Abstract
Vitamin D (VitD) has a role in the regulation of calcium and phosphate metabolism and in addition impacts the activity of the immune system. VitD deficiency might be linked to increased susceptibility to respiratory tract infection. The aim of the present study was to characterize the impact of VitD deficiency on the susceptibility to bacterial infection in murine models. C57BL/6N mice were fed a diet with or without VitD for 10 weeks. The VitD-deficient or -sufficient mice were infected with Pseudomonas aeruginosa or Streptococcus pneumoniae. The colonization and inflammatory response in the lung were analyzed at defined time points. The serum 25-hydroxy-VitD concentration was significantly lower in mice on the VitD-deficient diet. In infection experiments with Pseudomonas aeruginosa or Streptococcus pneumoniae, no differences could be observed in the numbers of viable bacteria or in differential cell counts in the bronchoalveolar lavage fluids. Measurements of inflammatory cytokines (KC and interleukin-1β [IL-1β]) did not show significant differences between the groups. In conclusion, VitD-deficient animals did not show significantly increased susceptibility to infection or an altered course of infection. The immune systems of humans and mice likely respond differently to VitD. Murine models are likely not appropriate for drawing conclusions on the role of VitD in human pulmonary host defense.
INTRODUCTION
The increased incidence of respiratory infections during wintertime has been recognized for many decades (1). The underlying mechanisms are largely speculative. It appears reasonable that limited exposure to sunlight during this period of the year contributes to the increased susceptibility to infection. It is possible that vitamin D (VitD) could be involved in this phenomenon because the metabolism of this bioactive molecule is highly dependent on exposure of the skin to sunlight (2).
VitD is photosynthesized in the skin or is taken up from food and has to undergo metabolic activation (3). 7-Dehydrocholesterol is the precursor of cholecalciferol, and its conversion into cholecalciferol depends on UVB exposure in the skin. In the liver, cholecalciferol is hydroxylated into calcifediol [25-hydroxyvitamin D3; 25(OH)D3] by the 25-hydroxylase enzymes (gene names CYP27A1 and/or CYP2R1). In the kidney, calcifediol is then hydroxylated at position 1 by the mitochondrial cytochrome P450 enzyme 25-hydroxyvitamin-D-1α-hydroxylase (gene name CYP27B1) and becomes calcitriol [1,25-dihydroxyvitamin D3; 1,25(OH)2D3].
Epidemiological and mechanistic studies show that the VitD metabolism is relevant for the development of pulmonary disease (3–5). This extends the earlier view that VitD is solely responsible for the regulation of calcium and phosphate metabolism. VitD deficiency is associated with respiratory tract infections such as tuberculosis (6, 7), bronchiectasis (8), or pneumonia (9, 10). Also, chronic obstructive pulmonary disease (COPD) and asthma are associated with low serum concentrations of VitD (11–13).
Data from recent years showed that structural cells of the lung and macrophages are able to metabolize VitD derivatives and that VitD is involved in the regulation of pulmonary host defense and inflammation. Macrophages express VitD-metabolizing enzymes as well as the VitD receptor (VDR) and respond to VitD exposure (14, 15). In airway epithelial cells, VitD induces the expression of the antimicrobial peptide cathelicidin (16, 17). VitD deficiency in mice causes alterations in the structure of the lung with decreased functional capacity (18), and deletion of the VDR leads to premature emphysema (19). Surprisingly, a recent paper showed that dietary VitD deficiency did not result in a frank change of the pulmonary response to lipopolysaccharide (LPS) in a mouse model (20).
The aim of the present study was to determine whether VitD deficiency results in a breach of pulmonary host defense. To address this question, we applied murine models of VitD deficiency and pneumonia with Gram-positive Streptococcus pneumoniae and Gram-negative Pseudomonas aeruginosa. Both pathogens are commonly found in cystic fibrosis and COPD, where low VitD serum concentrations correlate with disease severity (21–23).
MATERIALS AND METHODS
Bacterial species.
Pseudomonas aeruginosa PAO1 was cultured on LB agar plates (Roth, Karlsruhe, Germany) overnight and transferred to 35 ml LB medium (Roth, Karlsruhe, Germany) for cultivation for 2 h at 37°C. The bacteria were centrifuged at 1,885 × g for 10 min, and the pellet was washed with phosphate-buffered saline (PBS). The suspension was adjusted to an optical density at 600 nm (OD600) of 1.00. For infection experiments, the suspension was diluted 1:10 with PBS to reach a concentration of 1.3 × 106 to 4.95 × 106 CFU/animal. For heat inactivation (h.i.), the undiluted solution was incubated for 5 min at 95°C and stored in aliquots at −20°C (3.56 × 107 CFU/animal). Streptococcus pneumoniae D39 was grown in THB medium (Roth, Karlsruhe, Germany) to an OD600 of 0.5. The bacteria were centrifuged at 1,885 × g for 10 min, and the pellet was washed with 35 ml PBS. The pellet was dissolved in 4 ml PBS (3.2 × 105 to 2.94 × 106 CFU/animal). S. pneumoniae and P. aeruginosa are common representatives of Gram-positive and -negative bacterial infections in humans, respectively.
