Abstract
Salmonella enterica serovar Typhi, the causative agent of typhoid fever in humans, forms biofilms encapsulated by an extracellular matrix (ECM). Biofilms facilitate colonization and persistent infection in gallbladders of humans and mouse models of chronic carriage. Individual roles of matrix components have not been completely elucidated in vitro or in vivo. To examine individual functions, strains of Salmonella enterica serovar Typhimurium, the murine model of S. Typhi, in which various ECM genes were deleted or added, were created to examine biofilm formation, colonization, and persistence in the gallbladder. Studies show that curli contributes most significantly to biofilm formation. Expression of Vi antigen decreased biofilm formation in vitro and virulence and bacterial survival in vivo without altering the examined gallbladder pro- or anti-inflammatory cytokines. Oppositely, loss of all ECM components (ΔwcaM ΔcsgA ΔyihO ΔbcsE) increased virulence and bacterial survival in vivo and reduced gallbladder interleukin-10 (IL-10) levels. Colanic acid and curli mutants had the largest defects in biofilm-forming ability and contributed most significantly to the virulence increase of the ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant strain. While the ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant was not altered in resistance to complement or growth in macrophages, it attached and invaded macrophages better than the wild-type (WT) strain. These data suggest that ECM components have various levels of importance in biofilm formation and gallbladder colonization and that the ECM diminishes disseminated disease in our model, perhaps by reducing cell attachment/invasion and dampening inflammation by maintaining/inducing IL-10 production. Understanding how ECM components aid acute disease and persistence could lead to improvements in therapeutic treatment of typhoid fever patients.
INTRODUCTION
Salmonella enterica serovar Typhi, a human-specific pathogen and the etiologic agent of typhoid or enteric fever, is a globally rampant disease agent reported to cause over 20 million infections and 200,000 deaths annually (1, 2). Infection by S. Typhi is most commonly caused by consumption of contaminated food or water. Once ingested, S. Typhi crosses the intestinal epithelial barrier, where it is phagocytosed by macrophages. This allows the bacteria to spread systemically to common sites of infection: bone marrow, spleen, liver, and pancreas. From the liver, the bacterium is able to transit into the gallbladder, where it can either induce inflammation (cholecystitis) and an acute infection or persist chronically, creating a carrier state (3, 4). Chronic carriers are a threat to public health as they are able to live asymptomatically while shedding bacteria in their feces, thereby maintaining the pathogen within the population.
Bacterial biofilms are of high medical significance, as they are responsible for 60 to 80% of human infections (5). Bacterial cells within a biofilm are encapsulated in a mucoid substance known as an extracellular matrix (ECM), comprised of polysaccharides, proteins, and nucleic acids. The primary function of the matrix in human infections is protection of the bacterial community against the hazards of external (e.g., antibiotics) and internal (e.g., innate immune system) factors (6). While these general functions are associated with most microbial biofilms, the individual ECM components often possess unique properties for the bacterial community and with regard to the host (7).
An important factor in the development of chronic gallbladder carriage is cholesterol gallstones, the presence of which correlates with 80 to 90% of chronic carriers (8). Without gallbladder removal as a means of bacterial eradication, these patients become a critical reservoir for continued spread of disease (9, 10). We have demonstrated that biofilm formation is observed on the surface of gallstones, both in humans and in a mouse model of carriage (11, 12). In this model, 129×1/SvJ mice are fed a lithogenic diet to induce gallstone development, mimicking the condition of the majority of human carriers. This facilitates colonization of the gallbladder and increases shedding. Additionally, biofilm formation on and invasion of the gallbladder epithelium aid in the establishment and maintenance of carriage (11). Salmonella biofilm ECM components have been determined and include proteins (curli fimbriae and BapA), polysaccharides (extracellular DNA, cellulose, O-antigen capsule, colanic acid [in nontyphoidal serovars], and Vi antigen [ViAg] [in serovars Typhi, Dublin, and Paratyphi C]) (7, 13–17). Individual mutations in these ECM factors have been tested by various groups for their roles in biofilm formation, and some have also been examined for phenotypes in vertebrate models of infection (18–24). However, combinations of mutations and direct comparisons in vitro or in vivo have not been made.
In the present study, we further examine the role of selected ECM components (both individual and combined mutants) in biofilm formation in vitro and gallbladder colonization in our gallstone carriage mouse model. Our results indicate that, under the conditions studied, the ECM components play various individual roles both in vitro and in vivo that impact host colonization and virulence.
MATERIALS AND METHODS
Ethics statement.
Mice were housed and cared for in accordance with determined guidelines established by the Ohio State University (OSU) Institutional Animal Care and Use Committee (IACUC). All work was approved by OSU IACUC. The Ohio State University Animal Care and Use Program is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Research activities conformed to the statutes of the Animal Welfare Act and guidelines of the Public Health Service as required in the Guide for the Care and Use of Laboratory Animals (25).
Bacterial strains and growth conditions.
Wild-type (WT) Salmonella enterica serovar Typhimurium ATCC 14028 (JSG210) and its derivatives were used in these studies (Table 1). Cultures were grown in either Luria-Bertani (LB) broth or tryptic soy broth (TSB). When grown in the presence of bile, sodium choleate from ox bile (MP Biomedicals, LLC, Solon, OH) was included at 0.1% in 1:20 TSB. Antibiotics, when needed, were used at the following concentrations: chloramphenicol, 25 μg/ml; ampicillin, 100 or 200 μg/ml; kanamycin, 45 μg/ml; and streptomycin, 100 μg/ml.
TABLE 1.
Strains used in this study
| Strain | Genotype or relevant phenotypea | Reference or source |
|---|---|---|
| JSG210 | Wild-type S. Typhimurium ATCC 14028 | ATCC |
| JSG224 | phoN2 zxx::6251Tnd10-Cam | 38 |
| JSG3540 | ΔcsgA::Kan | 39 |
| JSG3672 | ΔyihO | 24 |
| JSG3712 | ΔwcaM::Kan | 39 |
| JSG3736 | ΔcsgA | This study |
| JSG3742 | ΔwcaM | This study |
| JSG3738 | JSG210(pTH122) | 40 |
| JSG3808 | ΔyihO::Cam | 24 |
| JSG3836 | ΔbcsE::Cam | This study |
| JSG3838 | ΔbcsE | This study |
| JSG3790 | ΔwcaM ΔcsgA | This study |
| JSG3829 | ΔwcaM ΔcsgA ΔyihO | This study |
| JSG3839 | ΔwcaM ΔcsgA ΔyihO ΔbcsE::Cam | This study |
| JSG3841 | ΔwcaM ΔcsgA ΔyihO ΔbcsE | This study |
| JSG3934 | JSG210 Strepr | This study |
Cam, chloramphenicol; Kan, kanamycin; Strepr, streptomycin resistant.
