Abstract
Hypoplastic right heart syndrome is a rare cyanotic congenital heart disease with under-development of the right ventricle, tricuspid, and pulmonary valves leading to right-to-left shunting of the blood through inter-atrial septal defect. Perinatal mortality is high with very few patients surviving to adulthood without corrective surgery. This report describes a 26-year-old young woman, who had recurrent abortions and stillbirths and detected to have marked cyanosis with hypoplastic right heart, sub-arterial ventricular septal defect, absent pulmonary valve, non-compaction of the left ventricle, and bicuspid aortic valve with aortic regurgitation. The patient died owing to progressive heart failure 4 years after the diagnosis was made.
Keywords: Hypoplastic right heart syndrome, Absent pulmonary valve, Bicuspid aortic valve, Non-compaction of the left ventricle
1. Introduction
Hypoplastic right heart syndrome (HRHS) is characterized by under-development of the tricuspid and/or pulmonary valves and of the right ventricle (RV) with right to left shunting through an inter-atrial communication.1 The extreme forms are associated with tricuspid and/or pulmonary atresia. There may be other multiple associated congenital cardiac defects.2 There are a very few reports of HRHS with patent but severely under-developed right-sided inflow and outflow tracts surviving into adulthood without any repair.2, 3, 4 This report describes a 26-year-old young lady, who was diagnosed to have a cyanotic congenital heart disease (CHD) in childhood but sought medical attention for recurrent abortions and stillbirths in adult life. She was detected to have hypoplastic tricuspid valve and markedly under-developed inlet and trabecular portion of the RV, restrictive subarterial ventricular septal defect, absent pulmonary valve with severely hypoplastic main pulmonary artery, bicuspid aortic valve with moderate aortic regurgitation, markedly dilated but non-compacted left ventricle with ejection fraction of 20% and severe mitral regurgitation.
2. Case report
A 26-year-old woman presented for evaluation of an elevated hemoglobin level of 18 g/dL with normal platelet and white blood cell counts. She had one abortion and two stillbirths in the recent past. The patient had been diagnosed with a heart murmur and cyanosis in childhood; however, her only symptom was mild dyspnea on exertion. She had not undergone prior cardiac evaluation. Her physical examination was notable for good physical development (weight 61 kg, height 162 cm, BSA of 1.6), moderate clubbing and cyanosis with an oxygen saturation of 78% on room air, supine blood pressure of 100/80 mmHg, pulse rate of 110/min, engorged jugular veins and suffused face. Cardiac auscultation revealed a quiet precordium, a normal S1, single S2, and apical S3. A grade 3/6 systolic murmur was heard at the apex with a basal early diastolic murmur. Hematologic evaluation revealed a hemoglobin level of 17.8 g/dL, hematocrit of 52%, a white blood cell count of 6.2 × 109/L, and a platelet count of 260 × 109/L. A 12-lead electrocardiogram revealed situs solitus, sinus rhythm, normal atrio-ventricular conduction, and an intra-ventricular conduction defect resembling left bundle branch block. The chest skiagram revealed pulmonary oligemia and cardiomegaly. A transthoracic echocardiogram was obtained for further evaluation of the murmur, hypoxemia, and erythrocytosis (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5). The echocardiogram revealed situs solitus, normally related great vessels, D-loop of the ventricles, and normal systemic and pulmonary venous drainage. The inferior vena cava was dilated with reduced respiratory variation. The right atrium was dilated with inter-atrial septum pushed to the left during inspiration (Fig. 1). The tricuspid annulus measured 14 mm (Z score −2.5); inlet portion of the right ventricular cavity had end-diastolic diameter of 20 mm and the trabecular portion of 12 mm.
Fig. 1.
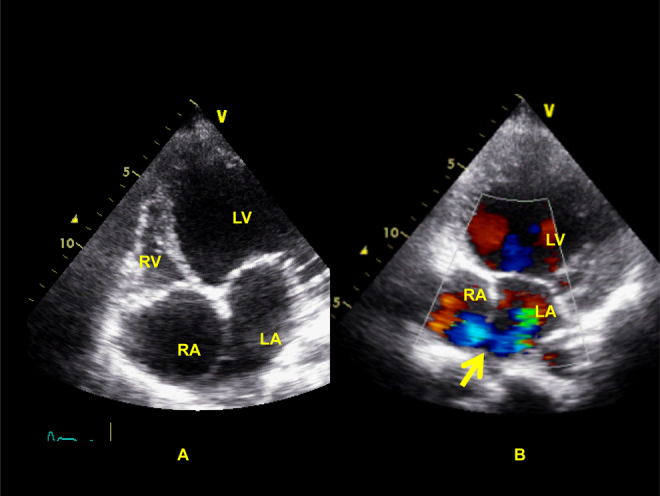
Modified apical 4-chamber view on trans-thoracic echocardiographic examination showing dilated right atrium (RA), rudimentary right ventricle (RV), dilated left ventricle (LV) (Panel A) with inter-atrial right-to-left shunting (Panel B, arrows).
