Abstract
Global evidence shows that children's growth deteriorates rapidly during/after illness if foods and feeding practices do not meet the additional nutrient requirements associated with illness/convalescence. To inform policies and programmes, we conducted a review of the literature published from 1990 to 2014 to document how children 0–23 months old are fed during/after common childhood illnesses. The review indicates that infant and young child feeding (IYCF) during common childhood illnesses is far from optimal. When sick, most children continue to be breastfed, but few are breastfed more frequently, as recommended. Restriction/withdrawal of complementary foods during illness is frequent because of children's anorexia (perceived/real), poor awareness of caregivers' about the feeding needs of sick children, traditional beliefs/behaviours and/or suboptimal counselling and support by health workers. As a result, many children are fed lower quantities of complementary foods and/or are fed less frequently when they are sick. Mothers/caregivers often turn to family/community elders and traditional/non‐qualified practitioners to seek advice on how to feed their sick children. Thus, traditional beliefs and behaviours guide the use of ‘special’ feeding practices, foods and diets for sick children. A significant proportion of mothers/caregivers turn to the primary health care system for support but receive little or no advice. Building the knowledge, skills and capacity of community health workers and primary health care practitioners to provide mothers/caregivers with accurate and timely information, counselling and support on IYCF during and after common childhood illnesses, combined with large‐scale communication programmes to address traditional beliefs and norms that may be harmful, is an urgent priority to reduce the high burden of child stunting in South Asia.
Keywords: child feeding, common childhood illnesses, diarrhoea, pneumonia, South Asia
Introduction
About a quarter (26%) of the world's children under five live in South Asia. Thirty‐eight per cent of them have stunted growth (UNICEF 2015). Stunting, or linear growth retardation during early childhood, is an outcome of biological and/or psychosocial deprivation (Stewart et al. 2013). The short‐term and long‐term consequences of stunting include impaired survival, physical growth and cognitive development in pre‐school age children; poor school readiness, school enrolment and learning outcomes in school‐age children; increased risk of obstetric complications and mortality in women; and reduced height, productivity and earnings in adults (Grantham‐McGregor et al. 2007; Walker et al. 2007; de Onis et al. 2013).
A significant proportion of stunting can happen prenatally. However, evidence indicates that most stunting in low‐income and middle‐income countries occurs during the first 24 months of life as a result of suboptimal breastfeeding and complementary feeding practices, often in combination with recurrent infections (Stewart et al. 2013; Jones et al. 2014). Furthermore, children's nutritional status can deteriorate rapidly during/after illness if the additional nutrient requirements associated with illness/convalescence are not met and nutrients are diverted from growth and development towards the immune response. Children's poor appetite induced by illness can contribute to perpetuate the vicious cycle of infection and stunting (Brown 2003; Ramachandran & Gopalan 2009; Gulati 2010; Neumann et al. 2012; Richard et al. 2014). Additionally, in low‐income and middle‐income countries, infant and young child feeding (IYCF) practices during and after common childhood illnesses can be particularly poor owing to harmful traditional practices and the low coverage/quality of primary health care services (Bhutta & Salam 2012; de Onis et al. 2012; Stewart et al. 2013).
Recognizing the importance of optimal IYCF practices for child survival, growth and development, the World Health Organization (WHO) launched in 2003 the Global Strategy for Infant and Young Child Feeding and issued in 2003 the Guiding Principles for Complementary Feeding of Breastfed and Non‐Breastfed Children (WHO/UNICEF 2003; WHO 2003a,2003b). These global frameworks highlight the importance of optimal IYCF practices during and after common childhood illnesses such as diarrhoea and pneumonia and emphasize the need to increase fluid intake during illness while feeding is maintained and increase food intake during convalescence. In addition, appropriate IYCF during and after illness is part of the WHO‐led Global Strategy for the Integrated Management of Childhood Illnesses (WHO 2005). The definition and measurement of the indicators for assessing IYCF practices – beyond the scope of this paper – are comprehensively detailed elsewhere (WHO 2008, 2010).
In South Asia, breastfeeding is a quasi‐universal practice. An estimated 96% of children are breastfed at some point in their lives, and most (80%) continue to be breastfed at 2 years of age (Dibley et al. 2010; UNICEF 2015). However, data from household surveys across the region indicate that the majority of South Asian children are not fed as per the internationally agreed upon recommendations: only a quarter (27%) of newborns start breastfeeding within 1 h of birth; less than half (48%) of infants 0–5 months old are exclusively breastfed; only about half (56%) of infants 6–8 month olds are fed soft, semi‐solid or solid foods; and a mere 21% of children 6–23 months old are fed a diet that meets the minimum requirements in terms of feeding frequency and diet diversity (Senarath et al. 2012; UNICEF 2015). In view of this situation, researchers and practitioners have not hesitated to refer to IYCF in South Asia as a crisis (Memon 2012). There is evidence that the incidence and severity of common childhood diseases are high in this region (Walker et al. 2013). However, less is known about IYCF practices during and after common childhood illnesses in South Asia.
Thus, the objective of this paper is threefold: (1) document the current IYCF practices during and after common childhood illnesses – particularly diarrhoea, fever and pneumonia – and their trends since 1990 in South Asia; (2) document caregiver's behaviours and health providers' practices with respect to IYCF during and after common childhood illnesses in South Asia; and in light of the preceding objectives, (3) identify priorities in terms of policy formulation, programme design, research and advocacy to protect, promote and support optimal IYCF practices during and after common childhood illnesses in South Asia post 2015.
Key messages.
Information on infant and young child feeding (IYCF) behaviors and practices during common childhood illnesses in South Asia is limited. Information of IYCF after illnesses is virtually inexistent. The evidence available indicates that IYCF practices during common childhood illnesses are far from optimal.
When sick, most children (up to 98%) continue to be breastfed although a significant proportion (up to 49%) are breastfed less frequently than usual. Few sick children (<20%) are breastfed more frequently than usual, as is recommended, to compensate for the additional fluid and nutrient requirements associated with illnesses.
When sick, many children (up to 75%) see their complementary foods restricted in frequency, quantity and/or quality due to children's anorexia (perceived or real), lack of awareness of caregivers' about the feeding needs of sick children, traditional beliefs, or sub‐optimal counselling and support by health workers.
In general, health providers do not advise mothers to increase breastfeeding frequency while encouraging sick children to eat soft, varied, and favourite foods during illness, as is recommended. Important policy, programme and capacity gaps exist with respect to IYCF for children during and after common childhood illnesses in many South Asian countries.
Methods
We reviewed data and information from two primary sources: Demographic Health Surveys (DHS) and peer‐reviewed publications. DHS collects information on care‐seeking and care‐giving practices during diarrhoea, fever and pneumonia using standardized sampling methodologies, interview tools and data analyses procedures, with minor country‐specific adaptations. We reviewed the national DHS surveys conducted in South Asia between 1990 and 2014 to document the prevalence of common childhood illnesses – diarrhoea, fever and pneumonia – in children 0–23 months old, the frequency and type of medical advice that caregivers sought, the type of treatment and/or advice that children received and how children were fed during common childhood illnesses. For countries with two data points, trends in IYCF practices during and after common childhood illness were estimated as well as the average annual rate of improvement to quantify the average improvement in a given indicator per year between the base year and end year.
We also conducted a comprehensive review of the peer‐reviewed literature published between January 1990 and December 2014. Peer‐reviewed articles were identified through an online PubMed search using the following search terms and search filters: (1) search terms: <feeding>, <sick>, <morbidity>, <pneumonia>, <diarrhea/diarrhoea> and <IMCI/IMNCI>, each term combined with <Afghanistan>, <Bangladesh>, <Bhutan>, <India>, <Maldives>, <Nepal>, <Pakistan>, <Sri Lanka> and/or <Asia>; (2) search filters: age <child 0–59 months>; language: <English>; text availability: <abstract>; species :<human>; and search fields: <title/abstract>. Although children 0–23 months old are the focus of our analysis, we expanded our ‘child age’ search criteria to 0–59 months to capture additional publications that, while focusing on ‘children under five’ or ‘preschool‐age children’, also address IYCF practices in the first 2 years of life.
The PubMed search identified 367 publications with one or more of the search terms in the title and/or abstract. In‐depth scrutiny of the titles excluded 158 publications as not relevant to our review and identified 209 as potentially relevant. In‐depth scrutiny of the abstracts of these 209 publications excluded 126 as not relevant to our review and identified 83 as likely relevant. Lastly, full‐text scrutiny of these 83 publications excluded 54 as not relevant to our review and identified 29 articles that were relevant to our review. In addition, we reviewed the bibliographic references of these 29 papers to identify any additional publication that could have been missed by our online search and found three additional publications that were relevant to our analysis. Hence, 32 publications were included in our analysis as they focused specifically on IYCF practices during diarrhoea, fever and/or pneumonia in South Asian countries (Fig. 1).
Figure 1.
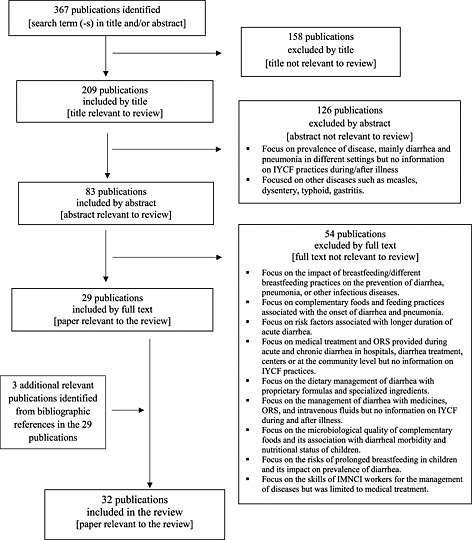
Flow diagram of literature review.
In addition, we conducted interviews with 13 key informants. The purpose of the key informant interviews was not to collect key informants' views, opinions or recommendations but rather to help the authors of the paper identify the existing national policies, guidelines and programmes related to IYCF during and after common childhood illnesses in the eight countries included in the analysis. In the five large countries (Afghanistan, Bangladesh, India, Nepal and Pakistan), we interviewed two UNICEF staff by country, namely the Chief of Health and the Chief of Nutrition, while in the three smaller countries (Bhutan, Maldives and Sri Lanka), we interviewed one UNICEF staff per country, namely the Chief of the Health and Nutrition programme. This made a total of 13 key informants who in turn consulted with relevant national counterparts to complete the information‐gathering process.
Findings
Household survey evidence on infant and young child feeding and care practices during and after illness
Six countries – Bangladesh, India, Maldives, Nepal, Pakistan and Sri Lanka – had at least one DHS survey that included information on common childhood illnesses and IYCF practices (Afghanistan's 2010 DHS did not include data collection on child morbidity, and no DHS survey was available for Bhutan). DHS survey data indicate that in the countries included in the analysis, children 0–23 months old suffer from common childhood illnesses frequently. Up to 20–30% of the mothers/caregivers interviewed reported that their children had suffered from diarrhoea or pneumonia in the 2 weeks prior to the survey. The prevalence of common childhood illnesses – diarrhoea, fever and pneumonia – was highest in Pakistan. In all countries, the prevalence of fever was higher than the prevalence of diarrhoea or pneumonia. Similarly, in all countries, the prevalence of common childhood illnesses was lowest during the exclusive breastfeeding period (0–5 months) and highest during the early complementary feeding period (6–11 months) (Table 1).