Generation of mutants and recombination procedures.
ECM mutants were created by the λ-Red mutagenesis method (26) with specific primers designed for the gene of interest (Table 2). Marked gene deletions were transduced into the WT or a mutant strain with bacteriophage P22HTint to create double, triple, and quadruple mutants starting with ΔwcaM, followed by ΔcsgA, ΔyihO, and finally ΔbcsE. Mutants were verified throughout the process via PCR and electrophoretic gel analysis, while the final mutant was confirmed with sequencing through the OSU Plant-Microbe Genomics Facility. Plasmid pTH122 containing the entire Vi antigen (viaB locus) (21) was electroporated into S. Typhimurium and was verified Vi antigen positive by serum agglutination.
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequence | Purpose |
|---|---|---|
| JG2093 JMM | 5′-CTTCACGTTCTTCCGTAAGGTCTC-3′ | Forward yihO |
| JG2094 JMM | 5′-GTTTTCTACATAAGCGCCAGCA-3′ | Reverse yihO |
| JG2674 | 5′-CCAATATTACCCAGTACGGTACGCAGAAAACAGC-3′ | Forward csgA |
| JG2675 | 5′-GGTTCGTTTAATGTGACCTGAGGGATCACCG-3′ | Reverse csgA |
| JG2676 | 5′-GCTTGTTTTCGCCAGCATGAATTACTCTGC-3′ | Forward wcaM |
| JG2677 | 5′-CGTGGTATCTACCGTGCATAGCGGTATTCC-3′ | Reverse wcaM |
| JG2682 | 5′-GGCAGCATTACGCCCCGTCGTCAATACGGG-3′ | Forward bcsE |
| JG2683 | 5′-CGGCGCAGAGTTCCGGCTGGTTTTACATAACG-3′ | Reverse bcsE |
| JG2700 | 5′-AACTGATAAACAGTTAAAATAGTGACATGAGTGTAGGCTGGAGCTGCTTC-3′ | λ-Red deletion of forward bcsE |
| JG2701 | 5′-CGATATCGCTGATGGTCATCATGATGCACGCATATGAATATCCTCCTTAG-3′ | λ-Red deletion of reverse bcsE |
Biofilm growth and crystal violet assays.
Biofilm attachment was tested on polypropylene microtiter wells coated by evaporation with 5 mg of cholesterol (diluted in 1:1 ethanol-isopropanol [Sigma-Aldrich, St. Louis, MO]). Microtiter wells were inoculated with a 1:10 dilution of 2 × 109 bacteria in 1:20 TSB with bile and incubated for 24 h at 30°C. Attached biofilms were then washed twice in double-distilled water (ddH2O), heat fixed for 1 h at 60°C, and stained with 0.33% crystal violet for 5 min. After two subsequent washes in ddH2O, the dye was released using 33% acetic acid, and the optical density at 570 nm (OD570) was measured in a SpectraMax spectrophotometer with SoftMax Pro software (Molecular Devices, Sunnyvale, CA) to determine the amount of dye retained, which correlates to the amount of biofilm present. All biofilm experiments were performed in triplicate.
Mouse infections.
Because S. Typhi is a human-restricted pathogen, in vivo studies of S. Typhi pathogenesis are performed using a mouse model with infection by S. Typhimurium. Naturally resistant NRAMP1 (SLC11A1)+/+ 129×1/SvJ mice (n = 128) (Jackson Laboratory, ME) were fed a lithogenic diet (1% cholesterol and 0.5% cholic acid [Envigo/Harlan Laboratory, IN]) to induce gallstone formation. After 8 weeks on the diet, mice were infected intraperitoneally with 104, 105, or 106 S. Typhimurium bacteria and sacrificed at 7 or 8 days postinfection (p.i.). The gallbladder, bile, and gallstones were collected, homogenized, and/or diluted to enumerate the bacteria using LB plates with or without antibiotics. For bacterial CFU enumeration of Salmonella in competition experiments, mice were given intraperitoneal injections containing 104 CFU of an equal mixture of WT cells marked with streptomycin resistance and cells of a mutant strain marked with either kanamycin or chloramphenicol resistance and sacrificed at 7 or 8 days postinfection. Gallbladder, bile, and gallstones were collected, homogenized, and/or diluted to enumerate the bacteria using LB plates with appropriate antibiotics. The competitive index was calculated by determining the output (mutant divided by the WT CFU) and dividing that value by the input (initial dose in CFU).
IL-10 and TNF-α binding ELISA.
Tissue lysates from the euthanized mice were prepared as described earlier (OptEIA set; BD Biosciences, San Diego, CA) and frozen at −20°C until cytokine analysis by enzyme-linked immune sorbent assay (ELISA). Tissue lysates were analyzed for anti-inflammatory (interleukin-10 [IL-10]) and proinflammatory (tumor necrosis factor alpha [TNF-α]) cytokines by ELISA per the manufacturer's instructions (BD OptEIA set protocol [BD Biosciences, San Diego, CA]). Briefly, Maxisorp 96-well plates were coated with purified anti-IL-10 and anti-TNF-α capture antibody (100 μl/well) in 0.2 M sodium phosphate (pH 6.5) and incubated overnight at 4°C. The plates were blocked for 1 h at room temperature (RT) with 200 μl of blocking buffer (phosphate-buffered saline [PBS] with 10% fetal bovine serum [FBS]) and then washed four times with PBS plus 0.05% Tween 20 (PBST). Samples and respective recombinant standards for IL-10 (1,000 pg/ml to 31.25 pg/ml) and TNF-α (1,000 pg/ml to 15.62 pg/ml) were added (100 μl/well) in duplicate, and the plates were incubated for 2 h at RT. Plates were washed with PBST, 100 μl of biotinylated detection antibody with streptavidin-horseradish peroxidase (working detector) was added to each well, and the mixture was incubated for 1 h at RT. Plates were washed and further developed using substrate solution (tetramethyl benzidine [TMB] and hydrogen peroxide) followed by adding 50 μl of 1 M phosphoric acid (H3PO4) to stop the reaction. The optical density of the reaction at 450 nm (OD450) was measured with a microplate reader SpectraMax spectrophotometer with SoftMax Pro software (Molecular Devices) within 30 min with a λ correction at 570 nm.
Intramacrophage survival assays.
J774.1 cells were seeded at 2 × 105 cells/well in a 24-well plate format for 24 h in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The cells were then infected with either the WT or mutant (multiplicity of infection [MOI] of 20). Infection was synchronized by centrifugation at 233 × g for 5 min at RT. The plates were incubated for 1 h at 37°C with 5% CO2. The extracellular bacteria were removed by adding gentamicin (50 μg/ml) for 45 min followed by three subsequent washes with warm medium. The cells were lysed using 0.1% Triton X-100 (Sigma-Aldrich). The intracellular bacteria at different time points were enumerated by serial plating. To obtain information on attachment and invasion, wells were also deprived of gentamicin, washed three times, and enumerated alongside bacteria immediately after exposure to gentamicin for 45 min as described above. Attached bacteria were calculated by subtracting the total bacteria recovered in the absence of gentamicin from those with gentamicin.