Fig. 2.
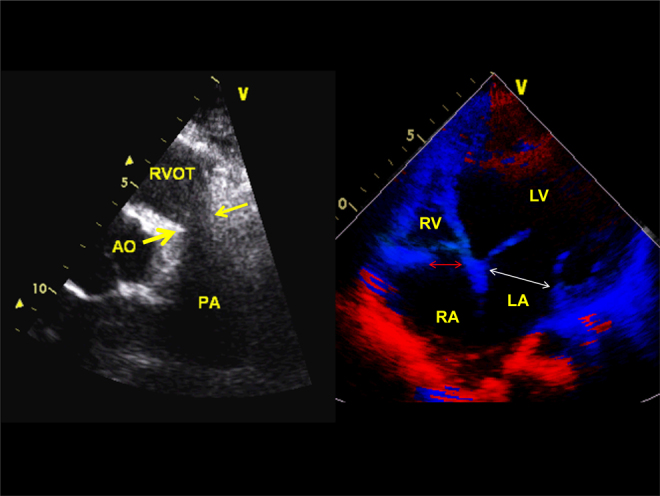
Parasternal short-axis view (left panel) showing absent pulmonary valve and hypoplastic pulmonary annulus and the main pulmonary artery. The distal pulmonary arteries are markedly dilated. The right panel shows hypo-plastic tricuspid annulus (14 mm) compared to 29 mm mitral annulus in diastole.
Fig. 3.
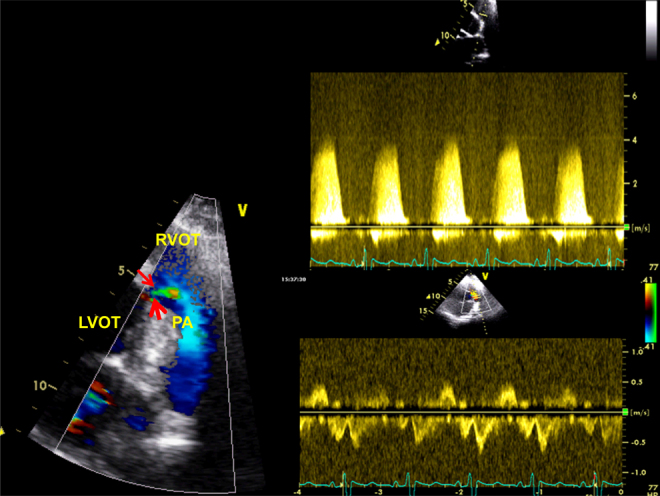
Left panel shows small sub-pulmonic ventricular septal defect (red arrows) with a peak trans-ventricular pressure gradient of 70 mmHg (upper right panel). The lower right panel shows low velocity to-and-fro flow across the right ventricular outflow tract.
Fig. 4.
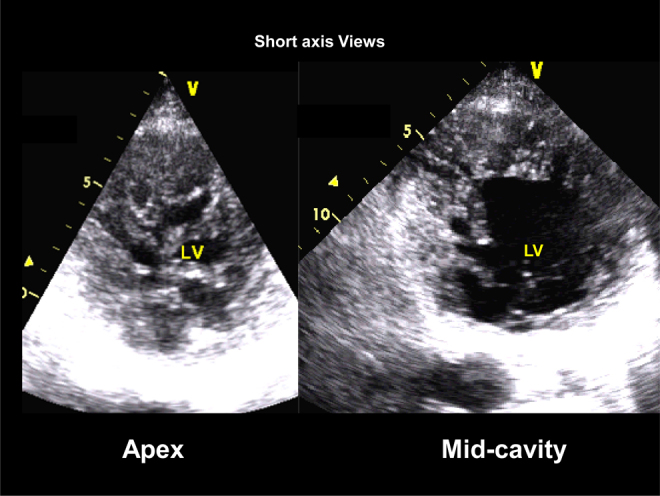
2D-echocardiographic parasternal short-axis views of the left ventricle. Note extensive trabeculations, inter-trabecular recesses and the thin myocardial walls.
Fig. 5.