To induce a persistent (chronic) infection, we used the alginate-overproducing strain P. aeruginosa NH57388A (24). The bacteria were grown in Trypticase soy agar II with 5% sheep blood (BD Bioscience, Heidelberg, Germany) for 2 days. The suspension was diluted to an OD600 of 0.4, and 6 ml was centrifuged at 14,000 rpm at 4°C for 30 min. The supernatant was incubated at 80°C for 30 min to inactivate bacteria. The cotton-like alginate was separated and washed once with ice-cold ethanol and three times with NaCl 0.9%. The alginate was dissolved in 1.5 ml of 0.9% NaCl and stored at 4°C. The pellet of bacterial cells was then dissolved in 300 μl alginate solution.
Animal experiments.
C57BL/6N mice were purchased from Charles River (Cologne, Germany). The animals were used at the age of 8 weeks and were fed with a control (containing 500 IU VitD/kg of body weight) or 25(OH)D3-deficient diet (Altromin, Lage, Germany) for 10 weeks (animals termed here “VitD deficient” or “VitD depleted”). Animals were housed at the animal facility of Saarland University. All animal experiments were approved by the Landesamt für Soziales, Gesundheit und Verbraucherschutz of the State of Saarland according to the national guidelines for animal treatment. Mice were maintained under a pathogen-free condition.
To determine the influence of VitD on the susceptibility to infection, the mice were inoculated with h.i. or viable bacteria, and PBS was used as a control. The mice were anesthetized with a mixture of ketamine (7 mg/kg of body weight; Bayer, Leverkusen, Germany) and xylazine (105 mg/kg of body weight; Bayer) and infected intranasally with 40 μl of the bacterial solution or PBS alone. The mice were euthanized at 6 h, 24 h, 3 days, or 5 days postinfection by an overdose of ketamine (35 mg/kg of body weight) and xylazine (525 mg/kg of body weight). Blood was taken directly from the heart, mixed with a drop of EDTA, and centrifuged for 10 min at 10,000 × g. The plasma was collected and stored at −80°C. The trachea was cannulated, and the lungs were rinsed three times with 1 ml PBS to obtain bronchoalveolar lavage fluid (BALF). To determine the number of viable bacteria in the bronchoalveolar compartments, serial dilutions of the BALF were plated on agar plates and incubated overnight. The BALF was centrifuged for 10 min at 300 × g, and the supernatants were stored at −80°C. The cell pellet was dissolved in an appropriate volume of PBS, and a differential cell count was done on cytospins after cell staining with Diff-Quick (Medion Diagnostic, Gräfelfing, Germany). The lungs were removed, and one lung was directly frozen into liquid nitrogen and stored at −80°C. The left lung was homogenized in 1 ml PBS. Serial dilutions were plated on agar plates.
Determination of 25(OH)D3 concentration in mouse serum.
The levels of serum 25(OH)D3 were measured by the central laboratory of the Saarland University Medical Center by chemiluminescence using the Liaison 25-OH vitamin D total assay (DiaSorin Inc., Stillwater, MN, USA).
Measurement of inflammatory mediators.
The concentrations of the inflammatory mediators KC (murine functional homologue to CXCL8/interleukin-8 [IL-8]) and IL-1β were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA) using a Tecan Ultra 384 ELISA reader and Magellan software (Tecan, Mainz, Germany).
Statistical analysis.
Values are displayed as means ± standard errors of the means (SEM). The data were analyzed using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA). Comparisons were analyzed by the t test (two-sided) between two groups or by analysis of variance (ANOVA) for more than two groups. Results were considered statistically significant for P values less than 0.05.
RESULTS
Dietary 25(OH)D3 depletion leads to reduced serum VitD concentrations in a murine model.
To investigate the influence of VitD on the immune system, we generated a VitD-deficient mouse model. Eight-week-old mice were fed with the control or the VitD-deficient diet as described in Materials and Methods. The average levels of 25(OH)D3 in serum were 30.8 (±2.1) ng/ml in the control group and below the detectable minimum of 4 ng/ml in the VitD-deficient mice except for two measurements (4.21 ng/ml and 15.7 ng/ml).