Table 1.
Number and percentage of children 0–23 months old who experienced diarrhoea, fever or pneumonia in the 2 weeks preceding the survey (South Asia, Demographic and Health Surveys)
| Prevalence of common childhood diseases | ||||
|---|---|---|---|---|
| n | Diarrhoea (%) | Fever (%) | Pneumonia (%) | |
| Bangladesh, 2011 | ||||
| 0–5 months | 816 | 3.1 | 35.1 | 6.2 |
| 6–11 months | 864 | 8.4 | 49.2 | 7.4 |
| 12–23 months | 1 547 | 7.1 | 42.6 | 6.9 |
| 0–23 months | 3 227 | 6.4 | 42.5 | 6.9 |
| India, 2006 | ||||
| 0–5 months | 5 127 | 10.6 | 11.6 | 6.2 |
| 6–11 months | 5 276 | 18.1 | 21.1 | 8.1 |
| 12–23 months | 10 419 | 13.8 | 19.1 | 7.1 |
| 0–23 months | 20 822 | 14.1 | 17.8 | 7.1 |
| Maldives, 2009 | ||||
| 0–5 months | 406 | 2.5 | 21.8 | — |
| 6–11 months | 441 | 6.9 | 34.4 | — |
| 12–23 months | 822 | 6.7 | 33.7 | — |
| 0–23 months | 1 669 | 5.7 | 31.0 | <1 |
| Nepal, 2011 | ||||
| 0–5 months | 531 | 12.9 | 17.1 | 3.9 |
| 6–11 months | 491 | 24.1 | 29.7 | 7.5 |
| 12–23 months | 1 000 | 23.9 | 24.2 | 7.9 |
| 0–23 months | 2 022 | 21.1 | 23.7 | 6.8 |
| Pakistan, 2012 | ||||
| 0–5 months | 1 164 | 25.8 | 33.8 | 15.3 |
| 6–11 months | 1 024 | 35.3 | 49.8 | 21.2 |
| 12–23 months | 2 074 | 32.9 | 46.4 | 20.2 |
| 0–23 months | 4 262 | 31.5 | 43.8 | 19.1 |
| Sri Lanka, 2007 | ||||
| 0–5 months | 634 | 1.5 | 9.5 | 2.2 |
| 6–11 months | 739 | 9.1 | 22.8 | 4.9 |
| 12–23 months | 1 438 | 4.7 | 21.5 | 5.0 |
| 0–23 months | 2 811 | 5.1 | 19.1 | 4.3 |
The proportion of caregivers who sought medical advice in the event of diarrhoea was highest in India and Pakistan (>60%); the proportion of caregivers who sought medical advice in the event of fever was highest in Maldives and Sri Lanka (>80%); lastly, the proportion of caregivers who sought medical advice in the event of pneumonia was highest in India and Pakistan (>65%). The lowest proportion of caregivers seeking medical advice for common childhood illnesses was recorded in Bangladesh (30.1%, 31.4% and 41.4% for diarrhoea, fever and pneumonia, respectively (Table 2).
Table 2.
Among children 0–23 months old who experienced diarrhoea, fever or pneumonia in the 2 weeks preceding the survey, number and percentage for whom advice/treatment was sought from a health facility or health provider (South Asia, Demographic and Health Surveys)
| Bangladesh 2011 | India 2006 | Maldives 2009 | Nepal 2011 | Pakistan 2012 | Sri Lanka 2007 | |
|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Diarrhoea | ||||||
| 0–5 months | 25 (43.6) | 542 (57.1) | — | 68 (32.6) | 300 (60.0) | — |
| 6–11 months | 73 (30.1) | 956 (60.3) | — | 118 (41.6) | 361 (60.8) | — |
| 12–23 months | 109 (27.0) | 1434 (66.1) | — | 239 (40.2) | 682 (68.0) | — |
| 0–23 months | 207 (30.1) | 2932 (62.5) | — | 425 (39.4) | 1343 (64.3) | — |
| Fever | ||||||
| 0–5 months | 155 (36.0) | 593 (71.0) | 88 (79.9) | 91 (34.2) | 394 (66.7) | 60 (62.5) |
| 6–11 months | 284 (32.8) | 1113 (76.4) | 152 (86.2) | 146 (45.9) | 51 (64.9) | 169 (87.5) |
| 12–23 months | 466 (29.0) | 1991 (71.4) | 277 (84.5) | 242 (46.2) | 962 (66.0) | 310 (85.5) |
| 0–23 months | 905 (31.4) | 3697 (72.8) | 517 (84.2) | 479 (43.8) | 1407 (66.2) | 539 (83.6) |
| Pneumonia | ||||||
| 0–5 months | 51 (39.8) | 319 (70.7) | — | — | 178 (70.7) | 14 (0.0) |
| 6–11 months | 64 (42.8) | 427 (76.9) | — | — | 217 (62.7) | 37 (67.6) |
| 12–23 months | 106 (41.4) | 743 (69.0) | — | — | 420 (65.5) | 71 (65.7) |
| 0–23 months | 221 (41.4) | 1489 (71.6) | — | — | 815 (65.9) | 122 (58.7) |
Data on the type of treatment/care provided to children 0–23 months old who experienced diarrhoea, fever or pneumonia in the 2 weeks preceding the survey and for whom advice or treatment was sought from a health facility or health provider were available for Bangladesh, India, Nepal and Pakistan. The proportion of children who received oral rehydration solutions (ORS) or increased fluids was highest in Bangladesh (>75%) and lowest in India (<20%) (Table 3). Similarly, the proportion of children who received antibiotic therapy for the treatment of fever and pneumonia was highest in Bangladesh (>66%) and lowest in India (<15%) (Table 4).
Table 3.
Among children 0–23 months old who experienced diarrhoea in the 2 weeks preceding the survey and for whom advice or treatment was sought from a health facility or health provider, percentage according to the type of treatment/care that they were provided during the diarrhoea episode (South Asia, Demographic and Health Surveys)
| ORS | RHF | Children given ORS or RHF | Children given increased fluids | Zn treatment | ||||
|---|---|---|---|---|---|---|---|---|
| Zn syrup | Zn tablet | Zn supplements | Zn + ORS | |||||
| Bangladesh, 2011 | ||||||||
| 0‐5 months | 46.1 | 0.0 | 46.1 | 1.7 | 13.9 | 7.2 | — | 8.8 |
| 6–11 months | 73.4 | 9.3 | 76.2 | 23.0 | 39.4 | 21.9 | — | 35.8 |
| 12–23 months | 75.7 | 7.1 | 77.7 | 25.5 | 32.0 | 23.2 | — | 39.7 |
| 0–23 months | 71.3 | 9.8 | 73.4 | 21.7 | 32.4 | 21.5 | — | 34.6 |
| India, 2006 | ||||||||
| 0–5 months | 13.7 | 15.6 | 15.6 | 2.6 | — | — | 0.0 | — |
| 6–11 months | 21.3 | 15.9 | 31.8 | 7.9 | — | — | 0.5 | — |
| 12–23 months | 34.6 | 23.6 | 48.0 | 11.1 | — | — | 0.3 | — |
| 0–23 months | 26.4 | 20.8 | 36.7 | 8.5 | — | — | 0.3 | — |
| Nepal, 2011 | ||||||||
| 0–5 months | 5.8 | — | 5.8 | 13.6 | — | — | 1.8 | 0.0 |
| 6–11 months | 35.2 | — | 35.2 | 9.3 | — | — | 5.7 | 4.6 |
| 12–23 months | 48.2 | — | 48.2 | 17.7 | — | — | 6.3 | 6.2 |
| 0–23 months | 37.8 | — | 37.8 | 14.7 | — | — | 5.4 | 4.8 |
| Pakistan, 2012 | ||||||||
| 0–5 months | 25.9 | 3.3 | 27.2 | 7.9 | — | — | 0.6 | — |
| 6–11 months | 38.8 | 10.5 | 42.7 | 5.3 | — | — | 0.3 | — |
| 12–23 months | 44.4 | 11.0 | 48.5 | 8.2 | — | — | 2.7 | — |
| 0–23 months | 38.8 | 23.6 | 42.2 | 7.4 | — | — | 1.6 | — |
ORS, oral rehydration solution; RHF, recommended home fluids.
Table 4.
Among children 0–23 months old who experienced fever or pneumonia in the 2 weeks preceding the survey and for whom advice or treatment was sought from a health facility or health provider, percentage according to the type of treatment/care that they were provided during the fever/pneumonia episode (South Asia, Demographic and Health Surveys)
| No. of children with fever | Percentage of children with fever who received antimalarial drugs | Percentage of children with fever who received antibiotic drugs | No. of children with pneumonia | % of children with pneumonia who received antibiotic drugs | |
|---|---|---|---|---|---|
| Bangladesh, 2011 | |||||
| 0–5 months; | 286 | 1.8 | 54.3 | 51 | 69.1 |
| 6–11 months | 425 | 0.1 | 66.9 | 64 | 81.8 |
| 12–23 months | 659 | 0.9 | 70.7 | 106 | 78.0 |
| 0–23 months | 1 370 | 0.8 | 66.1 | 221 | 77.0 |
| India, 2006 | |||||
| 0–5 months | 5 127 | 7.5 | 14.6 | 319 | 14.6 |
| 6–11 months | 5 276 | 7.2 | 14.9 | 427 | 11.9 |
| 12–23 months | 10 419 | 9.2 | 13.8 | 743 | 12.7 |
| 0–23 months | 20 822 | 8.3 | 14.3 | 1489 | 12.9 |
| Nepal, 2011 | |||||
| 0–5 months | 91 | 1.8 | 29.4 | — | — |
| 6–11 months | 146 | 0 | 34.4 | — | — |
| 12–23 months | 242 | 1.5 | 38.1 | — | — |
| 0–23 months; | 479 | 1.1 | 35.3 | — | — |
| Pakistan, 2012 | |||||
| 0–5 months | 394 | 2.4 | 32 | 178 | 41.4 |
| 6–11 months | 51 | 5.3 | 41.1 | 217 | 44.1 |
| 12–23 months | 962 | 4.0 | 40.0 | 420 | 43.5 |
| 0–23 months | 1 407 | 3.6 | 37.8 | 815 | 43.2 |
In these four countries – Bangladesh, India, Nepal and Pakistan – DHS information on IYCF practices during/after common childhood illnesses focused only on feeding practices during diarrhoea (Table 5). No information was available on IYCF practices when children had fever or pneumonia or after episodes of diarrhoea, fever or pneumonia. The proportion of mothers/caregivers who fed their children more/same fluids as usual was highest in Bangladesh (72.6%) and lowest in India (Table 5).
Table 5.
