Abstract
BACKGROUND
Almonds contain considerable amounts of potential prebiotic components, and the roasting process may alter these components. The aim of this study was to compare the in vitro fermentation properties and in vivo prebiotic effect of raw and roasted almonds.
RESULTS
In vitro, predigested raw and roasted almonds promoted the growth of Lactobacillus acidophilus (La‐14) and Bifidobacterium breve (JCM 1192), and no significant differences were found between these two nuts. In a 4‐week animal trial, daily intake of raw or roasted almonds promoted the population of Bifidobacterium spp. and Lactobacillus spp. and inhibited the growth of Enterococcus spp. in faeces and caecal contains of rats. Compared with roasted almonds, raw almonds had a greater bifidobacteria promotion effect. Besides, significantly higher β‐galactosidase activity and lower β‐glucuronidase and azoreductase activities in faeces or caecal contents of rats were observed with raw almonds than with roasted almonds. While, in terms of metabolic effects, the ingestion of roasted almonds resulted in significantly greater intestinal lipase activities.
CONCLUSION
Both raw and roasted almonds exhibit potential prebiotic effects, including regulation of intestinal bacteria and improved metabolic activities. The roasting process may slightly reduce the prebiotic effects of almonds but significantly improve the metabolic effects.© 2016 The Authors. Journal of the Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: almond, roasting, prebiotic effect, fermentation profile, bacterial enzyme activities, digestive enzyme activities
INTRODUCTION
A complex community of microorganisms inhabits the mammalian gastrointestinal tract from mouth to anus. The colon is the main site of microbial colonisation. The large intestine contains a complex and dynamic microbial ecosystem with high densities of living bacteria, which achieve concentrations of up to 1011 or 1012 cells g−1 of luminal contents.1 The gut microbiota play important nutritional and physiological roles such as protection against pathogens, development of the immune system, and positive effects on colonic health and host nutrition.2 With a view toward improving colonic function, the influence of nutrition on the intestinal ecosystem has been a subject of great interest. The dietary use of prebiotics is among the most well‐known food‐based strategies to promote gut microbiota modulation towards a health‐promoting profile. The most important bacterial genera targeted for selective stimulation by prebiotics are the indigenous bifidobacteria and lactobacilli.3 Several in vivo studies have shown the ability of bifidobacteria and lactobacilli to ferment prebiotic carbohydrates, which provides for their selective enrichment in the gastrointestinal tract.3, 4, 5 Bifidobacteria and lactobacilli are able to inhibit the growth of clostridia and pathogenic Enterobacteriaceae by the production of short‐chain fatty acids and antimicrobial compounds, as well as by competition for growth substrate and adhesion sites.6, 7
Almonds are the most commonly consumed tree nuts in the world. The global production of almonds is greater than 1 million metric tons, with California producing about 80% of the world's almonds (Almond Almanac, Almond Board of California, 2014). From the United States Department of Agriculture (USDA) nutrient database, almonds have a healthy nutrient profile, providing a nutrient‐dense source of protein (21.15%), monounsaturated fatty acids (31.55%), polyunsaturated fatty acids (12.33%), dietary fibre (12.5%), polyphenols (0.195%), α‐tocopherol, essential minerals and other phytochemicals. Regular consumption of almonds has been associated with nutritional benefits. Numerous studies have demonstrated the nutritional functions of almonds, including lipid regulation,8, 9 antioxidant activity,10, 11, 12 weight control,9, 13 and potential prebiotic effects.14 The significant amounts of fibre and polyphenols in almonds form an important substrate for microbial fermentation in the gut and thus might facilitate the maintenance or selection of a healthy microbiota composition. Mandalari et al. 14, 15, 16 demonstrated the potential prebiotic effect of almond seed and almond skin in vitro. In our previous study, the prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans were evaluated, and the results indicated that almonds and almond skins possess potential prebiotic properties which were similar to the commercial prebiotic of fructooligosaccharides.17
In China, almonds also are a popular snack food, and are mostly consumed in roasted forms. The conditions of almond roasting generally used by processors are heating to 130–180 °C and a residence time of 10 to 60 min. Roasting enhances the flavour and alters the colour and texture due to dehydration, browning, oxidation and diverse structural changes. However, the nutritional properties of almond may be modified during thermal processes. Amrein et al. 18 found a higher acrylamide concentration in dark roasted almonds than in raw almonds. Zhang et al. 19 analysed the five common advanced glycation end products in raw and roasted almonds and found that the roasting process significantly increased carboxymethyl‐lysine, carboxyethyl‐lysine and pyralline formation. Thermal treatments promote protein denaturation, lipid hydrolysis, carbohydrate degradation and structure disruption, and therefore have a potential to modify the prebiotic properties of almonds. Currently, no comparative data exist on the prebiotic effect of raw and roasted almonds. Thus research is required to achieve a better understanding of the changes in the efficacy of almonds in terms of prebiotic properties after roasting.
The present study compared the in vitro fermentation profile of predigested raw and roasted almonds by three typical intestinal bacteria species and the in vivo modulation effect of raw and roasted almond intake on faecal and caecal bacteria in Wistar rats. The effects of raw and roasted almond intake on faecal and caecal bacterial and digestive enzyme activities in rats were also compared.
MATERIALS AND METHODS
Almond samples
Almonds (Prunus amygdalus; variety Nonpareil, with skins), raw and roasted, were kindly provided by the Almond Board of California (Modesto, CA, USA). A hot air (dry) roasting process was used to produce the roasted almonds: hot air (171 °C) was directed through a batch of raw almonds for 10 min.
