Abstract
Yersinia enterocolitica evades the immune response by injecting Yersinia outer proteins (Yops) into the cytosol of host cells. YopH is a tyrosine phosphatase critical for Yersinia virulence. However, the mucosal immune mechanisms subverted by YopH during in vivo orogastric infection with Y. enterocolitica remain elusive. The results of this study revealed neutrophil recruitment to Peyer's patches (PP) after infection with a YopH-deficient mutant strain (Y. enterocolitica ΔyopH). While the Y. enterocolitica wild-type (WT) strain in PP induced the major neutrophil chemoattractant CXCL1 mRNA and protein levels, infection with the Y. enterocolitica ΔyopH mutant strain exhibited a higher expression of the CXCL1 receptor, CXCR2, in blood neutrophils, leading to efficient neutrophil recruitment to the PP. In contrast, migration of neutrophils into PP was impaired upon infection with Y. enterocolitica WT strain. In vitro infection of blood neutrophils revealed the involvement of YopH in CXCR2 expression. Depletion of neutrophils during Y. enterocolitica ΔyopH infection raised the bacterial load in PP. Moreover, the clearance of WT Y. enterocolitica was improved when an equal mixture of Y. enterocolitica WT and Y. enterocolitica ΔyopH strains was used in infecting the mice. This study indicates that Y. enterocolitica prevents early neutrophil recruitment in the intestine and that the effector protein YopH plays an important role in the immune evasion mechanism. The findings highlight the potential use of the Y. enterocolitica YopH-deficient strain as an oral vaccine carrier.
INTRODUCTION
The genus Yersinia includes three human-pathogenic species: Yersinia pseudotuberculosis, Y. pestis, and Y. enterocolitica. Y. pestis causes plague, and Y. pseudotuberculosis and Y. enterocolitica cause gastroenteritis (1). Y. enterocolitica infections are characterized by fever, abdominal pain, and diarrhea resulting in gastroenteritis and lymphadenitis, which are commonly self-limiting in humans (2). The bacteria are usually ingested through contaminated food or water and travel to the terminal ileum, where they attach to, and invade via, the M cells of Peyer's patches (PP). Y. enterocolitica then survives, undergoes extracellular replication in the PP, and may eventually disseminate to deeper tissues of the mesenteric lymph nodes, liver, spleen, and lung (3).
For survival in host tissues, pathogenic Yersinia species have a plasmid-encoded type 3 secretion system (T3SS) that translocates virulence proteins, the so-called Yersinia outer proteins (Yops), into the cytosol of target cells, suppressing the host immune response and enabling extracellular replication of the bacteria in lymphatic tissue (4). Yops include YopH, which is a tyrosine phosphatase critical for virulence of Yersinia (5–7) and targets tyrosine kinases and their adapters in a variety of cell types. The substrates of YopH thus far identified include the following: the adapters p130Cas and paxilin in epithelial cells (8, 9); the adapters ADAP, SKAP-HOM, and the tyrosine kinase FAK in macrophages (10, 11); and the tyrosine kinase LcK and ZAP-70 and the adapters SLP-76 and LAT in T cells (12, 13). Rolán et al. have recently demonstrated that PRAM-1/SKAP-HOM and SLP-76 are molecular targets of YopH in polymorphonuclear neutrophils (PMN) during animal infection (14). As a result, YopH promotes inhibition of phagocytosis (15), blocks specific bactericidal function of PMN (16), inhibits cytokine production by T cells and T-cell proliferation, and prevents the expression of the costimulatory receptor CD86 on B cells (17, 18). In addition, YopH inhibits the phosphatidylinositol 3 kinase (PI3K)/Akt signaling pathway in macrophages, preventing the expression of the chemokine monocyte chemoattractant protein-1 (MCP-1, CCL2), an important chemotactic factor for macrophages (17). Different reports have shown that the lack of YopH reduces Y. enterocolitica virulence in mice, underlining the relevance of YopH for the full virulence of Y. enterocolitica (5–7, 19, 20). However, the immune mechanisms involved in the control of YopH-deficient Y. enterocolitica strain have yet to be fully clarified.
In previous studies, we demonstrated that the Y. enterocolitica ΔsycH mutant strain, which is unable to secrete the virulence protein YopH, is reduced in virulence, colonizes PP (7), and induces mucosal and systemic Yersinia-specific IgA levels (21). In the present study, we show that a YopH deletion mutant (Y. enterocolitica ΔyopH) is avirulent after orogastric infection. Interestingly, while mRNA and protein levels of CXCL1, the major neutrophil chemoattractant, associated with a higher bacterial load of the Y. enterocolitica wild-type (WT) strain in PP, circulating blood neutrophils expressed a higher level of the CXCL1 receptor, CXCR2, in Y. enterocolitica ΔyopH-infected mice. Indeed, we observed higher CXCR2 expression after in vitro infection of blood neutrophils with the mutant strain than with the wild type. Moreover, the elimination of Y. enterocolitica ΔyopH was impaired in neutrophil-depleted mice, supporting the contribution of recruited neutrophils in the intestinal defense against Y. enterocolitica. Finally, the clearance of virulent Y. enterocolitica WT was improved when mice were coinfected with Y. enterocolitica WT and Y. enterocolitica ΔyopH. The data shed new light on the role of YopH in the context of animal infection and support the notion of a protective response induced by the Y. enterocolitica ΔyopH mutant when mice are coinfected with the virulent Y. enterocolitica strain. These findings highlight the potential of this attenuated strain as an oral vaccine vector.
MATERIALS AND METHODS
Mice.
C57BL/6 wild-type mice were purchased from the Animal Facilities of the National University of La Plata (La Plata, Argentina). Breeding colonies were established at the Animal Facility of the National University of San Luis (San Luis, Argentina). Mice were kept in a positive-pressure cabinet (Ehret, Emmendingen, Germany) and provided with sterile food and water ad libitum. Six- to 8-week-old mice were used for the experiments. All animal procedures were performed according to the rules and standards for the use of laboratory animals of the U.S. National Institutes of Health. Animal experiments were approved by the Institutional Committee of Care and Use of Animals (CICUA) of the Faculty of Chemistry, Biochemistry and Pharmacy, at the National University of San Luis (San Luis, Argentina) (protocol number B-163/13).
Bacterial strains and infection.
The following strains were used in this study: Y. enterocolitica WA-314 wild-type (pYV+, serotype O:8; clinical isolate; WA-314 pYVO8+; Nalr; Y. enterocolitica WT) (22), and Y. enterocolitica WA-314 deficient in YopH (pYV+, WA-C pYV yopH Δ17–455 Nalr Kanr; Y. enterocolitica ΔyopH) (23). Bacteria were cultured and prepared as previously described (7). For infection, mice were first starved for 3 h before and after orogastric infection with 5 × 108 yersiniae in 0.2 ml phosphate-buffered saline (PBS) using a gastric tube. Control mice received PBS. In coinfection experiments, Y. enterocolitica ΔyopH was administered in combination with Y. enterocolitica WT or with Y. enterocolitica WT-green fluorescent protein (GFP) (24) with an equal dose of 2.5 × 108 or 1 × 1010 CFU, respectively. The number of inoculated bacteria was controlled by plating of serial dilutions of the inoculated suspension on Trypticase soy agar (TSA) and determining the CFU after incubation at 27°C for 48 h. To determine the bacterial burden after infection, spleen, PP, and feces were obtained and homogenates were prepared in isotonic saline solution or in a cold extraction buffer (50 mM EDTA, 30 mg/ml soybean trypsin inhibitor, 1% bovine serum albumin in PBS) for feces. Serial dilutions were plated on Irgasan-MacConkey agar plates for PP and feces or on TSA plates for spleen samples. Plates were incubated for 48 h at 27°C, and CFU were determined. In coinfection experiments, the Y. enterocolitica WT clearance was calculated as the differential CFU number between plates with and without kanamycin, since Y. enterocolitica ΔyopH is kanamycin resistant. The limit of CFU detection is log10 of 25 CFU = 1.4 (7).
Cell preparation and flow cytometry.