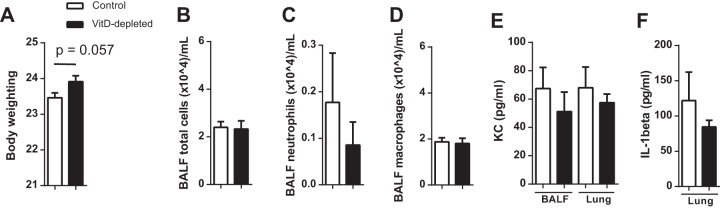
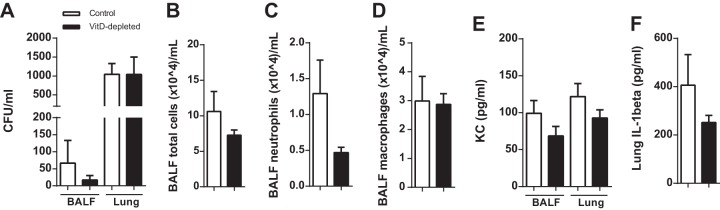
To investigate whether VitD depletion results in changes of the gross phenotype, we determined the body weight, BALF differential counts, and BALF cytokine levels. The medians of body weight did not differ significantly between the two groups (Fig. 1A). The numbers of leukocytes in the BALF (Fig. 1B to D) and the levels of KC and IL-1β were not altered in the VitD-depleted animals (Fig. 1E and F).
FIG 1.
VitD deficiency does not cause a change in body weight and does not induce pulmonary inflammation. Mice were fed with a standard or VitD-deficient diet and analyzed after 10 weeks (n = 20, two replications). (A) All mice were weighed after 10 weeks on diets, and no significant differences were found (n = 110). (B to D) The cells of the BALF were counted and differentiated. The two groups exhibited similar numbers of total cells, neutrophils, and macrophages in the BALF. (E and F) The levels of cytokines in BALF were measured by ELISA. No differences were observed in the concentrations of KC in BALF or lung homogenates (E) and of IL-1β in lung homogenates (F). Data are expressed as means ± standard errors from 10 (A) or two (B to F) independent experiments. Statistical analysis was performed by Mann-Whitney U test. No statistically significant differences between the groups were detected.
VitD deficiency has no impact on the course of acute infection with P. aeruginosa PAO1.
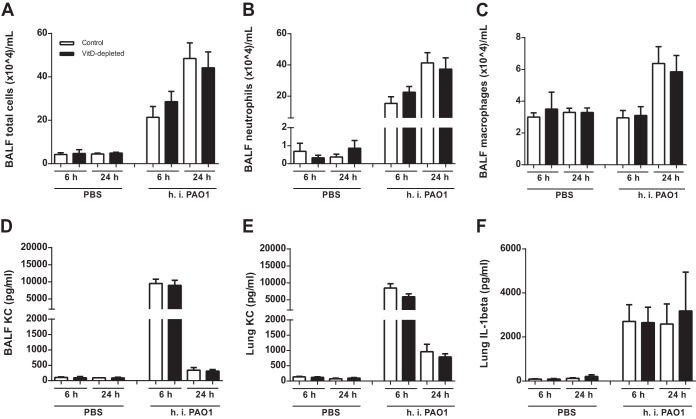
Next, we aimed to determine if VitD depletion leads to an altered disease course in the early stages of acute infection. VitD-deficient and control mice were exposed to h.i. or viable P. aeruginosa PAO1 and euthanized after 6 h or 24 h. The application of h.i. bacteria resulted in an increase of the total BALF cell number (Fig. 2A), neutrophils (Fig. 2B), and macrophages (Fig. 2C) after 6 h and 24 h (Fig. 2A to C). The levels of KC and IL-1β in BALF and lungs were elevated 6 h after application (Fig. 2D to F). No differences in these parameters could be detected between the control and the VitD-deficient groups (Fig. 2).
FIG 2.
VitD deficiency does not alter the inflammatory response after stimulation with heat-inactivated P. aeruginosa PAO1. h.i. P. aeruginosa PAO1 (n = 10) was inoculated into animals, which were analyzed after 6 h or 24 h (2 replications). (A to C) The two groups exhibited similar numbers of total cells, neutrophils, and macrophages in the BALF. (D to F) The levels of cytokines were measured by ELISA. No differences were observed in the concentrations of KC in BALF or lung homogenates (D and E) and of IL-1β in lung homogenates (F). Statistical analysis was performed by ANOVA and t test between control and malnutrition groups with no significant differences.
To investigate whether the course of pneumonia is worse in VitD-deficient animals, mice were infected with viable P. aeruginosa PAO1. The numbers of BALF cells, the levels of inflammatory mediators, and the numbers of viable bacteria were determined. Infection with P. aeruginosa PAO1 resulted in increased numbers of BALF total cells, neutrophils, and macrophages and increased levels of KC and IL-1β (Fig. 3A to G) in both groups. Also, this model did not uncover a significant difference between the VitD-sufficient and -deficient groups (Fig. 3).
FIG 3.