In vitro study
Simulated digestion of raw and roasted almonds
The raw and roasted almonds used for the in vitro study were previously hydrolysed under simulated gastric and duodenal digestion. Simulated digestion of almonds was performed using the protocol described by Gil‐Izquierdo et al. 20 and with slight modifications. Briefly, 10 g of ground raw or roasted almonds were mixed with 200 mL sterile distilled water and blended for 5 min at normal speed using a homogeniser. About 10 mg of α‐amylase (activity 380 U mg−1; Sigma Chemical Co., St Louis, MO, USA) was dissolved in 200 mL of filter‐sterilised CaCl2 solution (1 mmol L−1, pH 7.0); 10 mL of this solution and the blended almonds were placed in a 500 mL beaker. The mixture was incubated at 37 °C for 30 min on a laboratory shaker (IKA Labortechnik KS501, Staufen, Germany), and then acidified to pH 2 with 6 mol L−1 HCl. Approximately 500 mg of pepsin (activity 3200 U mg−1; Sigma Chemical Co.) was added to the almond mixture and incubated at 37 °C for a further 2 h, with shaking; the pH of the mixture was then adjusted to 6.5 with 6 mol L−1 NaOH. A solution made by dissolving 0.08 g of pancreatin (Sigma Chemical Co.) and 0.5 g of bile extract (Sigma Chemical Co.) in 20 mL of 0.5 mol L−1 NaHCO3 solution was dispensed into the mixture and incubated at 37 °C for 3 h, with shaking. Subsequently, the mixture was boiled for 15 min to destroy enzyme activities, and the semi‐fluid mixture was collected for use in fermentation property assays.
Fermentation profile of almonds by intestinal bacteria
The fermentation profile of predigested raw and roasted almonds by three typical intestinal bacteria species, namely Lactobacillus acidophilus, Bifidobacterium breve and Escherichia coli, was determined in this study. Bacterial strains were obtained from the China General Microbiological Culture Collection Center, Beijing, China (L. acidophilus LA‐14 and B. breve JCM 1192) or Fujian Center for Disease Control and Prevention, Fuzhou, China (E. coli JM103). The bacterial strains were pre‐cultured in peptone–yeast extract (PY) broth. The inoculated media were incubated anaerobically in an Anaero‐Pack (Mitsubishi Gas Chemical Co., Inc., Tokyo, Japan)21 at 37 °C for 24 h. Bacterial strains were collected by centrifugation (1500 × g, 20 min, 4 °C) and washed with sterile phosphate buffered peptone. Subsequently, the strains were diluted to an optical density of 0.5 at 600 nm with sterile phosphate buffered peptone to generate the working suspensions of the three bacteria. The digested semi‐fluid mixtures of raw or roasted almonds were dissolved in a basal growth medium (Luria–Bertani; Beijing Land Bridge Technology Co. Ltd., Beijing, China) to give final concentrations of 0, 2.5, 5, 10 and 15% (v/v), prior to autoclaving. The basal medium was maintained at a final concentration of 85% (v/v). After sterilisation, the three working suspensions were used to inoculate the various almond‐medium concentrations, with an inoculation volume of 1%, and the medium without digested almonds served as the control. After anaerobic incubation at 37 °C for 24 h in an Anaero‐Pack, a series of 10‐fold dilutions (10−4 to 10−7) was prepared in sterile physiological saline, and 0.1 mL of the dilutions was spread in quadruplicate onto plates of MRS agar for L. acidophilus, MRS agar supplemented with 50 µg mL−1 lithium mupirocin (Li‐MUP) for B. breve, and Luria–Bertani agar for E. coli. All the agars were purchased from Beijing Land Bridge Technology Co. Ltd. Culture plates were incubated at 37 °C for 24 h for L. acidophilus and E. coli, and incubated anaerobically at 37 °C for 48 h for B. breve. The numbers of viable bacteria were determined by counting colony‐forming units (CFU).
In vivo study
Animals and diets
A total of 30 10‐week‐old male specific‐pathogen‐free (SPF) Wistar rats (initial body weight of 180–200 g) were obtained from Fuzhou General Hospital of Nanjing Military Command (Fuzhou, China). The rats were housed in an SPF animal laboratory at conditions of 18–29 °C and relative humidity (50–80% RH) with alternating 12‐h light and 12‐h dark periods. All animal feed was sterilised, and the drinking water was sterile water. Rats, matching in weight, were randomly divided into three groups (10 rats per group) according to feeding regime (control, raw almond and roasted almond groups). Once a day, via intragastric application, rats in these three groups received either 3 mL of sterile water, 3 mL of raw almond slurry, or 3 mL of roasted almond slurry, respectively, for 4 weeks. The Almond Board of California suggests customers have a daily intake of 2 oz (56 g) of almonds, which is around 1 g kg−1 of body weight. In the present study, we used a five‐times dosage of the recommended amount of human for rats, which was 5 g (kg d)−1. The average body weight of rats were around 200 g, thus a dose of 1.00 g was used to feed the rats. To prepare the almond slurries, ground raw or roasted almonds were sieved through a 60‐mesh sieve, and 1.00‐g samples each were suspended in about 2 mL of distilled water.
Enumeration of faecal and caecal bacteria
On days 0, 14 and 28 of the experimental period, the faecal samples of each rat were collected in an individual sterilised specimen cup, and then sealed and removed to an anaerobic environment soon after collection for later enumeration of faecal bacteria, respectively. Under anaerobic conditions, 1.00 g of the faecal sample was weighted and dissolved in 99 mL of sterile physiological saline and mixed thoroughly to generate a faecal suspension. On day 28 of the experimental period, rats were anaesthetised with sodium pentobarbital (60 mg kg−1 of body weight, i.p.) and the caecum of each rat was removed. The caecal contents from each rat were aseptically emptied into a sterile plastic bag and removed to an anaerobic environment soon after collection for enumeration of caecal bacteria. Under anaerobic conditions, 1.00 g of the caecal contents was weighed and dissolved in 99 mL of sterile physiological saline and mixed thoroughly to generate a caecal content suspension. A series of 10‐fold dilutions (10−2 to 10−7) of the faecal suspension or caecal content suspension were prepared, and 0.1‐mL aliquots of each dilutions were spread in quadruplicate onto four selective agar plates: raffinose‐Bifidobacterium agar for Bifidobacterium spp.; lactobacilli select agar for Lactobacillus spp.; alizarin‐β‐galactosidase agar for E. coli and Pfizer selective enterococcus agar for Enterococcus spp.. All of the media were purchased from Beijing Land Bridge Technology Co. Ltd. The inoculated plates were incubated anaerobically at 37 °C for 48 h in an Anaero‐Pack for Bifidobacterium spp. and Lactobacillus spp. and for 24 h for E. coli and Enterococcus spp. After incubation, plates were examined for bacterial colonies and the results of the microbial counts were expressed as CFU per gram of wet faeces or per gram of caecal contents.