Serum sensitivity.
Serum sensitivity was assessed by following the protocol described by Wilson et al. (19). Bacterial cells (1 × 107) were incubated in 10% human serum complement (Quidel, San Diego, CA) diluted in 1× PBS. At the indicated time points, survival was quantified by enumeration of serial dilutions on LB agar plates.
RESULTS
Salmonella ECM components impact biofilm formation.
In order to assess the involvement of the ECM components in biofilm formation, we performed a biofilm assay on cholesterol-coated polypropylene plates in the presence of 0.1% bile for 24 h. These conditions mimic the environment within the gallbladder, where biofilm formation by Salmonella is biologically significant. The strains examined include WT S. Typhimurium, a WT strain expressing S. Typhi Vi antigen from a plasmid (WT+ViAg), mutants with single-gene deletions of colanic acid (ΔwcaM), curli (ΔcsgA), O-antigen capsule (ΔyihO), and cellulose (ΔbcsE), as well as double (ΔwcaM ΔcsgA), triple (ΔwcaM ΔcsgA ΔyihO), and quadruple (ΔwcaM ΔcsgA ΔyihO ΔbcsE) ECM mutants (Fig. 1). Interestingly, we observed a reduction in biofilm formation for the WT+ViAg strain, although this did not reach statistical significance. All of the individual mutants showed a decrease in biofilm formation, with the ΔcsgA strain demonstrating a significant reduction of 45%. The double, triple, and quadruple mutants containing the ΔcsgA mutation were at a level similar to that of the ΔcsgA single mutant alone, suggesting a major contribution of curli fimbriae and a minor contribution of the other ECM components to biofilms in this in vitro model.
FIG 1.
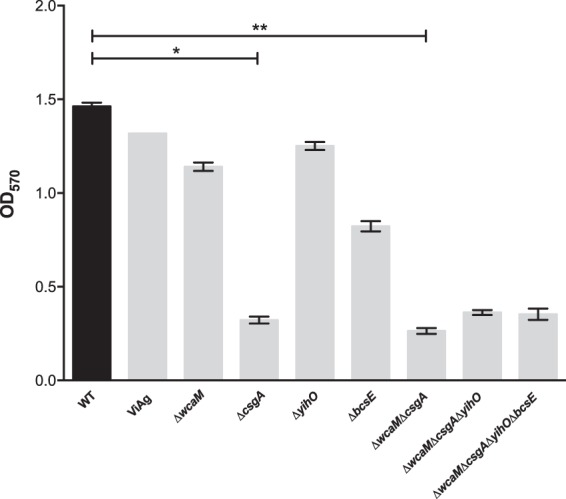
Mutations in components of the ECM showed variations in biofilm formation compared to WT S. Typhimurium exposed to 0.1% bile on cholesterol-coated surfaces in vitro. Biofilm screening was performed in untreated microtiter plates coated with 5 mg/ml cholesterol at 24 h post-bacterial inoculation. The crystal violet staining method was used to estimate biofilm production. Experiments were performed in triplicate and repeated 3 times. A one-way analysis of variance (ANOVA) with Dunnett's multiple comparison test correction was used to determine significant differences between mutant strains and the WT. *, P < 0.05; **, P < 0.01. The error bars indicate standard errors of the means.
ViAg expression in S. Typhimurium results in reduced virulence and gallbladder colonization.
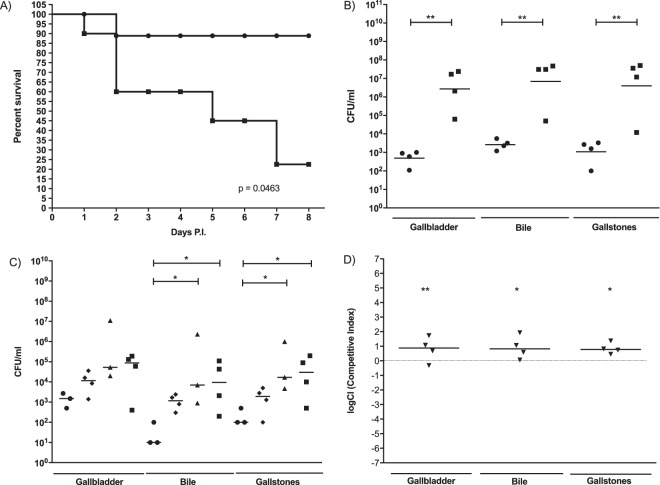
Since the addition of ViAg to WT S. Typhimurium resulted in decreased biofilm formation and because ViAg has been demonstrated to mediate anti-inflammatory effects (18, 19, 21), we tested whether it had an effect on virulence using a mouse model of infection. 129X1/SvJ mice fed a lithogenic diet (to induce gallstone development) for 8 weeks were infected with the WT and WT+ViAg strains at a dose of 1 × 106, 2 logs higher than typical in the gallstone mouse model. Mice given the WT+ViAg strain showed 100% survival at day 7 postinfection, while the WT strain-infected mice demonstrated only 45% survival (Fig. 2). At the typical dose of 1 × 104, mice given the WT+ViAg or WT strain had 100% survival. Commensurate with decreased virulence, those infected with WT+ViAg had significantly lower bacterial loads in the gallbladder of these mice at day 7 postinfection than those infected with the WT (Fig. 3A). The same trend was observed at 14 and 21 days postinfection for surviving mice (see Fig. S1 in the supplemental material). To determine if ViAg expression made the strain less fit in vivo, a competition assay was performed. 129×1/SvJ mice were coinfected with an equal mixture of marked WT and WT+ViAg strains. As hypothesized, based on the independent mouse experiments, the WT strain significantly outcompeted WT+ViAg at day 7 (Fig. 3B). Growth curves in both rich and minimal media verified that this phenotype was likely not due to a growth defect (see Fig. S2 in the supplemental material). Interestingly, when the organ tissues were examined for cytokine levels, there was no significant difference in levels of proinflammatory (TNF-α) or anti-inflammatory (IL-10) cytokine expression between the two strains in the examined tissues (Fig. 4).
FIG 2.