PP were finely cut and digested for 10 min at 37°C in Hanks' balanced salt solution containing collagenase (0.5 mg/ml; type IV; Sigma-Aldrich) and DNase I (15 μg/ml; Roche). Flow-cytometric staining was conducted as previously described (25). Cells were first incubated with anti-mouse CD16/32 (Fc block) for 15 min at 4°C and then stained with anti-F4/80-FITC (clone CI:A3-1), anti-CD11b-APC (clone M1/70), anti-CD11b PerCp-Cy5.5 (clone M1/70), anti-CD11c-APC (clone HL3), and anti-Ly6G-PE (clone 1A8) for 30 min at 4°C. Anti-CD16/32 and anti-CD11b were from BD Biosciences (San Jose, CA, USA), and anti-Ly6G and anti-F4/80 were from Biolegend (San Diego, CA, USA). CXCR2 staining was performed with anti-CXCR2-PE (clone 242216; R&D, Minneapolis, MN, USA) generously provided by Cristina Pistoresi (Córdoba, Argentina) or with anti-CXCR2-Alexa Fluor 647 (clone SA045E1) from Biolegend with their appropriate matched isotype control antibodies. To exclude dead cells, 7-aminoactinomycin D (7-AAD; Sigma-Aldrich, St. Louis, MO, USA) was used. Neutrophil cells were identified as Ly6G+ CD11b+, and macrophages were identified as F4/80+ CD11b+ cells. Data were acquired on a FACSCalibur flow cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star, Ashland, OR). A total of 1 × 106 to 2 × 106 events were acquired.
Determination of chemokines by bead array or ELISA.
The PP were homogenized in PBS containing 0.5% bovine serum albumin, 0.4 M NaCl, 1 mM EDTA, 0.05% Tween 20, and 1% protease inhibitor cocktail (Sigma-Aldrich) and centrifuged at 10,000 × g for 10 min (adapted from reference 26). CXCL1 was quantified in the homogenates using the cytometric bead assay (CBA; BD Biosciences), and the level of CXCL2 was quantified using the specific enzyme-linked immunosorbent assay (ELISA) kit (Peprotech, Mexico) according to the manufacturer's instructions. These antibodies were generously provided by Eva Acosta (Córdoba, Argentina). The chemokine levels were normalized to protein concentration, which was determined by Qubit fluorometric quantification (Invitrogen, San Diego, CA, USA).
Reverse transcription (RT)-PCR and qPCR analysis.
The total RNA from the PP of the vehicle-treated and infected mice was isolated using the TRIzol reagent (Invitrogen), according to the instructions of the manufacturer. Each total RNA sample was treated with the RQ1 RNase-free DNase according to the manufacturer's instructions (Promega, Madison, WI, USA). First-strand cDNA was synthesized using ProtoScript M-MuLV First Strand cDNA synthesis kit according to the manufacturer's instructions (New England Biolab, Ipswich, MA, USA). Quantitative PCR (qPCR) analysis was performed using an ABI PRISM 7500 instrument (Applied Biosystems, Pleasanton, CA, USA) with SBR Green PCR master mix (Applied Biosystems). The following primers were used for PCR amplification: for mouse β-actin cDNA, sense, 5′-CGTTGACATCCGTAAAGACCT-3′, and antisense, 5′-CTTGATCTTCATGGTGCTAGGAG-3′; for mouse cxcl1, sense, 5′-TCCAGCACTCCAGACTCC-3′, and antisense, 5′-TGACAGCGCAGCTCATTG-3′. Forty cycles of PCR amplification were performed in duplicate for each primer set. The fold change in the quantity of gene transcripts was measured and compared to the β-actin gene using the comparative 2−ΔΔCT method (where CT is threshold cycle).
Histological evaluation.
The histological examination of PP was carried out after routine fixation and paraffin embedding. Five-micrometer-thick sections were cut, stained with hematoxylin and eosin, and examined under a light microscope. Photographs were taken using an Olympus BX40 light microscope equipped with a Sony SSC-DC5OA camera.
Immunofluorescence studies.
The PP were embedded in the Tissue-Tek OCT compound (Sakura, Zoeterwoude, Netherlands) and frozen at −80°C, and 7-μm cryostat sections were prepared. Tissue sections were fixed for 10 min at room temperature with 4% paraformaldehyde, washed three times with PBS, and then incubated with 50 mM ammonium chloride. The tissue sections were washed twice with PBS and permeabilized with 0.1% Triton X-100. After incubation with anti-mouse CD16/32 (BD Biosciences) and a biotin blocking kit (Vector Laboratories, Burlingame, CA, USA), the sections were washed three times, incubated 30 min at room temperature with a biotin anti-Gr1 antibody (100 μg/ml in PBS–10% fetal bovine serum [FBS]; generously provided by Cristina Pistoresi, Córdoba, Argentina), washed, and then incubated with streptavidin-Alexa Fluor 594 (1 μg/ml in PBS–10% FBS) for 30 min at room temperature. The slides were mounted in Mowiol (Carl Roth, Karlsruhe, Germany). Labeled cells were visualized with an Axiovert 40 CFL fluorescence microscope (Zeiss, Esslingen, Germany).
Expression of CXCR2 after in vitro infection.
Y. enterocolitica WT and Y. enterocolitica ΔyopH were grown overnight at 27°C in Trypticase soy broth (TSB) supplemented with 20 mM magnesium chloride and 20 mM sodium oxalate. A 1:20 dilution of the overnight bacterial culture was incubated for an additional 3 h at 37°C. The bacteria were washed once with saline, and the optical density at 600 nm was determined.
Whole blood was collected from uninfected mice, and erythrocytes were removed by treatment with a lysis buffer containing 0.15 M NH4Cl, 10 mM K2CO3, and 0.1 mM EDTA, followed by centrifugation. The remaining leukocytes containing 11% of neutrophils were suspended in RPMI 1640 (Invitrogen) medium supplemented with 10% FBS (Sigma), 2 mM glutamine (Invitrogen), 50 mM 2-mercaptoethanol (Sigma), and 1 mM sodium pyruvate (Invitrogen) without antibiotics and infected with Y. enterocolitica WT or Y. enterocolitica ΔyopH at a multiplicity of infection (MOI) of 50:1. The bacteria were sedimented onto the cells at 400 × g for 5 min. After 30 min of infection, the cells were washed with gentamicin (100 μg/ml; Invitrogen) diluted in saline. The CXCR2 expression in Ly6G+ CD11b+ cells was analyzed by flow cytometry.
Neutrophil depletion and bacterial counting.
To deplete neutrophils, mice were injected intraperitoneally with 100 μg of the monoclonal anti-Gr1antibody (clone RB6-8C5; generously provided by Cristina Pistoresi, Córdoba, Argentina) diluted in 100 μl of sterile saline 1 day before and on days 2 and 3 after Y. enterocolitica ΔyopH infection. Control mice received the same dosage of saline. Neutrophil depletion was measured by counting the Ly6G+ CD11b+ cells in peripheral blood using flow cytometric analysis. To further monitor the effect of neutrophil depletion on Y. enterocolitica ΔyopH infection, the CFU was determined in PP and spleen of depleted and control mice 3 days after infection, 4 h after the last anti-Gr1 antibody dose.
Statistical analysis.
Multiple comparisons were tested using one-way analysis of variance (ANOVA), followed by Bonferroni's posttest. For a comparison of data of two groups, Student's t test was used. Statistical analysis of survival was performed by using the log-rank test. Results with P values of <0.05 were considered statistically significant. Data were analyzed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA, USA).
RESULTS
Deletion of YopH decreases Y. enterocolitica virulence in mice.