Bacterial enzyme assays
The collected faeces and caecal contents were also used for the determination of β‐galactosidase, β‐glucuronidase, nitroreductase and azoreductase enzyme activities. To prepare samples for enzyme assay, 1 g of faeces or caecal contents was diluted 10‐fold in 0.1 mol L−1 potassium phosphate buffer. The specimens were homogenised in a blender, then sonicated for 15 min, and centrifuged at 13 800 × g for 10 min at 4 °C. The supernatant fractions were used for measuring the bacterial enzyme activities, following the instructions of the respective commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). One unit of β‐galactosidase, β‐glucuronidase, and nitroreductase was defined as the amount that released 1 µmol of o‐nitrophenyl, p‐nitrophenyl, and p‐aminobenzoic acid per minute, respectively. One unit of azoreductase activity was defined as 1 µmol of amaranth metabolised per minute. Results were expressed as units per gram (U g−1) of wet faeces or caecal contents.
Digestive enzyme assays
The supernatant fractions of faeces or caecal contents mentioned above were also used for the quantitative determinations of protease, amylase and lipase activities by spectrophotometry. The protease activity was assayed in the following mixture: 1 mL of 1% (w/v) casein (Sigma Chemical Co.) dissolved in 0.1 mol L−1 Tris‐HCl (pH 7.5), 450 μL buffer (0.05 mol L−1 Tris‐HC1, pH 7.6), and 100 μL faecal or caecal specimen suspension. The mixture was incubated at 30 °C for 45 min. After incubation, the reaction was stopped by adding 3 mL of 5% (w/v) trichloroacetic acid. After 15 min, the reaction mixture was centrifuged at 9600 × g for 5 min, and the absorbance was measured at 280 nm. α‐Amylase activity was assayed with a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China): 4‐chloro‐2‐nitrophenol was generated from the hydrolysis of 2‐chloro‐4‐nitrophenol‐α‐d‐maltotrioside and absorbance was measured at 405 nm and 37 °C. Lipase activity was assayed in the following enzymatic reaction mixture: 100 μL faecal or caecal specimen suspension and 4‐nitrophenyloctanoate as substrate (0.35 mmol L−1) in 0.05 mol L−1 Tris‐HCl buffer (pH 7.4), 6 mmol L−1 sodium taurocholic acid and 1 mol L−1 NaCl. Nitrophenol generation was measured at 400 nm and 30 °C. For all enzymatic determinations, digestive enzyme activity was expressed as the number of digestive enzyme units per gram (U g−1) of wet faeces or caecal contents; one unit of digestive enzyme was defined as the amount of enzyme required to increase 0.01 absorbance units per millilitre per minute.
Statistical analysis
The in vitro fermentation profile of the predigested raw and roasted almond by three typical intestinal bacteria and the in vivo modulation effect of raw and roasted almond intake on the faecal and caecal bacteria, bacterial and digestive enzyme activities in rats were analysed using Duncan's multiple range tests. Mean values were considered significantly different at P < 0.05. Statistical analyses were performed with SPSS 19.0 software (IBM Inc., Armonk, NY, USA). Data were expressed as mean ± standard deviation (SD).
RESULTS AND DISCUSSION
Effects of hydrolysed raw and roasted almonds on bacterial growth in vitro
Both the raw and roasted almonds hydrolysed under simulated gastric and duodenal digestion were utilised by L. acidophilus and B. breve (Table 1). L. acidophilus populations reached the highest level in media containing 5% and 10% hydrolysed raw or roasted almonds. For B. breve populations, no significant differences were observed in all raw or roasted almond‐supplemented broth, but they were significantly higher than in the control. For E. coli, no significant changes were observed between growth in the control and almond‐supplemented broth. In general, predigested raw or roasted almonds stimulated the growth of the L. acidophilus and B. breve in vitro, and no suppression effect on E. coli was observed in this study.
Table 1.
Effect of predigested raw and roasted almonds on the growth of Lactobacillus, Bifidobacterium and Escherichia coli in Luria–Bertani broth
| Predigested raw almond concentration (%) | Predigested roasted almond concentration (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Organism | Control | 2.5 | 5 | 10 | 15 | 2.5 | 5 | 10 | 15 |
| L. acidophilus (La‐14) | 7.52 ± 0.18a | 7.67 ± 0.23a | 8.12 ± 0.30b | 8.01 ± 0.21 b | 7.89 ± 0.26a | 7.76 ± 0.19a | 8.07 ± 0.22b | 7.96 ± 0.27b | 7.88 ± 0.18a |
| B. breve (JCM 1192) | 5.88 ± 0.13a | 6.37 ± 0.11b | 6.40 ± 0.18b | 6.55 ± 0.11 b | 6.42 ± 0.14b | 6.21 ± 0.26b | 6.41 ± 0.21b | 6.42 ± 0.17b | 6.35 ± 0.22b |
| E. coli (JM103) | 9.57 ± 0.24a | 9.85 ± 0.37a | 9.90 ± 0.31a | 9.92 ± 0.23 a | 9.87 ± 0.19a | 9.70 ± 0.18a | 9.82 ± 0.22a | 9.85 ± 0.40a | 9.83 ± 0.31a |
Values are expressed as the mean of the log10 number ± SD (CFU) per mL of Luria–Bertani broth with or without raw or roasted almonds.