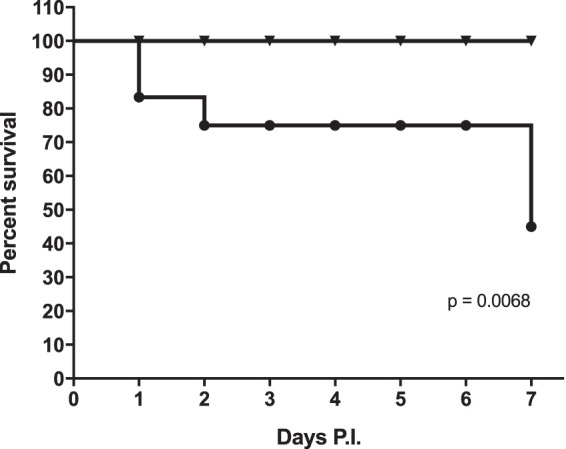
Survival plot of mice given intraperitoneal injections containing 106 WT (●) or WT+ViAg (▼) cells. No further mice died after day 7, and the surviving animals were sacrificed at day 13 postinfection (P.I.). n = 8 mice per group.
FIG 3.
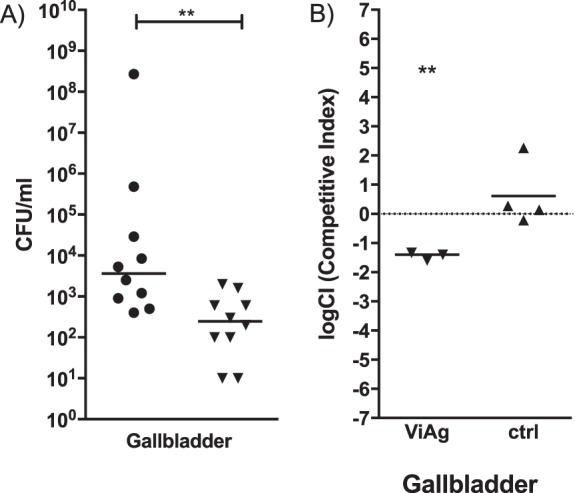
Bacterial gallbladder CFU enumeration of Salmonella at 7 days postinfection in the presence of gallstones. (A) Mice were given intraperitoneal injections of WT (circles) or WT+ViAg (triangles) cells. (B) In the competition experiment, mice were given intraperitoneal injections containing a mixture of WT cells marked with streptomycin resistance and WT+ViAg cells marked with kanamycin resistance. In both cases, the resulting final dose was 104, and the limit of detection was 100 CFU/ml. The dotted line represents a completely neutral competitive index. An unpaired t test with Welch's correction was used to determine significant differences between mutant strains and the WT in each organ. **, P < 0.01. Bars indicate means. The WT Camr versus WT Strepr comparison (ctrl) showed a neutral competitive index (CI) with a median value of 0.2059.
FIG 4.
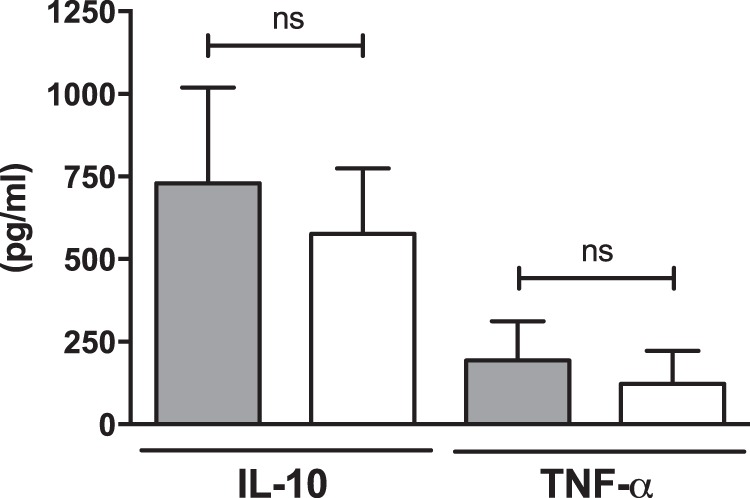
IL-10 and TNF-α production of mouse gallbladder samples infected with either the WT (gray bars) or WT+ViAg (white bars). Each bar consists of the combined data from 10 separate samples. Error bars indicate standard errors. ns, not significant.
Loss of ECM components enhances virulence.
In order to observe the importance of the ECM components on virulence in the gallstone mouse model, 129×1/SvJ mice were infected with a strain missing four major ECM biofilm contributors: colanic acid, curli, O-antigen capsule, and cellulose (ΔwcaM ΔcsgA ΔyihO ΔbcsE). We observed a significant increase in mouse morbidity for mice infected with the ΔwcaM ΔcsgA ΔyihO ΔbcsE strain at 8 days postinfection compared to mice infected with the WT, suggesting that without these extracellular components, bacteria are hypervirulent (Fig. 5A). Following this trend, the bacterial loads recovered from mice infected with the ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant were significantly higher than those infected with the WT (Fig. 5B). Additionally, when mice were infected with the WT or the double (ΔwcaM ΔcsgA), triple (ΔwcaM ΔcsgA ΔyihO), or quadruple (ΔwcaM ΔcsgA ΔyihO ΔbcsE) mutants, we observed an increasing trend of persistence upon the deletion of each additional gene, culminating in a 2- to 3-log increase in the quadruple mutant versus the WT (Fig. 5C). Growth curves in both rich and minimal media verified that these increases in virulence and bacterial load were likely not due to enhanced growth rates but rather represent a true increase in persistence or immune evasion (see Fig. S2 in the supplemental material). Furthermore, competitive infection experiments were performed between the ΔwcaM ΔcsgA ΔyihO ΔbcsE and WT strains (Fig. 5D). Consistent with the single-mutant strain virulence and CFU results, the ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant outcompeted the WT strain in the gallbladder.
FIG 5.
(A) Survival plot of mice given intraperitoneal injections containing 105 WT (●) or ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant (■) cells. All mice were sacrificed at day 8 postinfection. n = 8 mice/group. (B) CFU enumeration in gallbladder or bile or on gallstones from mice given intraperitoneal injections containing 104 WT (●) or ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant (■) cells at 8 days postinfection in the presence of gallstones. The limit of detection was 100 CFU/ml. An unpaired t test with Welch's correction was used to determine significant differences between the mutant strain and WT (**, P < 0.01). Bars indicate means. (C) CFU enumeration in gallbladder or bile or on gallstones from mice given intraperitoneal injections containing 104 WT (●) or ΔwcaM ΔcsgA (◆), ΔwcaM ΔcsgA ΔyihO (▲), or ΔwcaM ΔcsgA ΔyihO ΔbcsE (■) mutant cells at 8 days postinfection in the presence of gallstones. The limit of detection was 100 CFU/ml. One-way ANOVA with Dunnett's multiple comparison test was used to determine significant differences between mutant strains and the WT (*, P < 0.05). Bars indicate means. (D) Bacterial CFU enumeration of Salmonella at 8 days postinfection in the presence of gallstones. Mice were challenged with intraperitoneal injections containing a 104-CFU mixture of WT cells marked with streptomycin resistance and ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant cells marked with chloramphenicol. The limit of detection was 100 CFU/ml. The dotted line represents a completely neutral competitive index. Wilcoxon's signed rank test was used to determine significant differences between mutant strains and the WT (*, P < 0.05; **, P < 0.01). Bars indicate means. Positive values represent the mutant outcompeting the WT.