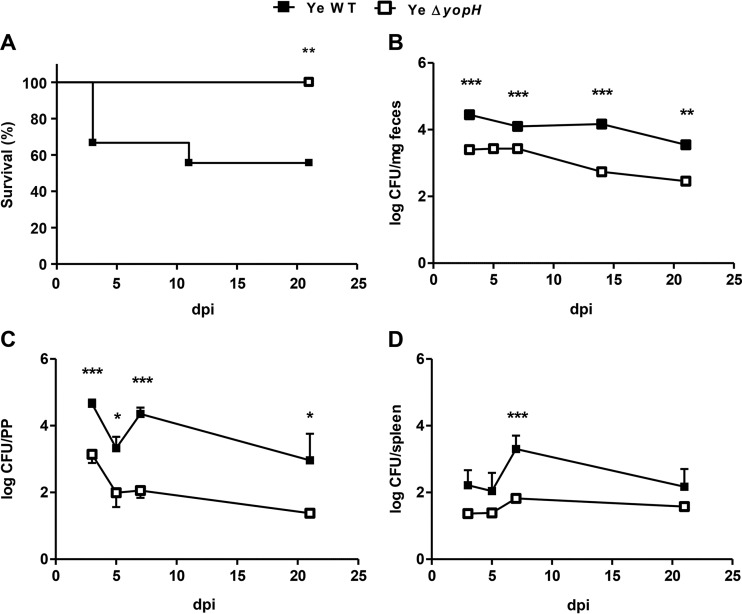
To analyze how YopH deletion affects Y. enterocolitica infection, C57BL/6 mice were orogastrically infected with the Y. enterocolitica WT strain or Y. enterocolitica ΔyopH mutant strain (5 × 108 CFU/mice). While 45% of the mice infected with the Y. enterocolitica WT strain died, all the mice infected with the Y. enterocolitica ΔyopH mutant strain survived the infection (Fig. 1A), demonstrating that YopH is critical for Y. enterocolitica virulence after oral infection. When the intestinal elimination of these strains was examined in feces at different days after infection, we found significantly higher intestinal clearance of this mutant strain than Y. enterocolitica WT strain on days 3, 7, 14, and 21 (P < 0.001 at days 3, 7, and 14; P < 0.01 at day 21) (Fig. 1B and Table 1). We previously reported that Y. enterocolitica WT colonization of gut tissues continues at high levels (4 to 5 log CFU/mg feces) for at least 5 weeks (21). Therefore, we monitored for how long Y. enterocolitica ΔyopH was shed from the intestinal tract and found that this mutant strain had been completely eliminated from the intestine by day 42 after infection (see Fig. S1 in the supplemental material). Next, the bacterial loads in PP and in the spleen were analyzed in both groups of mice on days 3, 5, 7, and 21 after infection. The bacterial burden was significantly higher in the PP of mice infected with the Y. enterocolitica WT throughout the period of infection (and on day 7 in the spleen) than in mice infected with Y. enterocolitica ΔyopH mutant strain (Fig. 1C and D). The bacterial load in PP decreased significantly after Y. enterocolitica ΔyopH infection on days 3, 5, and 7 (P < 0.05 on day 5; P < 0.001 on days 3 and 7 compared with Y. enterocolitica WT) to undetectable CFU on day 21 (P < 0.05). Moreover, after infection with Y. enterocolitica WT, we observed that the bacterial load increased from day 5 to day 7 in the mice that survived (Fig. 1C and D).
FIG 1.
Survival curves and bacterial load following oral infection of mice with Y. enterocolitica strains. (A) Survival curves of C57BL/6 mice infected with 5 × 108 CFU Y. enterocolitica wild-type (Ye WT) strain or with a similar dose of the mutant that lacks YopH (Ye ΔyopH). Results shown are the summary results of 3 experiments. n = 12 mice per group. The log rank test was used (**, P < 0.01). (B to D) Kinetics of bacterial clearance in feces (B) and the bacterial load in Peyer's patches (PP) (C) and spleen (D) of mice infected with WT or ΔyopH strains at the indicated days postinfection (dpi). Data in panels B to D are means and standard errors of the means (SEM) of the summary results from 2 experiments. n = 3 to 5 mice per day for each group of mice (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
TABLE 1.
Comparison of kinetics of elimination of Y. enterocolitica ΔyopH and WT strains
| Sample | No. of days | % eliminationa |
P value | Ratio of elimination of ΔyopH strain to WT strain | |
|---|---|---|---|---|---|
| Y. enterocolitica WT | Y. enterocolitica ΔyopH | ||||
| PP | 3 | 40.1 ± 1.7 | 64.2 ± 7.9 | <0.05 | 1.6 |
| 7 | 45.5 ± 5.5 | 79.9 ± 5 | <0.01 | 1.8 | |
| 21 | 63.3 ± 5.5 | 100 | <0.001 | 1.6 | |
| Feces | 3 | 44.4 ± 2.3 | 60.3 ± 2.1 | <0.001 | 1.4 |
| 7 | 48.8 ± 2.8 | 57 ± 2.2 | <0.01 | 1.2 | |
| 21 | 55.6 ± 0.3 | 69.3 ± 1.9 | <0.01 | 1.3 | |
Ratio of the bacterial load at the final time point to the load at the initial time point.
Neutrophil recruitment is increased in Y. enterocolitica ΔyopH-infected mice.
Since Y. enterocolitica ΔyopH was eliminated soon after infection and phagocytes of the innate immune system contribute to an early antibacterial defense (27), we analyzed the recruitment of neutrophils in PP 3 days postinfection (dpi) with Y. enterocolitica WT or Y. enterocolitica ΔyopH mutant strain. Notably, although the bacterial burden was lower after the infection with Y. enterocolitica ΔyopH mutant strain, a significantly higher frequency and absolute number of CD11b+ Ly6G+ neutrophils was detected in PP of Y. enterocolitica ΔyopH-infected mice than in Y. enterocolitica WT-infected mice (P < 0.001) (Fig. 2). Moreover, we did not detect differences in CD11b expression in Ly6G+ cells in PP of mice infected with Y. enterocolitica ΔyopH or Y. enterocolitica WT (see Fig. S2 in the supplemental material). Therefore, our results reveal that the activation state of neutrophils is not different after Y. enterocolitica ΔyopH infection. These findings indicate that the mutant strain Y. enterocolitica ΔyopH induces early recruitment of neutrophils to the PP and point to the involvement of YopH in neutrophil migration to the infection site.
FIG 2.
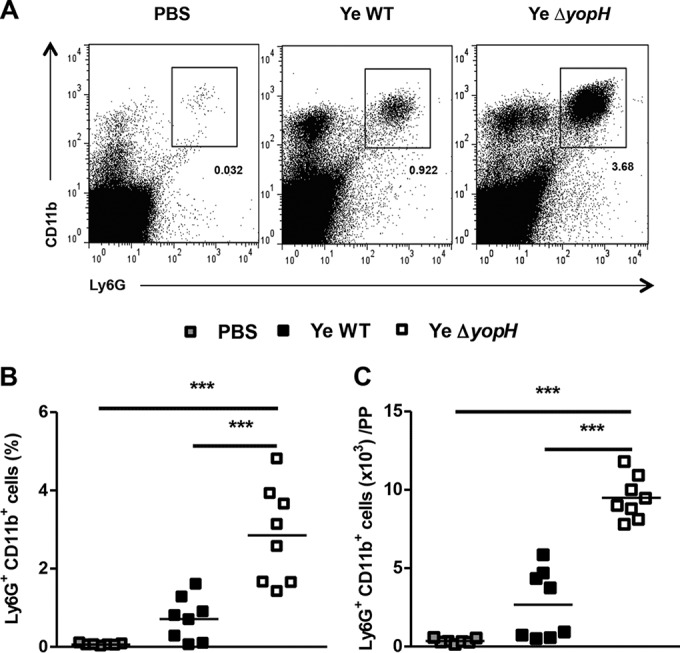
Neutrophil infiltration in PP following Y. enterocolitica WT or Y. enterocolitica ΔyopH infection. C57BL/6 mice were infected orally with 5 × 108 CFU of Y. enterocolitica WT strain or ΔyopH mutant strain. Control mice received PBS. Cells were collected from Peyer's patches (PP), stained for the neutrophil markers Ly6G and CD11b, and subjected to flow cytometry analysis. (A) Representative dot plot showing analysis of neutrophils in PP from control (PBS) and Y. enterocolitica WT- and ΔyopH-infected mice. The numbers in the plots indicate the percentages of labeled cells. Percentages (B) and absolute neutrophil numbers (C) in the PP of mice at day 3 after infection with WT or ΔyopH strains are presented. The data in panels B and C are the summary results of 3 experiments. Each symbol represents an individual mouse; horizontal lines indicate the means; ***, P < 0.001.
YopH is not associated with CXCL1 mRNA and protein expression.