Percentage distribution of children 0–23 months old who had diarrhoea in the 2 weeks preceding the survey by amount of liquids and food offered during the diarrhoea episode compared with normal practice as reported by the mother/primary caregiver (South Asia, Demographic and Health Surveys)
| Bangladesh 2011 | India 2006 | Nepal 2011 | Pakistan 2012 | |||||
|---|---|---|---|---|---|---|---|---|
| Liquids | Solids | Liquids | Solids | Liquids | Solids | Liquids | Solids | |
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | |
| Children 0–5 months | ||||||||
| More | 1.7 | 0.0 | 2.6 | 1.7 | 13.6 | 0.0 | 7.9 | 0.9 |
| Same as usual | 71.9 | 50.1 | 58.7 | 23.3 | 69.0 | 10.0 | 59.4 | 12.4 |
| Somewhat less | 16.5 | 16.5 | 19.4 | 11.9 | 2.2 | 6.0 | 19.2 | 8.8 |
| Much less | 5.3 | 5.3 | 7.0 | 6.9 | 0.0 | 3.4 | 6.7 | 6.9 |
| None | 4.6 | 7.7 | 12.0 | 0.7 | 15.1 | 0.7 | 6.5 | 0.7 |
| Never gave food | — | — | — | 54.4 | — | 79.9 | — | 70.2 |
| Do not know or missing | — | — | — | 1.0 | — | 0.0 | — | 0.0 |
| Children 6–11 months | ||||||||
| More | 23.0 | 14.9 | 7.9 | 1.9 | 9.3 | 5.5 | 5.3 | 1.4 |
| Same as usual | 44.5 | 40.0 | 48.3 | 30.3 | 78.3 | 58.4 | 53.7 | 34.6 |
| Somewhat less | 25.1 | 29.4 | 28.4 | 25.2 | 5.3 | 8.2 | 32.0 | 23.5 |
| Much less | 7.0 | 9.3 | 9.7 | 7.4 | 0.0 | 1.9 | 6.5 | 6.4 |
| None | 0.4 | 1.1 | 5.4 | 5.6 | 7.1 | 0.7 | 1.5 | 7.7 |
| Never gave food | — | 20.4 | — | 29.2 | — | 25.3 | — | 25.5 |
| Do not know or missing | — | — | 0.4 | 0.3 | 0.0 | 0.0 | 0.8 | 0.8 |
| Children 12–23 months | ||||||||
| More | 25.5 | 12.5 | 11.1 | 1.7 | 17.7 | 10.1 | 8.2 | 2.7 |
| Same as usual | 50.3 | 54.7 | 45.7 | 39.7 | 67.5 | 65.5 | 52.8 | 44.5 |
| Somewhat less | 21.8 | 23.8 | 29.8 | 34.2 | 11.4 | 21.8 | 31.3 | 36.1 |
| Much less | 2.4 | 4.4 | 10.6 | 13.6 | 1.1 | 2.1 | 7.5 | 9.9 |
| None | — | 4.5 | 2.4 | 4.0 | 2.4 | 0.5 | 0.3 | 3.9 |
| Never gave food | — | 0.2 | — | 6.0 | — | 0.0 | — | 3.0 |
| Do not know or missing | — | — | 0.4 | 0.9 | 0.0 | 0.0 | 0.0 | 0.0 |
| Children 0–23 months | ||||||||
| More | 21.7 | 11.8 | 8.5 | 1.8 | 14.7 | 7.2 | 7.4 | 1.9 |
| Same as usual | 50.9 | 49.0 | 49.0 | 33.6 | 70.7 | 54.6 | 54.5 | 34.7 |
| Somewhat less | 22.3 | 24.9 | 27.4 | 27.1 | 8.2 | 15.5 | 28.8 | 26.6 |
| Much less | 4.4 | 6.2 | 9.6 | 10.3 | 0.6 | 2.3 | 7.1 | 8.3 |
| None | 0.7 | 3.4 | 5.2 | 5.1 | 5.7 | 1.0 | 2.0 | 5.6 |
| Never gave food | — | 1.1 | — | 0.4 | — | 0.4 | — | 0.3 |
| Do not know or missing | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
We were able to examine time trends in four countries – Bangladesh, India, Nepal and Pakistan, where three DHS surveys were available for the period 1990–2014. Bangladesh and Nepal made significant progress in reducing the prevalence of diarrhoea, fever and pneumonia in children 0–23 months old, mirrored by significant increases in care‐seeking behaviour for these common childhood illnesses. Improvements in India were low to nil, while surveys in Pakistan reported a significant deterioration (Table 6).
Table 6.
Percentage of children 0–23 months old who experienced diarrhoea, fever or pneumonia in the 2 weeks preceding the survey and for whom advice/treatment was sought from a heath facility or health provider (Demographic Health Surveys, 1990–2013)
| Bangladesh | 1994 | 2004 | 2011 | AARI* |
| Children 0–23 months old with diarrhoea (%) | 13.2 | 10.1 | 6.4 | −0.40 |
| Children with diarrhoea seeking medical advice (%) | 20.6 | 18.8 | 30.1 | +0.56 |
| Children 0–23 months old with fever (%) | — | 46.4 | 42.5 | −0.56 |
| Children with fever seeking medical advice (%) | — | 22.8 | 31.4 | +1.23 |
| Children 0–23 months old with pneumonia (%) | 26.8 | 26.9 | 6.9 | −1.17 |
| Children with pneumonia seeking medical advice (%) | 29.3 | 25.4 | 41.4 | +0.71 |
| India | 1992 | 1998 | 2006 | AARI* |
| Children 0–23 months old with diarrhoea (%) | 13.2 | 21.1 | 14.1 | +0.06 |
| Children with diarrhoea seeking medical advice (%) | 61.9 | 61.5 | 62.5 | +0.04 |
| Children 0–23 months old with fever (%) | 22.8 | 30.3 | 17.8 | −0.36 |
| Children with fever seeking medical advice (%) | 67.7 | — | 72.8 | +0.36 |
| Children 0–23 months old with pneumonia (%) | 7.4 | 20.2 | 7.1 | −0.02 |
| Children with pneumonia seeking medical advice (%) | 68.3 | 63.6 | 71.6 | +0.24 |
| Nepal | 1996 | 2006 | 2011 | AARI* |
| Children 0–23 months old with diarrhoea (%) | 31.2 | 18.2 | 21.1 | −0.67 |
| Children with diarrhoea seeking medical advice (%) | 14.1 | 27.1 | 39.4 | +1.69 |
| Children 0–23 months old with fever (%) | 41.2 | 21.5 | 23.7 | −1.17 |
| Children with fever seeking medical advice (%) | — | 33.1 | 43.8 | +2.14 |
| Children 0–23 months old with pneumonia (%) | 37.8 | 7.2 | 6.8 | −2.07 |
| Children with pneumonia seeking medical advice (%) | 18.3 | 42.8 | — | +2.45 |
| Pakistan | 1991 | 2007 | 2012 | AARI* |
| Children 0–23 months old with diarrhoea (%) | 19.2 | 31.6 | 31.5 | +0.59 |
| Children with diarrhoea seeking medical advice (%) | 51.0 | 57.4 | 64.3 | −0.01 |
| Children 0–23 months old with fever (%) | 35.6 | 36.4 | 43.8 | +0.39 |
| Children with fever seeking medical advice (%) | 66.4 | 68.6 | 66.2 | −0.01 |
| Children 0–23 months old with pneumonia (%) | 19.1 | 15.9 | 74.1 | +0.00 |
| Children with pneumonia seeking medical advice (%) | 68.4 | 74.1 | 65.9 | −0.12 |
Average annual rate of improvement (AARI) quantifies the average rate of change between base year and end year.
Table 7 summarizes the trends in feeding and care practices for children 0–23 during diarrhoea episodes. Over the 1990–2014 period, the proportion of children with diarrhoea who were given Oral Rehydration Solution (ORS) increased in Bangladesh, India and Nepal, while there was no improvement in Pakistan. The proportion of children who were not given ORS/recommended home fluids/increased fluids declined in all countries. The highest average annual rate of reduction was recorded in Bangladesh (0.41) and the lowest in India (0.11). Detailed information on trends in IYCF during diarrhoea was available only for Nepal (2006–2011) and Pakistan (2007–2012). In both countries, most mothers reported that the amount of liquids offered to their infants during the diarrhoea episode was ‘same as usual’ in both base year and end year. Only about half the mothers in Nepal and one‐third of mothers in Pakistan reported that the amount of food offered to their children was ‘same than usual’ – with no improvement between base year and end year.
Table 7.
Percentage of children 0–23 months old who experienced diarrhoea in the 2 weeks preceding the survey, by type of treatment/care they were provided and amount of liquids/food offered compared with normal practice (Demographic Health and Surveys, 1990–2013)
| Bangladesh | 1994 | 2004 | 2011 | AARI |
| Children given ORS (%) | 47.8 | 65.2 | 71.3 | 1.38 |
| Children given recommended home fluids (%) | 14.3 | 17.9 | 7.0 | −0.43 |
| Children given increased fluids (%) | 48.8 | 48.6 | 21.7 | −1.59 |
| Children not given ORS/recommended home fluids /increased fluids (%) | 31.5 | 16.9 | 24.5 | −0.41 |
| India | 1992 | 1996 | 2006 | |
| Children given ORS (%) | 17.8 | 25.5 | 26.4 | 0.61 |
| Children given recommended home fluids (%) | 18.1 | 12.8 | 17.4 | −0.05 |
| Children given increased fluids (%) | 13.8 | 20.7 | 8.5 | −0.38 |
| Children not given ORS/ recommended home fluids /increased fluids (%) | 61.4 | 54.3 | 59.8 | −0.11 |
| Nepal | 1996 | 2006 | 2011 | |
| Children given ORS (%) | 23.8 | 25.5 | 37.8 | 0.93 |
| Children given recommended home fluids (%) | 4.2 | — | — | — |
| Children given increased fluids (%) | 33.1 | 20.1 | 14.7 | −1.23 |
| Children not given ORS/recommended home fluids/increased fluids (%) | 53.8 | 62.6 | 51.6 | −0.15 |
| Feeding practices: amount of liquids offered to children (%) | ||||
| More | 20.1 | 14.7 | −1.08 | |
| Same as usual | 63.4 | 70.7 | 1.46 | |
| Less than usual | 13.4 | 8.8 | −0.92 | |
| None | 3.1 | 5.7 | 0.52 | |
| Feeding practices: amount of food offered to children (%) | ||||
| More | 4.7 | 7.2 | 0.50 | |
| Same as usual | 53.3 | 54.6 | 0.26 | |
| Less than usual | 25.1 | 17.8 | −1.46 | |
| None | 2.6 | 1.0 | −0.32 | |
| Never gave food | 0.2 | 0.4 | 0.04 | |
| Pakistan | 1991 | 2007 | 2012 | |
| Children given ORS (%) | 38.8 | 40.3 | 38.8 | 0.0 |
| Children given recommended home fluids (%) | 14.7 | 15.8 | 9.1 | −0.27 |
| Children given increased fluids (%) | 9.4 | 18.2 | 7.4 | −0.10 |
| Children not given ORS/recommended home fluids/increased fluids (%) | 54.9 | 46.7 | 49.7 | −0.25 |
| Feeding practices: amount of liquids offered to children (%) | ||||
| More | 18.2 | 7.4 | −2.16 | |
| Same as usual | 43.3 | 54.5 | 2.24 | |
| Less than usual | 33.1 | 35.9 | 0.56 | |
| None | 5.2 | 2.0 | −0.64 | |
| Do not know or missing | 0.2 | 0.0 | 0.0 | |
| Feeding practices: amount of food offered to children (%) | ||||
| More | 6.3 | 1.9 | −0.88 | |
| Same as usual | 34.2 | 34.7 | 0.10 | |
| Less than usual | 31.1 | 34.9 | 0.76 | |
| None | 4.8 | 5.6 | 0.16 | |
| Never gave food | 23.3 | 0.3 | −4.6 | |
| Do not know or missing | 2.0 | 0.0 | 0.0 |
AARI, average annual rate of improvement; ORS, oral rehydration solution.