Within the same row, mean values with different letters are significantly different at P < 0.05, n = 3.
Almonds are rich in protein, monounsaturated fatty acids, dietary fibre and mineral elements, which are favourable substrates for the growth of microorganisms. Through hydrolysis under simulated gastric and duodenal digestion the peptides, amino acids, fatty acids, dextrin, oligosaccharides and dietary fibre are released from raw or roasted almonds, which may correlate to the stimulation of the growth of bifidobacteria and lactobacilli. These results were consistent with in vitro studies by Mandalari et al. 15, 16 In the current study, E.coli growth was high in the control group (>9.5 log CFU mL−1) so the stimulation effect of predigested raw or roasted almonds on E. coli could not be assessed. Thus, in further studies for evaluation of the effect of predigested almonds on this bacterial growth, a medium without carbon should be used, so that the dietary fibres from almonds could act as the sole carbon source for the growth of the organism.
The stimulatory effect of raw and roasted almonds on L. acidophilus and B. breve was not significantly different when growth at the same almond concentration was compared. According to the USDA nutrient database, an ounce of raw almonds is nutritionally comparable to an ounce of dry roasted almonds, such as protein (21.15% for raw almond; 20.96% for roasted almond), lipid (49.93% for raw almond; 52.54% for roasted almond) and carbohydrate (21.55% for raw almond; 21.01% for roasted almond). The similar nutrition components might be the reason of the similar promoting effect on the intestinal microorganisms in vitro of these two types of almond.
Effect of raw and roasted almond intake on faecal and caecal bacterial populations in rats
The effects of raw and roasted almond intake on faecal bacterial populations in rats are compared in Fig. 1. The viable counts of Lactobacillus spp. and Bifidobacterium spp. increased significantly (P < 0.05) during the whole trial period for the rats fed raw or roasted almond (1 g per day over 4 weeks) as compared with the control group. In stimulating the growth of bifidobacteria and lactobacilli, raw almonds were more effective than the roasted almond (P < 0.05 for bifidobacteria on day 14 and 28; P < 0.05 for lactobacilli on day 28). By contrast, the Enterococcus spp. populations in the faeces of rats after ingestion of raw or roasted almond decreased significantly (P < 0.05) by day 14 or day 28, respectively. Compared with roasted almond group, the numbers of faecal Enterococcus spp. populations in raw almond group were significantly lower (P < 0.05) on day 14, but there was no significant difference on day 28. No significant difference in viable counts of E. coli was observed among the control, raw and roasted groups.
Figure 1.
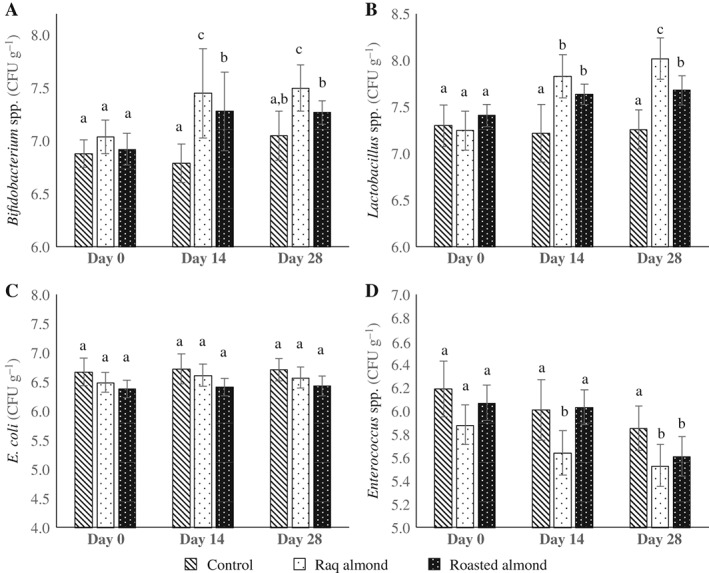
Effect of raw and roasted almond intake on faecal intestinal bacteria of rats. Faecal samples were collected at three time points: Days 0, 14 and 28 for the enumeration of Bifidobacterium spp. (A), Lactobacillus spp. (B), E. coli (C) and Enterococcus spp. (D). Values are expressed as the mean of the log10 number ± SD (CFU) per gram of wet faeces. Different letters of a–c indicate significantly different means at P < 0.05, n = 10.
To further confirm the effect of raw and roasted almonds on intestinal microorganisms, the populations of bacteria in ceca were also counted and the results are shown in Fig. 2. Compared with the control, both almond types promoted the growth of Bifidobacterium spp. and Lactobacillus spp. significantly (P < 0.05); E. coli and Enterococcus spp. populations were reduced by up to 1 log and 0.6 log, respectively. The viable counts of Bifidobacterium spp. from the caecal contents of rats fed with roasted almond for 28 days were lower than those fed with raw almond (P < 0.05), which suggested that raw almonds had a slightly stronger bifidobacteria promotion effect. No significant differences between the two almond types were observed for populations of Lactobacillus spp., E. coli and Enterococcus spp.
Figure 2.
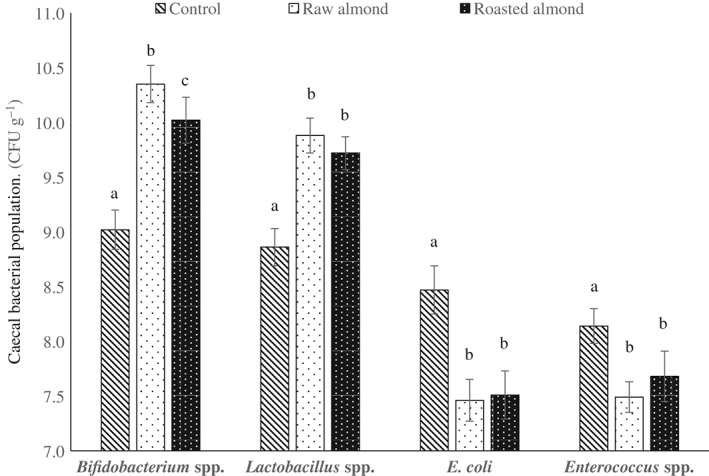
Effect of raw and roasted almond intake on caecal intestinal bacteria of rats. Caecal contents were collected after sacrificing the animals on day 28 for the enumeration of Bifidobacterium spp., Lactobacillus spp., E. coli and Enterococcus spp. Values are expressed as the mean of the log10 number ± SD (CFU) per gram of caecal contents. Within the same bacterium, different letters of a–c indicate significantly different means at P < 0.05, n = 10.