Loss of ECM components results in reduced levels of IL-10.
To determine whether cytokine expression levels in the gallbladder differ in response to infection with the ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant and WT, IL-10 and TNF-α levels were measured at days 7 to 8 postinfection. The levels of the proinflammatory cytokine TNF-α were significantly lower in bile but did not statistically differ between the strains in gallbladder tissue. However, the anti-inflammatory cytokine IL-10 was both significantly lower in the bile and 2-fold reduced in gallbladder tissue from those mice infected with the ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant (Fig. 6).
FIG 6.
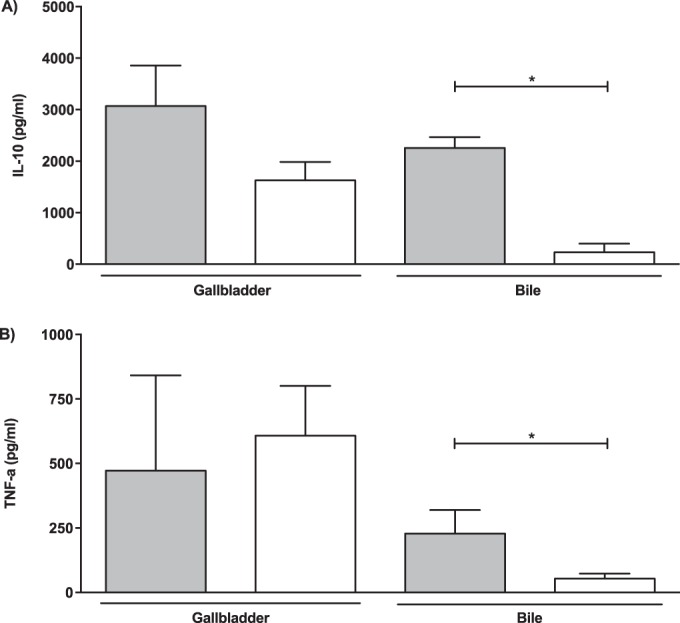
(A) IL-10 and (B) TNF-α levels in gallbladder tissue or bile from mice infected with either WT (gray bars) or ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant (white bars) cells. The data from gallbladder tissue or bile are from 10 separate samples. An unpaired t test was used to determine significant differences between the mutant strain and WT (*, P < 0.05). Error bars indicate standard errors.
Loss of ECM components results in enhanced macrophage attachment/invasion but not intracellular survival or complement resistance.
To determine if the ECM components contribute to cell attachment, invasion, intracellular survival, or resistance to innate immune factors, in vitro assays were performed with the ΔwcaM ΔcsgA ΔyihO ΔbcsE strain versus the WT. Assays with J774.1 macrophages demonstrated the ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant to have a 47% enhancement in attachment and a 40% increase in invasion versus the WT strain. However, once in the macrophage, there was no difference in growth of the bacteria over a 24-h period (Fig. 7A).
FIG 7.
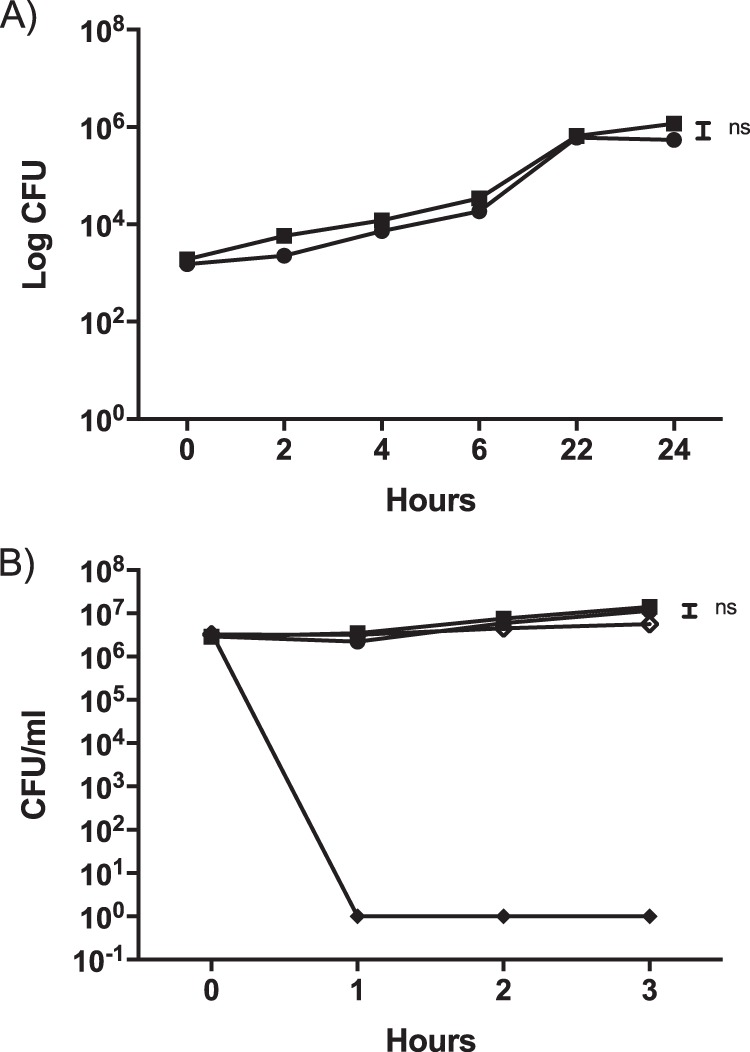
(A) Macrophage cell line J774.1 was infected with either WT (●) or ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant (■) cells. Numbers of bacteria per cell (both inside and membrane associated) were recovered at 1 h postinfection and after 45 min of gentamicin treatment. An unpaired t test was used to determine significant differences between mutant strains and the WT in each organ (ns, not significant). Bars indicate means. (B) Human serum sensitivity assays were performed on WT (●) and ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant (■) cells. E. coli (◆) was used as a positive control to ensure complement killing, and E. coli with heat-inactivated serum (♢) served as a negative control. The limit of detection was 100 CFU/ml. Experiments were repeated 3 times. An unpaired t test with Welch's correction was used to determine significant differences between mutant strain and WT (ns, no significance).