VitD deficiency does not cause changes in response to acute infection with viable P. aeruginosa PAO1. Mice were infected intranasally with viable P. aeruginosa PAO1 (1.3 × 106 to 4.95 × 106 CFU/mouse; n = 10, two replications). (A) The numbers of viable bacteria in BALF and lungs were counted after 6 h or 24 h. No differences between the two groups were found. (B to D) The two groups exhibited similar numbers of total cells, neutrophils, and macrophages in BALF. (E to G) The levels of cytokines were measured by ELISA, and no differences were observed in the concentrations of KC in BALF or in lung homogenates (E and F) and of IL-1β in lung homogenates (G). Statistical analysis was performed by ANOVA and t test between control and malnutrition groups with no significant differences.
VitD deficiency has no impact on the course of subacute infection with P. aeruginosa.
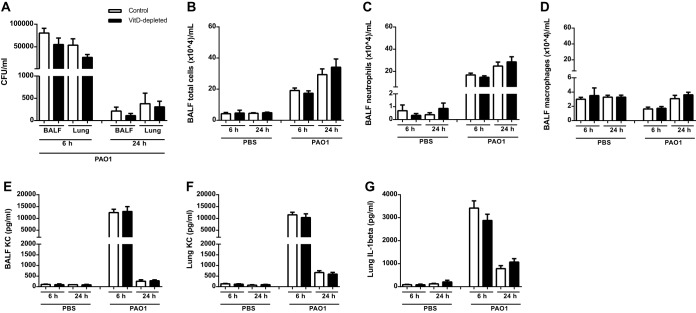
To investigate if a chronic infection with P. aeruginosa is able to uncover a breach in host defense in the VitD-deficient mice, we applied two models of long-term infection. In the first model, we infected the control and the VitD-depleted mice with viable P. aeruginosa PAO1 (8.4 × 106 CFU/mouse) or PBS. After 3 days, most bacteria were cleared from the lungs (Fig. 4A). The numbers of leukocytes and the levels of KC and IL-1β were elevated after infection (Fig. 4B to G). In all data sets, no significant differences between the VitD-depleted and the control group were found (Fig. 4).
FIG 4.
VitD deficiency does not cause alterations of the host defense reaction after subacute infection with P. aeruginosa PAO1. Mice were analyzed 3 days after intranasal infection with viable P. aeruginosa PAO1 (8.4 × 106 CFU/mouse; n = 8, two replications). (A) The numbers of viable bacteria in BALF and lungs were counted 24 h after the end of the experiment. No differences between the two groups were found. (B to D) The two groups exhibited similar numbers of total cells, neutrophils, and macrophages in BALF. (E to G) The levels of cytokines were measured by ELISA, and no differences were observed in the concentrations of KC in BALF or in lung homogenates (E and F) and of IL-1β in lung homogenates (G). Statistical analysis was performed by ANOVA and t test between control and malnutrition groups with no significant differences.
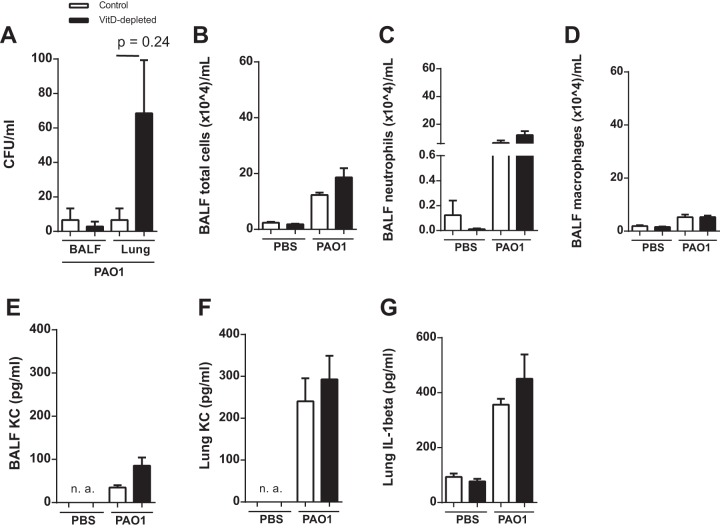
In a different pneumonia model, the mice were infected with P. aeruginosa NH57388A, an alginate-overproducing strain associated with subacute pulmonary infections in murine models (24). The mice were infected intranasally with 1.316 × 107 CFU/mouse and analyzed after 5 days. The changes of body weight were not different between the two groups (data not shown). After 5 days, a significant number of bacteria were found in the lung homogenate (Fig. 5A) with no differences between the experimental groups. In the BALF, no differences in the number of leukocytes and the levels of KC and IL-1β (lung homogenate) could be found between the VitD-sufficient and -deficient groups (Fig. 5B to F).
FIG 5.
