are antagonised and LTP if 5‐HT4‐receptors are activated (Kemp and Manahan‐Vaughan, 2005). These findings indicate that the 5‐HT4‐receptor exhibits frequency‐dependent properties, thereby regulating the direction of change of synaptic strength. Given the distinct functional roles of LTP and LTD in spatial memory (Kemp and Manahan‐Vaughan, 2004, 2007, 2008; Etkin et al., 2006; Hagena and Manahan‐Vaughan, 2011; Goh and Manahan‐Vaughan, 2012a), 5‐HT4‐receptors may thus also influence the encoding and content of information stored. In line with this, it has been reported that in the CA1 region, 5‐HT4‐receptor activation prevents learning‐induced facilitation of LTD (Kemp and Manahan‐Vaughan, 2004), and activation of 5‐HT4‐receptors prevents depotentiation of LTP in both CA1 and dentate gyrus (DG) (Kulla and Manahan‐Vaughan, 2002). This suggests that 5‐HT4‐receptors may serve to prioritise synaptic encoding through LTP, at the expense of LTD.
Here, we examined the role of the 5‐HT4‐receptor in synaptic plasticity at perforant path‐dentate gyrus synapses and mf‐CA3 synapses of freely behaving adult rats. We observed that in the DG, using ligand doses that do not affect basal synaptic transmission, receptor activation prevents LTD (>24 h), whereas LTP is insensitive to 5‐HT4‐receptor manipulation. In contrast, in mf‐CA3 synapses, receptor activation prevents both, LTP and LTD. In the CA1 region, 5‐HT4‐activation promotes LTP and prevents LTD (Kemp and Manahan‐Vaughan, 2004). Together, these data suggest that 5‐HT4‐receptors play a pivotal role in regulating synaptic information encoding in the hippocampus. When activated, this receptor will drive hippocampal synapses towards favouring synaptic encoding through LTP, and suppress persistent encoding at mossy fiber synapses, and thus strongly influence the content and encoding of stored information.
MATERIALS AND METHODS
Surgery
Seven‐to eight week old male Wistar rats (Charles River, Germany) were prepared as described previously (Manahan‐Vaughan and Reymann, 1997; Hagena and Manahan‐Vaughan, 2011). Briefly, under sodium pentobarbital anaesthesia (52 mg/kg, i.p., Merial, Halbermoos, Germany), animals underwent implantation of a monopolar recording and a bipolar stimulation electrode. A cannula was implanted into the lateral cerebral ventricle that was ipsilateral to electrode placements (0.5 mm posterior to bregma, 1.6 mm lateral to the midline; size: 5.6 mm length, 0.8 mm diameter, 4.5 mm depth).
For DG recordings, the stimulation electrode was implanted into the medial perforant path (6.9 mm posterior to bregma and 4.1 mm lateral to the midline) and a recording electrode was placed in the granule cell layer of the DG (3.1 mm posterior to bregma and 1.9 mm lateral to the midline). The correct placement of the electrodes was verified using electrophysiological criteria that distinguish medial perforant path‐DG potentials from lateral perforant path‐DG potentials (Abraham & McNaughton, 1984). For mossy fiber (mf)‐CA3 recordings, a bipolar stimulation electrode was implanted at coordinates corresponding to mf projections (3.5 mm posterior to bregma and 2.5 mm lateral to the midline) and a recording electrode was placed above the CA3 pyramidal layer of the dorsal hippocampus (3.0 mm posterior to bregma and 3.2 mm lateral to the midline). After surgery, the animals were housed in single cages. Animals were treated pre‐ and postoperatively with the analgesic Meloxicam (“Metacam,” 0.2 mg/kg, i.p., Boehringer Ingelheim, Ingelheim am Rhein, Germany), and the skull incision was treated both with local anaesthetic (lidocaine) and a topical antibiotic. The animals were allowed 7–10 days to recover from surgery before experiments were conducted. Post‐mortem histological analysis was also conducted to verify correct electrode placement.
Measurement of Evoked Potentials
The field excitatory post‐synaptic potential (fEPSP) slope was employed as a measure of excitatory synaptic transmission in the CA3 region, and was detected from maximal slope through the five steepest points obtained on the first negative deflection of the potential. To assess synaptic activity in the DG, analysis of both the population spike (PS) amplitude and fEPSP slope was conducted. PS amplitude was measured from the peak of the first positive deflection of the potential to the peak of the subsequent negative deflection. The fEPSP was measured as the maximal slope through the five steepest points obtained on the first positive deflection of the potential.
To obtain these measurements, an evoked response was generated by stimulating at low frequency (0.025 Hz) with single biphasic square wave pulses of 0.2 ms duration per half wave, generated by a constant current isolation unit. On the morning of each experiment, by means of input/output curve (evaluation of evoked responses elicited by increasing stimulation in steps from 100 to 900 µA) the maximum response was found, and during experiments, all potentials employed as baseline criteria were evoked at a stimulus intensity that produced 40% of this maximum. For each time‐point measured during the experiments, five records of evoked responses were averaged. The first six time‐points recorded at 5 min interval were used as baseline and all time‐points were calculated as a percentage of the average of these six points.
Since, in the DG experiments, the changes in fEPSP corresponded with changes in PS amplitude, fEPSP data are shown in Figure 1 only. Cortical EEG was continuously monitored throughout the experiments. Here, no disturbances were identified as being provoked by the experimental protocols.
Figure 1.

5‐HT4‐receptor ligands neither affect basal synaptic transmission at perforant path‐DG synapses, nor at mossy fiber synapses in vivo. (A) and (B) Test‐pulse stimulation in vehicle‐injected animals showed no change in evoked responses. Injection of RS67333 (10 µg) or RS39604 (50 µg), had no effect on test‐pulses recorded for 24 h at perforant path‐DG and mf–CA3 synapses, respectively. The time‐point of vehicle/ligand treatment is indicated by the arrow. Line breaks indicate change in time scale. (C), Analogs represent perforant path responses recorded during an control experiment (upper traces), in an experiment in which RS67333 was injected (middle traces) and in an experiment in which RS39604 was injected (bottom traces). Traces were taken pre‐stimulation (i), 5 min post‐stimulation (ii) and 24 h post‐stimulation (iii). Vertical scale bar: 2 mV, horizontal scale bar: 8 ms. (D), Analogs represent mossy fiber responses that were taken during an control experiment (upper traces), in an experiment in which RS67333 was injected (middle traces) and in an experiment in which RS39604 was injected (bottom traces). Traces were taken pre‐stimulation (i), 5 min post‐stimulation (ii) and 24 h post‐stimulation (iii). Vertical scale bar: 2 mV, horizontal scale bar: 8 ms.
LTP in the CA3 region was evoked using high‐frequency stimulation (HFS, four trains with 100 pulses at 100 Hz). This protocol induces robust LTP in this structure that lasts for over 24 h (Manahan‐Vaughan et al., 1998; Hagena and Manahan‐Vaughan, 2012). In the DG, LTP was evoked using a higher frequency of HFS (200 Hz given as 10 bursts of 15 pulses, with an interburst interval of 10s) (Manahan‐Vaughan et al., 1998). Short‐term potentiation (STP) was evoked using three bursts of 15 pulses at 200 Hz with an interburst interval of 10s (wHFS) (Manahan‐Vaughan and Reymann, 1996). Low‐frequency stimulation (LFS) at 1 Hz was used to evoked LTD (900 pulses), whereas 1 Hz stimulation given 600 times (wLFS) was applied to induce STD (Klausnitzer et al., 2004). To test the effect of afferent stimulation at Θm frequencies, 10 Hz LFS comprising 450 pulses at 10 Hz, or 5 Hz stimulation (450 pulses) was used.
Following HFS or LFS, three recordings at 5 min intervals were made, following recordings at 15 min intervals until 4 h had elapsed. A further 1 h of recordings were made 24 h later.
Pharmacological Treatment
The 5‐HT4‐receptor agonist 1‐[4‐Amino‐5‐chloro‐2‐methoxyphenyl]‐3‐[1‐butyl‐4‐ piperidinyl]‐1‐propanone; RS67333 and the 5‐HT4‐receptor antagonist 1‐[4‐Amino‐5‐chloro‐2‐(3,5‐dimethoxyphenyl)methyloxy]‐3‐[1‐[2‐methylsulphonylamino]ethyl]piperidin‐4‐yl]propan‐1‐one; RS39604 (Biotrend, Germany) were dissolved in double‐distilled water. Thirty minutes prior to, or 2 h after LFS or HFS, a volume of 5 µl was injected via the implanted cannula over a period of 5 min. Animals in control experiments received 0.9% NaCl. Strain‐dependent differences in the sensitivity of neurotransmitter receptors to pharmacological manipulation have been reported (Manahan‐Vaughan and Braunewell, 2005). Furthermore, intrastrain differences (Hilgers et al., 1985) impact on the robustness of synaptic plasticity that is elicited by equivalent experimental conditions (Ranson et al., 2012). In a previous study we reported that intracerebral application of 10 µg of RS67333 reduces fEPSPs evoked by test‐pulse stimulation in Wistar rats of the Schönwalde substrain. Here, we chose to use the same dose of agonist in Charles River Wistar rats, because it had no effect on PS or fEPSP profiles in the current study (Fig. 1). In a previous study we showed that intracerebral application of 25µg of the antagonist RS39604 significantly prevents the effects of RS67333 on synaptic transmission in the DG, but that 50 µg of the agonist is required to facilitate STD into LTD in the CA1 region in vivo (Kemp and Manahan‐Vaughan, 2005). Here, we chose to use the dose of 50 µg, so that we could compare effects between the DG and CA3 regions.
Data Analysis
The results were expressed as the mean percentage ± standard error of the mean (S.E.M.). For analysis of differences between groups (between factor), a two‐way, or factorial analysis of variance (ANOVA) with repeated measures was applied. Statistical differences between individual time‐points were assessed using a post‐hoc Student's t‐test. The level of significance was set at P < 0.05.
RESULTS
5‐HT4‐Receptor Ligands Have No Effect on Basal Synaptic Transmission at Perforant Path‐Dentate Gyrus, or at Mossy Fiber Synapses In Vivo
In order to exclude that possible effects of 5‐HT4‐receptor ligands on synaptic plasticity were mediated by direct effects on excitability, we assessed basal synaptic transmission. Neither the 5‐HT4‐agonist, RS67333 (10 µg, n = 6), nor the antagonist RS39604 (50 µg, n = 6), affected field excitatory postsynaptic potentials (fEPSPs) evoked by test‐pulse stimulation at perforant path‐DG synapses compared to vehicle‐treated controls (n = 6).