Research evidence on caregivers' behaviours and health providers' practices on infant and young child feeding during and after common childhood illnesses
The bibliographic search identified 32 peer‐reviewed publications that met the inclusion criteria for this review. One study (3%) was from Nepal, eight studies (25%) were from Bangladesh, seven studies (22%) were from Pakistan and 15 studies (47%) were from India.
The majority of the studies (n = 31; 97%) reported IYCF practices during common childhood illness, while only one study reported IYCF practices both during and after illness.
Thirty studies (94%) reported IYCF practices for children with diarrhoea, eight studies (25%) reported IYCF practices for children with pneumonia and five studies (16%) reported IYCF practices for children with fever. Most studies (n = 29; 91%) reported caregivers' IYCF behaviours when children were sick, while only six studies (19%) reported health providers' IYCF counselling to mothers of sick children. Twenty‐eight (88%) were observational studies. Only four (13%) studies – one in Bangladesh and three in India – assessed the impact of one or more interventions to improve IYCF practices during/after illness.
The findings of our review are organized around the seven key focus areas (Table 8).
Table 8.
Summary table of findings from review of evidence on infant and young child feeding during and after common childhood illnesses in South Asia (1990–2014)
| Findings | Disease | Breastfeeding (BF) | Fluid intake | Complementary foods (CF) & feeding practices | Traditional beliefs and their role in feeding practices | Community elders/traditional practitioners' advice | Health professionals’ advice | Interpersonal and group counselling |
|---|---|---|---|---|---|---|---|---|
| Study | ||||||||
| Agha et al., 2007 Pakistan | Diarrhea ARI | NA | ‐ 88% mothers continued fluids, | ‐ Continued feeding: 17% for Diarrhea & 56% for pneumonia, | NA | NA | NA | NA |
| ‐ 11.7% mothers gave less fluid for diarrhea and lesser for pneumonia, | ‐ Less food: 43.9% children (diarrhea) and 83% with pneumonia, | |||||||
| ‐ Caregivers are less resistant to giving more fluids than to food (diarrhea). This is due to emphasis of fluids than food in media campaigns. | ‐ Mothers gave “butter” to help cure diarrhea. | |||||||
| Ahmed et al., 1992 Bangladesh | Diarrhea | ‐ EBF is increased for children < 1 years from 14.8% to 35.2%, | NA | ‐ 22% had normal family diets, | ‐ 50% mothers withheld some food believing ‐ “Some foods cause diarrhea, others increase frequency of loose motion, & withholding or adding some food cures illnesses.” | ‐ Elders in the family advised mothers on care of sick child. | NA | NA |
| ‐ Mothers partially BF decreased 62.5% to 43.2%, | ‐ 23.7% mothers believed that withholding or adding certain foods can cure diarrhea. | |||||||
| ‐ Increase in exclusive BF due to withholding of other foods. | ||||||||
| ‐ These children were given special diets. | ||||||||
| Badruddin et al., 1991 Pakistan | Diarrhea | ‐ >95% BF continued. | NA | ‐ Withholding CF: None, | ‐ Buffalo milk commonly given, | NA | NA | NA |
| ‐ 25% decreased food intake due to poor appetite and decreased food intake, | ‐ Home remedies, herbal medicines & teas were given to children with diarrhea. | |||||||
| ‐ Nature & amount of fluids & foods varied according to disease intensity & duration. | ||||||||
| Badruddin et al., 1997 Pakistan | Diarrhea | ‐ ~98% BF continued. | NA | NA | NA | NA | ‐ 46% doctors provide no nutritional advice, ‐ Treatment given with expensive medications & intravenous fluids. | NA |
| Becker et al., 1991 Bangladesh | Diarrhea Fever | NA | NA | ‐ Food restriction common | NA | NA | NA | NA |
| Benakappa & Shivamurthy, 2012 India | ARI Fever | ‐ 97% BF continued, | NA | ‐ 57% continued CF, | “Special diets” | ‐ 19% gave home remedies on elders’ advice. | ‐ Doctors asked to avoid "cold" food, like curds, butter milk, fruit juices and bananas in pneumonia (18%). Fever: avoid rice – 15%, | NA |
| ‐ 3% increased BF, | ‐ 12.5% diluted the CF; 70.5% no changes in consistency, | ‐ Firmly rooted beliefs about “hot” and “cold“ foods lead to restriction of food available at home, | ||||||
| ‐ 38.2% decreased BF, | ||||||||
| ‐ 3% stopped BF as they believed that illness would be transmitted to the child, | ‐ 43% caregivers reduced CF, | |||||||
| ‐ Reasons for decreased feeds: child is tired,” or “child cannot digest during illness. | ‐ Preferred foods were ‘idli’ (26.41%), rice (18.46%) and bread (16.98%). 17% ‐ no preference. Oily (49%) foods, spicy foods (45.28%) and food considered as "cold" were avoided. | |||||||
| ‐ Reasons for decreased BF: “child cannot suck”; or ”Mother is sick“. | ||||||||
| ‐ 6% restrict diet to rice and butter milk (diarrhea), | ||||||||
| ‐ 62%: not to restrict any kind of food and to follow the usual diet during recovery. | ||||||||
| Bharti et al., 2006 India | Pneumonia | NA | NA | ‐ When children were sick with pneumonia, there were feeding restrictions in a large number of cases. | NA | 16.5% sought advice from unqualified practitioners. | NA | NA |
| Bhuiya & Streatfield, 1995 Bangladesh | Diarrhea Fever | ‐ BF is continued in a majority, | NA | ‐ ~ 50% continued CF for diarrhea & fever, | NA | ‐ Consulting traditional health care providers was quite common. | ‐ 100% doctors provide no nutritional advice, | NA |
| ‐ No mother reported increase in BF, | ‐ 39% reduced and 10% discontinued CF, | |||||||
| ‐ 16% reduced BF, | ‐ None gave more CF, | ‐ Depending on illness, practitioners advised on foods to eat or restrict. | ‐ Advice by doctors mainly on medicine or use of ORS (diarrhea) : 9%, | |||||
| ‐ Reduction in BF, highest for fever + cough, fever & diarrhea, | ‐ Reasons for discontinuing CF: (1) refusal to eat/ anorexia (2) considered harmful (3) imposition by caregiver, | |||||||
| ‐ 5‐10% advised to reduce or stop feeding depending on the illness. | ||||||||
| ‐ Reasons to reduce or discontinue BF are: “refusal to eat” or “considered harmful”. | ‐ In diarrhea, normal food is believed to be harmful. | |||||||
| Das et al., 2013 Bangladesh | Diarrhea | NA | ‐ 61.3% gave same amount, | ‐ No child was given more food, | NA | ‐ 39.5% mothers with 0–11 m babies and 20% mothers with 1–2 y children sought advice from uncertified. traditional providers. | NA | NA |
| ‐ 10.8% offered less, | ‐ 71.4% gave same amount, | |||||||
| ‐ 27.6% gave homemade fluids like thin watery porridge of maize, rice, or wheat, soup, sugar salt water solution & yogurt. | ‐ 28.7% gave less food, | |||||||
| ‐ Younger 0‐1‐y were encouraged to drink/eat more, | ||||||||
| ‐ Few older children (11–24 m) were encouraged to eat more, | ||||||||
| Ansari et al., 2009 Nepal | Diarrhea | NA | Few mothers gave more fluids. | Few mothers gave more food. | NA | NA | NA | NA |
| Dhadave et al., 2012 India | Diarrhea | ‐ 87.2% continued BF, | NA | ‐ 78.6% did not restrict CF, | ‐ 55.7% of mothers gave home based care. | NA | NA | NA |
| ‐ 8.5% BF more, | ‐ 21.4% restricted CF, | |||||||
| ‐ 4.3% BF less / stopped. | ‐ No mother gave more CF. | |||||||
| Dongre et al., 2010 India | Diarrhea Fever ARI | ‐ 69.7% continued BF. | ‐ ~50% continued fluids, | ‐ Reduced CF: 50%, | Special diets given to sick children include: | ‐ 5.7% mothers went to “faith healers”. | NA | NA |
| ‐ 73% continued BF children (<1 y) compared to 75% with children >1y, | ‐ 43.5% <1y and 53.9% >1 year olds were given extra fluids. | ‐ For children with diarrhea only dry food items are given to eat to reduce stress on baby's stomach & the frequency of loose stool, | ‐ Food items containing oil & sour food were avoided: leads to difficulty in breathing & cause cough, | |||||
| ‐ 73.2% working mothers continued BF. | ‐ Few mothers responded that children had reduced appetite & eat less. | ‐ A large number of sick children were given herbal tea, honey, ginger etc. to provide relief from cough. | ||||||
| ‐ More children < 1 y given extra CF compared to children > 2 y, | ‐ Hot foods (such as papaya, egg, apple, chikku) & cold foods (such as curd, banana, guava, pomegranate, lemon & custard apple) were avoided, | |||||||
| Lower proportion of children from scheduled tribes/ nomadic tribes given increased CF. | Medicines, syrup, injections & tablets are preferred over home remedies. | |||||||
| Giri & Phalke, 2014 India | Diarrhea | ‐ 60% continued BF, | NA | ‐ 91% continued CF, | ‐ 71%: cold food be restricted in cold/cough, | NA | NA | NA |
| ‐ 21% decreased BF, | ‐ 26.5%: decreased CF, | ‐ 89%: curd be restricted in ARI, | ||||||
| ‐ 17% increased BF, | ‐ 9%: stopped CF, | ‐ 75%: heavy food be restricted in diarrhea, | ||||||
| ‐ 2% stopped BF. | ‐ Among those who continued: 32% preferred thinner consistency and 8% preferred thick consistency of food. | ‐ 72% oily food be restricted in fever, | ||||||
| ‐ Preferred “special diets” during illness: | ||||||||
| 1) Feeding khichadi (81.5%), milk (67.5%) & biscuits (59%) for ARI, | ||||||||
| 2) Banana (95%), sago (92.5%) & rice water (89%) during diarrhea. | ||||||||
| Gupta & Gupta, 2000 India | Diarrhea Fever | ‐ > 75% continued BF, | ‐ 92.2%: continued fluids, | NA | ‐ Household remedies such as rice gruel, spices, onion juice were adopted by 8.6% mothers. | ‐ Mother‐in‐law advised mothers on care of the sick child, | ‐ 47.3% mothers prefer private doctors, | NA |
| ‐ 7.8% stopped BF. | ‐ 7.8%: stopped fluids, | |||||||
| ‐ 8.7% children < 1 years stopped fluids compared to 6.8% children > 1 yr, | ||||||||
| ‐ Home remedies were generally advised. | ‐ 20.4% mothers prefer govt doctors, | |||||||
| ‐ Majority mothers: | ||||||||
| ‐ 15.9% gave home‐based fluids & ORS as first action. These include readymade ORS, sugar‐salt solution, Lassi or Shikkanji. | not given nutritional advice | |||||||
| ‐ 71% govt. doctors laid emphasis on use of home based fluids/ORS as compared to 27% private practitioners. | ||||||||
| Gupta et al., 2007 India | Diarrhea ARI | NA | ‐ 42% : continued fluids, | 50%: Complementary feeding (CF) continued | NA | ‐ Home remedies were first line of treatment. | NA | |
| ‐ 20% : stopped fluids, | 30%: gave home cooked family foods. | |||||||
| ‐ 42% gave home‐based fluids. | ||||||||
| Hirani, 2012 Pakistan | Diarrhea | NA | NA | ‐ CF restriction common | NA | ‐ Mothers prefer traditional practitioners. | NA | NA |
| ‐ For ARI: milk & rice restricted, | ||||||||
| Huffman & Combest, 1990 Bangladesh | Diarrhea | ‐ In majority BF continued | NA | ‐ >75%infants often refuse other foods. | NA | NA | NA | NA |