To examine the potential role of the ECM components in innate immune resistance, complement resistance assays were performed. In 10% human serum, the WT and ΔwcaM ΔcsgA ΔyihO ΔbcsE strains demonstrated no significant difference in survival (Fig. 7B). The control of Escherichia coli DH5α was efficiently killed by the serum, demonstrating its potency.
Competition experiments reveal that colanic acid and curli fimbriae contribute most significantly to the ΔwcaM ΔcsgA ΔyihO ΔbcsE virulence phenotype.
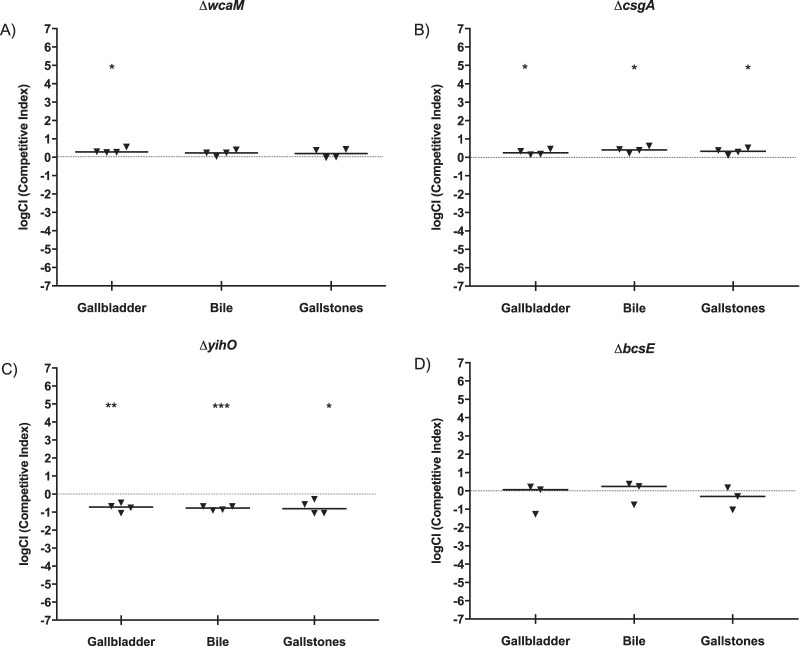
To investigate the individual extracellular components and their roles in the observed ability of the ΔwcaM ΔcsgA ΔyihO ΔbcsE strain to outcompete the WT, 129×1/SvJ mice were infected with an equal mixture of WT and differentially marked individual mutant strains (ΔwcaM, colanic acid; ΔcsgA, curli fimbriae; ΔyihO, O-antigen capsule; or ΔbcsE, cellulose), and the recovered bacterial enumerations were compared (Fig. 8). Bacterial loads recovered from the ΔwcaM and ΔcsgA mutants versus WT competitions showed a slight but significant outcompetition favoring the mutant in the gallbladder for both the ΔwcaM and ΔcsgA mutants and in bile and on gallstones for the ΔcsgA mutant, which is consistent with the ΔwcaM ΔcsgA ΔyihO ΔbcsE strain competition results (Fig. 5D). Contrary to those assays, the ΔyihO and ΔbcsE mutants were unable to outcompete the WT strain, with the ΔbcsE mutant equally competitive to and the ΔyihO mutant less competitive than the WT. Thus, the absence of either curli fimbriae or colanic acid resulted in the gain of a competitive advantage similar to that of the ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant.
FIG 8.
Bacterial CFU enumeration of Salmonella in competition experiments at 8 days postinfection in the presence of gallstones. Mice were given intraperitoneal injections containing 104 CFU that represent an equal mixture of WT cells marked with streptomycin resistance and (A) ΔwcaM::Kan, (B) ΔcsgA::Kan, (C) ΔyihO::Cam, or (D) ΔbcsE::Cam mutant cells. The limit of detection was 100 CFU/ml. The dotted line represents a completely neutral competitive index. Wilcoxon's signed rank test was used to determine significant differences between mutant strains and the WT (*, P < 0.05; **, P < 0.01; ***, P < 0.001). The bars indicate the means of the groups. Positive values represent the mutant outcompeting the WT.
DISCUSSION
Microbial biofilm formation is an important component of persistent/chronic infections. S. Typhi biofilm formation on gallstone surfaces aids the development and establishment of gallbladder chronic carriage. The key component of mature biofilms is the ECM that encases and protects the bacteria from microenvironmental insults. The specific components of the ECM in Salmonella spp. include curli fimbriae, cellulose, O-antigen capsule, colanic acid, and Vi antigen (ViAg). Many of these components are intimately related in both regulation and expression, and some are only present in certain serovars. How they interact and their roles in vivo versus in vitro are still not fully understood. In an effort to elucidate their roles, we hypothesized that one or more of these components influence biofilm formation, gallbladder colonization, and subsequent disease persistence.
In this study, we first examined the role of each ECM component in biofilm formation in vitro. Independent disruption of curli fimbriae (ΔcsgA) alone resulted in significantly impaired biofilm formation. Deletion of cellulose, O-antigen capsule, and colanic acid also each decreased biofilm development, but not to the same extent as deletion of curli. As filamentous appendages, curli fimbriae have highly adhesive properties and are an important component in the ECM of many Enterobacteriaceae. These fibers, along with cellulose expression, have been previously shown to be critical in initial adhesion of the bacterial cells to surfaces, cell aggregation, pellicle formation, and biofilm formation (27–31). Combinations of mutations resulted in only modest further decreases in biofilm formation, except when combined with ΔcsgA, which reduced biofilms to the level of the ΔcsgA mutant alone. These data suggest that while all components contribute to biofilm formation, curli fimbriae are clearly the most important under our in vitro conditions simulating the gallbladder environment. In fact, curli is a key element in community structure as it forms a highly hydrophobic matrix containing cells tightly aligned in parallel formation, an important method of construction for the tolerance of biofilms to environmental insults (30).
ViAg is expressed in S. Typhi but not S. Typhimurium. It has been demonstrated that ViAg dampens several aspects of host immunity, allowing S. Typhi to disseminate systemically (32–34). Heterologous expression of ViAg in S. Typhimurium enables this strain to also suppress immunity in both a bovine ligated ileal loop and the streptomycin-pretreated mouse model (35). We demonstrated that expression of ViAg in S. Typhimurium reduced biofilms in vitro and decreased virulence and gallbladder colonization in the gallstone mouse model. While a growth defect (tested in a rich and minimal medium) was likely not responsible for the observed phenotypes, the biofilm defect may be due to altered ECM interactions (with each other or the surface) caused by the addition of a new polysaccharide on the bacterium. The virulence phenotype may be related to the defect in biofilm formation allowing immune or mechanical clearance of bacteria from the gallbladder to occur. No differences in tested pro- or anti-inflammatory cytokines was noted, thus making altered immune responses in the gallbladder less likely as an explanation of the observed colonization and virulence defect of the S. Typhimurium ViAg-positive strain. Additional immunological and gene expression experiments are under way and will shed more light on the mechanism(s) at play.