(ANOVA; fEPSP slope F 2,15 = 0.16, not significant (NS); interaction effect: F 44,330 = 0.81, NS; PS amplitude: F 2,15 = 1.90, NS; interaction effect: F44 ,330 = 0.77, NS; Figs. 1A,C).
Similarly, using the same ligand doses, no effect of the same doses of the agonist (n = 4), or the antagonist (n = 4) was observed with regard to basal synaptic transmission of the mossy fibers compared to animals that received vehicle (n = 4), (ANOVA: F2 ,8 = 1.15, NS; interaction effect: F 44,176 = 1.1, NS; Figs. 1B,D).
Agonist Activation of 5‐HT4‐Receptors Prevents Dentate Gyrus LTD, Does Not Affect Weak Potentiation (< 2 h) and Curtails LTP (> 24 h) In Vivo
In the DG of control animals (n = 7), LFS of perforant path (pp)‐DG synapses at 1 Hz (900 pulses) resulted in robust LTD that lasted for at least 24 h in vehicle‐treated animals (Figs. 2A–C). Treatment with the 5‐HT4‐receptor agonist, RS67333 (10 µg, n = 7), significantly impaired LTD (PS amplitude: F 1,12 = 22.15; P < 0.01; interaction effect: F 22,264 = 2.09; P < 0.01; fEPSP slope: F 1,12 = 11.87; P < 0.01; interaction effect: F 22,264 = 0.84, NS; Figs. 2A–C).
Figure 2.

Agonist activation of 5‐HT4‐receptors prevents dentate gyrus LTD and curtails LTP (>24 h), but does not affect short‐term potentiation in the DG. (A) and (B) Low‐frequency stimulation (LFS, 900 pulses at 1 Hz) resulted in LTD that lasted for over 24 h in the DG of vehicle‐injected animals. Application of RS67333 (50 µg), 30 min prior to stimulation led to a significant inhibition of both LTD of (A) the PS and of (B) the field excitatory postsynaptic potential (fEPSP). The time‐points of vehicle/agonist treatment, and of LFS are indicated by the arrows. Line breaks indicate change in time scale. (C) Left traces show analogs recorded (i) 5 min pre‐LFS, (ii) 5 min post‐LFS and (iii) 24 h post‐LFS from a control animal. Right traces show analogs recorded at the same time‐points from an animal that received RS67333. Vertical scale bar: 2 mV, horizontal scale bar: 8 msec. (D) Weak high‐frequency stimulation (wHFS, 3 bursts of 200 Hz) resulted in STP in vehicle‐injected animals. RS67333 had no effect on STP. (E) The stronger high‐frequency stimulation (HFS) protocol comprising 200 Hz, given 10 times, resulted in a robust form of LTP that lasted for over 24 h. Application of RS67333 curtailed LTP compared to vehicle‐treated controls, although potentiation was still evident for almost 4 h after HFS. The time‐points of vehicle/agonist treatment, and of wHFS/HFS application are indicated by the arrows. Line breaks indicate change in time scale. (F) Analog traces were recorded (i) 5 min pre‐wHFS, (ii) 5 min post‐wHFS and (iii) 24 h post‐wHFS from an animal that received vehicle (left traces) and from an animal that received RS67333 (right traces). Vertical scale bar: 2 mV, horizontal scale bar: 8 msec. (G) Analog traces were recorded (i) 5 min pre‐HFS, (ii) 5 min post‐HFS and (iii) 24 h post‐HFS from an animal that received vehicle (left traces) and from an animal that received RS67333 (right traces). Vertical scale bar: 2 mV, horizontal scale bar: 8 msec.
In a previous study, we reported that the same dose of RS67333 alters the maintenance of weak potentiation in the DG of freely behaving rats, such that a robust form of LTP results that lasts for over 24 h (Kulla and Manahan‐Vaughan, 2002). Here, we explored if weaker forms of LTP are sensitive to agonist activation of 5‐HT4‐receptors using the same agonist and dose. We observed that potentiation that lasts for ca. 2 h is unaffected by 5‐HT4‐receptor activation (n = 6) (PS amplitude: F 1,10 = 0.14, NS; interaction effect: F 22,220 = 0.61, NS; fEPSP slope: F 1,10 = 0.84, NS; interaction effect: F 22,220 = 1.60; P < 0.05, compared to vehicle–treated controls, n = 6; Figs. 2D,F). We also assessed the effect of the agonist on a more robust form of LTP that persisted for over 24 h. Here, similar to previous findings (Kulla and Manahan‐Vaughan, 2002), the persistency of LTP was significantly curtailed by 5‐HT4‐receptor activation (n = 7) (PS amplitude: F 1,12 = 7.55; P < 0.05; interaction effect: F 22,264 = 1.79; P < 0.05; fEPSP slope: F 1,12 = 8.04; P < 0.05; interaction effect: F 22,264 = 1.74; P < 0.05, compared to vehicle–treated controls, n = 7; Figs. 2D,G).
Antagonism of 5‐HT4‐Receptors Strengthens Weak Synaptic Depression in the Dentate Gyrus But Has No Effect on Weak Potentiation
We observed that activation of 5‐HT4‐receptors prevented LTD but not LTP (< 4 h) in pp‐DG synapses. Therefore, we explored whether synaptic plasticity in this hippocampal subfield is affected by antagonism of these receptors. We induced short‐term depression (STD) in vehicle–treated animals (n = 9) by giving 1 Hz weak LFS (wLFS) at 600 pulses (Figs. 3A,D). Short‐term depression was sustained for ca. 60 min compared to test‐pulse stimulated controls (n = 9) (PS amplitude: F 1,16 = 56.93; P < 0.001; interaction effect: F 5,80 = 2.39; P < 0.05; fEPSP slope: F 1,16 = 7.69; P < 0.05; interaction effect: F 5,80 = 0.77, NS). Treatment with the 5‐HT4‐receptor antagonist, RS39604 (50 µg, n = 9), strengthened the synaptic depression such that 24 h after wLFS, LTD was still evident (PS amplitude: F 1,16 = 31.67; P < 0.001; interaction effect: F 22,352 = 4.06; P < 0.01; fEPSP slope: F 1,16 = 6.98; P < 0.05; interaction effect: F 22,352 = 2.24; P < 0.01; Figs. 3A,D).
Figure 3.

Antagonism of 5‐HT4‐receptors facilitates short‐term depression into LTD in the DG, but has no effect on short‐term and long‐term potentiation. (A) Weak low‐frequency stimulation (wLFS, 600 pulses at 1 Hz) resulted in STD in the DG of vehicle‐injected animals. Application of RS39604, 30 min prior to stimulation facilitated LTD. (B) High‐frequency stimulation (HFS, 15 trains of 200 Hz) resulted in LTP lasting for at least 24 h. Application of RS39604 had no effect on LTP. (C) Weak high‐frequency stimulation (wHFS, 3 bursts of 200 Hz) leads to STP in vehicle‐injected animals. Application of RS39604 had no effect on STP compared to controls. The time‐points of vehicle/antagonist treatment, and of wLFS/wHFS/HFS application are indicated by the arrows. Line breaks indicate change in time scale. (D) Left traces show analogs recorded from a control animal, right traces show analogs recorded at the same time‐points from an animal that received RS39604 (i) 5 min pre‐wLFS, (ii) 5 min post‐wLFS and (iii) 24 h post‐wLFS. Vertical scale bar: 2 mV, horizontal scale bar: 8 msec. (E) Traces show analogs of evoked responses in the DG recorded (i) 5 min‐pre stimulation, (ii) 5 min post‐stimulation and (iii) 24 h post‐stimulation in a vehicle‐injected animal (left traces), in an animal that received RS39604 (right traces) Vertical scale bar: 2 mV, horizontal scale bar: 8 msec. (F) Left traces show analogs recorded from an animal that received vehicle, right traces show analogs recorded at the same time‐points from an animal that received RS39604 (i) 5 min pre‐wHFS, (ii) 5 min post‐wHFS and (iii) 24 h post‐wHFS. Vertical scale bar: 2 mV, horizontal scale bar: 8 msec.
We then tested effects on persistent LTP in pp‐DG synapses that lasts for over 24 h. Here, no change in the profile of LTP was seen when the 5‐HT4‐receptor antagonist (50 µg, n = 6) was applied (PS amplitude: F 1,10 = 0.54, NS; interaction effect F 1,22 = 1.29, NS; fEPSP slope: F 1,10 = 3.96, NS; interaction effect: F 1,22 = 1.12, NS; Figures 3B,E compared to vehicle‐treated controls, n = 6).
Similarly, weak potentiation that lasted for ca. 2 h in controls (n = 6) was unaffected by the antagonist (50 µg, n = 6) (ANOVA: PS amplitude: F 1,10 = 4.0, NS; interaction effect: F 22,220 = 0.68, NS; fEPSP slope: F 1,10 = 0.35, NS; interaction effect: F 22,220 = 1.61; P < 0.05; Figs. 3C,F).
5‐HT4 Receptor Activation in the DG Has No Effect on Established LTD But Curtails Established LTP
Neurotransmitter receptors that couple to adenylyl cyclase (AC), such as dopaminergic receptors, have been reported to modulate late phases of LTP (Frey et al., 1989, 1990). Although typically, effects are most potent when a ligand of, e.g., dopamine receptors is present during the tetanus Frey et al., 1991), there are suggestions that the modulation of the cAMP/PKA‐dependent cascade through AC‐coupled neurotransmitter receptors may influence late associative processing that contributes to the stabilisation of plasticity phenomena (Sajikumar and Frey, 2004). Therefore, having observed that 5‐HT4 receptor activation in the DG (prior to, and during, HFS) significantly prevents late LTP (LTP > 24 h), but does not affect weak potentiation (LTP < 2 h) (Fig. 2), we explored whether agonist activation of 5‐HT4 receptors has any effect on established LTP and LTD.