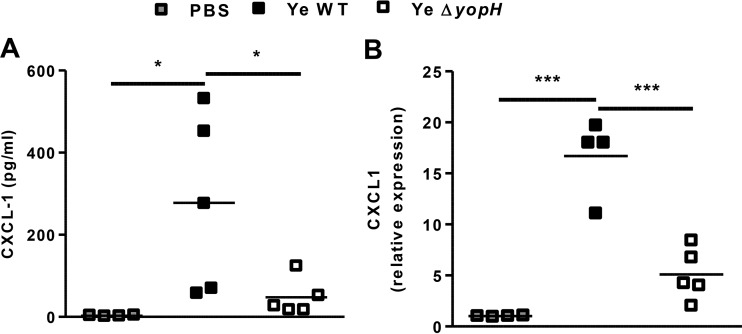
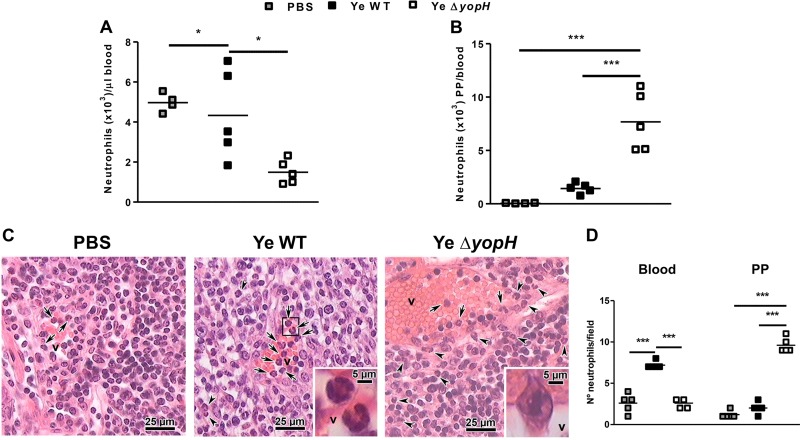
The increase of neutrophils in PP induced by Y. enterocolitica ΔyopH suggested that YopH may be involved in the early suppression of chemoattractants in this organ. Because the chemokines CXCL1 and CXCL2 are of central importance for neutrophil recruitment, we explored the expression of these chemokines. Although we did not detect the induction of CXCL2 protein in PP within infected mice (the levels were lower than the limit of detection, 0.1 pg/ml), both CXCL1 protein and mRNA expressions were higher in PP of Y. enterocolitica WT- than in Y. enterocolitica ΔyopH-infected mice (Fig. 3A and B). These results correlate with the bacterial load in PP as a stimulus for chemokine expression (Fig. 1C). Previous studies have also demonstrated that CXC chemokine secretion is triggered through recognition of bacterial peptidoglycan or lipopolysaccharide (LPS), causing the migration of neutrophils to the infection site (27–30). Since resident macrophages and newly recruited monocyte-derived macrophages are the main source of CXCL1 (31), we analyzed the number of macrophages in PP of infected mice. Accordingly, we detected increased macrophage (F4/80+ CD11b+) influx in PP 3 dpi with Y. enterocolitica WT compared to infection with Y. enterocolitica ΔyopH mutant strain (Fig. 4). However, these results did not explain the massive neutrophil recruitment in PP after Y. enterocolitica ΔyopH infection. Therefore, since in response to infection, neutrophils are rapidly mobilized from the bone marrow, resulting in a rise in circulating cell numbers and followed by rapid trafficking of neutrophils into the infected tissue (32), we compared the neutrophil number in blood of mice infected with both strains. Neutrophil numbers in the blood of Y. enterocolitica WT-infected mice were significantly higher than in PBS-treated and Y. enterocolitica ΔyopH-infected mice (Fig. 5A). However, the levels of neutrophils infiltrating into the PP (calculated as the absolute number of PP neutrophils to blood neutrophils in each mouse) were augmented after Y. enterocolitica ΔyopH infection (Fig. 5B). Furthermore, histological studies analyzing the migration of neutrophils into PP revealed that in noninfected mice the neutrophils were predominantly located in the blood vessels, while low neutrophil numbers were in the tissue at 3 dpi with the Y. enterocolitica WT strain (Fig. 5C and D). In contrast, increased neutrophil numbers were observed in the tissue of Y. enterocolitica ΔyopH-infected mice (Fig. 5C and D).
FIG 3.
CXCL1 expression in PP. C57BL/6 mice were infected with 5 × 108 CFU Y. enterocolitica WT or with the same dose of the avirulent ΔyopH strain. At 3 days postinfection (dpi), the concentrations of CXCL1 protein in homogenates of uninfected (PBS) and infected Peyer's patches (PP) were quantified by flow cytometry using the cytometric bead assay (CBA) (A). In addition, RNA was purified from the PP and subjected to quantitative PCR (qPCR) analysis of CXCL1 gene expression (B). The data are the summary results of 2 experiments. Each symbol represents an individual mouse; horizontal lines indicate the means (*, P < 0.05; ***, P < 0.001).
FIG 4.
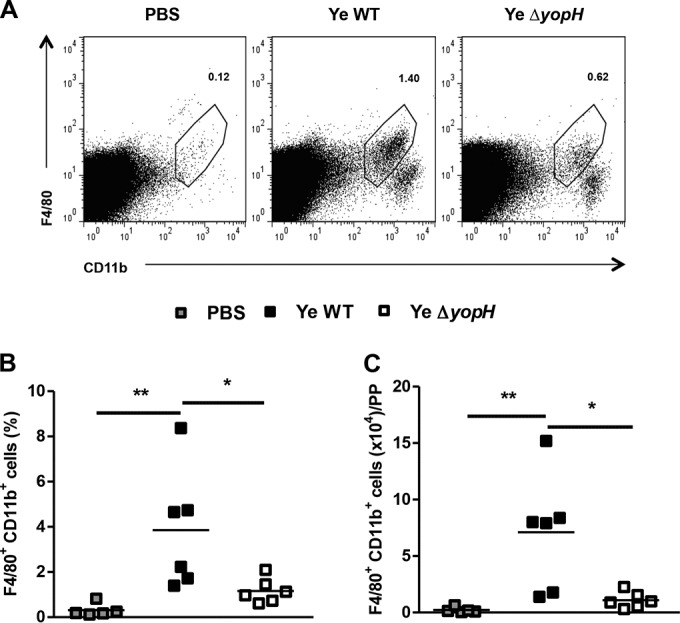
Macrophage infiltration in PP following Y. enterocolitica WT or Y. enterocolitica ΔyopH infection. Macrophages in Peyer's patches (PP) of infected mice (day 3 after infection) were stained with F4/80 and CD11b and subjected to flow cytometry analysis. (A) Representative dot plots showing analysis of macrophages in PP from control (PBS), Y. enterocolitica WT-, and Y. enterocolitica ΔyopH-infected mice. The numbers in the plots indicate the percentages of labeled cells in representative mice. Percentages (B) and absolute numbers (C) of macrophages in the PP of Y. enterocolitica WT- and ΔyopH-infected mice are presented. The data are the summary results of 2 experiments. Each symbol represents an individual mouse; horizontal lines indicate the means (*, P < 0.05; **, P < 0.01).
FIG 5.
Neutrophil homing from blood to Peyer's patches. (A) Absolute number of neutrophils in the blood of mice at day 3 after infection with Y. enterocolitica WT or ΔyopH strains. (B) Relation of absolute number of neutrophils in Peyer's patches (PP) to absolute number of neutrophils in blood of each infected mouse at 3 days postinfection (dpi). (C) Histological analysis of migration of neutrophils. Sections of PP from mice that received PBS (control) and from infected mice at 3 dpi were stained with hematoxylin and eosin. Photographs show blood neutrophils (arrows) and neutrophils in PP (arrowhead) (magnification, ×400; insets, ×1,000). In the infection by the WT strain, the inset shows an amplification of two neutrophils in blood vessels (v). In the ΔyopH strain infection, the inset shows at greater magnification an extravasing neutrophil. Photographs are representative of 1 of 4 mice per group of 2 independent experiments. (D) Quantification of results from multiple independent images (n = 5 per group). The data in panels A and B are the summary results of 2 experiments. Each symbol represents an individual mouse in panels A and B or a single image in panel D; horizontal lines indicate the means (*, P < 0.05; ***, P < 0.001).
YopH prevents CXCR2 expression in blood circulating neutrophils.
