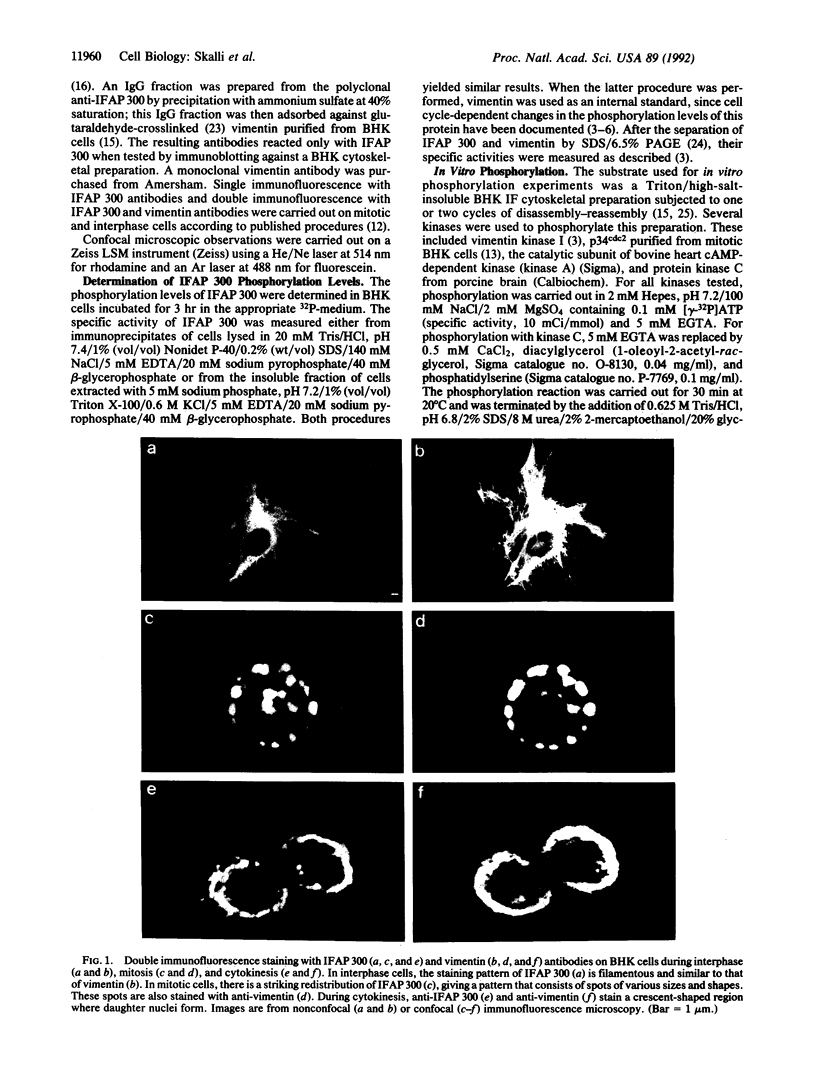
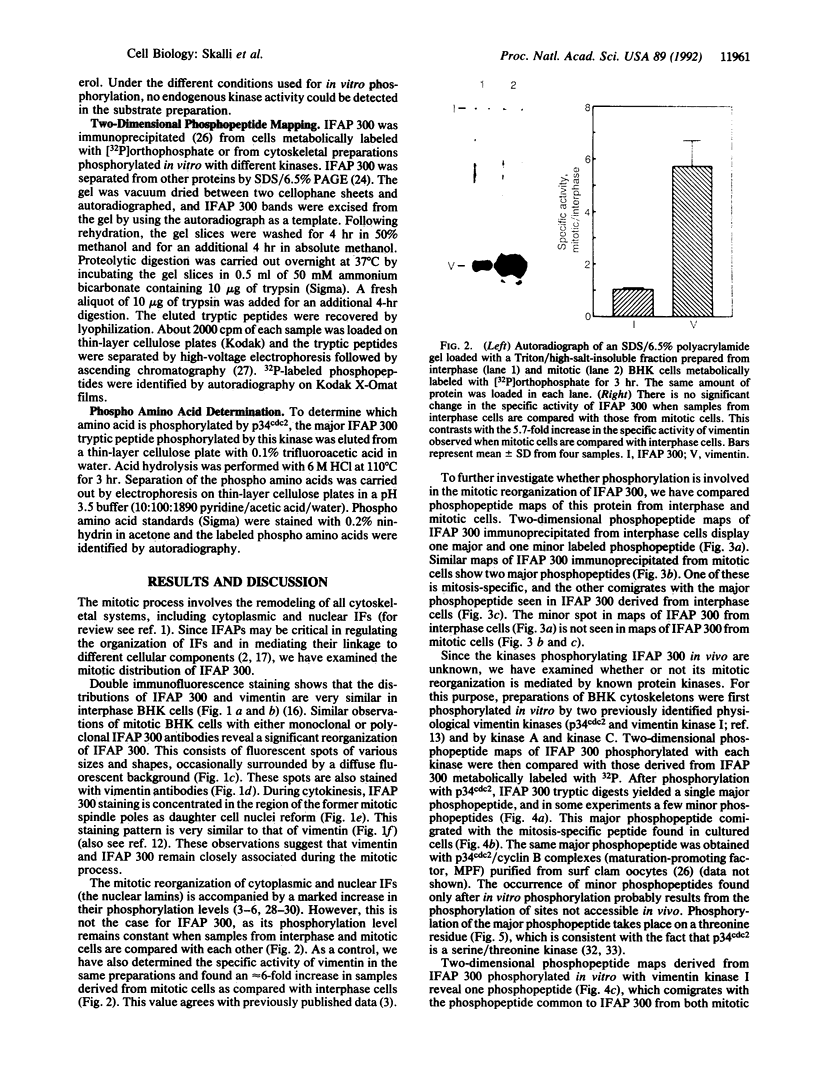
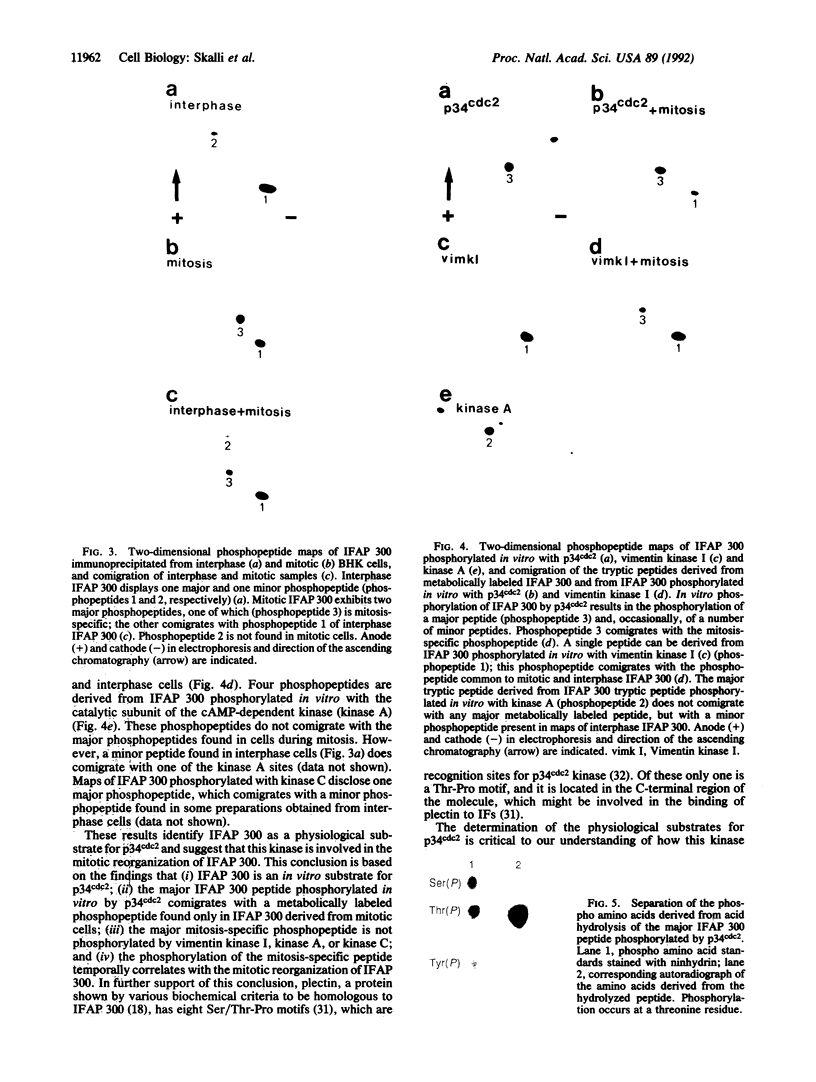
Abstract
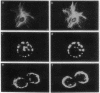
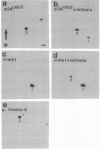
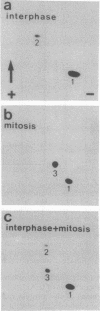
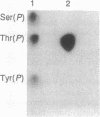
During mitosis in BHK-21 baby hamster kidney cells the hyperphosphorylation of the type III intermediate filament (IF) protein vimentin is accompanied by the disruption of the IF network into punctate, protofilamentous structures. In this study, the morphological and biochemical changes of IFAP 300, a 300-kDa IF-crossbridging protein, are examined during mitosis. Double-label immunofluorescence shows that the distribution of IFAP 300 coincides with the typical filamentous pattern displayed by vimentin in interphase cells, whereas in mitotic cells it is reorganized into a punctate, nonfilamentous pattern. Accompanying these latter morphological changes, IFAP 300 is phosphorylated at a unique, mitosis-specific site. Comparison of the sites phosphorylated in cultured cells with those phosphorylated in vitro by various kinases suggests that IFAP 300 is phosphorylated by the same two kinases that phosphorylate vimentin during mitosis. One of these is p34cdc2 protein kinase, which appears to be responsible for the phosphorylation of the mitosis-specific site. The other kinase phosphorylates IFAP 300 in vitro at a site that is also found in the protein immunoprecipitated from either mitotic or interphase cells. In contrast to vimentin, the phosphorylation levels of IFAP 300 are not obviously altered between interphase and mitosis. Our results show that IFAP 300 is a physiological substrate for p34cdc2 and that this kinase may be involved in the mitotic reorganization of IFAP 300 by phosphorylating a mitosis-specific site. Taken together with our previous results, this study suggests that the activation of p34cdc2 coordinates the mitotic reorganization of the vimentin IF network both by severing IF-IF connections mediated by IFAP 300 and by disassembling individual IFs into protofilaments.