|
Kasi et al, 1995 Pakistan |
Diarrhea | NA | NA | NA | NA | NA |
‐ 71% doctors gave no nutritional advice instead prescribed drugs & ORS, ‐ 46% doctors provided advice on BF and not CF, ‐ 7% introduction of CF after illness. |
NA |
| Kaur et al, 1994 India | Diarrhea |
‐ 85.5% continued BF, ‐ Very few stopped BF. |
‐ 95.8% : Fluids continued, ‐ 39.6 %: Fluids usual amounts, ‐ 50%: Fluids restricted, ‐ 4.1%: Fluids stopped. |
‐ ~60%: usual amounts CF, ‐ 35.4%: restricted CF, ‐ ~33% stopped CF due to less appetite, ‐ Diets of sick children were modified. |
NA | NA | NA | ‐ CF restriction favored by 98.1% earlier, now is favored by 35%.Very few withheld BF. |
| Kaur & Singh, 1994 India | Diarrhea | NA |
‐ Homemade fluids given, ‐ Few restricted fluids. |
‐ 38.2% : same amount CF, ‐ 61.8%: CF restricted. |
NA | NA | NA |
‐ Health education programme: Giving salt sugar solution increased from 2% to 29.6%; only 23.8% gave 3‐4 times/day, ‐ Less CF improved from 55% to 29%. |
| Kaushal et al, 2005 India | Diarrhea Fever |
‐ >75% continued BF, ‐ Refusal to feed was considered “normal during illness” & as a marker of a sickness by most grandmothers & mothers. They believed that health‐seeking for poor feeding could be delayed for 1 day. |
NA | NA | NA |
‐ Grandmothers influenced mother's caregiving practices, ‐ They advised mothers on home‐based care and patterns of feeding, ‐ Helped mothers to recognize danger signs such as poor activity, poor feeding, hypothermia, and respiratory distress, ‐ Grandmothers had homemade remedies for common ailments, some of which could be harmful. |
NA | NA |
|
Malik et al, 1991 Pakistan |
Diarrhea | ‐ 70% continued BF, | NA | ‐ 78%‐87%: were given normal family foods. | Sick children also received solid & semi‐solid diet which was either "Khitchri" or banana as mentioned by more than half of the respondents. | NA | NA | NA |
|
Mangala et al, 2000 India |
Diarrhea |
‐ 92.4% continued BF, ‐ 15.1% BF more frequently, ‐ 8.6% ceased BF. |
‐ 47% aware of increased fluids. |
‐ 30%: Continued CF, ‐ 2.4%: gave “special diets” i.e., cooking practices modified i.e., food mashed or ground food for easier digestion (modification in food quality), ‐ 16.7% more CF, ‐ Educational intervention: increase of mothers modifying food to make it soft & more easily digestible 2.4% to 26.2% ‐ Increased feeding after illness: None. |
NA | NA | NA |
After an educational intervention improvements in ‐ BF frequency (15.1% to 47.2%) ‐ Modification of CF preparation (2.4% to 26.2%) ‐ Increased CF after illness (1.2% to 20%) |
| Memon et al, 2010 Pakistan |
Diarrhea ARI Fever |
NA |
‐ Most mothers continued fluids, ‐ 40%: aware of increased fluids. |
‐ 53%: gave more CF as home cooked meals. | NA | NA | NA | NA |
| Mishra et al, 1990 India | Diarrhea | NA | NA |
‐ 60‐66% CF was given as usual, ‐ 27‐30% cases CF modified to make food soft as normal foods can trigger diarrhea, ‐ 6.25% CF was stopped to help child recuperate and restricting food reduces stools. |
‐ 27% to 30% cases: special diets were given to sick children, ‐ “Hot” foods are avoided. |
NA | NA | NA |
| Piechulek et al, 1999 Bangladesh | Diarrhea & ARI |
‐ BF continued in a majority, ‐ 22.2% discontinued BF for diarrhea, ‐ Reasons for not BF & giving animal milk: 1) Mother's belief that fluids are harmful, cannot be absorbed. Another reason for restricting breastmilk 2) Improvements seen in diminished stool volume. |
Diarrhea ‐ None: Increased fluids, ‐ 91.5%: continued fluids, ‐ 0.8%: stopped fluids, ‐ 87% Fluid restriction (diarrhea) and 9.3% (pneumonia). ARI ‐ 14.8%: Restricted fluids until full recovery. |
‐ 38%: continued CF, ‐ None: increased CF, ‐ >54.6%: restricted/withheld CF, ‐ 59.1% restricted for diarrhea & 22.4% for pneumonia, ‐ ~30%: stopped CF for > 24 hrs; of this a small proportion stopped until child's recovery, ‐ Mothers withhold food because 1) medical advice; 2) own belief of “keeping bowels at rest”; 3) poor appetite, ‐ Educated mothers less likely to withhold food, ‐ Food quality modified to “cure illness”. |
‐ 97% mothers gave special diet to their ill child. According to type of illness, certain foods were avoided or preferred, ‐ In all illnesses foods like fish, meat & vegetables are “avoided” as they increase loose motions or prolong disease effects, ‐ Special diet to cure cold: warm milk & a syrup of basil “tulsi” leaves (hot foods), ‐ Special foods to cure ARI/pneumonia: foods that aggravate symptoms of cold like fish, duck or pigeon meat; and banana, green papaya, green coconut and some vegetables are avoided, ‐ Special foods to cure diarrhea: raw banana & coconut reduce abdominal discomfort & diminish frequency of stools, ‐ Fish, milk, meat, or vegetables avoided by ~98% others as they “increase the frequency of loose motions” (diarrhea). |
‐ NA | ‐ Many mothers withheld food because of doctors’ advice. | NA |
|
Rashid et al, 2001 Bangladesh |
ARI | NA | NA | ‐ CF restricted and given once a day. |
‐ Most mothers modified children's diets, ‐ ‘Cold foods’ i.e., left over or stale foods were avoided as “aggravate pneumonia”, ‐ Once a day: only rice & salt or dry bread was provided and are deprived of vegetables & fruits. |
‐ 16% mothers were advised by mothers‐in law to restrict certain types of food, ‐ Traditional and allopathic care was sought depending on the perceived severity of the illness. |
NA | NA |
| Shah et al, 2011 India | Diarrhea |
‐ 50% BF continued, ‐ 49.3% decreased/stopped, ‐ Reasons for stopping BF: Energy dense foods that the mothers consume is secreted in the breastmilk that the child cannot digest. |
NA | NA | NA | 29% mothers consulted traditional medical practitioners or quacks. | ||
| Sharma & Thakur, 1995 India | Diarrhea Fever | NA | NA | ‐ CF is commonly restricted. |
‐ CF quality is modified, ‐ Foods preferred during cough and fever: Cold & light foods i.e., curd, fruits, rice, sago, barley, and biscuits. |
NA | NA | |
| Singh et al, 1994 India | Diarrhea | ‐ BF continued in most cases. | NA | NA |
‐ Feeding was not withheld but changes made in the nature of foods given which varied by illness type, Special diets given in diarrhea: ‐ 1) daliya & khitchri because ‘intestines become weak & children are unable to digest heavy foods”; ‐ 2) Diluted tea & banana to frequency of stools; ‐ 3) Cow's milk as “evil eye had contaminated breast milk. ‐ 4) Foods avoided are “wheat flour bread” & milk as it is “too heavy”; and “Hot foods' like apple, mango, jaggery, nearly all pulses as these could enhance the frequency & intensity of diarrhea. |
NA | NA | NA |
| Zeitlyn et al, 1993 Bangladesh | Diarrhea | NA | NA |
‐ >36%: restrict CF and 64% gave normal home cooked meals, ‐ Withholding food is a first measure to treat diarrhea, ‐ 10% stopped CF to give bowels a rest, ‐ <25%: gave CF as usual. ‐ Food is restricted as mothers recognize that children have reduced appetites & are reluctant to force feed to eat. Working mothers do not want to force feed the child due to “time constraints” ‐ Soft foods given children <10‐months as mothers believe “illness weakens a child's digestive power & soft diets in the form of gruels & soups are easier to digest”. |
‐ Special diets: Normal family diets are modified to soft foods to aid digestion, ‐ Foods are restricted/modified due to cultural notions “digestive power in illness”, ‐ Soft foods given children <10‐months as mothers believe “illness weakens a child's digestive power & soft diets in the form of gruels & soups are easier to digest”, ‐ Maternal or family's perceptions of “hot or cold foods” and its perceived beneficial/ harmful effects. ‐ Fish is avoided: vehicle attracting “evil” forces that perpetuate illness. ‐ Cold & stale foods i.e., foods cooked several hours earlier are considered breeding grounds for bacteria. |
‐ A few mothers reported traditional practitioners advised them to withhold foods when their child had diarrhea, including breastmilk, ‐ Mothers in law was the main source of advice to mothers on home remedies. |
‐ Health providers provided nutrition related advice on feeding a sick child during illness, ‐ A few mothers reported that health providers advised them to withhold foods when their child had diarrhea, including breastmilk. |
BF, breast feeding; ARI, acute respiratory inspection; NA, not applicable.
Breastfeeding during and after common childhood illnesses
Sixteen studies (50%) investigated whether children continued to be breastfed while they were sick. All the studies reported that most mothers (range 69.7–98.0%) continued to breastfeed their sick children irrespective of children's age or the nature of their illness (Huffman & Combest 1990; Malik et al. 1991; Badruddin et al. 1991, 1997; Kaur et al. 1994; Singh 1994; Bhuiya & Streatfield 1995; Piechulek et al. 1999; Gupta & Gupta 2000; Mangala et al. 2000; Kaushal et al. 2005; Shah et al. 2011; Benakappa & Shivamurthy 2012; Dhadave et al. 2012; Dongre et al. 2010; Giri & Phalke 2014). Three studies reported that some mothers (range 8.5–17.0%) breastfed their children more frequently when children were sick (Mangala et al. 2000; Dhadave et al. 2012; Giri & Phalke 2014); conversely, four studies reported that some mothers (range 4.3–49.3%) breastfed their sick children less frequently (Piechulek et al. 1999; Shah et al. 2011; Benakappa & Shivamurthy 2012; Giri & Phalke 2014); lastly, six studies reported that some mothers (range 1–9%) ceased to breastfeed when children were sick (Kaur et al. 1994; Gupta & Gupta 2000; Mangala et al. 2000; Shah et al. 2011; Dhadave et al. 2012; Giri & Phalke 2014). The three main reasons given by mothers for reducing or ceasing breastfeeding while children were sick are: (1) the belief that infants could not digest breast milk when they were sick (two studies; Piechulek et al. 1999; Shah et al. 2011); (2) the perception that children were anorexic/had no appetite and/or refused to be fed (two studies; Bhuiya & Streatfield 1995; Benakappa & Shivamurthy 2012); and/or (3) the belief that breast milk had become harmful to the child because of mystical/evil forces and/or that the illness had been transmitted by the mother to the child through mother's milk (three studies) (Bhuiya & Streatfield 1995; Kaushal et al. 2005; Benakappa & Shivamurthy 2012). Two studies reported that a significant proportion of mothers (range 35–61%) – particularly among those with young infants 0–11 months old and/or children with diarrhoea – switched back to predominant or exclusive breastfeeding when children were sick (Ahmed et al. 1992; Shah et al. 2011).