VitD deficiency does not result in alterations of the host defense response after subacute infection with P. aeruginosa NH57388A. Mice were analyzed 5 days after intranasal infection with viable P. aeruginosa NH57388A (1.316 × 107 CFU/mouse; n = 10, two replications). (A) The numbers of viable bacteria in BALF and lungs were counted after 6 h or 24 h. No differences between the two groups were found. (B to D) The two groups exhibited similar numbers of total cells, neutrophils, and macrophages in BALF. (E and F) The levels of cytokines were measured by ELISA, and no differences were observed in the concentrations of KC in BALF or in lung homogenates (E) and of IL-1β in lung homogenates (F). Statistical analysis was performed by ANOVA and t test between control and malnutrition groups with no significant differences.
VitD depletion has no impact on the course of acute infection with S. pneumoniae D39.
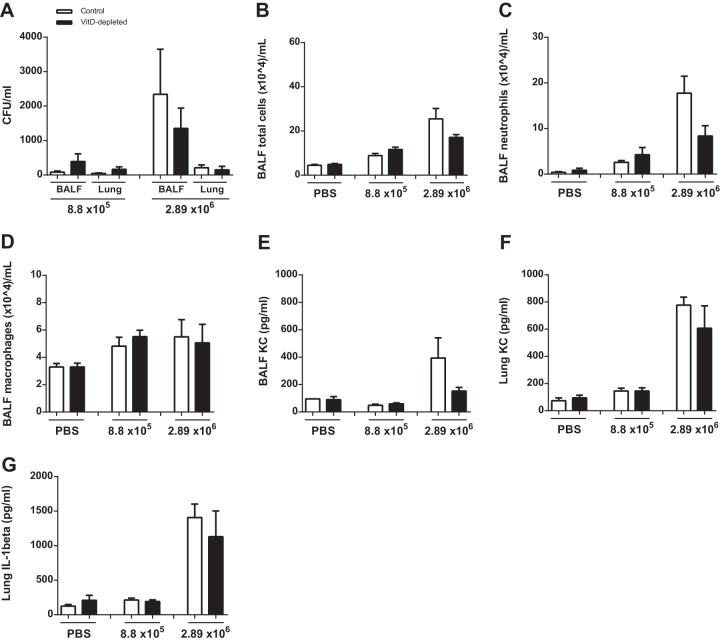
VitD depletion had little effect on models of infection with Gram-negative bacteria. Next, we aimed to investigate whether the response to Gram-positive bacteria depends on VitD status. S. pneumoniae D39 was used in two different doses to reflect different intensities of infection, and the outcomes after 24 h were analyzed. No significant differences could be found in the numbers of viable bacteria between the groups (Fig. 6A). Also, the numbers of inflammatory cells and the levels of cytokines KC and IL-1β were not different between the VitD-depleted and control animals (Fig. 6B to G).
FIG 6.
VitD deficiency does not result in alterations of the response after subacute infection with S. pneumoniae D39. Mice were analyzed 24 h after intranasal stimulation with two different doses of viable S. pneumoniae D39 (8.8 × 105 or 2.94 × 106 CFU/mouse; n = 10, two replications). (A) The numbers of viable bacteria in BALF and lungs were counted. No differences between the VitD-deficient and -sufficient animals were found. (B to D) The two groups exhibited similar numbers of total cells, neutrophils, and macrophages in BALF. (E to G) The levels of cytokines were measured by ELISA, and no differences were observed in the concentrations of KC in BALF or in lung homogenates (E and F) and of IL-1β in lung homogenates (G). Statistical analysis was performed by ANOVA and t test between control and malnutrition groups with no significant differences.
DISCUSSION
The main finding of the current study was that VitD depletion does not cause a significant breach of the host defense against pulmonary bacterial infections in mice.
We applied a mouse model of VitD starvation induced by feeding the animals with a low-VitD diet. To exclude any influence of low VitD serum levels on lung structure (18), we started feeding the low-VitD diet to animals older than 6 weeks, in which alveolarization was completely finished (25). Animals on this diet had less than 80% of the serum VitD level of mice on the control diet (31 ng/ml versus 4 ng/ml), which is in a range achieved by other groups using a VitD-deficient diet (26, 27). VitD depletion did not cause a gross phenotype, as shown by the absence of significant alterations of the body weight or pulmonary inflammation, as determined by measuring BALF cells and cytokine levels. We used Gram-positive and -negative bacteria to account for any dose-dependent effects and also applied acute and subacute models of pneumonia.