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Larsen P. M., Fey S. J., Celis A. Phosphorylation of keratin and vimentin polypeptides in normal and transformed mitotic human epithelial amnion cells: behavior of keratin and vimentin filaments during mitosis. J Cell Biol. 1983 Nov;97(5 Pt 1):1429–1434. doi: 10.1083/jcb.97.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y. H., Bischoff J. R., Beach D., Goldman R. D. Intermediate filament reorganization during mitosis is mediated by p34cdc2 phosphorylation of vimentin. Cell. 1990 Sep 21;62(6):1063–1071. doi: 10.1016/0092-8674(90)90384-q. [DOI] [PubMed] [Google Scholar]
- Chou Y. H., Rosevear E., Goldman R. D. Phosphorylation and disassembly of intermediate filaments in mitotic cells. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1885–1889. doi: 10.1073/pnas.86.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessev G., Goldman R. Meiotic breakdown of nuclear envelope in oocytes of Spisula solidissima involves phosphorylation and release of nuclear lamin. Dev Biol. 1988 Dec;130(2):543–550. doi: 10.1016/0012-1606(88)90349-1. [DOI] [PubMed] [Google Scholar]
- Dessev G., Iovcheva-Dessev C., Bischoff J. R., Beach D., Goldman R. A complex containing p34cdc2 and cyclin B phosphorylates the nuclear lamin and disassembles nuclei of clam oocytes in vitro. J Cell Biol. 1991 Feb;112(4):523–533. doi: 10.1083/jcb.112.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M., Fink L. M. An alteration in the phosphorylation of vimentin-type intermediate filaments is associated with mitosis in cultured mammalian cells. Cell. 1982 May;29(1):43–52. doi: 10.1016/0092-8674(82)90088-5. [DOI] [PubMed] [Google Scholar]
- Fey S. J., Larsen P. M., Celis J. E. Evidence for coordinated phosphorylation of keratins and vimentin during mitosis in transformed human amnion cells. Phosphate turnover of modified proteins. FEBS Lett. 1983 Jun 27;157(1):165–169. doi: 10.1016/0014-5793(83)81138-7. [DOI] [PubMed] [Google Scholar]