Thus, we applied the 5‐HT4 receptor after LTP or LTD had stabilised. The stimulation protocols used were those that elicit LTP, or LTD, that last for over 24 h. The 5‐HT4 agonist RS67333 (10 µg) was injected 2 h after HFS (n = 5) or LFS (n = 6). Compared to vehicle‐injected controls, LTD was not affected by 5‐HT4 receptor activation (ANOVA; fEPSP slope F 1,10 = 0.12, NS; interaction effect: F 22,220 = 1.18, NS; PS amplitude F 1,10 = 0.09, NS; interaction effect F 22,220 = 1.11, NS; Figs. 4A–C). Interestingly, a mild impairment of LTP was evident in agonist‐treated animals compared to controls (ANOVA; fEPSP slope F 1,8 = 4.66, NS; interaction effect: F 22,176 = 1.4, NS; PS amplitude F 1,8 = 1.37, NS; interaction effect: F 22,176 = 1.69; P < 0.05) Figs. 4D–F). Nonetheless, LTP persisted for over 24 h in the drug‐treated group.
Figure 4.

5‐HT4 receptor activation in the DG has no effect on established LTD and weakly affects established LTP. (A,B) Low‐frequency stimulation (LFS, 900 pulses with 1 Hz) resulted in LTD in the DG of vehicle‐injected animals. Injection of RS67333 2 h after stimulation elicited no change in evoked responses compared to control animals. The time‐points of vehicle/agonist treatment, and of LFS application are indicated by the arrows. Line breaks indicate change in time scale. (C) Left traces show analogs of the fEPSP slope and PS amplitude recorded from an animal that received a vehicle injection, right traces depict analog responses from an animal that was injected with the 5‐HT4 receptor agonist RS67333 (i) 5 min pre‐LFS, (ii) 5 min post‐LFS and (iii) 24 h post‐LFS. Vertical scale bar: 2 mV, horizontal scale bar: 8 msec. (D,E) High‐frequency stimulation (HFS, 15 trains of 200 Hz) resulted in persistent LTP in vehicle‐injected animals. Injection of RS67333 2 h after HFS elicited only a mild depression of evoked responses. LTP remained intact however. The time‐points of vehicle/agonist treatment, and of HFS application are indicated by the arrows. Line breaks indicate change in time scale. (F) Analog responses of the fEPSP slope and PS amplitude were recorded (i) 5 min pre‐HFS, (ii) 5 min post‐HFS and (iii) 24 h post‐HFS from an animal that received vehicle (left traces) and from the same animal that received RS67333 (right traces). Vertical scale bar: 2 mV, horizontal scale bar: 8 msec.
Stimulation of Dentate Gyrus Synapses Using Frequencies That Are Close to Θm, Do Not Affect Evoked Responses
In Schaffer collateral‐Stratum radiatum synapses of the CA1 region in vivo, afferent stimulation at 10 Hz (450 pulses) neither results in LTP nor LTD (Kemp and Manahan‐Vaughan, 2005). However, agonist activation of 5‐HT4‐receptors prior to this stimulation, results in the expression of robust LTP, whereas antagonism of the receptors results in robust LTD (Kemp and Manahan‐Vaughan, 2005). Here, we explored if similar properties are evident in the DG.
In contrast to CA1 synapses, 10 Hz stimulation of the perforant path (450 pulses) resulted in an initial depression of synaptic responses in the DG (n = 6), that was followed by synaptic potentiation (ANOVA for time‐points 1‐8: PS amplitude: F 1,10 = 5.04; P < 0.05; interaction effect: F 8,80 = 4.44; P < 0.001; fEPSP slope: F 1,10 = 3.79, NS; interaction effect: F 8,80 = 3.45; P < 0.01; for time‐points 9‐22: PS amplitude: F 1,10 = 5.40; P < 0.05; interaction effect: F 13,130 = 2.80; P < 0.001; fEPSP slope: F 1,10 = 4.53, NS; interaction effect: F 13,130 = 2.91; P < 0.001; Figs. 5A,C).
Figure 5.
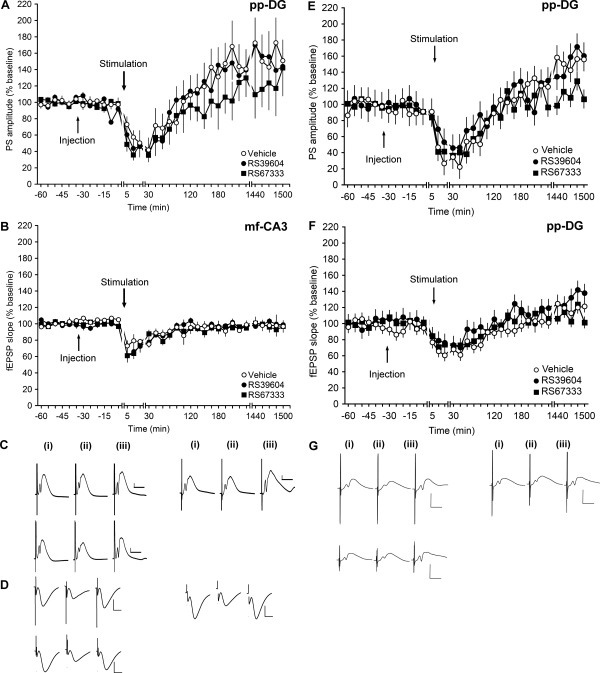
5‐HT4‐receptor manipulation has no effect on evoked responses elicited by afferent stimulation at Θm‐frequencies of perforant path‐DG or mossy fiber‐CA3 synapses. (A) In the DG of vehicle‐treated animals, 10 Hz stimulation led to an initial depression that was followed by synaptic potentiation ca. 2 h after 10 Hz stimulation was applied. Neither the application of the 5‐HT4‐receptor agonist RS67333 nor the 5‐HT4‐receptor antagonist RS39604 modified the evoked responses. (B) In vehicle‐treated animals, 10 Hz stimulation resulted in STD in mossy fiber(mf)‐CA3 synapses. Evoked responses were unaffected by treatment with the 5‐HT4‐receptor agonist RS67333, or the 5‐HT4‐receptor antagonist, RS39604. The time‐points of vehicle/ligand treatment, and of 10 Hz stimulation are indicated by the arrows. Line breaks indicate change in time scale. (C) Traces show analogs of the fEPSP slope and PS amplitude, respectively recorded (i) 5 min‐pre stimulation, (ii) 5 min post‐stimulation and (iii) 24 h post‐stimulation in a vehicle‐injected animal (top left traces), in an animal that received RS39604 (top right traces) and an animal that was injected with RS6733 (lower left traces). Vertical scale bar: 2 mV, horizontal scale bar: 8 msec. (D) Traces show analogs recorded (i) 5 min‐pre stimulation, (ii) 5 min post‐stimulation and (iii) 24 h post‐stimulation in a vehicle‐injected animal (top left traces), in an animal that received RS67333 (top right traces) and an animal that was injected with RS39604 (lower left traces). Vertical scale bar: 2 mV, horizontal scale bar: 8 msec. (E,F) Compared to responses evoked in the DG of vehicle‐treated animals, changes in evoked potentials that were elicited by 5 Hz stimulation were unaffected by the application of the 5‐HT4‐receptor agonist RS67333, or the 5‐HT4‐receptor antagonist RS39604. (G) Traces show analogs of the fEPSP slope and PS amplitude recorded (i) 5 min‐pre stimulation, (ii) 5 min post‐stimulation and (iii) 24 h post‐stimulation in a vehicle‐injected animal (top left traces), in an animal that received RS39604 (top right traces) and an animal that was injected with RS6733 (lower left traces). Vertical scale bar: 2 mV, horizontal scale bar: 8 msec.
Treatment with either the 5‐HT4‐receptor agonist, RS67333 (10 µg, n = 6), or the antagonist RS39604 (50 µg, n = 6), failed to change the profile of responses elicited by 10 Hz (ANOVA: PS amplitude: F 2,15 = 0.61, NS; interaction effect: F 44,330 = 0.83, NS; fEPSP slope: F 2,15 = 0.50, NS; interaction effect: F 44,330 = 0.86, NS; Figs. 5A,C).
To clarify if the lack of effect related to the dose of the ligand used, we increased the dose of the agonist to 20 µg (n = 6), but this treatment also failed to change the profile of responses elicited with 10 Hz LFS (not shown) (ANOVA for fEPSP: F 1,10 = 0.10, NS; interaction effect. F 22,220 = 1.09, NS; PS slope: F 1,10 = 0.17, NS; interaction effect: F 22,220 = 0.80, NS).
Similar results were observed when we increased the dose of the antagonist to 100 µg (n = 6) (not shown) (ANOVA for fEPSP: F 1,10 = 0.34, NS; interaction effect: F 22,220 = 0.79, NS; PS amplitude: F 1,10 = 0.54, NS; interaction effect: F 22,220 = 0.88, NS).
The 10 Hz protocol elicited more robust effects on synaptic responses than those seen in CA1 synapses (Kemp and Manahan‐Vaughan, 2004). We cannot exclude the possibility that this protocol may have been to “near” to the threshold for inducing LTP at pp‐DG synapses. Thus, we repeated the experiments, but this time used 5 Hz stimulation (450 pulses). We chose this protocol because we had previously observed that this has no lasting effect on synaptic strength, but under specific circumstances can serve to depotentiate LTP (Kulla and Manahan‐Vaughan, 2008).
Here, LFS at 5 Hz (450 pulses, n = 7) also resulted in an initial depression followed by a potentiation of DG responses (ANOVA: for timepoint 1‐8: fEPSP slope F 1,12 = 23.65; P < 0.001; interaction effect: F 7,84 = 2.34; P < 0.05; PS amplitude F 1,12 = 36.43; P < 0.001; interaction effect F 7,84 = 3.35; P < 0.01; for time‐point 9‐22: fEPSP slope treatment effect: F 1,12 = 0.06, NS; interaction effect: F 14,168 = 4.06; P < 0.001; Figs. 5E–G (baseline data not shown)).
In the presence of the 5‐HT4 receptor agonist, RS67333, or antagonist, RS39604, the response to 5 Hz stimulation did not differ from vehicle‐treated controls (n = 7 for both groups) (ANOVA: fEPSP slope F 2,18 = 0.99, NS; interaction effect: F 44,396 = 0.82, NS; PS amplitude F 2,18 = 0.62, NS; interaction effect: F 44,396 = 1.02; P = 0.45, Figs. 5E–G). Thus, in contrast to the CA1 region in vivo (Kemp and Manahan‐Vaughan, 2005). Modulation of the activation state of the 5‐HT4‐receptor has no influence on the direction of change in synaptic strength in the DG in vivo that is elicit at afferent frequencies in the range of Θm.