Fluid intake during and after common childhood illnesses
Ten studies (31%) investigated whether children continued to be given fluids when they experienced common illnesses and/or whether fluid intake increased or decreased when children were sick. Nine studies reported that most mothers (range 40–92%) continued to administer fluids to their sick children (Kaur & Singh 1994; Kaur et al. 1994; Piechulek et al. 1999; Gupta & Gupta 2000; Agha et al. 2007; Gupta et al. 2007; Dongre et al. 2010; Memon et al. 2010; Das et al. 2013). Two studies reported that some mothers (range 6–28%) gave additional liquids/fluids to their children during illness (Kaur et al. 1994; Das et al. 2013). Three studies reported that, in addition to water, mothers fed sick children home‐made fluids such as watery porridges made from maize, rice or wheat; soups; sugar–salt–water solutions; and/or yogurt (Gupta & Gupta 2000; Gupta et al. 2007; Das et al. 2013). Six studies reported that mothers restricted the amount of liquids/fluids given to sick children (Kaur & Singh 1994; Kaur et al. 1994; Piechulek et al. 1999; Agha et al. 2007; Ansari et al. 2009; Das et al. 2013). Fluid restriction was more frequent during diarrhoea episodes (range 4–87%) (Kaur & Singh 1994; Kaur et al. 1994; Piechulek et al. 1999; Agha et al. 2007; Das et al. 2013) than during episodes of fever or pneumonia (range 8.3–15%) (Piechulek et al. 1999; Gupta & Gupta 2000; Agha et al. 2007). Only one study reported the reasons given by mothers for restricting children's fluid intake during sickness (Piechulek et al. 1999); these were: (1) the belief that fluids could not be absorbed during diarrhoea and thus were harmful; and (2) the perception that a reduction in the stool volume in children with diarrhoea was an improvement of the child's condition. Two studies reported that the proportion of mothers who were aware that children need more fluids during sickness ranged between 40% and 47% (Mangala et al. 2000; Memon et al. 2010).
Complementary foods and feeding practices during and after common childhood illnesses
Twenty studies (63%) investigated whether children were fed lower, similar or larger amounts of soft, semi‐solid or solid foods when they suffered from common childhood illnesses. Thirteen studies reported that children (range 25–79%) continued to be fed regular family foods as usual, with no restrictions/changes in frequency and/or quantity (Becker et al. 1991; Badruddin et al. 1991; Malik et al. 1991; Ahmed et al. 1992; Zeitlyn et al. 1993; Kaur et al. 1994; Bhuiya & Streatfield 1995; Gupta et al. 2007; Mangala et al. 2000; Dongre et al. 2010; Memon et al. 2010; Dhadave et al. 2012; Das et al. 2013).
However, a significant number of studies (18) indicated that feeding restrictions during common childhood illnesses were frequent; these restrictions affected feeding frequency (4 studies: Mangala et al. 2000; Dongre et al. 2010; Benakappa & Shivamurthy 2012; Giri & Phalke 2014); food quality (11 studies: Mishra et al. 1990; Badruddin et al. 1991; Ahmed et al. 1992; Zeitlyn et al. 1993; Sharma & Thakur 1995; Piechulek et al. 1999; Mangala et al. 2000; Agha et al. 2007; Dongre et al. 2010; Benakappa & Shivamurthy 2012; Giri & Phalke 2014); and/or food quantity (7 studies: Kaur et al. 1994; Bhuiya & Streatfield 1995; Piechulek et al. 1999; Mangala et al. 2000; Agha et al. 2007; Dhadave et al. 2012; Das et al. 2013). Food restrictions seemed to be more common during diarrhoea episodes (up to 83% of the mothers interviewed) than during episodes of pneumonia and fever (up to 70% and 47% of the mothers interviewed, respectively). The reasons most commonly reported by mothers for restricting food intake when children were sick were: (1) caregivers' perception that children had less appetite or refused to eat/be fed (five studies: Zeitlyn et al. 1993; Kaur et al. 1994; Bhuiya & Streatfield 1995; Piechulek et al. 1999; Dongre et al. 2010); (2) mothers' reluctance to ‘force’ the child to eat (two studies: Zeitlyn et al. 1993; Bhuiya & Streatfield 1995); (3) mothers' inability to feed the children more food/more frequently owing to resources (fuel) or time constraints (one study: Zeitlyn et al. 1993); (4) mothers' belief that illness ‘disturbed’ the digestive system and that feeding ‘normal’ foods was harmful to the sick child as the child's digestive power was ‘diminished’ and ‘normal foods’ would trigger diarrhoea, produce cough and/or put stress on the child's stomach (nine studies: Mishra et al. 1990; Ahmed et al. 1992; Zeitlyn et al. 1993; Bhuiya & Streatfield 1995; Sharma & Thakur 1995; Mangala et al. 2000; Dongre et al. 2010; Benakappa & Shivamurthy 2012; Giri & Phalke 2014); (5) mothers' belief that withholding certain foods would help to cure diarrhoea whereas introducing normal foods before the child was cured would have a detrimental effect on the development of the child or would lead to a ‘big belly’ (three studies: Mishra et al. 1990; Ahmed et al. 1992; Zeitlyn et al. 1993); and/or (6) the belief that restricting food intake was a first measure to manage diarrhoea at home and reduce the frequency of loose stools (five studies: Mishra et al. 1990; Zeitlyn et al. 1993; Piechulek et al. 1999; Dongre et al. 2010). Two studies that measured food intake in sick children 6–23 months old reported that the mean energy intake of children was significantly lower than that of healthy children and up to 70% below WHO recommendations (Becker et al. 1991; Benakappa & Shivamurthy 2012). Two studies reported that some mothers (range 10–30%) ceased feeding their child for 24 h or longer following medical advice, because mothers perceived that the children had poor/no appetite and/or because social norms advised ‘to keep bowels at rest’ (Zeitlyn et al. 1993; Piechulek et al. 1999). None of the studies reported an increase in children's food intake (frequency, quantity and/or quality). Only four studies assessed mothers' knowledge about the feeding needs of sick children; few mothers (range 17–38%) recognized the importance of feeding sick children nutritious diets comprising vegetables, pulses, small fish and/or other nutrient‐rich foods (Zeitlyn et al. 1993; Piechulek et al. 1999; Agha et al. 2007; Benakappa & Shivamurthy 2012).
Traditional beliefs and their role in IYCF during and after common childhood illnesses
Thirteen studies (41%) explored the importance of traditional beliefs and perceptions on IYCF practices during and after common childhood illnesses. Nine studies reported that when children were sick, caregivers (range 13–98%) replaced children's usual diets with ‘special diets’ owing to the belief that children's usual diets need to be modified to aid digestion ‘because intestines become weak’ (Mishra et al. 1990; Ahmed et al. 1992; Zeitlyn et al. 1993; Dongre et al. 2010; Benakappa & Shivamurthy 2012; Giri & Phalke 2014). Young children were often fed home remedies, herbal medicines and teas, and ‘soft foods’ in the form of soups and gruels (Badruddin et al. 1991; Zeitlyn et al. 1993; Piechulek et al. 1999; Gupta & Gupta 2000; Dongre et al. 2010; Benakappa & Shivamurthy 2012; Giri & Phalke 2014) because they were perceived to ‘be lighter on the stomach’, ‘be easier to digest’, ‘reduce abdominal pain’ and/or ‘diminish the frequency of stools’. Conversely, foods like fish, milk, meat, food items containing oil or even vegetables were avoided because they were considered ‘difficult to digest’ or ‘too heavy to digest’ (Ahmed et al. 1992; Zeitlyn et al. 1993; Piechulek et al. 1999; Giri & Phalke 2014).
Seven studies (Mishra et al. 1990; Zeitlyn et al. 1993; Piechulek et al. 1999; Rashid et al. 2001; Dongre et al. 2010; Benakappa & Shivamurthy 2012; Giri & Phalke 2014) reported that caregivers avoided giving specific ‘hot’ or ‘cold’ foods to sick children because these foods were considered inappropriate for certain diseases. Foods commonly avoided in case of diarrhoea were eggs/meat (range 25% to >95%), roti/chapatti/wheat flour breads (~70%) and milk (47–50%); in case of pneumonia, commonly avoided foods were fish/duck/pigeon (>90%) or curd/buttermilk (range 76% to 93%); in case of fever, commonly avoided foods included rice (40%) and curd/butter milk (range 60–70%). Two studies reported that some caregivers (range 16–23%) avoided giving sick children foods like fish, meat or eggs because ‘they attract evil forces’ and thus they were harmful to children (Zeitlyn et al. 1993; Piechulek et al. 1999).
Community elders/traditional practitioners' advice on IYCF during/after childhood illnesses
Five studies (16%) reported on the role of mothers‐in‐law (four studies: Zeitlyn et al. 1993; Rashid et al. 2001; Kaushal et al. 2005; Gupta et al. 2007) or other family elders (Ahmed et al. 1992) on decision regarding IYCF practices when children were sick. Common advice given by family elders to mothers when children were sick included the following: (1) to opt for home remedies as a first line of treatment (Zeitlyn et al. 1993; Gupta et al. 2007); (2) to accept children's refusal to eat/be fed as ‘normal’ and delay feeding by one day (Kaushal et al. 2005); and/or (3) to refrain from giving sick children certain foods such as fish, meat, vegetables or milk (Zeitlyn et al. 1993). Nine studies (28%) reported that a varying proportion of mothers (range 5.3–84%) sought help from traditional/unqualified practitioners when their children were sick (Zeitlyn et al. 1993; Kaur et al. 1994; Bhuiya & Streatfield 1995; Rashid et al. 2001; Bharti et al. 2006; Dongre et al. 2010; Shah et al. 2011; Hirani 2012; Das et al. 2013). However, only one study described the role of traditional/unqualified practitioners on IYCF counselling to mothers when children were sick (Bhuiya & Streatfield 1995). This study reported that traditional/unqualified practitioners advised mothers to restrict children's food intake; however, it did not provide specific details on the types of foods that a mother should avoid when her child was sick.