- Foisner R., Traub P., Wiche G. Protein kinase A- and protein kinase C-regulated interaction of plectin with lamin B and vimentin. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3812–3816. doi: 10.1073/pnas.88.9.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisner R., Wiche G. Structure and hydrodynamic properties of plectin molecules. J Mol Biol. 1987 Dec 5;198(3):515–531. doi: 10.1016/0022-2836(87)90297-x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Grund C., Geiger B. Intermediate filament proteins in nonfilamentous structures: transient disintegration and inclusion of subunit proteins in granular aggregates. Cell. 1982 Aug;30(1):103–113. doi: 10.1016/0092-8674(82)90016-2. [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Chou Y. H., Dessev C., Dessev G., Eriksson J., Goldman A., Khuon S., Kohnken R., Lowy M., Miller R. Dynamic aspects of cytoskeletal and karyoskeletal intermediate filament systems during the cell cycle. Cold Spring Harb Symp Quant Biol. 1991;56:629–642. doi: 10.1101/sqb.1991.056.01.072. [DOI] [PubMed] [Google Scholar]
- Heald R., McKeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell. 1990 May 18;61(4):579–589. doi: 10.1016/0092-8674(90)90470-y. [DOI] [PubMed] [Google Scholar]
- Herrmann H., Wiche G. Plectin and IFAP-300K are homologous proteins binding to microtubule-associated proteins 1 and 2 and to the 240-kilodalton subunit of spectrin. J Biol Chem. 1987 Jan 25;262(3):1320–1325. [PubMed] [Google Scholar]
- Horwitz B., Kupfer H., Eshhar Z., Geiger B. Reorganization of arrays of prekeratin filaments during mitosis. Immunofluorescence microscopy with multiclonal and monoclonal prekeratin antibodies. Exp Cell Res. 1981 Aug;134(2):281–290. doi: 10.1016/0014-4827(81)90427-4. [DOI] [PubMed] [Google Scholar]
- Jones J. C., Goldman A. E., Yang H. Y., Goldman R. D. The organizational fate of intermediate filament networks in two epithelial cell types during mitosis. J Cell Biol. 1985 Jan;100(1):93–102. doi: 10.1083/jcb.100.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb N. J., Cavadore J. C., Labbe J. C., Maurer R. A., Fernandez A. Inhibition of cAMP-dependent protein kinase plays a key role in the induction of mitosis and nuclear envelope breakdown in mammalian cells. EMBO J. 1991 Jun;10(6):1523–1533. doi: 10.1002/j.1460-2075.1991.tb07672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Watrin A., Labbé J. C., Cavadore J. C. Microinjection of p34cdc2 kinase induces marked changes in cell shape, cytoskeletal organization, and chromatin structure in mammalian fibroblasts. Cell. 1990 Jan 12;60(1):151–165. doi: 10.1016/0092-8674(90)90725-t. [DOI] [PubMed] [Google Scholar]