Agonist Activation of 5‐HT4‐Receptors Prevents Both Mossy Fiber LTD And Mossy Fiber LTP In the CA3 Region In Vivo
Given the striking difference in 5‐HT4 receptor‐mediated regulation of LTP and LTD in the CA1 (Kemp and Manahan‐Vaughan, 2005) and DG subregions (Figs. 2, 3, 4, 5), we were interested to clarify to what extent 5‐HT4 receptors influence synaptic information processing in the CA3 region. Here, we examined the mossy fiber (mf)‐CA3 synapses.
In vehicle‐treated animals (n = 7), 1 Hz LFS (900 pulses) resulted in robust LTD that lasted for at least 24 h (Figs. 6A,C). Treatment with the 5‐HT4‐receptor agonist, RS67333 (10 µg, n = 4), significantly impaired LTD (ANOVA: F 1,3 = 18.993; P < 0.05; interaction effect: F 22,66 = 11.013; P < 0.001; Figs. 6A,C).
Figure 6.

Agonist activation of 5‐HT4‐receptors prevents both mossy fiber LTD and mossy fiber LTP, whereas antagonism strengthens weak synaptic depression and weak synaptic potentiation. (A) Low‐frequency stimulation (LFS, 900 pulses at 1 Hz) resulted in LTD that lasted for over 24 h in vehicle‐injected animals. Application of RS67333, 30 min prior to stimulation led to a significant inhibition of evoked responses. (B) High‐frequency stimulation (HFS, 4 × 100 pulses at 100 Hz) facilitated LTP for over 24 h in vehicle‐injected animals. Application of RS67333 significantly inhibited LTP at mf–CA3 synapses. The time‐points of vehicle/agonist treatment, and of LFS/HFS application are indicated by the arrows. Line breaks indicate change in time scale. (C) Left traces show fEPSP analogs recorded (i) 5 min pre‐LFS, (ii) 5 min post‐LFS and (iii) 24 h post‐LFS from a control animal. Right traces show analogs recorded at the same time‐points from an animal that received RS67333. Vertical scale bar: 2 mV, horizontal scale bar: 8 msec. (D) Analog traces were recorded (i) 5 min‐pre‐HFS, (ii) 5 min post‐HFS and (iii) 24 h post‐HFS from an animal that received vehicle (left traces) and from an animal that received RS67333 (right traces). Vertical scale bar: 2 mV, horizontal scale bar: 8 msec. (E) Weak low‐frequency stimulation (wLFS, 600 pulses at 1 Hz) resulted in STD in control animals. Injection of RS39604 facilitated LTD that lasted for over 24 h. (F) Weak high‐frequency stimulation (wHFS, 2 × 100 pulses at 100 Hz) lead to STP in vehicle‐injected animals. Application of RS39604 facilitated LTP for over 24 h. The time‐points of vehicle/antagonist and of wLFS/wHFS application are indicated by the arrows. Line breaks indicate change in time scale. (G) Left analog traces show fEPSPs recorded (i) 5 min pre‐wLFS, (ii) post‐wLFS and (iii) 24 h post‐wLFS from control animals and from animals that received an injection of RS39604 (right traces). Vertical scale bar: 2 mV, horizontal scale bar: 8 msec. (H) Left analog traces show fEPSPs recorded (i) 5 min pre‐wHFS, (ii) post‐wHFS and (iii) 24 h post‐wHFS from control animals and from animals that received an injection of RS39604 (right traces). Vertical scale bar: 2 mV, horizontal scale bar: 8 msec.
HFS of mf‐CA3 synapses of control animals (n = 7) at 100 Hz (4 trains of 100 pulses at 100 Hz) resulted in robust LTP that persisted for at least 24 h (Figs. 6B,D). Strikingly, treatment with the 5‐HT4‐receptor agonist, RS67333 (10 µg, n = 4), also significantly impaired LTP (ANOVA: F 1,3 = 11.47; P < 0.05; interaction effect: F 22,88 = 3.41; P < 0.001; Figs. 6B,D). Thus, in contrast to pp‐DG synapses, both LTD and LTP are impaired by agonist activation of 5‐HT4‐receptors.
Antagonism of 5‐HT4‐Receptors Strengthens Weak Synaptic Depression and Weak Synaptic Potentiation at mf‐CA3 Synapses
Given the fact that we found that agonist activation of 5‐HT4‐receptors prevents mf‐CA3 LTP and LTD, we explored if antagonism of the receptors could strengthen weak potentiation or weak depression. Vehicle‐treated animals (n = 4) received 1 Hz weak LFS (wLFS) at 600 pulses (Figs. 6E,G) to induce STD. Injection of the 5‐HT4‐receptor antagonist, RS39604 (50 µg, n = 4), facilitated STD into LTD that lasted for over 24 h (ANOVA: F 1,6 = 18.89; P < 0.01; interaction effect: F 22,132 = 1.77; P < 0.05; n = 4; Figs. 6E,G).
To evaluate the effect of 5‐HT4‐receptor antagonism on STP, RS39604 (50 µg) was injected in animals that received weak HFS (wHFS) consisting of 4 trains of 100 pulses at 100 Hz. In vehicle‐treated animals, wHFS resulted in STP that lasted for ca. 2 h. Application of RS39604 significantly strengthened STP, resulting in LTP that lasted for over 24 h (ANOVA: F 1,6 = 13.02; P < 0.05; interaction effect: F 22,132 = 1.34, NS; n = 4; Figs. 6F,H).
5‐HT4‐Receptor Activation Has No Effect on Established Persistent Plasticity at mf‐CA3 Synapses
Given the strong regulation by 5‐HT4‐receptors of LTP and LTD at mf‐CA3 synapses, we explored whether receptor agonism could affect established LTP and LTD. Here, RS67333 (10 µg) was applied 2 h after patterned stimulation, using protocols that typically generate LTP and LTD that last for over 24 h. Application of the 5‐HT4‐receptor agonist RS67333, had no effect on the profile of LTD compared to controls (ANOVA: F 1,8 = 4.63, NS; interaction effect: F22,176 = 1.88; P < 0.05; n = 5; Figs. 7A,C). Similarly, agonist‐treatment with RS67333 (10 µg) applied 2 h after HFS, elicited LTP that was not significantly different from that observed in control animals (ANOVA: F 1,6 = 0.01, NS; interaction effect: F22,132 = 1.68; P < 0.05; n = 4; Figs. 7B,D).
Figure 7.

5‐HT4‐receptor activation has no effect on established persistent plasticity at mf‐CA3 synapses. (A) Robust LTD that lasted for over 24 h was observed in control animals that received a vehicle injection 2 h after low‐frequency stimulation (LFS, 1 Hz, 900 pulses). Injection of RS67333 2 h after stimulation, resulted in LTD that was not significantly different from responses observed in the control group. (B) Application of HFS (4 × 100 pulses at 100 Hz) resulted in LTP in animals that received vehicle 2 h after stimulation. Injection of RS67333 2 h after stimulation showed no change of the evoked responses. The time‐points of vehicle/agonist and of LFS/HFS application are indicated by the arrows. Line breaks indicate change in time scale. (C) Traces show analogs recorded (i) 5 min‐pre LFS, (ii) 5 min post‐LFS and (iii) 24 h post‐LFS in a vehicle‐injected animal (left traces), and in an animal that received RS67333 (right traces) Vertical scale bar: 2 mV, horizontal scale bar: 8 msec. (D) Traces show analogs recorded (i) 5 min‐pre HFS, (ii) 5 min post‐HFS and (iii) 24 h post‐HFS in a vehicle‐injected animal (left traces), and in an animal that received RS67333 (right traces) Vertical scale bar: 2 mV, horizontal scale bar: 8 msec.
Stimulation of mf‐CA3 Synapses Using Frequencies That Are Close to Θm, Do Not Affect Evoked Responses
To compare responses to those examined at pp‐DG synapses, we explored whether 5‐HT4‐receptor modulation affects synaptic responses that are elicited at frequencies that are close to Θm. Here, brief 10 Hz stimulation resulted in a potent, albeit transient depression of synaptic responses (Figs. 5B,D) that lasted for ca. 90 min. Treatment with either the 5‐HT4‐receptor agonist, RS67333 (10 µg, n = 4), or the antagonist RS39604 (50 µg, n = 4), failed to alter the profile of responses elicited by 10 Hz (ANOVA: F 2,9 = 0.22, NS; interaction effect: F 44,198 = 0.92, NS; (Figs. 5B,D)
DISCUSSION
The results of this study indicate that 5‐HT4‐receptors exert a highly specific regulation of the direction of change in synaptic strength in the hippocampus. We observed that when 5‐HT4‐receptors are activated, information encoding by means of LTD is suppressed in mossy fiber (mf‐)CA3 and perforant path (pp)‐DG synapses. Mf‐LTP is also inhibited, suggesting that activation of 5‐HT4‐receptors generally suppress long‐term information storage at mf‐CA3 synapses. By contrast DG‐LTP remains intact following 5‐HT4‐receptor activation, with only very long‐lasting forms of LTP being affected. These data suggest that 5‐HT4‐receptors may shape information storage across the hippocampal circuitry and specify the nature of experience‐dependent synaptic encoding.
This possibility is corroborated by the observation that, although LTD is prevented by activation of 5‐HT4‐receptors in all main subfields of the trisynaptic hippocampal circuitry (pp‐DG and mf‐CA3 synapses, as shown in this study, Schaffer collateral‐CA1 synapses as shown by Kemp and Manahan‐Vaughan, 2004, 2005), clear subregional differences in the regulation of LTP by 5‐HT4‐receptors are evident. At pp‐DG synapses, LTP‐inducing, afferent tetanisation in the presence of a 5‐HT4‐receptor agonist curtails late‐LTP (>24 h), without preventing LTP (>4 h) and completely prevents LTP at mf‐CA3 synapses, whereas at Schaffer collateral‐CA1 synapses LTP is unaffected by receptor activation. Further evidence for subfield‐specific and highly tuned regulation by 5‐HT4‐receptors, of hippocampal LTP, derives from observations with regard to the response of hippocampal synapses to pharmacological manipulations of these receptors during afferent stimulation in the range of Θm.