Oppositely, the strain missing all four ECM components under study showed increased gallbladder colonization and virulence. The observed phenotype increased in a stepwise pattern as additional mutations were added, with the largest increase in recovered CFU occurring after the edition of the csgA mutation to the wcaM mutant. This double mutant also showed the biggest defect in biofilm formation. It is perhaps counterintuitive to attribute increased colonization of the gallbladder to decreased biofilm formation, but immune alterations were also noted. In general, in response to the ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant, IL-10 production was decreased in the gallbladder tissue and bile while TNF-α was reduced only in bile. This host response selectively favors the mutant, although as competition infections with the WT still showed an advantage to the ECM mutant, not to both strains, the WT was not able to add a function to the gallbladder environment that reversed the ΔwcaM ΔcsgA ΔyihO ΔbcsE phenotype. Individually, competition experiments showed that the ΔcsgA mutant consistently outcompeted the WT in bile, gallbladder tissue, and on gallstones, while the ΔwcaM strain only outcompeted the WT in the gallbladder tissue. All other single mutants showed equal or defective survival versus the WT strain. Thus, consistent with biofilm formation in vitro and CFU data in vivo, curli fimbriae, in association with colanic acid, affect colonization and persistence in the gallbladder. In fact, curli fimbriae, recognized by Toll-like receptor 1 (TLR1)/TLR2, induce IL-10 production (36). Consistent with this finding, as mentioned above, we observed decreased IL-10 in the gallbladder in vivo in response to the ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant, which is likely attributed to the lack of curli fimbriae. Perhaps the lack of sufficient IL-10 alters the immune balance in the gallbladder that favors ECM mutant survival and persistence.
In addition to the cytokine alterations that were observed, the ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant demonstrated increased adherence and invasion into macrophages. This may also hold true for other cell types, including gallbladder epithelial cells. There was no significant difference in intracellular survival rates in macrophages or difference in levels of resistance to another innate immune factor, complement. Thus, in addition to cytokine alterations of the local environment, increased numbers of intracellular salmonellae may also help account for the observed enhanced presence of the ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant in the gallbladder in the gallstone mouse model.
The role of the ECM components in altering host gallbladder colonization and persistence remains an active area of investigation. It is important to understand the relative abundance of ECM components in biofilms in the gallbladder, and we recently developed a gallstone sectioning technique to allow for ECM detection by microscopy (37). Knowing the major ECM constituents in vivo coupled with the above-described data regarding their functions in vitro and in biofilms in the gallbladder will allow unique insight into this biofilm-mediated infection. Based on their roles established here and elsewhere, future work will focus on immunomodulatory properties of curli and ViAg and their participation in gallbladder colonization and persistent gallbladder infection.
Supplementary Material
ACKNOWLEDGMENTS
The work presented here was funded by grants AI109002 and AI116917 from the National Institutes of Health to J.S.G.
We thank A. Bäumler for his strain contribution.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00532-16.
REFERENCES
- 1.Crump JA, Luby SP, Mintz ED. 2004. The global burden of typhoid fever. Bull World Health Organ 82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 2.Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Döpfer D, Fazil A, Fischer-Walker CL, Hald T, Hall AJ, Keddy KH, Lake RJ, Lanata CF, Torgerson PR, Havelaar AH, Angulo FJ. 2015. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med 12:e1001921. doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez-Escobedo G, Marshall JM, Gunn JS. 2010. Chronic and acute infection of the gall bladder by Salmonella Typhi: understanding the carrier state. Nat Rev Microbiol 9:9–14. doi: 10.1038/nrmicro2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunn JS, Marshall JM, Baker S, Dongol S, Charles RC, Ryan ET. 2014. Salmonella chronic carriage: epidemiology, diagnosis, and gallbladder persistence. Trends Microbiol 22:648–655. doi: 10.1016/j.tim.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romling U, Balsalobre C. 2012. Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med 272:541–561. doi: 10.1111/joim.12004. [DOI] [PubMed] [Google Scholar]
- 6.Parsek MR, Fuqua C. 2004. Biofilms 2003: emerging themes and challenges in studies of surface-associated microbial life. J Bacteriol 186:4427–4440. doi: 10.1128/JB.186.14.4427-4440.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunn JS, Bakaletz LO, Wozniak DJ. 2016. What's on the outside matters: the role of the extracellular polymeric substance of Gram-negative biofilms in evading host immunity and as a target for therapeutic intervention. J Biol Chem 291:12538–12546. doi: 10.1074/jbc.R115.707547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venneman NG, van Erpecum KJ. 2010. Pathogenesis of gallstones. Gastroenterol Clin North Am 39:171–183. doi: 10.1016/j.gtc.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Freitag JL. 1964. Treatment of chronic typhoid carriers by cholecystectomy. Public Health Rep 79:567–570. doi: 10.2307/4592192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai CW, Chan RC, Cheng AF, Sung JY, Leung JW. 1992. Common bile duct stones: a cause of chronic salmonellosis. Am J Gastroenterol 87:1198–1199. [PubMed] [Google Scholar]
- 11.Gonzalez-Escobedo G, Gunn JS. 2013. Gallbladder epithelium as a niche for chronic Salmonella carriage. Infect Immun 81:2920–2930. doi: 10.1128/IAI.00258-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawford RW, Rosales-Reyes R, de la Luz Ramírez-Aguilar M, Chapa-Azuela O, Alpuche-Aranda C, Gunn JS. 2010. Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc Natl Acad Sci U S A 107:4353–4358. doi: 10.1073/pnas.1000862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ledeboer NA, Jones BD. 2005. Exopolysaccharide sugars contribute to biofilm formation by Salmonella enterica serovar Typhimurium on HEp-2 cells and chicken intestinal epithelium. J Bacteriol 187:3214–3226. doi: 10.1128/JB.187.9.3214-3226.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prouty AM, Schwesinger WH, Gunn JS. 2002. Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infect Immun 70:2640–2649. doi: 10.1128/IAI.70.5.2640-2649.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson DL, White AP, Snyder SD, Martin S, Heiss C, Azadi P, Surette M, Kay WW. 2006. Salmonella produces an O-antigen capsule regulated by AgfD and important for environmental persistence. J Bacteriol 188:7722–7730. doi: 10.1128/JB.00809-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonas K, Tomenius H, Kader A, Normark S, Römling U, Belova LM, Melefors O. 2007. Roles of curli, cellulose and BapA in Salmonella biofilm morphology studied by atomic force microscopy. BMC Microbiol 7:70. doi: 10.1186/1471-2180-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solano C, García B, Valle J, Berasain C, Ghigo J-M, Gamazo C, Lasa I. 2002. Genetic analysis of Salmonella enteritidis biofilm formation: critical role of cellulose. Mol Microbiol 43:793–808. doi: 10.1046/j.1365-2958.2002.02802.x. [DOI] [PubMed] [Google Scholar]