- Lane E. B., Goodman S. L., Trejdosiewicz L. K. Disruption of the keratin filament network during epithelial cell division. EMBO J. 1982;1(11):1365–1372. doi: 10.1002/j.1460-2075.1982.tb01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieska N., Yang H. Y., Goldman R. D. Purification of the 300K intermediate filament-associated protein and its in vitro recombination with intermediate filaments. J Cell Biol. 1985 Sep;101(3):802–813. doi: 10.1083/jcb.101.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miake-Lye R., Kirschner M. W. Induction of early mitotic events in a cell-free system. Cell. 1985 May;41(1):165–175. doi: 10.1016/0092-8674(85)90071-6. [DOI] [PubMed] [Google Scholar]
- Moreno S., Nurse P. Substrates for p34cdc2: in vivo veritas? Cell. 1990 May 18;61(4):549–551. doi: 10.1016/0092-8674(90)90463-o. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- O'Connor C. M., Gard D. L., Lazarides E. Phosphorylation of intermediate filament proteins by cAMP-dependent protein kinases. Cell. 1981 Jan;23(1):135–143. doi: 10.1016/0092-8674(81)90278-6. [DOI] [PubMed] [Google Scholar]
- Ottaviano Y., Gerace L. Phosphorylation of the nuclear lamins during interphase and mitosis. J Biol Chem. 1985 Jan 10;260(1):624–632. [PubMed] [Google Scholar]
- Peter M., Nakagawa J., Dorée M., Labbé J. C., Nigg E. A. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990 May 18;61(4):591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- Rosevear E. R., McReynolds M., Goldman R. D. Dynamic properties of intermediate filaments: disassembly and reassembly during mitosis in baby hamster kidney cells. Cell Motil Cytoskeleton. 1990;17(3):150–166. doi: 10.1002/cm.970170303. [DOI] [PubMed] [Google Scholar]
- Shenoy S., Choi J. K., Bagrodia S., Copeland T. D., Maller J. L., Shalloway D. Purified maturation promoting factor phosphorylates pp60c-src at the sites phosphorylated during fibroblast mitosis. Cell. 1989 Jun 2;57(5):763–774. doi: 10.1016/0092-8674(89)90791-5. [DOI] [PubMed] [Google Scholar]