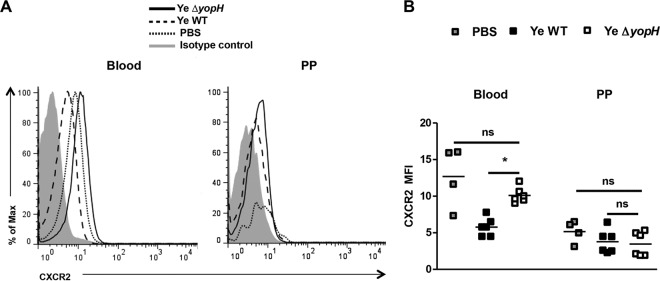
We then analyzed the CXC receptor 2 (CXCR2) expression on blood neutrophils of infected mice since CXC chemokines induce the migration of neutrophils to the site of infection predominantly through the signaling of CXCR2 (33). As depicted in Fig. 6, higher CXCR2 expression was detected on blood neutrophils from Y. enterocolitica ΔyopH-infected mice than on blood neutrophils from Y. enterocolitica WT-infected mice. However, no changes in CXCR2 expression were detected in PP upon infection with both strains (Fig. 6). Moreover, CXCR2 levels were higher on blood neutrophils following in vitro infection with Y. enterocolitica ΔyopH strain than following infection with Y. enterocolitica WT (Fig. 7). Together, these data suggest that neutrophil infiltration into PP upon Y. enterocolitica ΔyopH infection is presumably due to increased CXCR2 expression on circulating neutrophils in the blood.
FIG 6.
CXCR2 expression in blood circulation and Peyer's patches' neutrophils. Expression of cell surface CXCR2 on circulating neutrophils isolated from the peripheral blood and in neutrophils of Peyer's patches (PP) 3 days postinfection (dpi) with Y. enterocolitica WT or ΔyopH (5 × 108 CFU). (A) Representative overlaid flow cytometry histogram analysis showing CXCR2 expression on neutrophil (CD11b+ F4/80− CD11c−) gate compared with PBS mice. Isotype control (gray peak). (B) The average CXCR2 mean fluorescence intensity (MFI) levels are indicated. The data are the summary results of 2 experiments. Each symbol represents an individual mouse; horizontal lines indicate the means (*, P < 0.05; ns, not significant).
FIG 7.
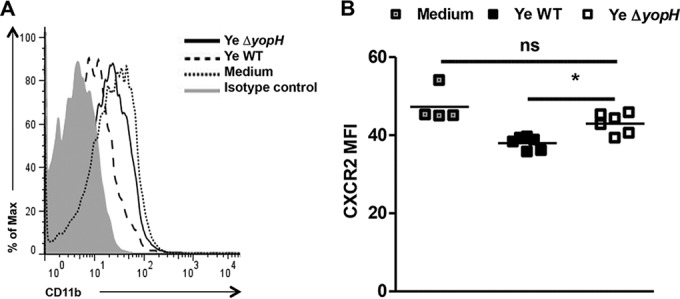
Expression of CXCR2 after in vitro infection. Blood neutrophils were in vitro infected with Y. enterocolitica WT or Y. enterocolitica ΔyopH at a multiplicity of infection (MOI) of 50:1 for 30 min. The cells were washed, and the CXCR2 expression in Ly6G+ CD11b+ cells was analyzed by flow cytometry. (A) Representative overlaid flow cytometry histogram analysis showing CXCR2 expression on neutrophil (Ly6G+ CD11b+) gate compared with uninfected cells (medium). Isotype control (gray peak). (B) The average CXCR2 mean fluorescence intensity (MFI) levels are indicated. The data are the summary results of 2 experiments. Each symbol represents cells from an individual mouse; horizontal lines indicate the means (*, P < 0.05; ns, not significant).
Neutrophils play a critical role in Y. enterocolitica ΔyopH elimination.
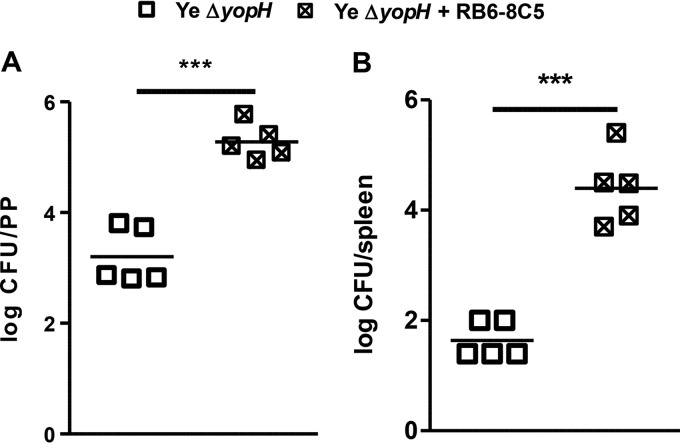
To study the function of neutrophils in the elimination of Y. enterocolitica ΔyopH, we depleted neutrophils in the mice with the monoclonal antibody RB6-8C5 prior to and during Y. enterocolitica ΔyopH infection. The analysis of peripheral blood of the mice showed that neutrophil depletion was achieved in more than 95% of mice treated with RB6-8C5 (see Fig. S3 in the supplemental material). On day 3 after infection, a significant increase in the bacterial load (more than 100-fold) was detected in PP of neutrophil-depleted mice (Fig. 8A). Remarkably, in contrast with control mice, high bacterial levels were detected in the spleen of mice treated with RB6-8C5 (Fig. 8B). These data indicate that neutrophils play a significant role in controlling Y. enterocolitica ΔyopH in PP and consequently in limiting the systemic spread of infection.
FIG 8.
Impact of neutrophil depletion on the outcome of Y. enterocolitica ΔyopH infection. Neutrophil-depleted mice were injected intraperitoneally with 100 μg of the monoclonal anti-Gr1antibody (clone RB6-8C5) 1 day before and on days 2 and 3 after intragastric Y. enterocolitica ΔyopH infection. Control mice received the same dosage of saline. Bacterial loads (CFU) in the Peyer's patches (PP) (A) and spleen (B) of neutrophil-depleted mice (Ye ΔyopH + RB6-8C5) and control mice (Ye ΔyopH) were assessed at day 3 after infection with Y. enterocolitica ΔyopH. Each symbol represents an individual mouse; horizontal lines indicate the means. ***, P < 0.001.
Promotion of Y. enterocolitica WT clearance during coinfection with Y. enterocolitica ΔyopH.
Neutrophils are important targets of Yop translocation (34); however, enhanced local recruitment of neutrophils improved bacterial clearance upon Y. enterocolitica infection (25). Therefore, we evaluated the clearance of Y. enterocolitica WT in mice infected with an equal mixture of Y. enterocolitica ΔyopH (2.5 × 108 of each strain). The colonies were dissected by culture on kanamycin agar due to Y. enterocolitica ΔyopH mutant strain resistance to kanamycin (23). Coinfection resulted in a complete elimination of the Y. enterocolitica WT strain. This contrasted with the high bacterial load after infection with Y. enterocolitica WT alone both in PP and in feces 3 dpi (Fig. 9A and B). Accordingly, immunofluorescence analyses of the PP revealed reduced numbers of GFP+ Y. enterocolitica colonies upon coinfection with GFP-expressing Y. enterocolitica WT and Y. enterocolitica ΔyopH compared with those obtained with the GFP-expressing Y. enterocolitica WT strain alone (Fig. 9C). Moreover, augmented neutrophil recruitment was detected after coinfection (Fig. 9C). Together, the data indicate that Y. enterocolitica ΔyopH improves rapid intestinal elimination of the Y. enterocolitica WT strain.
FIG 9.