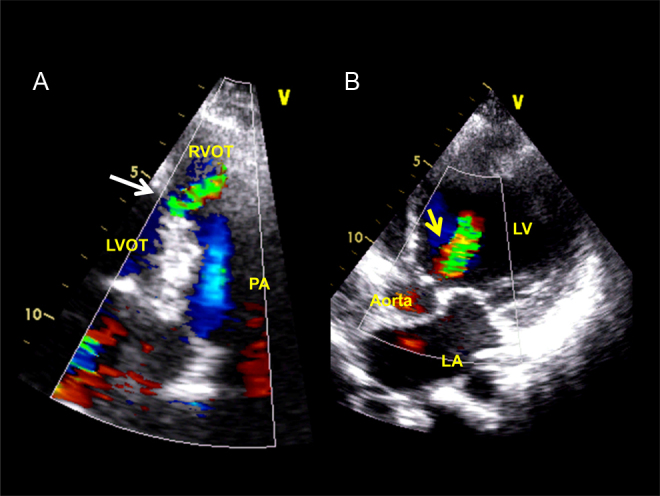
Panel A shows left-to-right flow of the sub-pulmonic ventricular septal defect (white arrow, systolic frame). Panel B shows aortic regurgitation jet in the left ventricular outflow and the cavity (yellow arrow, diastolic frame).
There was no moderator band seen in the RV. The sub-pulmonic part of the right ventricular outflow tract was well-developed (28 mm in diastole); the pulmonary valve was absent and virtual pulmonary annulus and the proximal part of the main pulmonary artery measured 14 mm (Z score −2). The tricuspid valve was rudimentary but showed normal motion and mild regurgitation (Fig. 2). The left ventricle was markedly dilated with severely reduced contractile function (ejection fraction 20%). There were prominent trabeculations in the mid and apical segments of the left ventricle with honey-comb appearance and compact ventricular wall was thin (Fig. 4). The aortic valve was bicuspid with moderate aortic regurgitation (Fig. 5). Moderately severe mitral regurgitation was detected on color Doppler interrogation. A small (4 mm) sub-pulmonic ventricular septal defect with left-to-right shunting was noted with a trans-ventricular peak gradient of 70 mmHg (Fig. 3, Fig. 5). Inter-atrial right-to-left shunting was detected on color flow map (Fig. 1). The pulsed wave Doppler examination of the right ventricular outflow tract was notable for pre-systolic and systolic forward flow of markedly reduced velocities and early diastolic retrograde flow (Fig. 3, videos 1 and 2). The tricuspid valve was patent with narrow antegrade color flow jet and a small jet of tricuspid regurgitation (Fig. 6).
Fig. 6.
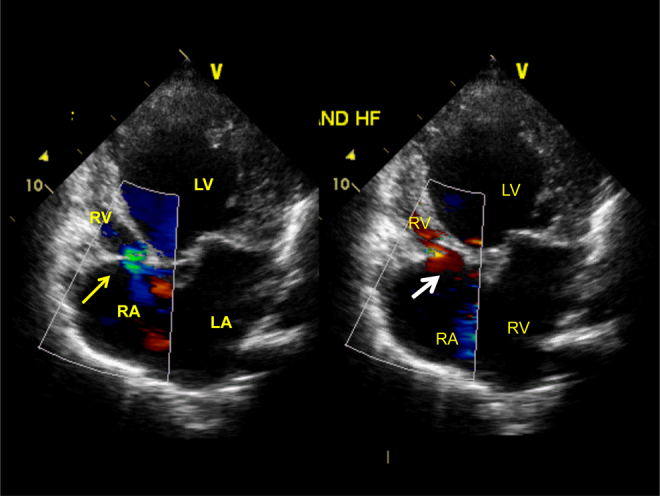
Patent tricuspid valve with color flow jet of tricuspid regurgitation in left panel and antegrade flow across the tricuspid valve shown in right panel in apical 4-chamber views.
The patient was managed with anti-failure therapy, followed by repeated phlebotomy and was advised contraception. Atrio-pulmonary and cavo-pulmonary shunts were considered, but there were obvious contraindications such as severely depressed left ventricular systolic function and aortic and mitral regurgitations. The patient survived for 4 years after the last stillbirth and finally succumbed to progressive heart failure.
3. Discussion
This minimally symptomatic adult patient of HRHS was notable for several peculiar features: (1) absent pulmonary valve with dilated pulmonary artery branches, (2) non-compaction of the left ventricle with severe dysfunction and (3) bicuspid aortic valve with moderate aortic regurgitation. She sought medical attention because of repeated mis-carriages. Such a presentation of repeated mis-carriages in a slightly symptomatic patient with HRHS has been described previously.3 Hypoxemia may be the major determinant of the miscarriages.