The results of the animal trial demonstrated a selective stimulatory effect on Lactobacillus spp. and Bifidobacterium spp., deemed as beneficial bacteria, and an inhibitory effect on E. coli and Enterococcus spp., deemed as detrimental bacteria. The bifidobacteria and lactobacilli promotion effects may derive from the residual component of almonds (and feed), including the digestible nutrients entrapped in unruptured almond cells and the indigestible carbohydrates such as pectin, hemicellulose, cellulose, and gums. The proliferation of bifidobacteria and lactobacilli could lead to an increased secretion of short‐chain fatty acids, folic acid, and some other organic acids, which could lower the pH of the digestive tract, forming a kind of acidic environment and promoting intestinal peristalsis. Thus, E. coli and Enterococcus spp. populations might be inhibited or reduced, and their metabolites (ammonia, hydrogen sulfide, indole, skatole and some other harmful substances) would also be reduced. Bifidobacteria and lactobacilli may secrete antibacterial agents that have an inhibitory effect on controlling pathogenic bacteria.22 In addition, bifidobacteria and lactobacilli may adhere tightly to the intestinal epithelial cells, take up the space of epithelial cells, and compete with pathogens in terms of nutrition and space, and form a barrier.23, 24
The results of the in vivo studies demonstrated that raw almonds had a greater promotion effect on the growth of bifidobacteria and lactobacilli than did the roasted almonds. The cell walls in almond tissue can behave as physical barriers that hinder the release of entrapped lipid and other nutrients, especially when almonds must be chewed before swallowing.25, 26 However, in this study almonds were ingested by rats after grinding and sieving, with the particle size around 250 µm. According to a theoretical prediction model developed by Grassby et al. 27 and Grundy et al.,28 the nutrient release percentages were similar between raw and roasted almonds at the particle size of 250 µm. In addition, as mentioned above, raw and roasted almonds had the similar protein, lipid and carbohydrate contents. Thus, the residual digestible nutrients in colon for intestinal bacteria were at the same levels, but the indigestible components, mainly fibre, were different between these two types of almonds. According to the USDA nutrient database, the dietary fibre content in raw almond is 12.5%, while for roasted almond it is 10.9%, which is 14.7% lower than that in the raw one. Also, Lin et al. 29 showed that roasting resulted in a substantial loss of phenolic components (total phenols, flavonoids, condensed tannins and phenolic acids) of almond. Bolling et al. 30 also found the skins of roasted almonds had 26% less total phenols than those of raw almonds. It is well known that some kinds of dietary fibre31 and phenols32 can serve as prebiotics to stimulate the growth of bifidobacteria or lactobacilli in the gut. The higher content of fibre and phenols in raw almonds might contribute to the extra stimulatory effect observed in this study. Another possibility for the reduced prebiotic effect of roasted almonds is the formation of Maillard reaction products. Jemmali33 reported that Maillard reaction products may extend the lag phases of lactobacilli and E. coli.
Effect of raw and roasted almond intake on faecal and caecal bacterial enzyme activities in rats
A 4‐week intake of raw or roasted almonds altered the activities of β‐galactosidase, β‐glucuronidase, nitroreductase and azoreductase in the faeces (Table 2) and caecal contents (Table 3). Raw almond intake resulted in a significant increase (P < 0.05) in faecal β‐galactosidase activity on day 14, and both raw and roasted almond intake resulted in increased caecal β‐galactosidase activity (day 28). Significant decreases in faecal and caecal β‐glucuronidase activity were observed after 4 weeks of raw almond ingestion (P < 0.05, on day 28). Significant decreases in faecal and caecal nitroreductase activities were observed on days 14 and 28, respectively, after raw and roasted almond ingestion (P < 0.05,). Faecal and caecal azoreductase activities decreased significantly on day 28, but only in rats in the raw almond group (P < 0.05). In general, 4 weeks of raw or roasted almond intake altered the bacterial enzyme activities in faeces and caecal contents in rats, and more significant changes were observed in the raw almond group than in the roasted almond group.
Table 2.
Effect of raw and roasted almonds intake on faecal bacterial and digestive enzyme activities in rats
| Day 0 | Day 14 | Day 28 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Enzyme | Control | Raw almond | Roasted almond | Control | Raw almond | Roasted almond | Control | Raw almond | Roasted almond |
| β‐Galactosidase | 13.42 ± 0.69a | 13.59 ± 0.71a | 13.39 ± 0.97a | 13.54 ± 0.69a | 14.22 ± 0.67b | 13.76 ± 0.77a | 13.74 ± 0.82a | 13.82 ± 0.15a | 13.97 ± 0.60a |
| β‐Glucuronidase | 13.85 ± 0.37a | 14.06 ± 0.50a | 13.90 ± 0.45a | 13.73 ± 0.46a | 13.83 ± 0.55a | 14.00 ± 0.48a | 13.80 ± 0.17a | 13.33 ± 0.19b | 13.79 ± 0.22a |
| Nitroreductase | 48.89 ± 0.80a | 49.11 ± 1.00a | 50.46 ± 1.10a | 48.86 ± 1.21a | 42.70 ± 1.38b | 43.32 ± 1.21b | 48.61 ± 2.10a | 43.10 ± 1.97b | 42.89 ± 1.38b |
| Azoreductase | 11.74 ± 0.61a | 12.06 ± 0.91a | 12.13 ± 1.04a | 11.72 ± 0.64a | 11.90 ± 0.80a | 12.06 ± 1.03a | 11.81 ± 0.39a | 9.64 ± 0.59b | 11.17 ± 0.72a |
| Protease | 100 ± 9a | 110 ± 17b | 104 ± 22a | 107 ± 11a | 108 ± 25a | 129 ± 37b | 110 ± 28a | 115 ± 13a | 112 ± 29a |
| Amylase | 443 ± 75a | 508 ± 94b | 460 ± 79a | 423 ± 102a | 464 ± 84a | 487 ± 91b | 456 ± 69a | 442 ± 113a | 471 ± 96a |
| Lipase | 933 ± 150a | 1030 ± 195a | 1250 ± 316b | 868 ± 137a | 1460 ± 323b | 1750 ± 568c | 963 ± 109a | 1300 ± 277b | 2150 ± 631c |
Values are expressed as the mean of the digestive enzyme activities (U) ± SD per gram of wet faeces.