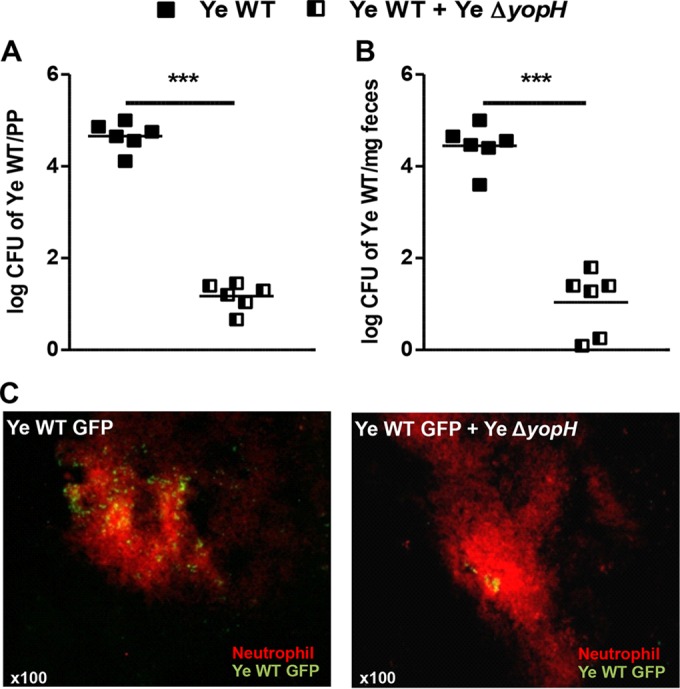
Y. enterocolitica ΔyopH promotes Y. enterocolitica WT clearance during coinfection. C57BL/6 mice were infected with 5 × 108 CFU Y. enterocolitica WT or with an equal mixture of the WT and ΔyopH strains (2.5 × 108 CFU of each strain). At day 3 after infection, the bacterial load of the WT was determined in Peyer's patches (PP) (A) and feces (B). The data in panels A and B are the summary results of 2 experiments. Each symbol represents an individual mouse; horizontal lines indicate the means (***, P < 0.001). (C) Immunofluorescence analysis of abscess at day 3 after Y. enterocolitica WT-GFP infection or Y. enterocolitica WT-GFP and Y. enterocolitica ΔyopH coinfection. Neutrophil infiltration was detected with Ly6G-PE antibody (red). Photographs are representative of 1 of 4 mice per group in 2 independent experiments.
DISCUSSION
Infections are recognized by the innate immune system, eliciting in this system an immediate defense, which works to promote long-lasting adaptive immunity (35). The ability of professional phagocytes to ingest and kill microorganisms is central to innate immunity and host defense. One strategy of bacterial pathogens is to evade phagocytosis during early immune response (36). Y. enterocolitica prevents phagocytosis by host cells and proliferates extracellularly in lymphatic tissue (15, 37). YopH plays a critical function in this process promoting intestinal colonization and persistence of Y. pseudotuberculosis (6).
We previously demonstrated that Y. enterocolitica ΔsycH, a functional YopH mutant, colonizes the PP without causing systemic infection, indicating that yersiniae lacking the YopH function can be eliminated by mechanisms that do not require an interleukin-12 (IL-12), IL-18-, and tumor necrosis factor receptor p55 (TNF-Rp55)-dependent immune defense (7). Further studies demonstrated that gamma interferon (IFN-γ) and IL-6 are not necessarily required for clearance of Y. enterocolitica ΔyopH (38). These results raised intriguing questions, namely, what early immune response controls Y. enterocolitica ΔyopH after oral infection. In this study, we also investigated whether this immune response would protect mice against the infection with the fully virulent Y. enterocolitica strain.
Our results clearly demonstrate the attenuation of the Y. enterocolitica ΔyopH strain after oral infection. When we compared the ΔyopH strain results with those from the Y. enterocolitica WT strain, we found that the survival rate of mice increased and bacterial colonization in PP decreased. Moreover, the bacterial dissemination to the spleen was strongly limited in the YopH-deficient mutant strain. These results are consistent with previous studies performed with Y. enterocolitica ΔsycH mutant strain (7, 21). Notably, infection with the Y. enterocolitica WT strain revealed a biphasic bacterial load in PP, indicating that a fully virulent Y. enterocolitica strain coping with the host immune response during early stages of infection would provoke a second increase of the bacterial load in PP and dissemination to the spleen. Moreover, recent studies in Y. pestis-infected mice have demonstrated a biphasic nature to the progression of the disease (28). In contrast, an early immune response induced by Y. enterocolitica ΔyopH infection was able to control this infection without a biphasic course of infection.
Neutrophils are fundamental cells in the primary innate immune defense, and they have been shown to be important for controlling systemic Yersinia infection (27). Yersinia species target neutrophils through the transfer of Yops (34, 39). YopH has been identified as one of the major contributors to the antiphagocytic capacity of Yersinia on neutrophils, and their target has been defined in in vitro assays and during in vivo systemic and oral murine infections (8–12, 14–16). Upon infection by invading microorganisms, neutrophils migrate to the infected tissue through circulation (40). Notably, we found dramatic increases of neutrophils in PP of mice infected with the YopH-deficient mutant strain. Consistent with this result, other authors have recently reported rapid neutrophil infiltration in the lungs of mice infected with an avirulent Y. pestis strain, but they observed no significant changes in the levels of neutrophils in the lungs after infection with a fully virulent Y. pestis (28). Moreover, we observed that depletion of neutrophils impaired Y. enterocolitica ΔyopH elimination in PP. Accordingly, Westermark et al. used neutrophil-depleted mice to demonstrate that the virulence-attenuated yopH mutant of Y. pseudotuberculosis was clearly more virulent in the absence of these cells (41).
Chemokines CXCL1 and CXCL2 are potent chemoattractants for neutrophils, and increased levels of these chemokines in serum are a hallmark of infection and inflammation in peripheral tissues (42). Our results are in line with previous studies that detected high CXCL1 levels in the sera of mice systemically infected with Y. enterocolitica WT, which correlated with the bacterial burden (43). The process of neutrophil recruitment within individual organs is dictated by inciting infection and the response of organ-specific tissue-resident cells (44). Moreover, resident macrophages are an important source of neutrophil-attracting chemokines in bacterial infections (31, 45). Increased recruitment of phagocytes to PP was observed in an oral Salmonella mouse infection model (46). Furthermore, purified monocytes from Salmonella-infected mice preferentially produced chemokines, and neutrophils from infected mice migrated toward these chemokines (47). Accordingly, a recent study using a mouse model of urinary tract infection with uropathogenic Escherichia coli nicely showed that tissue-resident and recruited macrophages work together to create an effective neutrophilic response to infection by producing CXCL1 (48). Moreover, splenic CD11b+ cells, which include macrophages, were shown, in contrast to the yopH mutant, to have increased CXCL-1 mRNA levels after systemic infection with Y. enterocolitica WT (38). In line with this, we also observed augmented numbers of macrophages in PP and increased CXCL-1 mRNA and protein levels after oral infection with Y. enterocolitica WT. Thus, our findings suggest that PP resident cells sense bacterial infection, leading to CXCL-1 production in relation to the bacterial load.
Although high CXCL1 levels were detected in PP after infection with Y. enterocolitica WT, neutrophil recruitment was lower than after infection with the YopH-deficient mutant strain. Our findings showed that infection with Y. enterocolitica WT decreased CXCR2 expression in blood neutrophils compared with noninfected and Y. enterocolitica ΔyopH-infected mice. Therefore, we assume that neutrophils rapidly mobilize from blood to PP after Y. enterocolitica ΔyopH infection and that the YopH virulence factor in the Y. enterocolitica WT strain targets CXCR2 expression on circulating neutrophils, affecting neutrophil influx into PP. Moreover, in other infections, including Y. pestis, the absence of CXCR2 resulted in increased colonization and decreased neutrophil recruitment to the infected site (29, 49–51). Additionally, consistent with the study reporting that phagocytosing neutrophils downregulate the expression of chemokine receptors (52), we detected that CXCR2 expression decreased when neutrophils reached PP. Correspondingly, other authors found that neutrophils from the PP of Salmonella-infected mice downregulated CXCR2 expression (46). In summary, our findings suggest that the YopH-dependent blocking of neutrophil recruitment into PP is a key event for Y. enterocolitica to evade the immune response at the intestinal mucosa. We are currently directing our studies to define the molecular targets of YopH involved in the downregulation of CXCR2 on neutrophils during Yersinia infection.
We hypothesized that YopH impairs neutrophil influx into PP to enhance survival of Y. enterocolitica. Notably, we observed that the Y. enterocolitica WT strain was eliminated when coinfection with Y. enterocolitica ΔyopH was performed. Moreover, by combining the findings that (i) Y. enterocolitica selectively delivers Yops to neutrophils (53), (ii) these cells are preferentially injected with YopH (34), (iii) Yop injection depends on bacterial adhesion on neutrophils (54), which leads to phagocytic uptake of pathogens (55), and (iv) Yop translocation increases when more neutrophils are present in PP (34), we can speculate that the Y. enterocolitica WT strain is preferentially phagocytosed by neutrophils after coinfection.