We could not identify a significantly increased susceptibility of the VitD-deficient animals to infection with Gram-positive and -negative bacteria. The numbers of viable bacteria were similar between the groups. We also applied h.i. bacteria to characterize the inflammatory responses, which were also found to be similar in VitD-deficient and -sufficient animals. This was surprising as data from clinical investigation and preclinical models indicated that VitD augments host defense through various mechanisms. Observational clinical studies showed that VitD is involved in inflammatory and infectious lung disease (3–5). Low serum concentrations of VitD metabolites were identified in patients with tuberculosis (6, 7), bronchiectasis (8), or pneumonia (9, 10, 23). Data from intervention trials with VitD as an investigational drug provided mixed results, with several studies providing positive outcomes in patients with pneumonia (28), sepsis (29), tuberculosis (30), or cystic fibrosis (31). These data underline that VitD has a complex impact on pulmonary immunity in patients.
Preclinical studies also indicate that the role of VitD in lung host defense is complex. VitD derivatives are able to decrease the release of inflammatory cytokines from macrophages, neutrophils, and epithelial cells (32–36). In a hamster model, the application of VitD leads to reduced neutrophil recruitment and blunted inflammation (32, 37). VitD deficiency resulted in an impairment of host defense and increased inflammation after challenge with Aspergillus fumigatus compared to mice with elevated VitD levels (38). Also, recent studies in mice show that VitD depletion leads to structural changes in the developing lung (18), increased susceptibility to cigarette smoke-induced emphysema (27), and decreased muscle function after cigarette smoke exposure (26). In contrast, VitD deficiency in mice did not cause frank modification of the inflammatory response after the application of LPS (20). In the present study, we were also unable to detect a major impact of VitD deficiency on susceptibility to bacterial infection. Several factors might cause the lack of increased susceptibility to infection in this animal model.
The infection models might not be appropriate, as we have used human pathogens in a murine model.
VitD is not related to pulmonary host defense in general. Based on the literature cited above, it is unlikely that VitD has no modulatory role in host defense. The underlying mechanisms are likely complex, as VitD regulates a multitude of genes and epigenetic mechanisms important for host defense (39). VitD also acts together with several other regulatory mediators or hormones, which also modulate immunity (40).
The host defense and immune response of lung cells are not regulated by VitD. Our models focused on the early stage of an immune response, even when the subacute models were applied. Preclinical models focusing on adaptive immune mechanisms showed consistent effects of VitD on the development of asthma (41) or on the activity of lymphocytes (42). Interestingly, also structural cells of the lung are known to respond to VitD (4, 17, 43).
The regulation of host defense by VitD is complex and involves regulatory mechanisms, some of which might be species specific. Murine cells likely respond differently to VitD than do human cells. One reason might be the differences in the binding site of the VDR in the promoter region of immune genes (44, 45). In mice, VitD deficiency results in increased production of parathyroid hormone (PTH) or PTH-related peptide, which also induces innate host defense reactions (40). These counteractions might mitigate the effect of VitD deficiency. Additionally, the VitD metabolisms are likely different between human and mouse.
In conclusion, VitD deficiency did not cause a breach of pulmonary host defense in murine models of pneumonia. As discussed above, numerous reasons might account for this finding. Based on the results from clinical and preclinical studies, a more detailed knowledge of the complex action of VitD on the host defense system is needed. There is a discrepancy between the mouse models used in the present study and clinical findings. Mouse models appear to be inappropriate to study the role of VitD in host defense of the human lung.
ACKNOWLEDGMENTS
We thank Anja Honecker and Andreas Kamyschnikow for excellent technical support.
Funding Statement
This work was supported by Mukoviszidose eV, 2013.
Footnotes
For a commentary on this article, see doi:10.1128/IAI.00679-16.
REFERENCES