Θm (Bienenstock et al, 1982) refers to a specific level of afferent (and postsynaptic) activity that is too strong to elicit LTD, but too weak to elicit LTP, whereby the net outcome of the afferent stimulation is that no persistent changes in synaptic strength result (Dudek and Bear, 1993; Coussens and Teyler, 1996; Manahan‐Vaughan, 2000; Kemp and Manahan‐Vaughan, 2005). Here, ambiguous patterned afferent information (in the range of Θm) that is delivered to the CA1 region under naïve conditions, (i.e., is received by animals/synapses that have not experienced this information before), elicits no lasting change in synaptic strength (Kemp and Manahan‐Vaughan, 2005). This kind of activity results in LTP in the CA1 region, however, when it occurs during 5‐HT4‐receptor activation (Kemp and Manahan‐Vaughan, 2005). In the present study, we observed that 5‐HT4‐receptor activation had no effect on responses elicited by Θm frequency stimulation of mf‐CA3 or pp‐DG synapses (see also summary of effects in Table 1). Taken together with our observation that receptor activation prevents both LTP and LTD at mf‐CA3 synapses, but leaves DG LTP intact, this suggests, on the one hand that 5‐HT4‐receptor activation selectively vetoes synaptic information encoding at mf‐CA3 synapses, whilst promoting information encoding by means of LTP in the CA1 region and DG. On the other hand, the CA1 region appears to profit most from this kind of regulation, whereby afferent activation patterns that normally do not elicit synaptic plasticity, result in LTP if they coincide with 5‐HT4‐receptor activation.
Table 1.
Summary of the Regulation by 5‐HT4 Receptor Ligands of Synaptic Plasticity in the Hippocampus
| 5‐HT4 agonist | 5‐HT4 antagonist | |||||
|---|---|---|---|---|---|---|
| DG | MF‐CA3 | CA1 | DG | MF‐CA3 | CA1 | |
| LTP | Moderation of non‐ decremental LTP at MPP synapsesa | Blocksb | No effectc | No effectb | No effectc | |
| No effect on decremental LTP at MPP synapsesb | ||||||
| No effect on LTP at LPP‐synapsesd | ||||||
| STP | No effectb | No effectb | ||||
| Depotentiation | Blocksa | Blocksc | Acceleratesd | |||
| LTD | Blocksb | Blocksb | Blocksc, e | |||
| STD | Enhances to LTDb | Enhances to LTDc | ||||
| 10 Hz | No effectb | No effectb | Results in LTPc | No effectb | No effectb | Results in LTDc |
The table describes what is currently known about the effects of agonism or antagonism of 5‐HT4 receptors on synaptic plasticity in the hippocampus of freely behaving adult rats. What is striking is that activation of 5‐HT4‐receptors prevents LTD in all hippocampal subfields.c ,b, e LTP in the CA1 region is unaffected by 5‐HT4‐receptor ligands.b Non‐decremental LTP (>24 h) in the DG is moderated to a decremental form (>24 h),a whereas decremental LTP (>24 h) and STP (<2 h) are unaffected by agonist activation.b By contrast, activation of 5‐HT4‐receptors prevents LTP in the CA3 region.b In the CA1 region, afferent stimulation at a frequency that does not elicit a change in synaptic strength (10 Hz) in the presence of a 5‐HT4‐receptor agonist results in LTP, and in the presence of a 5‐HT4‐receptor antagonist results in LTD.c 5‐HT4‐receptor ligands have no effects on synaptic responses elicited at 10 Hz in the CA3 region or DG.b
Kulla and Manahan‐Vaughan, 2002.
Present study.
Kemp and Manahan‐Vaughan, 2005.
Marchetti et al., 2004.
Kemp and Manahan‐Vaughan, 2004.
MPP: Medial perforant path, LPP: Lateral perforant path.
In both CA1 and DG synapses, the activation of 5‐HT4‐receptors, using doses that do not affect basal synaptic transmission, elicits a very specific control over LTD, without affecting LTP (>4 h), suggesting that in both subfields, the influence of these receptors on synaptic plasticity is highly tuned to the regulation of LTD. This possibility is corroborated by observations that activation of 5‐HT4‐receptors dose‐dependently inhibits depotentiation of LTP at both DG synapses (Kulla and Manahan‐Vaughan 2002) and CA1 synapses (Kemp and Manahan‐Vaughan, 2005). Whereas we examined medial perforant path synapses, Marchetti et al. (2004) reported that in freely behaving rats, agonist activation of 5‐HT4‐receptors during tetanisation of lateral perforant path synapses had no effect on LTP, but antagonism of the receptor accelerated depotentiation. Depotentiation of LTP is highly sensitive to the timing of afferent stimulation, as well as to the activity state of the targeted neurons (Staubli and Lynch, 1990; Stäubli and Chun, 1996; Kulla et al., 1999). It serves to rapidly terminate a recently initiated synaptic potentiation. By suppressing LTD and preventing depotentiation, the 5‐HT4‐receptor can thus not only modulate the magnitude but also the duration, of LTP.
In line with this, we observed that in the DG, 5‐HT4‐receptor activation appears to promote LTP expression, whilst at the same time constraining it within appropriate physiological ranges: late LTP that lasts for at least 24 h is curtailed by receptor activation, whereas LTP that lasts for at least 4 h remains intact. We believe that this could comprise a mechanism whereby flexibility in the nature of information storage at pp‐DG synapses is supported. Behavioral data indicate that this may indeed be the case: in olfactory association tasks, injection of a 5‐HT4‐receptor antagonist at the start of task acquisition results in very robust procedural and associative memories (Marchetti et al., 2004), an effect that could reflect inflexible information storage. Conversely, application of a 5‐HT4‐receptor agonist after initial task acquisition enhances short‐term memory (Letty et al., 1997). Furthermore, receptor activation improves olfactory discrimination in both young and old rodents (Marchetti et al, 2004, 2011), a task that requires a high level of learning flexibility. In many reported behavioral studies, the precise timing of 5‐HT4‐receptor manipulation appears to be highly decisive in terms of promoting or hindering subsequent memory performance (Letty et al., 1997; Meneses and Hong, 1997; Roman and Marchetti, 1998; Marchetti et al, 2000, 2004). We observed that activating 5‐HT4‐receptors downstream of the induction of robust LTP, or LTD, had no impact on the stability of these plasticity forms. However, as mentioned above, the relative degree of afferent activation at the time of 5‐HT4‐receptor activation can impact potently on the direction of change of synaptic strength, and thus, putatively, on subsequent memory.
Results from biochemical and electrophysiological studies point to possible neurobiological mechanisms for the abovementioned effects. 5‐HT4‐receptor activation leads to hippocampal elevations in protein kinase A and cAMP in the rat hippocampus, and one of the consequences is closure of K+ channels (Bockaert et al., 1998). The resultant increase in neuronal excitability (Andrade and Chaput, 1991; Fagni et al., 1992; Ansanay et al., 1995; Torres et al., 1995) can be expected to alter thresholds for the induction or stabilization of synaptic plasticity in the hippocampus. Thus, it was surprising to find that activation of the receptor resulted in an inhibition of synaptic plasticity in the hippocampal subfields studied here, that was particularly potent at mf‐CA3 synapses. This may relate to regulation by 5‐HT4 receptors of inhibitory GABAergic transmission. Activation of 5‐HT4 receptors on GABAergic interneurons in the DG results in their depolarization (Bijak and Misgeld, 1997). Furthermore, 5‐HT4 receptor‐activation enhances GABA(A) and GABA(B) receptor‐mediated hyperpolarization of DG granule cells (Bijak and Misgeld, 1997), modulates GABA release in the hippocampus (Bianchi et al., 2002), and regulates GABA(A) receptors in an activity‐dependent manner (Cai et al., 2002). GABAergic neurons are also present at mf‐CA3 synapses (Vida and Frotscher, 2000) and their activation results in a decrease of synaptic responses in the CA3 region (Vogt and Nicoll, 1999). Thus, 5‐HT4‐receptor‐mediated elevations of GABAergic inhibition may comprise a more plausible explanation for the inhibitory effects of the agonist on synaptic plasticity in at mf‐CA3 and pp‐DG synapses observed in the present study. It could also explain why agonist activation had a weak impairing effect on LTP in the DG, and why receptor antagonism (reduction of inhibitory tonus) promotes both LTP and LTD.
Brain 5‐HT‐levels fluctuate through the circadian cycle, with levels being at their lowest during REM‐ sleep (Portas et al., 1998). 5‐HT levels are not particularly affected by arousal (Cape and Jones, 1998), rather a relatively constant 5‐HT basal tonus occurs throughout the waking cycle (Trulson and Jacobs, 1979; Portas et al., 1998), that can also be expected to occur in the hippocampus (Kawata et al., 2001; Kehr et al., 2001). Under physiological conditions, basal 5‐HT‐tonus may support metaplasticity (Abraham and Bear, 1996; Anraham and Tate, 1997), by ensuring that information encoding by means of synaptic plasticity remains within a dynamic functional range. Given its role in spatial memory (Kemp and Manahan‐Vaughan, 2004, 2007), it is unlikely that LTD is suppressed during normal basal 5‐HT‐tonus. In line with this, it has been proposed (in a simulation study) that strong LTP, coupled with weak LTD, will promote pattern completion, but exacerbate pattern separation, whereas weak LTP coupled with strong LTD will impair pattern completion, whilst improving pattern separation (Hanson and Madison, 2010). Thus, a functional balance between these forms of plasticity is likely to be necessary for effective information discrimination and storage in the hippocampus. Correspondingly, although LTD is tightly linked to the encoding of spatial content (Kemp and Manahan‐Vaughan, 2004, 2007, 2008; Dong et al., 2012; Goh and Manahan‐Vaughan, 2013b), excessive LTD is detrimental to hippocampal function (Ondrejcak et al., 2010). The impairment of LTD that is mediated by 5‐HT4‐receptor activation may thus support effective hippocampal information processing under very specific learning conditions. That said, suppression of LTD is not always a good thing: object‐place and spatial content learning that are tightly coupled to induction of hippocampal LTD, fail when LTD is prevented during learning (Kemp and Manahan‐Vaughan, 2007, 2008; Goh and Manahan‐Vaughan, 2012b, 2013b), and agonist activation of 5‐HT4‐receptors prevents both learning‐facilitation of LTD and the associated memory of the learning event (Kemp and Manahan‐Vaughan, 2004). Thus, 5‐HT4‐receptors may enable the establishment of a functional balance between LTP and LTD that facilitates the learning of different kinds of experiences and the retention of different components, or forms, of hippocampus‐dependent memory.