In conclusion, in this study we reported differential neutrophil recruitment upon oral Y. enterocolitica WT or Y. enterocolitica ΔyopH infection. In addition, we argue that YopH of Y. enterocolitica may modulate neutrophil chemotaxis into the infection site. The mutant Y. enterocolitica ΔyopH contributed to the complete elimination of Y. enterocolitica WT. Therefore, our findings emphasize the importance of the early immune response in mucosa during intestinal infections and the potential use of the Y. enterocolitica ΔyopH strain as an oral vaccine carrier.
Supplementary Material
ACKNOWLEDGMENTS
We thank Fabian Mohamed, Angelina Bernardi, and Maria Elena Arce (National University of San Luis) for their collaboration in the histological and immunofluorescence studies.
This work was supported by grants from the National Agency for Promotion of Science and Technology (PICT-2008-763; PICT-2011-0732), the National University of San Luis (PROICO-2-1114), the Alexander von Humboldt Foundation, and the CONICET-DFG cooperation Project. R.J.E., V.P.F., C.V.G., and M.S.D.G. are members of the Scientific Career of the National Council of Scientific and Technical Investigations (CONICET); M.N.D., J.E.S., and M.B.J. are fellows from the CONICET.
We have no conflict of interest to declare.
Funding Statement
This work was supported by grants from the National Agency for Promotion of Science and Technology (PICT-2008-763; PICT-2011-0732), the National University of San Luis (PROICO-2-1114), Alexander von Humboldt Foundation, and CONICET-DFG cooperation Project. R.J.E., V.P.F., C.V.G., and M.S.D.G. are members of the Scientific Career of the National Council of Scientific and Technical Investigations (CONICET); M.N.D., J.E.S., and M.B.J. are fellows from the CONICET.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00568-16.
REFERENCES
- 1.Wren BW. 2003. The yersiniae—a model genus to study the rapid evolution of bacterial pathogens. Nat Rev Microbiol 1:55–64. doi: 10.1038/nrmicro730. [DOI] [PubMed] [Google Scholar]
- 2.Koornhof HJ, Smego RA Jr, Nicol M. 1999. Yersiniosis. II. The pathogenesis of Yersinia infections. Eur J Clin Microbiol Infect Dis 18:87–112. doi: 10.1007/s100960050237. [DOI] [PubMed] [Google Scholar]
- 3.Trülzsch K, Oellerich MF, Heesemann J. 2007. Invasion and dissemination of Yersinia enterocolitica in the mouse infection model. Adv Exp Med Biol 603:279–285. doi: 10.1007/978-0-387-72124-8_25. [DOI] [PubMed] [Google Scholar]
- 4.Cornelis GR. 2002. Yersinia type III secretion: send in the effectors. J Cell Biol 158:401–408. doi: 10.1083/jcb.200205077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trülzsch K, Sporleder T, Igwe EI, Russmann H, Heesemann J. 2004. Contribution of the major secreted Yops of Yersinia enterocolitica O:8 to pathogenicity in the mouse infection model. Infect Immun 72:5227–5234. doi: 10.1128/IAI.72.9.5227-5234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logsdon LK, Mecsas J. 2003. Requirement of the Yersinia pseudotuberculosis effectors YopH and YopE in colonization and persistence in intestinal and lymph tissues. Infect Immun 71:4595–4607. doi: 10.1128/IAI.71.8.4595-4607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Genaro MS, Waidmann M, Kramer U, Hitziger N, Bohn E, Autenrieth IB. 2003. Attenuated Yersinia enterocolitica mutant strains exhibit differential virulence in cytokine-deficient mice implications for the development of novel live carrier vaccines. Infect Immun 71:1804–1812. doi: 10.1128/IAI.71.4.1804-1812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black DS, Bliska JB. 1997. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J 16:2730–2744. doi: 10.1093/emboj/16.10.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black DS, Montagna LG, Zitsmann S, Bliska JB. 1998. Identification of an amino-terminal substrate-binding domain in the Yersinia tyrosine phosphatase that is required for efficient recognition of focal adhesion targets. Mol Microbiol 29:1263–1274. doi: 10.1046/j.1365-2958.1998.01014.x. [DOI] [PubMed] [Google Scholar]
- 10.Hamid N, Gustavsson A, Andersson K, McGee K, Persson C, Rudd CE, Fällman M. 1999. YopH dephosphorylates Cas and Fyn-binding protein in macrophages. Microb Pathog 27:231–242. doi: 10.1006/mpat.1999.0301. [DOI] [PubMed] [Google Scholar]
- 11.Persson C, Carballeira N, Wolf-Watz H, Fällman M. 1997. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J 16:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alonso A, Bottini N, Bruckner S, Rahmouni S, Williams S, Shoenberger SS, Mustelin T. 2004. Lck dephosphorylation at Tyr-394 and inhibition of T cell antigen receptor signaling by Yersinia phosphatase YopH. J Biol Chem 279:4922–4928. [DOI] [PubMed] [Google Scholar]
- 13.Gerke C, Falkow S, Chien YH. 2005. The adaptor molecules LAT and SLP-76 are specifically targeted by Yersinia to inhibit T cell activation. J Exp Med 201:361–371. doi: 10.1084/jem.20041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolán HG, Durand EA, Mecsas J. 2013. Identifying Yersinia YopH-targeted signal transduction pathways that impair neutrophil responses during in vivo murine infection. Cell Host Microbe 14:306–317. doi: 10.1016/j.chom.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grosdent N, Maridonneau-Parini I, Sory MP, Cornelis GR. 2002. Role of Yops and adhesins in resistance of Yersinia enterocolitica to phagocytosis. Infect Immun 70:4165–4176. doi: 10.1128/IAI.70.8.4165-4176.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruckdeschel K, Roggenkamp A, Schubert S, Heesemann J. 1996. Differential contribution of Yersinia enterocolitica virulence factors to evasion of microbicidal action of neutrophils. Infect Immun 64:724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sauvonnet N, van der Lambermont IBP, Cornelis GR. 2002. YopH prevents monocyte chemoattractant protein 1 expression in macrophages and T-cell proliferation through inactivation of the phosphatidylinositol 3-kinase pathway. Mol Microbiol 45:805–815. doi: 10.1046/j.1365-2958.2002.03053.x. [DOI] [PubMed] [Google Scholar]
- 18.Yao T, Mecsas J, Healy JI, Falkow S, Chien Y. 1999. Suppression of T and B lymphocyte activation by a Yersinia pseudotuberculosis virulence factor, yopH. J Exp Med 190:1343–1350. doi: 10.1084/jem.190.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adkins I, Köberle M, Gröbner S, Autenrieth SE, Bohn E, Borgmann S, Autenrieth IB. 2008. Y. enterocolitica inhibits antigen degradation in dendritic cells. Microbes Infect 10:798–806. doi: 10.1016/j.micinf.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Logsdon LK, Mecsas J. 2006. The proinflammatory response induced by wild-type Yersinia pseudotuberculosis infection inhibits survival of yop mutants in the gastrointestinal tract and Peyer's patches. Infect Immun 74:1516–1527. doi: 10.1128/IAI.74.3.1516-1527.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanco HM, Lacoste MG, Eliçabe RJ, Di Genaro MS. 2008. IgA response by oral infection with an attenuated Yersinia enterocolitica mutant: implications for its use as oral carrier vaccine. Vaccine 26:6497–6502. doi: 10.1016/j.vaccine.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 22.Heesemann J, Laufs R. 1983. Construction of a mobilizable Yersinia enterocolitica virulence plasmid. J Bacteriol 155:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adkins I, Köberle M, Gröbner S, Bohn E, Autenrieth I, Borgmann S. 2007. Yersinia outer proteins E, H, P, and T differentially target the cytoskeleton and inhibit phagocytic capacity of dendritic cells. Int J Med Microbiol 297:235–244. doi: 10.1016/j.ijmm.