- Skalli O., Goldman R. D. Recent insights into the assembly, dynamics, and function of intermediate filament networks. Cell Motil Cytoskeleton. 1991;19(2):67–79. doi: 10.1002/cm.970190202. [DOI] [PubMed] [Google Scholar]
- Starger J. M., Brown W. E., Goldman A. E., Goldman R. D. Biochemical and immunological analysis of rapidly purified 10-nm filaments from baby hamster kidney (BHK-21) cells. J Cell Biol. 1978 Jul;78(1):93–109. doi: 10.1083/jcb.78.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Tobey R. A. Production and characterization of mammalian cells reversibly arrested in G1 by growth in isoleucine-deficient medium. Methods Cell Biol. 1973;6:67–112. doi: 10.1016/s0091-679x(08)60048-5. [DOI] [PubMed] [Google Scholar]
- Tölle H. G., Weber K., Osborn M. Keratin filament disruption in interphase and mitotic cells--how is it induced? Eur J Cell Biol. 1987 Feb;43(1):35–47. [PubMed] [Google Scholar]
- Ward G. E., Kirschner M. W. Identification of cell cycle-regulated phosphorylation sites on nuclear lamin C. Cell. 1990 May 18;61(4):561–577. doi: 10.1016/0092-8674(90)90469-u. [DOI] [PubMed] [Google Scholar]
- Wiche G., Baker M. A. Cytoplasmic network arrays demonstrated by immunolocalization using antibodies to a high molecular weight protein present in cytoskeletal preparations from cultured cells. Exp Cell Res. 1982 Mar;138(1):15–29. doi: 10.1016/0014-4827(82)90086-6. [DOI] [PubMed] [Google Scholar]
- Wiche G., Becker B., Luber K., Weitzer G., Castañon M. J., Hauptmann R., Stratowa C., Stewart M. Cloning and sequencing of rat plectin indicates a 466-kD polypeptide chain with a three-domain structure based on a central alpha-helical coiled coil. J Cell Biol. 1991 Jul;114(1):83–99. doi: 10.1083/jcb.114.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiche G., Krepler R., Artlieb U., Pytela R., Denk H. Occurrence and immunolocalization of plectin in tissues. J Cell Biol. 1983 Sep;97(3):887–901. doi: 10.1083/jcb.97.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. Y., Lieska N., Goldman A. E., Goldman R. D. A 300,000-mol-wt intermediate filament-associated protein in baby hamster kidney (BHK-21) cells. J Cell Biol. 1985 Feb;100(2):620–631. doi: 10.1083/jcb.100.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]