- 18.Wilson RP, Raffatellu M, Chessa D, Winter SE, Tükel C, Bäumler AJ. 2008. The Vi-capsule prevents Toll-like receptor 4 recognition of Salmonella. Cell Microbiol 10:876–890. doi: 10.1111/j.1462-5822.2007.01090.x. [DOI] [PubMed] [Google Scholar]
- 19.Wilson RP, Winter SE, Spees AM, Winter MG, Nishimori JH, Sanchez JF, Nuccio S-P, Crawford RW, Tükel C, Bäumler AJ. 2011. The Vi capsular polysaccharide prevents complement receptor 3-mediated clearance of Salmonella enterica serotype Typhi. Infect Immun 79:830–837. doi: 10.1128/IAI.00961-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallo PM, Rapsinski GJ, Wilson RP, Oppong GO, Sriram U, Goulian M, Buttaro B, Caricchio R, Gallucci S, Tükel C. 2015. Amyloid-DNA composites of bacterial biofilms stimulate autoimmunity. Immunity 42:1171–1184. doi: 10.1016/j.immuni.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haneda T, Winter SE, Butler BP, Wilson RP, Tükel C, Winter MG, Godinez I, Tsolis RM, Bäumler AJ. 2009. The capsule-encoding viaB locus reduces intestinal inflammation by a Salmonella pathogenicity island 1-independent mechanism. Infect Immun 77:2932–2942. doi: 10.1128/IAI.00172-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White AP, Gibson DL, Collinson SK, Banser PA, Kay WW. 2003. Extracellular polysaccharides associated with thin aggregative fimbriae of Salmonella enterica serovar Enteritidis. J Bacteriol 185:5398–5407. doi: 10.1128/JB.185.18.5398-5407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White AP, Gibson DL, Kim W, Kay WW, Surette MG. 2006. Thin aggregative fimbriae and cellulose enhance long-term survival and persistence of Salmonella. J Bacteriol 188:3219–3227. doi: 10.1128/JB.188.9.3219-3227.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall JM, Gunn JS. 2015. The O-antigen capsule of Salmonella enterica serovar Typhimurium facilitates serum resistance and surface expression of FliC. Infect Immun 83:3946–3959. doi: 10.1128/IAI.00634-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC. [Google Scholar]
- 26.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnhart MM, Chapman MR. 2006. Curli biogenesis and function. Annu Rev Microbiol 60:131–147. doi: 10.1146/annurev.micro.60.080805.142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin JW, Sanders G, Kay WW, Collinson SK. 1998. Thin aggregative fimbriae enhance Salmonella enteritidis biofilm formation. FEMS Microbiology Lett 162:295–301. doi: 10.1111/j.1574-6968.1998.tb13012.x. [DOI] [PubMed] [Google Scholar]
- 29.Zogaj X, Bokranz W, Nimtz M, Römling U. 2003. Production of cellulose and curli fimbriae by members of the family Enterobacteriaceae isolated from the human gastrointestinal tract. Infect Immun 71:4151–4158. doi: 10.1128/IAI.71.7.4151-4158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zogaj X, Nimtz M, Rohde M, Bokranz W, Romling U. 2001. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol Microbiol 39:1452–1463. doi: 10.1046/j.1365-2958.2001.02337.x. [DOI] [PubMed] [Google Scholar]
- 31.Humphries AD, Townsend SM, Kingsley RA, Nicholson TL, Tsolis RM, Baumler AJ. 2001. Role of fimbriae as antigens and intestinal colonization factors of Salmonella serovars. FEMS Microbiol Lett 201:121–125. doi: 10.1111/j.1574-6968.2001.tb10744.x. [DOI] [PubMed] [Google Scholar]
- 32.Robbins JD, Robbins JB. 1984. Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi. J Infect Dis 150:436–449. doi: 10.1093/infdis/150.3.436. [DOI] [PubMed] [Google Scholar]
- 33.Janis C, Grant AJ, McKinley TJ, Morgan FJE, John VF, Houghton J, Kingsley RA, Dougan G, Mastroeni P. 2011. In vivo regulation of the Vi antigen in Salmonella and induction of immune responses with an in vivo-inducible promoter. Infect Immun 79:2481–2488. doi: 10.1128/IAI.01265-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atif SM, Winter SE, Winter MG, McSorley SJ, Bäumler AJ. 2014. Salmonella enterica serovar Typhi impairs CD4 T cell responses by reducing antigen availability. Infect Immun 82:2247–2254. doi: 10.1128/IAI.00020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raffatellu M, Santos RL, Chessa D, Wilson RP, Winter SE, Rossetti CA, Lawhon SD, Chu H, Lau T, Bevins CL, Adams LG, Bäumler AJ. 2007. The capsule encoding the viaB locus reduces interleukin-17 expression and mucosal innate responses in the bovine intestinal mucosa during infection with Salmonella enterica serotype Typhi. Infect Immun 75:4342–4350. doi: 10.1128/IAI.01571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oppong GO, Rapsinski GJ, Tursi SA, Biesecker SG, Klein-Szanto AJ, Goulian M, McCauley C, Healy C, Wilson RP, Tükel C. 2015. Biofilm-associated bacterial amyloids dampen inflammation in the gut: oral treatment with curli fibres reduces the severity of hapten-induced colitis in mice. NPJ Biofilms Microbiomes 1:15019. doi: 10.1038/npjbiofilms.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall JM, Flechtner AD, La Perle KM, Gunn JS. 2014. Visualization of extracellular matrix components within sectioned Salmonella biofilms on the surface of human gallstones. PLoS One 9:e89243. doi: 10.1371/journal.pone.0089243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller SI, Kukral AM, Mekalanos JJ. 1989. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A 86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porwollik S, Santiviago CA, Cheng P, Long F, Desai P, Fredlund J, Srikumar S, Silva CA, Chu W, Chen X, Canals R, Reynolds MM, Bogomolnaya L, Shields C, Cui P, Guo J, Zheng Y, Endicott-Yazdani T, Yang H-J, Maple A, Ragoza Y, Blondel CJ, Valenzuela C, Andrews-Polymenis H, McClelland M. 2014. Defined single-gene and multi-gene deletion mutant collections in Salmonella enterica sv Typhimurium. PLoS One 9:e99820. doi: 10.1371/journal.pone.0099820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsolis RM, Xavier MN, Santos RL, Baumler AJ. 2011. How to become a top model: impact of animal experimentation on human Salmonella disease research. Infect Immun 79:1806–1814. doi: 10.1128/IAI.01369-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.