Health professionals' advice on IYCF during and after common childhood illnesses
Six studies (19%) investigated the role of health professionals in providing IYCF advice to mothers when children were sick (Zeitlyn et al. 1993; Bhuiya & Streatfield 1995; Kasi et al. 1995; Badruddin et al. 1997; Piechulek et al. 1999; Benakappa & Shivamurthy 2012). In general, health professionals gave little or no advice to mothers on how to feed their children during or after the illness episode: three studies reported that health providers (range 46–100%) advised mothers to continue breastfeeding (Bhuiya & Streatfield 1995; Badruddin et al. 1997; Piechulek et al. 1999); two studies reported that health providers (range 9–71%) advised mothers to give oral rehydration solution/home‐based fluids to infants and young children suffering from diarrhoea (Bhuiya & Streatfield 1995; Kasi et al. 1995). No study reported that health providers advised mothers to increase fluid intake when children were sick. Similarly, no study reported that health providers advised mothers to encourage their sick children to eat soft, varied and favourite foods during illness, as recommended by WHO (WHO 2003a, 2003b). Conversely, two studies (Zeitlyn et al. 1993; Bhuiya & Streatfield 1995) reported that health providers advised mothers (proportion not reported) to withhold breast milk and foods such as rice and buttermilk (in case of diarrhoea), rice (in case of fever) or cold foods like curd, buttermilk, fruit juices and bananas (in case of pneumonia or acute respiratory infections). Two studies reported that health professionals (range 7–62%) advised mothers to feed their children soft and semi‐solid foods only after children had recovered from the illness (Kasi et al. 1995; Benakappa & Shivamurthy 2012).
Interpersonal and group counselling on IYCF during/after common childhood illnesses
Three studies (9%) – all in India – reported the impact of behaviour change communication interventions on mothers' IYCF practices when children had diarrhoea (Kaur & Singh 1994; Kaur et al. 1994; Mangala et al. 2000). Two studies reported the impact of home visits and group counselling by trained community health workers. In these studies, the proportion of mothers giving ‘less than usual’ amounts of food to their sick children declined from 98% to 35% and from 55% to 29% (Kaur & Singh 1994; Kaur et al. 1994). The third study assessed the impact of cooking demonstrations using locally available foods and interpersonal counselling sessions on mothers' IYCF practices when children were sick (Mangala et al. 2000). The results of the intervention indicated the following: (1) the proportion of mothers who breastfed more frequently while children had diarrhoea increased from 15% to 47%; (2) the proportion of mothers who modified family foods to make them soft and digestible (i.e. more palatable) increased from 2% to 26%; and (3) the proportion of mothers who fed their children additional food for at least 2 weeks after the diarrhoea episode increased from 0% to 20%.
Review of national policy and programme frameworks for infant and young child feeding during and after common childhood illnesses
We reviewed national policy and programme documents to assess whether national frameworks for maternal and child nutrition integrate IYCF during and after illness. In addition, we conducted interviews with 13 key informants to document the existing national programmes that protect, promote and support optimal IYCF practices for children during and after illness (Table 9).
Table 9.
Policies and programmes related to infant and young child feeding (IYCF) during and after illness in South Asian countries (1990–2014)
| Afghanistan | Bangladesh | Bhutan | India | Nepal | Maldives | Pakistan | Sri Lanka | |
|---|---|---|---|---|---|---|---|---|
| A national stand‐alone policy for the protection, promotion and support of optimal IYCF practices is available | Y | X | Y | Y | X | X | X | Y |
| A national nutrition and/or food security policy that includes IYCF is available | Y | X | Y | X | Y | X | X | X |
| The national IYCF/national nutrition/food security policy includes IYCF during and after illness | X | X | X | Y | Y | X | X | X |
| A national programme for the protection, promotion and support of optimal IYCF practices exists | Y | Y | Y | Y | Y | X | Y | Y |
| National guidelines for the protection, promotion and support of optimal IYCF practices are available | Y | Y | Y | Y | Y | Y | Y | Y |
| National guidelines for the protection, promotion and support of optimal IYCF include IYCF during and after illness | X | X | Y | Y | X | Y | Y | Y |
| The national training package for IYCF protection, promotion and support includes IYCF during/after illness | X | Y | Y | Y | Y | X | X | Y |
| A national programme for the integrated management of childhood illness (IMCI) exists | Y | Y | Y | Y | Y | Y | Y | Y |
| The national guidelines for IMCI include guidance on feeding children when they are sick | Y | Y | Y | Y | X | Y | Y | Y |
| The national guidelines for IMCI include guidance on feeding children after being sick | Y | Y | Y | Y | X | Y | Y | Y |
| The national training package on IMCI includes guidance on IYCF during and after illness | X | Y | X | Y | X | Y | X | Y |
Y: Yes, X: No
Five of the eight countries have a national IYCF policy, either as a stand‐alone policy framework on infant feeding or as part of a larger policy framework on nutrition/food security. However, only two countries – India and Nepal – have integrated the feeding needs of children during and after illness in their IYCF policy framework.
All countries have national guidelines on IYCF, and seven countries have a national programme for the protection, promotion and support of optimal IYCF. However, only five countries include in their national IYCF guidelines guidance on how children should be fed during and after illness, and only five countries have developed a training package on IYCF for programme staff that includes IYCF for children during and after illness.
All countries have a national programme for the integrated management of childhood illnesses (IMCI); six countries have national IMCI guidelines that include guidance on feeding children during and after illness; however, only four countries have developed an IMCI training package that includes guidance on how to feed children when they are sick and after being sick.
Discussion
We conducted a comprehensive review of the available evidence on IYCF practices during and after common childhood illnesses – diarrhoea, fever and pneumonia – in South Asia (1990–2014) to inform policy formulation, programme design, advocacy and research prioritization to protect, promote and support optimal IYCF practices during and after common childhood illnesses in South Asia post 2015.
Demographic Health Survey data on IYCF during common childhood illnesses were available only for Bangladesh, India, Nepal and Pakistan, which are home to ~96% of the children under 5 years of age in South Asia (UNICEF 2015). Similarly, the 32 publications that met the inclusion criteria of our review focused on these four countries. Furthermore, the available DHS data in these four countries were limited to IYCF during diarrhoea episodes. No survey data were available on IYCF practices after episodes of diarrhoea or during/after episodes of fever or pneumonia. Similarly, the published research was primarily focused on IYCF practices during diarrhoea and to a lesser extent during fever or pneumonia episodes. Research evidence on IYCF after common childhood illnesses was practically inexistent.
Demographic Health Survey data indicate that in the countries included in the analysis, children 0–23 months old suffer from common childhood illnesses frequently, as nearly one‐third of the mothers/caregivers reported that their children had suffered from diarrhoea or pneumonia in the 2 weeks prior to the survey. These findings are in line with reports indicating that 39% of child deaths in South Asia are due to diarrhoea and/or pneumonia (UNICEF 2012). Importantly, DHS data indicate that in all the countries included in our review, the occurrence of diarrhoea, pneumonia and fever was lowest during the exclusive breastfeeding period (0–5 months) and highest during the early complementary feeding period (6–11 months). This is most likely due to the well‐documented protective benefits of exclusive/predominant breastfeeding in the first 6 months of life and the higher levels of infection in late infancy and early childhood due to children's increased intake of complementary foods and fluids that may be contaminated as well as the ingestion of faecal bacteria through mouthing soiled fingers or household items when children begin to crawl and explore their environment (Kosek et al. 2003; Dewey & Mayers 2011; WHO 2013).
Our review shows that in South Asia, IYCF behaviours and practices during common childhood illnesses are far from optimal. Most infants and young children continue to be breastfed when they are sick; however, few children (<20%) are breastfed more frequently as recommended. Studies in other settings have reported a similar practice, as most mothers continue to breastfeed their sick children without altering the number of nursing episodes, total amount of time of suckling or energy derived from breast milk (Hoyle et al. 1980; Brown et al. 1990; Martz & Tomkins 1995; Brown 2003).
Similarly, most sick children continue to be fed fluids. However, few children (range 7.4–21.7% in Pakistan and Bangladesh, respectively) were fed fluids more frequently as recommended. Mothers' awareness about children's need for more fluids during sickness is low. This evidence is in line with reports indicating that in developing countries, less than a quarter (22%) of children are fed more fluids during illness (UNICEF/WHO 2009). Conversely, a significant proportion of mothers/caregivers in Bangladesh (26.7%), Pakistan (37.9%) and India (42.2%) fed their sick children less fluids than usual or no fluids at all in contrast with reports from other countries (Bani et al. 2002; Saha et al. 2013).
We find that food restrictions are frequent. Many children were fed lower quantities and/or less frequently when they were sick. As many as 36% of mothers/caregivers in Bangladesh, 41% in Pakistan and 43% in India reported that they fed their children less food than usual or no food at all during the last diarrhoea episode. Only one‐third (34%) of the mothers/caregivers in India to about half (55%) in Nepal reported that they fed their children same/similar amounts of food as usual during the diarrhoea episode. Studies in Latin America have indicated that anorexia is an important factor in the reduction of children's dietary intake during illness (particularly when diarrhoea or fever are present) as mothers/caregivers tend to give in when sick children send a ‘food reject’ signal (Bentley et al. 1991, 1995). The combined effects of anorexia and tradition‐driven withdrawal of complementary feeding during common childhood illnesses can be devastating (Scrimshaw & Sangiovanni 1997).
Our review indicates that in South Asian countries, mothers'/caregivers' knowledge about the feeding needs of sick children is limited and that feeding practices are often guided by traditional beliefs and norms that encourage the use of ‘special’ foods/diets to replace ‘usual diets’ when children are sick. Similarly, many caregivers seem to avoid giving certain ‘hot’ or ‘cold’ foods to sick children because these foods are considered inappropriate for specific diseases. Studies have reported that deeply held beliefs and traditions determine the types of foods or preparation methods that are ‘healthy’ or ‘unhealthy’ for sick children, when and what types of complementary foods are given to children and how to feed children who are sick and/or do not want to eat. These beliefs are heavily influenced by the individuals who surround mothers – that is, husbands, mothers‐in‐law, grandmothers and other family/community members – and the health care providers upon whom caregivers depend for support (Martz & Tomkins 1995; Stewart et al. 2013).
Care‐seeking practices in South Asia are said to be below global estimates for low‐income and middle‐income countries (Walker et al. 2012). However, the latest DHS data available for the countries included in our review indicate that a significant proportion of mothers/caregivers (ranging from 30% to 84% depending on country and illness) took their sick children for medical advice during the last episode of diarrhoea, fever or pneumonia. Our review shows that few published studies have investigated the quality of health providers' counselling on IYCF to mothers/caregivers when children are sick. The evidence reviewed indicates that when mothers/caregivers seek advice/support in the primary health care system, health professionals provide little or no advice to mothers/caregivers on how to feed children when they are sick/convalescent. In general, health providers do not advise mothers to increase children's fluid intake and encourage sick children to eat soft, varied and favourite foods during illness, while increasing breastfeeding frequency as is recommended. Moreover, there is indication that a non‐negligible proportion of health providers advise mothers to withdraw breast milk and/or specific nutritious foods/all complementary foods until children recover from illness.
Studies in other low‐income and middle‐income countries have found the following: (1) health workers do not maximize their contacts with women and children to support optimal IYCF; (2) there is poor knowledge among health practitioners on how to feed and/or manage sick children and manage children with poor appetite; (3) even when a national normative and guidance frameworks on IYCF for sick children are in place, a limited proportion of paediatricians and family practitioners follow them; and (4) the quality of care and advice among private practitioners is not necessarily better than among public health system providers (Bezerra et al. 1992; Bojalil et al. 1998; Baker et al. 2013; Lutter et al. 2013).