In line with this, it is becoming apparent that LTP and LTD may serve to encode different components of a cognitive representation. LTP occurs in tight association with novel spatial experience, particularly that which arises during changes in global space or scenery (Kemp and Manahan‐Vaughan, 2004, 2008; Hagena and Manahan‐Vaughan, 2011). In contrast, LTD is tightly associated with learning of the features or content of space (Kemp and Manahan‐Vaughan, 2007; Hagena and Manahan‐Vaughan, 2011). Activation of 5‐HT4‐receptors results in an inhibition of LTD‐related encoding, as seen in the present study for pp‐DG and mf‐CA3 synapses, and previously reported for the CA1 region (Kemp and Manahan‐Vaughan, 2004, 2005). This is coupled with a prioritisation of LTP‐related encoding in the DG (present study) and CA1(Kemp and Manahan‐Vaughan, 2005) (i.e., no agonist effect on LTP). 5‐HT4‐receptor activation also prevents depotentiation of LTP (Kulla and Manahan‐Vaughan, 2002; Kemp and Manahan‐Vaughan, 2005). Learning (and induction of synaptic plasticity) during 5‐HT4‐receptor activation would thus be expected to skew synaptic information storage in favour of memories that relate more to general spatial experience than the precise details of this experience. In line with this, 5‐HT4‐receptor activation improves learning of the platform position in the water maze (Fontana et al., 1997; Lelong et al., 2001), whereby a correlation between LTP and this kind of spatial memory has been suggested (Tsien et al., 1996; Moser et al., 1998). Furthermore, learning of object‐place associations is dependent upon LTD induction in both rats (Kemp and Manahan‐Vaughan, 2004, 2008) and mice (Goh and Manahan‐Vaughan, 2012a, 2012b, 2013b), and 5‐HT4‐receptor activation impairs this type of learning (Kemp and Manahan‐Vaughan, 2005).
Although 5‐HT4‐receptor activation prevents LTD and LTP in mf‐CA3 synapses, the 5‐HT4‐receptor does not modulate LTP in the DG and CA1 directly, rather it suppresses LTD and depotentiation, and moderates weaker forms of potentiation (Table 1). By suppressing LTD and depotentiation, two molecularly distinct phenomena (Lee et al., 2000), the 5‐HT4‐receptor acts permissively to support LTP‐related phenomena at DG synapses, without directly modulating LTP. In the CA1 region, a different picture emerges. Here, activation of 5‐HT4‐receptor changes the crossover‐point for LTD/LTP induction, θm (Bienenstock et al., 1982; Dudek and Bear, 1993), resulting in LTP expression at afferent frequencies that would normally not affect synaptic strength (Kemp and Manahan‐Vaughan, 2005). A bias, not only towards information encoding by means of LTP, but also towards information encoding in the CA1 region may thus be mediated by 5‐HT4‐receptor activation. This appears to occur to the disadvantage of information processing in mf‐CA3 synapses, whereby by 5‐HT4‐receptor activation suppresses persistent information encoding in the form of LTP or LTD.
Taken together, our data suggest that when 5‐HT4‐receptors are activated, learning that is mediated by LTP in the CA1 region and DG will be prioritised above learning that is supported by synaptic plasticity in mf‐CA3 synapses. A functional differentiation with regard to information processing has been postulated for the different hippocampal subfields. Whereas the DG may be important for the encoding of temporal relationships between spatial events (Morris et al., 2003), as well as spatial pattern separation (Kesner et al., 2004), the CA3 region may process information related to pattern completion, as well as short‐term memory (Kesner et al., 2004). The CA1 region may function as an integrator of information that has been pre‐processed by the other subfields (Hasselmo, 1997; Vinogradova, 2001; Lisman and Grace, 2005; Kumaran and Maguire, 2007; Schlichting et al., 2014) and engage in temporal pattern separation (Kesner, 2013). A specific role for CA3 in the processing of episodic memory has been proposed (Kesner et al., 2008; Li and Chao, 2008), whereby CA3 may be preferentially involved in the encoding of episodic memory for contextual fear (Daumas et al., 2004; Lee et al., 2004). The very specific suppression of synaptic plasticity in CA3 by 5‐HT4‐receptor activation, suggests that this receptor may play an important role in driving pattern separation in the DG and CA1, coupled with the suppression of (putatively) erroneous pattern completion in CA3.
Interestingly, 5‐HT4‐receptor binding sites studies revealed a difference in density, whereby CA3 < CA1 > DG (Manuel‐Apolinar et al., 2005). This could explain why DG LTP was the least vulnerable to inhibition by 5‐HT4‐receptor activation. Incidentally, memory consolidation after learning results in subfield‐specific differences in receptor expression, whereby expression increases in the CA1 region, decreases in the CA3, but remains relatively constant in the DG (Manuel‐Apolinar et al., 2005). Furthermore, 5‐HT4‐receptor expression levels also depend on the kind of memory task (for review see: Meneses, 2015). The subregional differences in the regulation of synaptic plasticity by 5‐HT4‐receptors observed in this, and other studies (Kemp and Manahan‐Vaughan, 2005) may moreover relate to the specific hippocampal expression of 5‐HT4‐receptors (Vilaró et al., 2005), or of 5‐HT4‐receptor isoforms (Pindon et al., 2002) within synaptic and somatic compartments. All regions express the receptor (Vilaró et al., 2005), whereby the molecular layer and granule cell layer of the DG exhibit 5‐HT4‐receptors, in the CA3 region both the pyramidal cell layer and Stratum lucidum express the receptor, whereas in the CA1 region the pyramidal cell layer, Stratum radiatum and Stratum oriens exhibit receptor expression. Given the fact that mossy fibers (Vilaró et al., 1996), and most specifically the Stratum lucidum (Vilaró et al., 2005), express very high levels of 5‐HT4‐receptors (Vilaró et al., 1996), this may comprise a means whereby selective information encoding by specific hippocampal subfields is regulated. This may comprise a functional mechanism whereby, under conditions of strong 5‐HT4‐receptor activation pattern separation is promoted (in CA1 and DG) at the expense of pattern completion in CA3. This in turn could explain why under circumstances where 5‐HT homeostasis is impaired (Heisler et al., 1998; Kusserow et al., 2004; Pennanen et al., 2013), inadequate 5‐HT4‐receptor activation leads to redundant or excessive encoding of details of a given experience (Moriya and Sugiura, 2012).
In conclusion, our data support a specific role for 5‐HT4‐receptors in fine‐tuning of the dynamic capacity of hippocampal synapses to express synaptic plasticity. 5‐HT4‐receptor activation suppresses synaptic information‐encoding by means of LTD in the CA1 and DG, and prevents depotentiation, thereby permissively supporting the expression of LTP in these hippocampal subfields. 5‐HT4‐receptor activation also completely suppresses persistent LTP and LTD in mf‐CA3 synapses. Afferent stimulation of mf‐CA3 or pp‐DG synapses at Θm frequencies does not result in synaptic plasticity via 5‐HT4‐receptor modulation. By contrast, the CA1 region is subjected to a very specific regulation by 5‐HT4‐receptors: afferent (Θm) stimulation frequencies that typically result in no persistent change in synaptic strength, result in LTP when 5‐HT4‐receptors are activated, suggesting that information encoding through this LTP structure is prioritised under these conditions. Taken together, these findings suggest that 5‐HT4‐receptor activation promotes information encoding through LTP in CA1 and DG, by suppressing LTD across the main hippocampal subfields, and altering the frequency‐dependency of synaptic plasticity to favour LTP. This is likely to support an optimisation of LTP‐mediated encoding of general spatial experience, at the expense of LTD‐mediated encoding of spatial content, a specific regulation of functional information encoding within the hippocampal subfields, and consequently modulation of the content of cognitive representations.
ACKNOWLEDGMENTS
The authors thank Jens Colitti‐Klausnitzer and Juliane Böge for technical assistance, and Silke Dirken and Nadine Kollosch for animal care.