Within the same row, mean values with different letters are significantly different at P < 0.05, n = 10.
Table 3.
Effect of raw and roasted almonds intake on caecal bacterial and digestive enzyme activities in rats (at day 28)
| Enzyme | Control | Raw almond | Roasted almond |
|---|---|---|---|
| β‐Galactosidase | 17.74 ± 0.39a | 21.03 ± 1.01b | 19.33 ± 1.15c |
| β‐Glucuronidase | 16.29 ± 0.61a | 14.28 ± 0.63b | 15.71 ± 0.74a |
| Nitroreductase | 52.77 ± 1.20a | 48.39 ± 1.41b | 48.20 ± 1.10b |
| Azoreductase | 15.41 ± 0.86a | 13.28 ± 0.49b | 14.50 ± 0.69a |
| Protease | 173 ± 31a | 234 ± 37b | 246 ± 42b |
| Amylase | 924 ± 84a | 1390 ± 165b | 1280 ± 129b |
| Lipase | 1700 ± 134a | 2580 ± 231b | 3520 ± 386c |
Values are expressed as the mean of the digestive enzyme activities (U) ± SD per gram of cecal contents.
Within the same row, mean values with different letters are significantly different at P < 0.05, n = 10.
Diet may alter the intestinal microbiota structure, which subsequently may alter the intestinal microbial enzymes, thus resulting in changes in the compounds generated by bacterial metabolism. Dietary fibre is one of the substrates that may alter intestinal microbial enzyme activities.34 In our other study, we found a significant increase in faecal β‐galactosidase activity and decreases in faecal β‐glucuronidase, nitroreductase and azoreductase activities after the consumption of almond or almond skin by healthy adult subjects.17
Similar enzyme activity results were observed in this animal trial. The faecal and caecal β‐galactosidase activities increased significantly over the 4 weeks of raw or roasted almond ingestion, and raw almonds seemed to be more effective in improving the intestinal β‐galactosidase activities. This enzyme plays a positive role in the human gut: the impairment of glycosidase activity might induce a failure in metabolism of unabsorbed carbohydrates, such as that reported in ulcerative colitis and Crohn's disease.35 It has been reported that β‐galactosidase can be synthesised by bifidobacteria and lactobacilli,36 thus elevated viable counts of bifidobacteria and lactobacilli may induce the increase of β‐galactosidase activities. In this study, faecal and caecal β‐glucuronidase, nitroreductase and azoreductase activities decreased significantly over the 4 weeks of raw almond ingestion, but with roasted almond ingestion, only nitroreductase activity changed significantly. It has been reported that the production of toxic bacterial enzymes, such as β‐glucuronidase, nitroreductase and azoreductase, could be influenced by feeding dietary fibre34 and some prebiotics, such as galactooligosaccharides.36 Compared with roasted almonds, raw almonds possessed greater modulation effect of intestinal bacterial enzymes, which might due to the greater stimulatory effect on Lactobacillus spp. and Bifidobacterium spp. and inhibitory effect on E. coli and Enterococcus spp. of raw almonds.
Effect of raw and roasted almond intake on faecal and caecal digestive enzyme activities in rats
The activities of protease, amylase and lipase in the rat faeces and caecal contents are shown in Table 2 and Table 3, respectively. The activities of protease and amylase in the faeces of rats fed almonds (raw and roasted) varied during the 3 days of determination. Whereas the lipase activities in the faeces of both almond‐fed groups were significantly higher than in the control group (P < 0.05), the lipase activities for the raw almond group were lower than those for roasted almond group on the same day of determination (P < 0.05). The caecal digestive enzyme activities for both almond groups were significantly higher than for the control (P < 0.05). Lipase activities in caecal contents for the roasted almond group were twice that of the control group.
The results suggest that almond ingestion for rats not only brought about changes in the composition of intestinal microbiota, but also caused alterations in the activities of digestive enzymes that play an essential role in the process of metabolism. The changes in activities of intestinal digestive enzymes might reflect the ability to digest food and access the energy value. Many factors can impact digestive enzyme activities, such as enteral chyme characters, pH, age and species of animals. It was also possible that the improvement of digestive enzyme activities was induced by the proliferation of bifidobacteria and lactobacilli. Many studies have demonstrated that probiotics,37 prebiotics38 and synbiotics39 ingestion could improve the activity level of digestive enzymes. For example, Soleimani et al. 38 found that digestive enzyme activities (amylase, lipase and protease) were significantly elevated with increasing levels of dietary prebiotic fructooligosaccharide (FOS) in Caspian roach (Rutilus rutilus) fry. In the current study, a healthier gastrointestinal microbial ecology formed by raw or roasted almond intake, which was similar to probiotics, prebiotics or synbiotics ingestion, may be the cause of the improvement of digestive enzyme activities.