- 1.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E. 2006. Epidemic influenza and vitamin D. Epidemiol Infect 134:1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White AN, Ng V, Spain CV, Johnson CC, Kinlin LM, Fisman DN. 2009. Let the sun shine in: effects of ultraviolet radiation on invasive pneumococcal disease risk in Philadelphia, Pennsylvania. BMC Infect Dis 9:196. doi: 10.1186/1471-2334-9-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herr C, Greulich T, Koczulla RA, Meyer S, Zakharkina T, Branscheidt M, Eschmann R, Bals R. 2011. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir Res 12:31. doi: 10.1186/1465-9921-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansdottir S, Monick MM. 2011. Vitamin D effects on lung immunity and respiratory diseases. Vitam Horm 86:217–237. doi: 10.1016/B978-0-12-386960-9.00009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rigo I, McMahon L, Dhawan P, Christakos S, Yim S, Ryan LK, Diamond G. 2012. Induction of triggering receptor expressed on myeloid cells (TREM-1) in airway epithelial cells by 1,25(OH)(2) vitamin D(3). Innate Immun 18:250–257. doi: 10.1177/1753425911399796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Pasvol G, Lalvani A, Wright D, Latif M, Davidson RN. 2000. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet 355:618–621. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 7.Wayse V, Yousafzai A, Mogale K, Filteau S. 2004. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr 58:563–567. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 8.Chalmers JD, McHugh BJ, Docherty C, Govan JR, Hill AT. 2013. Vitamin-D deficiency is associated with chronic bacterial colonisation and disease severity in bronchiectasis. Thorax 68:39–47. doi: 10.1136/thoraxjnl-2012-202125. [DOI] [PubMed] [Google Scholar]
- 9.Leow L, Simpson T, Cursons R, Karalus N, Hancox RJ. 2011. Vitamin D, innate immunity and outcomes in community acquired pneumonia. Respirology 16:611–616. doi: 10.1111/j.1440-1843.2011.01924.x. [DOI] [PubMed] [Google Scholar]
- 10.Pletz MW, Terkamp C, Schumacher U, Rohde G, Schutte H, Welte T, Bals R, CAPNETZ-Study Group. 2014. Vitamin D deficiency in community-acquired pneumonia: low levels of 1,25(OH)2 D are associated with disease severity. Respir Res 15:53. doi: 10.1186/1465-9921-15-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul G, Brehm JM, Alcorn JF, Holguin F, Aujla SJ, Celedon JC. 2012. Vitamin D and asthma. Am J Respir Crit Care Med 185:124–132. doi: 10.1164/rccm.201108-1502CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moberg M, Ringbaek T, Roberts NB, Vestbo J. 2014. Association between vitamin D status and COPD phenotypes. Lung 192:493–497. doi: 10.1007/s00408-014-9582-9. [DOI] [PubMed] [Google Scholar]
- 13.Black PN, Scragg R. 2005. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the third national health and nutrition examination survey. Chest 128:3792–3798. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. 2012. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 188:2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams JS, Sharma OP, Gacad MA, Singer FR. 1983. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest 72:1856–1860. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. 2007. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3). J Cyst Fibros 6:403–410. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansdottir S, Monick MM, Lovan N, Powers L, Gerke A, Hunninghake GW. 2010. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol 184:965–974. doi: 10.4049/jimmunol.0902840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zosky GR, Berry LJ, Elliot JG, James AL, Gorman S, Hart PH. 2011. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am J Respir Crit Care Med 183:1336–1343. doi: 10.1164/rccm.201010-1596OC. [DOI] [PubMed] [Google Scholar]
- 19.Sundar IK, Hwang JW, Wu S, Sun J, Rahman I. 2011. Deletion of vitamin D receptor leads to premature emphysema/COPD by increased matrix metalloproteinases and lymphoid aggregates formation. Biochem Biophys Res Commun 406:127–133. doi: 10.1016/j.bbrc.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klaff LS, Gill SE, Wisse BE, Mittelsteadt K, Matute-Bello G, Chen P, Altemeier WA. 2012. Lipopolysaccharide-induced lung injury is independent of serum vitamin D concentration. PLoS One 7:e49076. doi: 10.1371/journal.pone.0049076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. 2009. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med 179:630–636. doi: 10.1164/rccm.200810-1576PP. [DOI] [PubMed] [Google Scholar]
- 22.Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, Coolen J, Mathieu C, Decramer M, Lambrechts D. 2010. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 65:215–220. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 23.McCauley LA, Thomas W, Laguna TA, Regelmann WE, Moran A, Polgreen LE. 2014. Vitamin D deficiency is associated with pulmonary exacerbations in children with cystic fibrosis. Ann Am Thorac Soc 11:198–204. doi: 10.1513/AnnalsATS.201208-068OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann N, Rasmussen TB, Jensen PO, Stub C, Hentzer M, Molin S, Ciofu O, Givskov M, Johansen HK, Hoiby N. 2005. Novel mouse model of chronic Pseudomonas aeruginosa lung infection mimicking cystic fibrosis. Infect Immun 73:2504–2514. doi: 10.1128/IAI.73.4.2504-2514.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bäckström E, Hogmalm A, Lappalainen U, Bry K. 2011. Developmental stage is a major determinant of lung injury in a murine model of bronchopulmonary dysplasia. Pediatr Res 69:312–318. doi: 10.1203/PDR.0b013e31820bcb2a. [DOI] [PubMed] [Google Scholar]