REFERENCES
- Abraham WC, Bear MF. 1996. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci 19:126–130. [DOI] [PubMed] [Google Scholar]
- Abraham WC, McNaughton N. 1984. Differences in synaptic transmission between medial and lateral components of the perforant path. Brain Res 303:251–260. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Tate WP. 1997. Metaplasticity: A new vista across the field of synaptic plasticity. Prog Neurobiol 52:303–323. [DOI] [PubMed] [Google Scholar]
- Andrade R, Chaput Y. 1991. 5‐Hydroxytryptamine4‐like receptors mediate the slow excitatory response to serotonin in the rat hippocampus. J Pharmacol Exp Ther 257:930–937. [PubMed] [Google Scholar]
- Ansanay H, Dumuis A, Sebben M, Bockaert J, Fagni L. 1995. cAMP‐dependent, long‐lasting inhibition of a K+ current in mammalian neurons. Proc Natl Acad Sci USA 92:6635–6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. 1999. A review of central 5‐HT receptors and their function. Neuropharmacology 38:1083–1152. [DOI] [PubMed] [Google Scholar]
- Bianchi C, Rodi D, Marino S, Beani L, Siniscalchi A. 2002. Dual effects of 5‐HT4 receptor activation on GABA release from guinea pig hippocampal slices. Neuroreport 13:2177–2180. [DOI] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. 1982. Theory for the development of neuron selectivity: Orientation specificity and binocular interaction in visual cortex. J Neurosci 2:32–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijak M, Misgeld U. 1997. Effects of serotonin through serotonin1A and serotonin4 receptors on inhibition in the guinea‐pig dentate gyrus in vitro. Neuroscience 78:1017–1026. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Ansanay H, Letty S, Marchetti‐Gauthier E, Roman F, Rondouin G, Fagni L, Soumireu‐Mourat B, Dumuis A. 1998. 5‐HT4 receptors: Long‐term blockade of K+ channels and effects on olfactory memory. C R Acad Sci III 321:217–221. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Compan V, Dumuis A. 2004. 5‐HT4 receptors. Curr Drug Targets CNS Neurol Disord 3:39–51. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Compan V, Dumuis A. 2011. 5‐HT(4) receptors, a place in the sun: Act two. Curr Opin Pharmacol 11:87–93. [DOI] [PubMed] [Google Scholar]
- Buhot MC. 1997. Serotonin receptors in cognitive behaviors. Curr Opin Neurobiol 7:243–254. [DOI] [PubMed] [Google Scholar]
- Cai X, Flores‐Hernandez J, Feng J, Yan Z. 2002. Activity‐dependent bidirectional regulation of GABAA receptor channels by the 5‐HT4 receptor‐mediated signalling in rat prefrontal cortical pyramidal neurons. J Physiol 540:743–759. (Pt [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cape EG, Jones BE. 1998. Differential modulation of high‐frequency gamma‐electroencephalogram activity and sleep‐wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons. J Neurosci 18:2653–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti MZ, Vicini‐Chilovi B, Riva M, Zanetti M, Liberini P, Padovani A, Rozzini L. 2013. Odor identification deficit predicts clinical conversion from mild cognitive impairment to dementia due to Alzheimer's disease. Arch Clin Neuropsychol 28:391–399. [DOI] [PubMed] [Google Scholar]
- Coussens CM, Teyler TJ. 1996. Protein kinase and phosphatase activity regulate the form of synaptic plasticity expressed. Synapse 24:97–103. [DOI] [PubMed] [Google Scholar]
- Daumas S, Halley H, Lassalle J‐M. 2004. Disruption of hippocampal CA3 network: Effects on episodic‐like memory processing in C57BL/6J mice. Eur J Neurosci 20:597–600. [DOI] [PubMed] [Google Scholar]
- Dong Z, Gong B, Li H, Bai Y, Wu X, Huang Y, He W, Li T, Wang YT. 2012. Mechanisms of hippocampal long‐term depression are required for memory enhancement by novelty exploration. J Neurosci 32:11980–11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. 1993. Bidirectional long‐term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci 13:2910–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont GJH, de Visser SJ, Cohen AF, van Gerven JMA. Biomarker Working Group of the German Association for Applied Human Pharmacology. 2005. Biomarkers for the effects of selective serotonin reuptake inhibitors (SSRIs) in healthy subjects. Br J Clin Pharmacol 59:495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Alarcon JM, Weisberg SP, Touzani K, Huang Y‐Y, Nordheim A, Kandel ER. 2006. A role in learning for SRF: Deletion in the adult forebrain disrupts LTD and the formation of an immediate memory of a novel context. Neuron 50:127–143. [DOI] [PubMed] [Google Scholar]
- Fagni L, Dumuis A, Sebben M, Bockaert J. 1992. The 5‐HT4 receptor subtype inhibits K+ current in colliculi neurones via activation of a cyclic AMP‐dependent protein kinase. Br J Pharmacol 105:973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana DJ, Daniels SE, Wong EH, Clark RD, Eglen RM. 1997. The effects of novel, selective 5‐hydroxytryptamine (5‐HT)4 receptor ligands in rat spatial navigation. Neuropharmacology 36:689–696. [DOI] [PubMed] [Google Scholar]
- Frey U, Hartmann S, Matthies H. 1989. Domperidone, an inhibitor of the D2‐receptor, blocks a late phase of an electrically induced long‐term potentiation in the CA1‐region in rats. Biomed Biochim Acta 48:473–476. [PubMed] [Google Scholar]
- Frey U, Schroeder H, Matthies H. 1990. Dopaminergic antagonists prevent long‐term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res 522:69–75. [DOI] [PubMed] [Google Scholar]
- Frey U, Matthies H, Reymann KG, Matthies H. 1991. The effect of dopaminergic D1 receptor blockade during tetanization on the expression of long‐term potentiation in the rat CA1 region in vitro. Neurosci Lett 129:111–114. [DOI] [PubMed] [Google Scholar]
- Goh JJ, Manahan‐Vaughan D. 2012a. Spatial Object Recognition Enables Endogenous LTD that Curtails LTP in the Mouse Hippocampus. Cereb Cortex 23:1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JJ, Manahan‐Vaughan D. 2012b. Endogenous hippocampal LTD that is enabled by spatial object recognition requires activation of NMDA receptors and the metabotropic glutamate receptor, mGlu5. Hippocampus 23:129–138. [DOI] [PubMed] [Google Scholar]
- Goh JJ, Manahan‐Vaughan D. 2013a. Synaptic depression in the CA1 region of freely behaving mice is highly dependent on afferent stimulation parameters. Front Integr Neurosci 7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JJ, Manahan‐Vaughan D. 2013b. Hippocampal long‐term depression in freely behaving mice requires the activation of beta‐adrenergic receptors. Hippocampus 23:1299–1308. [DOI] [PubMed] [Google Scholar]
- Haahr ME, Fisher P, Holst K, Madsen K, Jensen CG, Marner L, Lehel S, Baaré W, Knudsen G, Hasselbalch S. 2013. The 5‐HT4 receptor levels in hippocampus correlates inversely with memory test performance in humans. Hum Brain Mapp 34:3066–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagena H, Manahan‐Vaughan D. 2011. Learning‐facilitated synaptic plasticity at CA3 mossy fiber and commissural‐associational synapses reveals different roles in information processing. Cereb Cortex 21:2442–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagena H, Manahan‐Vaughan D. 2012. Learning‐facilitated long‐term depression and long‐term potentiation at mossy fiber‐CA3 synapses requires activation of β‐adrenergic receptors. Front Integr Neurosci 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JE, Madison DV. 2010. Imbalanced pattern completion vs. separation in cognitive disease: Network simulations of synaptic pathologies predict a personalized therapeutics strategy. BMC Neurosci 11:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. 1997. Free recall and recognition in a network model of the hippocampus: Simulating effects of scopolamine on human memory function. Behav Brain Res 89:1–34. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. 1998. Elevated anxiety and antidepressant‐like responses in serotonin 5‐HT1A receptor mutant mice. Proc Natl Acad Sci U S A 95:15049–15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgers J, van Nie R, Ivanyi D, Hilkens J, Michalides R, de Moes J, Poort‐Keesom R, Kroezen V, von Deimling O, Kominami R, Holmes R. 1985. Genetic differences in BALB/c sublines. Curr Top Microbiol Immunol 122:19–30. [DOI] [PubMed] [Google Scholar]
- Hille C, Bate S, Davis J, Gonzalez MI. 2008. 5‐HT4 receptor agonism in the five‐choice serial reaction time task. Behav Brain Res 195:180–186. [DOI] [PubMed] [Google Scholar]
- Kawata Y, Okada M, Murakami T, Kamata A, Zhu G, Kaneko S. 2001. Pharmacological discrimination between effects of carbamazepine on hippocampal basal, Ca(2+)‐ and K(+)‐evoked serotonin release. Br J Pharmacol 133:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr J, Yoshitake T, Wang FH, Wynick D, Holmberg K, Lendahl U, Bartfai T, Yamaguchi M, Hoekfelt T, Ogren SO. 2001. Microdialysis in freely moving mice: Determination of acetylcholine, serotonin and noradrenaline release in galanin transgenic mice. J Neurosci Methods 109:71–80. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan‐Vaughan D. 2004. Hippocampal long‐term depression and long‐term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci USA 101:8192–8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan‐Vaughan D. 2005. The 5‐hydroxytryptamine4 receptor exhibits frequency‐dependent properties in synaptic plasticity and behavioural metaplasticity in the hippocampal CA1 region in vivo. Cereb Cortex 15:1037–1043. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan‐Vaughan D. 2007. Hippocampal long‐term depression: Master or minion in declarative memory processes? Trends Neurosci 30:111–118. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan‐Vaughan D. 2008. The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long‐term depression. Cereb Cortex 18:968–977. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Bright F, Trail B, Blackburn TP, Sanger GJ. 1997. Anxiolytic‐like actions of the selective 5‐HT4 receptor antagonists SB 204070A and SB 207266A in rats. Neuropharmacology 36:707–712. [DOI] [PubMed] [Google Scholar]
- Kesner RP. 2013. Role of the hippocampus in mediating interference as measured by pattern separation processes. Behav Process 93:148–154. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Lee I, Gilbert P. 2004. A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci 15:333–351. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR, Warthen MW. 2008. The CA3 subregion of the hippocampus is critical for episodic memory processing by means of relational encoding in rats. Behav Neurosci 122:1217–1225. [DOI] [PubMed] [Google Scholar]
- King MV, Marsden CA, Fone KCF. 2008. A role for the 5‐HT(1A), 5‐HT4 and 5‐HT6 receptors in learning and memory. Trends Pharmacol Sci 29:482–492. [DOI] [PubMed] [Google Scholar]
- Klausnitzer J, Kulla A, Manahan‐Vaughan D. 2004. Role of the group III metabotropic glutamate receptor in LTP, depotentiation and LTD in dentate gyrus of freely moving rats. Neuropharmacology 46:160–170. [DOI] [PubMed] [Google Scholar]
- Kulla A, Manahan‐Vaughan D. 2002. Modulation by serotonin 5‐HT(4) receptors of long‐term potentiation and depotentiation in the dentate gyrus of freely moving rats. Cereb Cortex 12:150–162. [DOI] [PubMed] [Google Scholar]