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Oellerich MF, Jacobi CA, Freund S, Niedung K, Bach A, Heesemann J, Trülzsch K. 2007. Yersinia enterocolitica infection of mice reveals clonal invasion and abscess formation. Infect Immun 75:3802–3811. doi: 10.1128/IAI.00419-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Autenrieth S, Warnke P, Wabnitz GH, Lucero Estrada C, Pasquevich KA, Drechsler D, Günter M, Hochweller K, Novakovic A, Beer-Hammer S, Samstag Y, Hämmerling GJ, Garbi N, Autenrieth IB. 2012. Depletion of dendritic cells enhances innate anti-bacterial host defense through modulation of phagocyte homeostasis. PLoS Pathog 8:e1002552. doi: 10.1371/journal.ppat.1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Souza DG, Cara DC, Cassali GD, Coutinho SF, Silveira MR, Andrade SP, Poole SP, Teixeira MM. 2000. Effects of the PAF receptor antagonist UK74505 on local and remote reperfusion injuries following ischaemia of the superior mesenteric artery in the rat. Br J Pharmacol 131:1800–1808. doi: 10.1038/sj.bjp.0703756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conlan JW. 1997. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect Immun 65:630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vagima Y, Zauberman A, Levy Y, Gur D, Tidhar A, Aftalion M, Shafferman A, Mamroud E. 2015. Circumventing Y. pestis virulence by early recruitment of neutrophils to the lungs during pneumonic plague. PLoS Pathog 11(5):e1004893. doi: 10.1371/journal.ppat.1004893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai W, Strieter R, Mehrad B, Newstead M, Zeng X, Standiford TJ. 2000. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect Immun 68:4289–4296. doi: 10.1128/IAI.68.7.4289-4296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tateda K, Moore T, Newstead M, Tsai W, Zeng X, Deng JC, Chen G, Reddy R, Yamaguchi K, Standiford TJ. 2001. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect Immun 69:2017–2024. doi: 10.1128/IAI.69.4.2017-2024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, Gunzer M, Roers A, Hogg N. 2013. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 121:4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 32.Vagima Y, Levy Y, Gur D, Tidhar A, Aftalion M, Abramovich H, Zahavy E, Zauberman A, Flashner Y, Shafferman A, Mamroud E. 2012. Early sensing of Yersinia pestis airway infection by bone marrow cells. Front Cell Infect Microbiol 2:143. doi: 10.3389/fcimb.2012.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reutershan J, Morris MA, Burcin TL, Smith DF, Chang D, Saprito MS, Ley K. 2006. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J Clin Invest 116:695–702. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durand EA, Maldonado-Aroncho FJ, Castillo C, Walsh RL, Mecsas J. 2010. The presence of professional phagocytes dictates the number of host cells targets for Yop translocation during infection. Cell Microbiol 12:1064–1082. doi: 10.1111/j.1462-5822.2010.01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwasaki A, Medzhitov R. 2015. Control of adaptive immunity by the innate immune system. Nat Immunol 16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen LAH. 2003. Mechanisms of pathogenesis: evasion of killing by polymorphonuclear leukocytes. Microbes Infect 5:1329–1335. doi: 10.1016/j.micinf.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Fallman M, Deleuil F, McGee K. 2002. Resistance to phagocytosis by Yersinia. Int J Med Microbiol 291:501–509. [DOI] [PubMed] [Google Scholar]
- 38.Matteoli G, Fahl E, Warnke P, Müller S, Bonin M, Autenrieth IB, Bohn E. 2008. Role of IFN-gamma and IL-6 in a protective immune response to Yersinia enterocolitica in mice. BMC Microbiol 8:153. doi: 10.1186/1471-2180-8-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marketon MM, DePaolo RW, DeBord KL, Jabri B, Schneewind O. 2005. Plague bacteria target immune cells during infection. Science 309:1739–1741. doi: 10.1126/science.1114580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nauseef WM, Borregaard N. 2014. Neutrophils at work. Nat Immunol 15:602–614. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 41.Westermark L, Fahlgren A, Fällmana M. 2014. Yersinia pseudotuberculosis efficiently escapes polymorphonuclear neutrophils during early infection. Infect Immun 82:1181–1191. doi: 10.1128/IAI.01634-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Day RB, Link DC. 2012. Regulation of neutrophil trafficking from the bone marrow. Cell Mol Life Sci 69:1415–1423. doi: 10.1007/s00018-011-0870-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuetz M, Weiss EM, Schindler M, Hallstroem T, Zipfe PF, Linke D, Autenrieth IB. 2010. Trimer stability of YadA is critical for virulence of Yersinia enterocolitica. Infect Immun 78:2677–2690. doi: 10.1128/IAI.01350-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.KimN D, Luster AD. 2015. The role of tissue resident cells in neutrophil recruitment. Trends Immunol 36:547–555. doi: 10.1016/j.it.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Filippo K, Henderson RB, Laschinger M, Hogg N. 2008. Neutrophil chemokines KC and macrophage inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J Immunol 180:4308–4315. doi: 10.4049/jimmunol.180.6.4308. [DOI] [PubMed] [Google Scholar]
- 46.Rydström A, Wick MJ. 2007. Monocyte recruitment, activation, and function in the gut-associated lymphoid tissue during oral Salmonella infection. J Immunol 178:5789–5801. doi: 10.4049/jimmunol.178.9.5789. [DOI] [PubMed] [Google Scholar]
- 47.Rydstroem A, Wick MJ. 2009. Monocyte and neutrophil recruitment during oral Salmonella infection is driven by MyD88-derived chemokines. Eur J Immunol 39:3019–3030. doi: 10.1002/eji.200939483. [DOI] [PubMed] [Google Scholar]
- 48.Schiwon M, Weisheit C, Franken L, Gutweiler S, Dixit A, Meyer-Schwesinger C, Pohl JM, Maurice NJ, Thiebes S, Lorenz K, Quast T, Fuhrmann M, Baumgarten G, Lohse MJ, Opdenakker G, Bernhagen J, Bucala R, Panzer U, Kolanus W, Gröne HJ, Garbi N, Kastenmüller W, Knolle PA, Kurts C, Engel DR. 2014. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell 156:456–468. doi: 10.1016/j.cell.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spehlmann M, Dann S, Hruz P, Hanson E, McCole D, Eckmann L. 2009. CXCR2-dependent mucosal neutrophil influx protects against colitis associated diarrhea caused by an attaching/effacing lesion-forming bacterial pathogen. J Immunol 183:3332–3343. doi: 10.4049/jimmunol.0900600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herbold W, Maus R, Hahn I, Ding N, Srivastava M, Christman JW, Mack M, Reutershan J, Briles DE, Paton JC, Winter C, Welte T, Maus UA. 2010. Importance of CXC chemokine receptor 2 in alveolar neutrophil and exudate macrophage recruitment in response to pneumococcal lung infection. Infect Immun 78:2620–2630. doi: 10.1128/IAI.01169-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eisele NA, Lee-Lewis H, Besch-Williford C, Brown CR, Anderson DM. 2011. Chemokine receptor CXCR2 mediates bacterial clearance rather than neutrophil recruitment in a murine model of pneumonic plague. Am J Pathol 178:1190–1200. doi: 10.1016/j.ajpath.2010.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doroshenko T, Chaly Y, Savitskiy V, Maslakova O, Portyanko A, Gorudko I, Voitenok NN. 2002. Phagocytosing neutrophils down-regulate the expression of chemokine receptors CXCR1 and CXCR2. Blood 100:2668–2671. doi: 10.1182/blood.100.7.2668. [DOI] [PubMed] [Google Scholar]
- 53.Köberle M, Klein-Günther A, Schütz M, Fritz M, Berchtold S, Tolosa E, Autenrieth IB, Bohn E. 2009. Yersinia enterocolitica targets cells of the innate and adaptive immune system by injection of Yops in a mouse infection model. PLoS Pathog 5:e1000551. doi: 10.1371/journal.ppat.1000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mejia E, Bliska JB, Viboud GI. 2008. Yersinia controls type III effector delivery into host cells by modulating Rho activity. PLoS Pathog 4:e3. doi: 10.1371/journal.ppat.0040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiedemann A, Linder S, Grassl G, Albert Autenrieth MI, Aepfelbacher M. 2001. Yersinia enterocolitica invasin triggers phagocytosis via β1 integrins, CDC42Hs and WASp in macrophages. Cell Microbiol 3:693–702. doi: 10.1046/j.1462-5822.2001.00149.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.