The higher lipase activities presented in faeces and caecal contents of rats treated with roasted almonds might due to the changes in physical structure and chemical composition of kernels during the roasting process. Kong and Singh40 revealed that roasting process significantly improved the digestion rates of almonds and might facilitate an effective digestion. However, the factors that affect digestive activity are complex, and the precise mechanisms of action have essentially remained elusive.
CONCLUSIONS
In summary, both raw and roasted almonds, hydrolysed under simulated gastric and duodenal digestion, similarly promoted the growth of L. acidophilus and B. breve in vitro. Raw and roasted almond intake appeared to modulate the intestinal microenvironment in rats via stimulating beneficial bacteria, inhibiting harmful bacteria, regulating the activities of various bacterial enzymes and improving metabolic activities. Thus, raw and roasted almonds may possess potential prebiotic properties. Raw almonds had a greater stimulatory effect on faecal Lactobacillus spp., and on faecal and caecal Bifidobacterium spp. and inhibitory effect on faecal Enterococcus spp. than did the roasted almonds. Raw almonds also possessed greater bacterial enzyme, including β‐galactosidase, β‐glucuronidase and azoreductase, regulation effects, which were correlated with the changes of intestinal bacteria. The higher content of dietary fibre and phenols might contribute to the greater prebiotic effect of raw almonds. In terms of the effect on metabolic activities, the almond roasting process might facilitate the release of nutrients from the nuts and thus result in increased intestinal lipase activity upon ingestion.
ACKNOWLEDGEMENTS
We would like to express sincere thanks to the Almond Board of California and the National Natural Science Foundation of China (Nos. 31501494, 31371820 and 31271859) for the financial support of this study. We would also like to express our appreciation to Fuzhou General Hospital of Nanjing Military Command for laboratory support and to Dr Karen Lapsley, Chief Scientific Officer of the Almond Board of California, for providing the almond products and helpful suggestions.
REFERENCES
- 1. Simon GL and Gorbach SL, Intestinal flora in health and disease. Gastroenterology 86:174–193 (1984). [PubMed] [Google Scholar]
- 2. Salminen S, Bouley C, Boutron‐Ruault MC, Cummings JH, Franck A, Gibson GR et al., Functional food science and gastrointestinal physiology and function. Br J Nutr 80:S147–S171 (1998). [DOI] [PubMed] [Google Scholar]
- 3. Gibson GR and Roberfroid MB, Dietary modulation of the human colonic microbiota – introducing the concept of prebiotics. J Nutr 125:1401–1412 (1995). [DOI] [PubMed] [Google Scholar]
- 4. Kaplan H and Hutkins RW, Fermentation of fructooligosaccharides by lactic acid bacteria and bifidobacteria. Appl Environ Microbiol 66:2682–2684 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schrezenmeir J and de Vrese M, Probiotics, prebiotics, and synbiotics – approaching a definition. Am J Clin Nutr 73:361s–364s (2001). [DOI] [PubMed] [Google Scholar]
- 6. Gibson GR and Wang X, Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol 77:412–420 (1994). [DOI] [PubMed] [Google Scholar]
- 7. Hudault S, Lievin V, BernetCamard MF and Servin AL, Antagonistic activity exerted in vitro and in vivo by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infection. Appl Environ Microbiol 63:513–518 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hyson DA, Schneeman BO and Davis PA, Almonds and almond oil have similar effects on plasma lipids and LDL oxidation in healthy men and women. J Nutr 132:703–707 (2002). [DOI] [PubMed] [Google Scholar]
- 9. Spiller GA, Jenkins DJA, Cragen LN, Gates JE, Bosello O, Berra K et al., Effect of a diet high in monounsaturated fat from almonds on plasma‐cholesterol and lipoproteins. J Am Coll Nutr 11:126–130 (1992). [PubMed] [Google Scholar]
- 10. Barreira JCM, Ferreira ICFR, Oliveira MBPP and Pereira JA, Antioxidant activity and bioactive compounds of ten Portuguese regional and commercial almond cultivars. Food Chem Toxicol 46:2230–2235 (2008). [DOI] [PubMed] [Google Scholar]
- 11. Sang SM, Lapsley K, Jeong WS, Lachance PA, Ho CT and Rosen RT, Antioxidative phenolic compounds isolated from almond skins (Prunus amygdalus batsch). J Agric Food Chem 50:2459–2463 (2002). [DOI] [PubMed] [Google Scholar]
- 12. Jia XD, Li N, Zhang WZ, Zhang XP, Lapsley K, Huang GW et al., A pilot study on the effects of almond consumption on DNA damage and oxidative stress in smokers. Nutr Cancer 54:179–183 (2006). [DOI] [PubMed] [Google Scholar]
- 13. Hollis J and Mattes R, Effect of chronic consumption of almonds on body weight in healthy humans. Br J Nutr 98:651–656 (2007). [DOI] [PubMed] [Google Scholar]
- 14. Mandalari G, Tomaino A, Faulks RM, Arcoraci T, Bisignano G, Saija A et al., Almonds (Amygdalus communis L.) as a possible source of prebiotic functional food. Faseb J 22:698.1 (2008). [Google Scholar]
- 15. Mandalari G, Faulks RM, Bisignano C, Waldron KW, Narbad A and Wickham MSJ, In vitro evaluation of the prebiotic properties of almond skins (Amygdalus communis L.). Fems Microbiol Lett 304:116–122 (2010). [DOI] [PubMed] [Google Scholar]
- 16. Mandalari G, Nueno‐Palop C, Bisignano G, Wickham MSJ and Narbad A, Potential prebiotic properties of almond (Amygdalus communis L.) seeds. Appl Environ Microbiol 74:4264–4270 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu ZB, Lin XC, Huang GW, Zhang W, Rao PF and Ni L, Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe 26:1–6 (2014). [DOI] [PubMed] [Google Scholar]