- Kulla A, Manahan‐Vaughan D. 2008. Modulation by group 1 metabotropic glutamate receptors of depotentiation in the dentate gyrus of freely moving rats. Hippocampus 18:48–54. [DOI] [PubMed] [Google Scholar]
- Kulla A, Reymann KG, Manahan‐Vaughan D. 1999. Time‐dependent induction of depotentiation in the dentate gyrus of freely moving rats: Involvement of group 2 metabotropic glutamate receptors. Eur J Neurosci 11:3864–3872. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. 2007. Match mismatch processes underlie human hippocampal responses to associative novelty. J Neurosci 27:8517–8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusserow H, Davies B, Hörtnagl H, Voigt I, Stroh T, Bert B, Deng DR, Fink H, Veh RW, Theuring F. 2004. Reduced anxiety‐related behaviour in transgenic mice overexpressing serotonin 1A receptors. Brain Res Mol Brain Res 129:104–116. [DOI] [PubMed] [Google Scholar]
- Lamirault L, Simon H. 2001. Enhancement of place and object recognition memory in young adult and old rats by RS 67333, a partial agonist of 5‐HT4 receptors. Neuropharmacology 41:844–853. [DOI] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. 2000. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405:955–959. [DOI] [PubMed] [Google Scholar]
- Lee I, Rao G, Knierim JJ. 2004. A double dissociation between hippocampal subfields: Differential time course of CA3 and CA1 place cells for processing changed environments. Neuron 42:803–815. [DOI] [PubMed] [Google Scholar]
- Lelong V, Dauphin F, Boulouard M. 2001. RS 67333 and D‐cycloserine accelerate learning acquisition in the rat. Neuropharmacology 41:517–522. [DOI] [PubMed] [Google Scholar]
- Letty S, Child R, Gale JD, Dumuis A, Bockaert J, Rondouin G. 1997. 5‐HT4 receptors improve social olfactory memory in rat. Neuropharmacology 36:681–687. [DOI] [PubMed] [Google Scholar]
- Li J‐S, Chao Y‐S. 2008. Electrolytic lesions of dorsal CA3 impair episodic‐like memory in rats. Neurobiol Learn Mem 89:192–198. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. 2005. The hippocampal‐VTA loop: Controlling the entry of information into long‐term memory. Neuron 46:703–713. [DOI] [PubMed] [Google Scholar]
- Lynch G, Rex CS, Gall CM. 2007. LTP consolidation: Substrates, explanatory power, and functional significance. Neuropharmacology 52:12–23. [DOI] [PubMed] [Google Scholar]
- Lynch G, Baudry M. 2015. Brain and memory: OLD arguments and new perspectives. Brain Res 1621:1–4. [DOI] [PubMed] [Google Scholar]
- Madsen K, Neumann W‐J, Holst K, Marner L, Haahr MT, Lehel S, Knudsen GM, Hasselbalch SG. 2011. Cerebral serotonin 4 receptors and amyloid‐β in early Alzheimer's disease. J Alzheimers Dis 26:457–466. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. 2004. LTP and LTD: An embarrassment of riches. Neuron 44:5–21. [DOI] [PubMed] [Google Scholar]
- Manahan‐Vaughan D. 2000. Long‐term depression in freely moving rats is dependent upon strain variation, induction protocol and behavioral state. Cereb Cortex 10:482–487. [DOI] [PubMed] [Google Scholar]
- Manahan‐Vaughan D, Braunewell K‐H. 2005. The metabotropic glutamate receptor, mGluR5, is a key determinant of good and bad spatial learning performance and hippocampal synaptic plasticity. Cereb Cortex 15:1703–1713. [DOI] [PubMed] [Google Scholar]
- Manahan‐Vaughan D, Reymann KG. 1996. Metabotropic glutamate receptor subtype agonists facilitate long‐term potentiation within a distinct time window in the dentate gyrus in vivo. Neuroscience 74:723–731. [DOI] [PubMed] [Google Scholar]
- Manahan‐Vaughan D, Reymann KG. 1997. Group 1 metabotropic glutamate receptors contribute to slow‐onset potentiation in the rat CA1 region in vivo. Neuropharmacology 36:1533–1538. [DOI] [PubMed] [Google Scholar]
- Manahan‐Vaughan D, Reymann KG, Brown RE. 1998. In vivo electrophysiological investigations into the role of histamine in the dentate gyrus of the rat. Neuroscience 84:783–790. [DOI] [PubMed] [Google Scholar]
- Manuel‐Apolinar L, Rocha L, Pascoe D, Castillo E, Castillo C, Meneses A. 2005. Modifications of 5‐HT4 receptor expression in rat brain during memory consolidation. Brain Res 1042:73–81. [DOI] [PubMed] [Google Scholar]
- Marchetti E, Dumuis A, Bockaert J, Soumireu‐Mourat B, Roman FS. 2000. Differential modulation of the 5‐HT(4) receptor agonists and antagonist on rat learning and memory. Neuropharmacology 39:2017–2027. [DOI] [PubMed] [Google Scholar]
- Marchetti E, Chaillan FA, Dumuis A, Bockaert J, Soumireu‐Mourat B, Roman FS. 2004. Modulation of memory processes and cellular excitability in the dentate gyrus of freely moving rats by a 5‐HT4 receptors partial agonist, and an antagonist. Neuropharmacology 47:1021–1035. [DOI] [PubMed] [Google Scholar]
- Marchetti E, Jacquet M, Escoffier G, Miglioratti M, Dumuis A, Bockaert J, Roman FS. 2011. Enhancement of reference memory in aged rats by specific activation of 5‐HT(4) receptors using an olfactory associative discrimination task. Brain Res 1405:49–56. [21745655] [DOI] [PubMed] [Google Scholar]
- Marchetti‐Gauthier E, Roman FS, Dumuis A, Bockaert J, Soumireu‐Mourat B. 1997. BIMU1 increases associative memory in rats by activating 5‐HT4 receptors. Neuropharmacology 36:697–706. [DOI] [PubMed] [Google Scholar]
- Meneses A. 1998. Physiological, pathophysiological and therapeutic roles of 5‐HT systems in learning and memory. Rev Neurosci 9:275–289. [DOI] [PubMed] [Google Scholar]
- Meneses A. 2015. Serotonin, neural markers, and memory. Front Pharmacol 6:143– [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses A, Hong E. 1997. A pharmacological analysis of serotonergic receptors: Effects of their activation of blockade in learning. Prog Neuropsychopharmacol Biol Psychiatry 21:273–296. [DOI] [PubMed] [Google Scholar]
- Moriya J, Sugiura Y. 2012. Impaired attentional disengagement from stimuli matching the contents of working memory in social anxiety. PLoS One 7:e47221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM, Moser EI, Riedel G, Martin SJ, Sandin J, Day M, O'Carroll C. 2003. Elements of a neurobiological theory of the hippocampus: The role of activity‐dependent synaptic plasticity in memory. Philos Trans R Soc Lond, B, Biol Sci 358:773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Krobert KA, Moser MB, Morris RG. 1998. Impaired spatial learning after saturation of long‐term potentiation. Sci. 281:2038–2042. [DOI] [PubMed] [Google Scholar]
- Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. 2014. Engineering a memory with LTD and LTP. Nature 511:348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin S, Murphy C. 1998. Odor memory in normal aging and Alzheimer's disease. Ann N Y Acad Sci 855:686–693. [DOI] [PubMed] [Google Scholar]
- Ondrejcak T, Klyubin I, Hu N‐W, Barry AE, Cullen WK, Rowan MJ. 2010. Alzheimer's disease amyloid beta‐protein and synaptic function. Neuromolecular Med 12:13–26. [DOI] [PubMed] [Google Scholar]
- Pennanen L, van der Hart M, Yu L, Tecott LH. 2013. Impact of serotonin (5‐HT)2C receptors on executive control processes. Neuropsychopharmacol 38:957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindon A, van Hecke G, van Gompel P, Lesage AS, Leysen JE, Jurzak M. 2002. Differences in signal transduction of two 5‐HT4 receptor splice variants: Compound specificity and dual coupling with Galphas‐ and Galphai/o‐proteins. Mol Pharmacol 61:85–96. [DOI] [PubMed] [Google Scholar]
- Portas CM, Bjorvatn B, Fagerland S, Gronli J, Mundal V, Ursin ES. R, 1998. On‐line detection of extracellular levels of serotonin in dorsal raphe nucleus and frontal cortex over the sleep/wake cycle in the freely moving rat. Neuroscience 83:807–814. [DOI] [PubMed] [Google Scholar]
- Ranson A, Cheetham CE, Fox K, Sengpiel F. 2012. Homeostatic plasticity mechanisms are required for juvenile, but not adult, ocular dominance plasticity. Proc Natl Acad Sci U S A 109:1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restivo L, Roman F, Dumuis A, Bockaert J, Marchetti E, Ammassari‐Teule M. 2008. The promnesic effect of G‐protein‐coupled 5‐HT4 receptors activation is mediated by a potentiation of learning‐induced spine growth in the mouse hippocampus. Neuropsychopharmacology 33:2427–2434. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Mason SL, Meldrum A, De Keczer S, Parnes H, Eglen RM, Wong EH. 1995. 5‐Hydroxytryptamine (5‐HT)4 receptors in post mortem human brain tissue: Distribution, pharmacology and effects of neurodegenerative diseases. Br J Pharmacol 114:993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman FS, Marchetti E. 1998. Involvement of 5‐HT receptors in learning and memory. Drugs 1:109–121. [PubMed] [Google Scholar]
- Sajikumar S, Frey JU. 2004. Late‐associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiol Learn Mem 82:12–25. [DOI] [PubMed] [Google Scholar]
- Schlichting ML, Zeithamova D, Preston AR. 2014. CA1 subfield contributions to memory integration and inference. Hippocampus 24:1248–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stäubli U, Chun D. 1996. Factors regulating the reversibility of long‐term potentiation. J Neurosci 16:853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Lynch G. 1990. Stable depression of potentiated synaptic responses in the hippocampus with 1–5 Hz stimulation. Brain Res 513:113–118. [DOI] [PubMed] [Google Scholar]
- Terry AV, Buccafusco JJ, Jackson WJ, Prendergast MA, Fontana DJ, Wong EH, Bonhaus DW, Weller P, Eglen RM. 1998. Enhanced delayed matching performance in younger and older macaques administered the 5‐HT4 receptor agonist, RS 17017. Psychopharmacologia 135:407–415. [DOI] [PubMed] [Google Scholar]
- Torres GE, Chaput Y, Andrade R. 1995. Cyclic AMPand protein kinase A mediate 5‐hydroxytryptamine type 4 receptor regulation of calciumactivated potassium current in adult hippocampal neurons. Mol Pharmacol 47:191–197. [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. 1979. Raphe unit activity in freely moving cats: Correlation with level of behavioral arousal. Brain Res 163:135–150. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. 1996. The essential role of hippocampal CA1 NMDA receptor‐dependent synaptic plasticity in spatial memory. Cell 87:1327–1338. [DOI] [PubMed] [Google Scholar]
- Vida I, Frotscher M. 2000. A hippocampal interneuron associated with the mossy fiber system. Proc Natl Acad Sci USA 97:1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilaró MT, Cortés R, Gerald C, Branchek TA, Palacios JM, Mengod G. 1996. Localization of 5‐HT4 receptor mRNA in rat brain by in situ hybridization histochemistry. Brain Res Mol Brain Res 43:356–360. [DOI] [PubMed] [Google Scholar]
- Vilaró MT, Cortés R, Mengod G. 2005. Serotonin 5‐HT4 receptors and their mRNAs in rat and guinea pig brain: Distribution and effects of neurotoxic lesions. J Comp Neurol 484:418–439. [DOI] [PubMed] [Google Scholar]
- Vinogradova OS. 2001. Hippocampus as comparator: Role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus 11:578–598. [DOI] [PubMed] [Google Scholar]
- Vogt KE, Nicoll RA. 1999. Glutamate and gamma‐aminobutyric acid mediate a heterosynaptic depression at mossy fiber synapses in the hippocampus. 96:1118–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. 2006. Learning induces long‐term potentiation in the hippocampus. Sci. 313:1093–1097. [DOI] [PubMed] [Google Scholar]