HRHS is not a commonly used generic term like hypoplastic left heart syndrome. It could be in the form of under-development of the tricuspid valve, RV and pulmonary artery or the more common variety i.e. tricuspid atresia.1 This underdevelopment is quite variable; when it is severe, patients with hypoplastic right-heart syndrome present in infancy or childhood. On the other hand, a less severe right-heart hypoplasia, as in our patient, can permit survival until adulthood. Development of pulmonary arteries and survival into adulthood depends upon the pulmonary blood flow either through a ventricular septal defect or through a patent ductus arteriosus. Presence of ventricular septal defect with left-to-right shunting in our case contributed to better survival. Hypoplastic RV can be an integral part of malformations such as pulmonary atresia with intact ventricular septum and tricuspid atresia and occasionally is associated with a variety of congenital heart defects, such as ventricular septal defect, atrial septal defect, atrioventricular septal defect, and other complex CHD.2, 5
The maternal and fetal risks of pregnancy in adults with CHD depend on many factors, including the type and complexity of the CHD, maternal hypoxemia, ventricular dysfunction, pulmonary hypertension, aortic aneurysm, or history of cardiac arrhythmias. Results of in vitro studies have shown that hypoxia has a deleterious effect on trophoblastic development, which probably explains our patient's repeated miscarriages.6
Successful repair of hypoplastic right-heart syndrome depends on the degree of hypoplasia of the tricuspid valve and the RV, and on the severity of pulmonary valve and pulmonary artery stenosis. The aim of surgically repairing hypoplastic right-heart syndrome is to offload the small RV by establishing a direct communication between the venous return from the vena cava and the pulmonary artery, thus bypassing the RV and closing the atrial septal defect. Atrial septal defect should be closed only if it can be demonstrated that the RV can adjust to the increased volume-load. Presence of inter-atrial communication prevents right ventricular failure in severely hypoplastic RV with myocardial fibrosis. The goals of repair can be achieved by means of a Glenn anastomosis (also referred to as a bidirectional cavopulmonary anastomosis) between the superior vena cava and right pulmonary artery; or a Fontan procedure, through which the inferior vena cava or right atrium is also surgically connected directly to the pulmonary artery or its branches. Possibility of raised pulmonary diastolic pressure secondary to severe mitral regurgitation and left ventricular dysfunction precluded cavo-pulmonary and/or atrio-pulmonary shunt surgery in our patient. Impaired function of the pumping ventricle is known to be a risk factor for mortality after the Fontan operation. The ‘Ten commandments’ by Choussat et al. mandate that normal ventricular function is one of the basic requirements for successful Fontan repair.7
Conflicts of interest
The authors have none to declare.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ihj.2016.03.030.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.der Hauwaert L.G., Michaelsson M. Isolated right ventricular hypoplasia. Circulation. 1971;44:466–474. doi: 10.1161/01.cir.44.3.466. [DOI] [PubMed] [Google Scholar]
- 2.Sadegpour A., Kyavar M., Yousefnia M.A., Chamanian Z., Khajali S., Sani Z.A. Hypoplastic right ventricle with multiple associated anomaly: a challenging case for biventricular repair or univentricular approach? Arch Cardiovasc Imaging. 2013;1:31–33. [Google Scholar]
- 3.Dib C., Araoz P.A., Davies N.P., Dearani J.A., Ammash N.M. Hypoplastic right-heart syndrome presenting as multiple miscarriages. Tex Heart Inst J. 2012;39:249–254. [PMC free article] [PubMed] [Google Scholar]
- 4.Prasad K., Singh M., Radhakrishnan S. Hypoplastic right ventricle with mild pulmonary stenosis in an adult. Int J Cardiol. 1992;37:260–262. doi: 10.1016/0167-5273(92)90219-s. [DOI] [PubMed] [Google Scholar]
- 5.Jacobstein M.D., Fletcher B.D., Goldstein S., Riemenschneider T.A. Magnetic resonance imaging in patients with hypoplastic right heart syndrome. Am Heart J. 1985;110:154–158. [PubMed] [Google Scholar]
- 6.Gultice A.D., Selesniemi K.L., Brown T.L. Hypoxia inhibits differentiation of lineage-specific Rcho-1 trophoblast giant cells. Biol Reprod. 2006;74:1041–1050. doi: 10.1095/biolreprod.105.047845. [DOI] [PubMed] [Google Scholar]
- 7.Choussat A., Fontan F., Besse P. Selection criteria for Fontan's procedure. In: Anderson R.H., Shinebourne E.A., editors. Pediatric Cardiology. Churchill Livingstone; Edingburgh: 1978. pp. 559–566. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