- 26.Cielen N, Heulens N, Maes K, Carmeliet G, Mathieu C, Janssens W, Gayan-Ramirez G. 2016. Vitamin D deficiency impairs skeletal muscle function in a smoking mouse model. J Endocrinol 229:97–108. doi: 10.1530/JOE-15-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heulens N, Korf H, Cielen N, De Smidt E, Maes K, Gysemans C, Verbeken E, Gayan-Ramirez G, Mathieu C, Janssens W. 2015. Vitamin D deficiency exacerbates COPD-like characteristics in the lungs of cigarette smoke-exposed mice. Respir Res 16:110. doi: 10.1186/s12931-015-0271-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manaseki-Holland S, Qader G, Isaq Masher M, Bruce J, Zulf Mughal M, Chandramohan D, Walraven G. 2010. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop Med Int Health 15:1148–1155. doi: 10.1111/j.1365-3156.2010.02578.x. [DOI] [PubMed] [Google Scholar]
- 29.Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, Urbanic Purkart T, Waltensdorfer A, Munch A, Warnkross H, Stojakovic T, Bisping E, Toller W, Smolle KH, Berghold A, Pieber TR, Dobnig H. 2014. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA 312:1520–1530. doi: 10.1001/jama.2014.13204. [DOI] [PubMed] [Google Scholar]
- 30.Grossmann RE, Zughaier SM, Kumari M, Seydafkan S, Lyles RH, Liu S, Sueblinvong V, Schechter MS, Stecenko AA, Ziegler TR, Tangpricha V. 2012. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: a randomized, controlled trial. Dermatoendocrinol 4:191–197. doi: 10.4161/derm.20332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coussens AK, Wilkinson RJ, Hanifa Y, Nikolayevskyy V, Elkington PT, Islam K, Timms PM, Venton TR, Bothamley GH, Packe GE, Darmalingam M, Davidson RN, Milburn HJ, Baker LV, Barker RD, Mein CA, Bhaw-Rosun L, Nuamah R, Young DB, Drobniewski FA, Griffiths CJ, Martineau AR. 2012. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc Natl Acad Sci U S A 109:15449–15454. doi: 10.1073/pnas.1200072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takano Y, Mitsuhashi H, Ueno K. 2011. 1α,25-Dihydroxyvitamin D(3) inhibits neutrophil recruitment in hamster model of acute lung injury. Steroids 76:1305–1309. doi: 10.1016/j.steroids.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Kuo YT, Kuo CH, Lam KP, Chu YT, Wang WL, Huang CH, Hung CH. 2010. Effects of vitamin D3 on expression of tumor necrosis factor-alpha and chemokines by monocytes. J Food Sci 75:H200–H204. doi: 10.1111/j.1750-3841.2010.01704.x. [DOI] [PubMed] [Google Scholar]
- 34.Karlsson J, Carlsson G, Larne O, Andersson M, Putsep K. 2008. Vitamin D3 induces pro-LL-37 expression in myeloid precursors from patients with severe congenital neutropenia. J Leukoc Biol 84:1279–1286. doi: 10.1189/jlb.0607437. [DOI] [PubMed] [Google Scholar]
- 35.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. 2004. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 36.Gerke AK, Pezzulo AA, Tang F, Cavanaugh JE, Bair TB, Phillips E, Powers LS, Monick MM. 2014. Effects of vitamin D supplementation on alveolar macrophage gene expression: preliminary results of a randomized, controlled trial. Multidiscip Respir Med 9:18. doi: 10.1186/2049-6958-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang M, Dong M, Liu W, Wang L, Luo Y, Li Z, Jin F. 2014. 1α,25-Dihydroxyvitamin D3 ameliorates seawater aspiration-induced acute lung injury via NF-kappaB and RhoA/Rho kinase pathways. PLoS One 9:e104507. doi: 10.1371/journal.pone.0104507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li P, Xu X, Cao E, Yu B, Li W, Fan M, Huang M, Shi L, Zeng R, Su X, Shi Y. 2014. Vitamin D deficiency causes defective resistance to Aspergillus fumigatus in mice via aggravated and sustained inflammation. PLoS One 9:e99805. doi: 10.1371/journal.pone.0099805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chun RF, Liu PT, Modlin RL, Adams JS, Hewison M. 2014. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Front Physiol 5:151. doi: 10.3389/fphys.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muehleisen B, Bikle DD, Aguilera C, Burton DW, Sen GL, Deftos LJ, Gallo RL. 2012. PTH/PTHrP and vitamin D control antimicrobial peptide expression and susceptibility to bacterial skin infection. Sci Transl Med 4:135ra66. doi: 10.1126/scitranslmed.3003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wittke A, Weaver V, Mahon BD, August A, Cantorna MT. 2004. Vitamin D receptor-deficient mice fail to develop experimental allergic asthma. J Immunol 173:3432–3436. doi: 10.4049/jimmunol.173.5.3432. [DOI] [PubMed] [Google Scholar]
- 42.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, Adikibi T, Pridgeon C, Dallman M, Loke TK, Robinson DS, Barrat FJ, O'Garra A, Lavender P, Lee TH, Corrigan C, Hawrylowicz CM. 2006. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest 116:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. 2008. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol 181:7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gombart AF, Borregaard N, Koeffler HP. 2005. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 45.Dimitrov V, White JH. 11 September 2015. Species-specific regulation of innate immunity by vitamin D signaling. J Steroid Biochem Mol Biol doi: 10.1016/j.jsbmb.2015.09.016. [DOI] [PubMed] [Google Scholar]