- 18. Amrein TM, Andres L, Schonbachler B, Conde‐Petit B, Escher F and Amado R, Acrylamide in almond products. Eur Food Res Technol 221:14–18 (2005). [Google Scholar]
- 19. Zhang G, Huang GW, Xiao L and Mitchell AE, Determination of advanced glycation end‐products by LC‐MS/MS in raw and roasted almonds (Prunus dulcis). J Agric Food Chem 59:12037–12046 (2011). [DOI] [PubMed] [Google Scholar]
- 20. Gil‐Izquierdo A, Zafrilla P and Tomás‐Barberán FA, An in vitro method to simulate phenolic compound release from the food matrix in the gastrointestinal tract. Eur Food Res Technol 214:155–159 (2002). [Google Scholar]
- 21. Delaney ML and Onderdonk AB, Evaluation of the AnaeroPack system for growth of clinically significant anaerobes. J Clin Microbiol 35:558–562 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gopal PK, Prasad J, Smart J and Gill HS, In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Escherichia coli . Int J Food Microbiol 67:207–216 (2001). [DOI] [PubMed] [Google Scholar]
- 23. Candela M, Perna F, Carnevali P, Vitali B, Ciati R, Gionchetti P et al., Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL‐8 production. Int J Food Microbiol 125:286–292 (2008). [DOI] [PubMed] [Google Scholar]
- 24. Turroni F, Ventura M, Buttó LF, Duranti S, O'Toole PW, Motherway MOC et al., Molecular dialogue between the human gut microbiota and the host: a Lactobacillus and Bifidobacterium perspective. Cell Mol Life Sci 71:183–203 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ellis PR, Kendall CW, Ren Y, Parker C, Pacy JF, Waldron KW et al., Role of cell walls in the bioaccessibility of lipids in almond seeds. Am J Clin Nutr 80:604–613 (2004). [DOI] [PubMed] [Google Scholar]
- 26. Grundy MM, Grassby T, Mandalari G, Waldron KW, Butterworth PJ, Berry SE et al., Effect of mastication on lipid bioaccessibility of almonds in a randomized human study and its implications for digestion kinetics, metabolizable energy, and postprandial lipemia. Am J Clin Nutr 101:25–33 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grassby T, Picout DR, Mandalari G, Faulks RM, Kendall CW, Rich GT et al., Modelling of nutrient bioaccessibility in almond seeds based on the fracture properties of their cell walls. Food Funct 5:3096–3106 (2014). [DOI] [PubMed] [Google Scholar]
- 28. Grundy MM, Wilde PJ, Butterworth PJ, Gray R and Ellis PR, Impact of cell wall encapsulation of almonds on in vitro duodenal lipolysis. Food Chem 185:405–412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin J‐T, Liu S‐C, Hu C‐C, Shyu Y‐S, Hsu C‐Y and Yang D‐J, Effects of roasting temperature and duration on fatty acid composition, phenolic composition, Maillard reaction degree and antioxidant attribute of almond (Prunus dulcis) kernel. Food Chem 190:520–528 (2016). [DOI] [PubMed] [Google Scholar]
- 30. Bolling BW, Mckay DL and Blumberg JB, The phytochemical composition and antioxidant actions of tree nuts. Asia Pac J Clin Nutr 19:117–123 (2010). [PMC free article] [PubMed] [Google Scholar]
- 31. Schley P and Field C, The immune‐enhancing effects of dietary fibres and prebiotics. Br J Nutr 87:S221–S230 (2002). [DOI] [PubMed] [Google Scholar]
- 32. Ávila M, Hidalgo M, Sánchez‐Moreno C, Pelaez C, Requena T and de Pascual‐Teresa S, Bioconversion of anthocyanin glycosides by Bifidobacteria and Lactobacillus . Food Res Int 42:1453–1461 (2009). [Google Scholar]
- 33. Jemmali M, Influence of the Maillard reaction products on some bacteria of the intestinal flora. J Appl Bacteriol 32:151–155 (1969). [DOI] [PubMed] [Google Scholar]
- 34. Shiau S‐Y and Chang GW, Effects of dietary fiber on fecal mucinase and beta‐glucuronidase activity in rats. J Nutr 113:138–144 (1983). [DOI] [PubMed] [Google Scholar]
- 35. Brigidi P, Vitali B, Swennen E, Bazzocchi G and Matteuzzi D, Effects of probiotic administration upon the composition and enzymatic activity of human fecal microbiota in patients with irritable bowel syndrome or functional diarrhea. Res Microbiol 152:735–741 (2001). [DOI] [PubMed] [Google Scholar]
- 36. Osman A, Tzortzis G, Rastall RA and Charalampopoulos D, BbgIV Is an important bifidobacterium β‐galactosidase for the synthesis of prebiotic galactooligosaccharides at high temperatures. J Agric Food Chem 60:740–748 (2012). [DOI] [PubMed] [Google Scholar]
- 37. Fang C, Ma M, Ji H, Ren T and Mims SD, Alterations of digestive enzyme activities, intestinal morphology and microbiota in juvenile paddlefish, Polyodon spathula, fed dietary probiotics. Fish Physiol Biochem 41:91–105 (2015). [DOI] [PubMed] [Google Scholar]
- 38. Soleimani N, Hoseinifar SH, Merrifield DL, Barati M and Abadi ZH, Dietary supplementation of fructooligosaccharide (FOS) improves the innate immune response, stress resistance, digestive enzyme activities and growth performance of Caspian roach (Rutilus rutilus) fry. Fish Shellfish Immun 32:316–321 (2012). [DOI] [PubMed] [Google Scholar]
- 39. Yang S‐C, Chen J‐Y, Shang H‐F, Cheng T‐Y, Tsou SC and Chen J‐R, Effect of synbiotics on intestinal microflora and digestive enzyme activities in rats. World J Gastroenterol 11:7413 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kong FB and Singh RP, Digestion of raw and roasted almonds in simulated gastric environment. Food Biophys 4:365–377 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]