Conclusion
Diarrhoea and pneumonia remain the leading infectious causes of childhood morbidity and mortality in South Asia (Fischer et al. 2013). Compelling evidence indicates that childhood diarrhoea and pneumonia deaths are avoidable and that scaling up optimal feeding behaviours and practices in combination with appropriate case management can avoid most of these deaths (Bhutta et al. 2013).
Our review shows that information of IYCF behaviours and practices during illnesses in South Asia is limited while information of IYCF after common childhood illnesses is virtually inexistent. The evidence reviewed indicates that in South Asia, IYCF behaviours and practices during common childhood illnesses are far from optimal. In general, sick children continue to be breastfed. However, few are breastfed more frequently to compensate for the additional fluid and nutrient requirements associated with illnesses, while a significant proportion of children is breastfed less frequently than usual. Restriction or withdrawal of complementary foods during illness is frequent because of children's anorexia (perceived or real), poor awareness by caregivers' about the feeding needs of sick children, traditional beliefs and behaviours, and/or suboptimal counselling and support by health workers. As a result, many sick children are fed less frequently and/or lower quantities of complementary foods.
Mothers/caregivers often turn to family/community elders and traditional/non‐qualified practitioners to seek advice on how to feed their sick children. Thus, traditional beliefs and behaviours often guide the use of ‘special’ feeding practices, foods and diets for sick children. Our review indicates that when children are sick, a significant proportion of families turn to the primary health care system for advice and support. In general, health professionals give little or no advice to mothers/caregivers on how to feed their children while they are sick. However, the few intervention studies available indicate that inter‐personal and group counselling as part of primary health care can substantially improve mothers' IYCF knowledge and practices during common childhood illnesses.
Global guidance and normative frameworks are in place to address the feeding of sick children during and after illness (WHO 2003a, 2003b; WHO/UNICEF 2003; WHO 2005). All the countries included in our review have national guidelines on IYCF and a national programme for the IMCI. However, there seem to be important policy, guidance and capacity building gaps in these frameworks with respect to IYCF when children are sick or convalescent. Our review indicates that a limited proportion of health practitioners follows all aspects of these guidance.
In light of our findings, it seems reasonable to recommend the following as a way forward to protect, promote and support optimal IYCF practices during and after common childhood illnesses in South Asia post 2015:
align national policy frameworks and programmatic guidance with internationally agreed upon recommendations on IYCF during and after common childhood illnesses, with a particular emphasis on diarrhoea, fever and pneumonia;
expand the DHS and National Nutrition Surveys to include quantitative information on IYCF during and after common childhood illnesses, with appropriate geographic, socio‐economic and gender disaggregation;
collect qualitative and quantitative information on caregivers' behaviours and health workers' practices related to IYCF during and after common childhood illnesses to identify the most important drivers of current behaviours/practices and bottlenecks to optimal IYCF when children are sick/convalescent;
build the capacity of facility‐based and community‐based health workers to provide mothers/caregivers with timely and accurate information, counselling and support on IYCF when children are sick/convalescent;
design and implement effective communication strategies that combine interpersonal communication and mass communication to address harmful beliefs and norms with respect to the nutrient and feeding needs of children during/after common illnesses; and
document the effectiveness, impact and lessons learned of the capacity building and communication strategies to improve IYCF during and after common childhood illnesses and their implications for programme scale up and universalization.
Source of funding
The UNICEF Regional Office for South Asia provided support for data analysis and paper writing. This research received no specific grant from any funding agency in the commercial sector.
Conflicts of interest
The authors declare that they have no conflicts of interest. The opinions expressed on this paper are those of the authors and do not necessarily represent an official position of UNICEF.
Contributions
VMA designed the study, KP led data analysis. Both authors contributed equally to data interpretation and manuscript writing and have read and approved the final submission.
Acknowledgements
The authors acknowledge inputs and feedback to the preliminary drafts of this paper by the following individuals: Aishnath Shahula Ahmed, Anirudra Sharma, France Begin, Gayatri Singh, Isabel Vashti Simbeye, Mohsin Ali, Pravin Khobragade, Renuka Jayatissa, Sherin Varkey, Tania Goldner and Wisal Khan.
Paintal, K. , and Aguayo, V. M. (2016) Feeding practices for infants and young children during and after common illness. Evidence from South Asia. Maternal & Child Nutrition, 12: 39–71. doi: 10.1111/mcn.12222.
Footnotes
For the purpose of this paper, South Asia refers to the eight member countries of the South Asia Association for Regional Cooperation, namely Afghanistan, Bangladesh, Bhutan, India, Maldives, Nepal, Pakistan and Sri Lanka.
References
- Agha A., White F., Younus M., Kadir M.M., Alir S. & Fatmi Z. (2007) Eight key household practices of integrated management of childhood illnesses (IMCI) amongst mothers of children aged 6 to 59 months in Gambat, Sindh, Pakistan. Journal of Pakistan Medical Association 57 (6), 288–293. [PubMed] [Google Scholar]
- Ahmed M.U., Rashid M. & Beguin S. (1992) Diarrhea and feeding practices of young children attending two selected urban clinics in Dhaka. Journal of Diarrheal Disease Research 10 (4), 217–220. [PubMed] [Google Scholar]
- Ansari M., Palaian S., Izham M. & Ibrahim M. (2009) The role of mothers in the management of childhood diarrhea in Nepal. Australasian Medical Journal 1 (14),235–238. [Google Scholar]
- Badruddin S.H., Islam A., Hendricks K.M., Bhutta Z.A., Shaikh S., Snyder J.D. et al (1991) Dietary risk factors associated with acute and persistent diarrhea in children in Karachi, Pakistan. American Journal of Clinical Nutrition 54, 745–749. [DOI] [PubMed] [Google Scholar]
- Badruddin S.H., Inam S.N., Ramzanali S. & Hendricks K. (1997) Constraints to adoption of appropriate breast feeding practices in a squatter settlement in Karachi, Pakistan. Journal of Pakistan Medical Association 47 (2), 63–68. [PubMed] [Google Scholar]
- Baker J., Sanghvi T., Hajeebhoy N., Martin L. & Lapping K. (2013) Using an evidence‐based approach to design large‐scale programs to improve infant and young child feeding. Food and Nutrition Bulletin 34 (3), S146–S155. [DOI] [PubMed] [Google Scholar]
- Bani I.A., Saeed A.A.W., Mohammed A.A. & Othman A.I. (2002) Diarrhea and child feeding practices in Saudi Arabia. Public Health Nutrition 5 (6), 727–731. [DOI] [PubMed] [Google Scholar]
- Becker S., Black R.E. & Brown K.H. (1991) Relative effects of diarrhea, fever, and dietary energy intake on weight gain in rural Bangladeshi children. American Journal of Clinical Nutrition 53, 1499–1503. [DOI] [PubMed] [Google Scholar]
- Benakappa A.D. & Shivamurthy P. (2012) Beliefs regarding diet during childhood illness. Indian Journal of Community Medicine 37, 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley M.E., Stallings R.Y., Fukumoto M. & Elder J.A. (1991) Maternal feeding behavior and child acceptance of food during diarrhea, convalescence, and health in the Central Sierra of Peru. American Journal of Public Health 81, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley M.E., Black M.M. & Hurtado E. (1995) Child‐feeding and appetite: what can programmes do? Food and Nutrition Bulletin 16 (2), 70–82. [Google Scholar]
- Bezerra J.A., Stathos T.H., Duncan B., Gaines J.A. & Udall J.N. (1992) Treatment of infants with acute diarrhea: what is recommended and what is practiced. Pediatrics 90, 1–4. [PubMed] [Google Scholar]
- Bharti B., Bharti S. & Verma V. (2006) Severe pneumonia in a remote hilly area. Integrated management of childhood illness. Indian Journal of Pediatrics 73 (1), 33–37. [DOI] [PubMed] [Google Scholar]
- Bhuiya A. & Streatfield K. (1995) Feeding, home‐remedy practices, and consultation with health care providers during childhood illness in rural Bangladesh. Journal of Diarrheal Disease Research 13 (2), 106–112. [PubMed] [Google Scholar]
- Bhutta Z.A. & Salam R.A. (2012) Global nutrition epidemiology and trends. Annals of Nutrition and Metabolism 61 , 19–27. [DOI] [PubMed] [Google Scholar]
- Bhutta Z.A., Das J.K., Walker N., Rizvi A., Campbell H., Rudan I., Black R.E. & for the Lancet Diarrhea and Pneumonia Interventions Study Group . (2013) Interventions to address deaths from childhood pneumonia and diarrhea equitably: what works and at what cost? Lancet 381, 1417–1429. [DOI] [PubMed] [Google Scholar]
- Bojalil R., Guiscafre H., Espinosa P., Martinez H., Palafox M., Romero G. et al (1998) The quality of private and primary health care management of children with diarrhea and acute respiratory infections in Tlaxcala, Mexico. Health Policy and Planning 13 (3), 323–331. [DOI] [PubMed] [Google Scholar]
- Brown K.H. (2003) Diarrhea and malnutrition. Journal of Nutrition 133, 328S–332S. [DOI] [PubMed] [Google Scholar]
- Brown K.H., Stallings R.Y., de Kanashiro H.C., de Romana G.L. & Black R.E. (1990) Effects of common illnesses on infants' energy intakes from breastmilk and other foods during longitudinal community‐based studies in Huascar (Lima), Peru. American Journal of Clinical Nutrition 52, 1005–1013. [DOI] [PubMed] [Google Scholar]
- Das S.K., Nasrin D., Ahmed S., Wu Y., Ferdous F., Farzana F.D. et al (2013) Health care‐seeking behavior for childhood diarrhea in Mirzapur, rural Bangladesh. American Journal of Tropical Medicine and Hygiene 89 (1), 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Onis M., Onyango A., Borghi E., Siyam A., Blössner M. & Lutter C. (2012) Worldwide implementation of the WHO Child Growth Standards. Public Health Nutrition 15, 1603–1610. [DOI] [PubMed] [Google Scholar]
- De Onis M., Dewey K.G., Borghi E., Onyango A.W., Blössner M., Daelmans B. et al (2013) The World Health Organization's global target for reducing childhood stunting by 2025: rationale and proposed actions. Maternal and Child Nutrition 9 (Suppl. 2), 6–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey K.G. & Mayers D.R. (2011) Early child growth: how do nutrition and infection interact? Maternal & Child Nutrition 7 (Suppl. 3), 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhadave M.M., Kumar G.A., Reddy S., Vijayanath V. (2012) A study on diarrhea related practices. Awareness of ORS among mothers of under‐five children attending OPD, CHTC, Rajapur. Journal of Pharmaceutical and Biomedical Sciences 19 (11), 1–5. [Google Scholar]
- Dibley M.J., Roy S.K., Senarath U., Patel A., Tiwari K., Agho K.E. et al (2010) Across‐country comparisons of selected infant and young child feeding indicators and associated factors in four South Asian countries. Food and Nutrition Bulletin 31 (2), 366–375. [DOI] [PubMed] [Google Scholar]
- Dongre A.R., Deshmukh P.R. & Garg B.S. (2010) Childhood morbidity, household practices and health care seeking for sick children in a tribal district of Maharashtra, India. Indian Journal of Medical Sciences 64, 7–16. [PubMed] [Google Scholar]
- Fischer W.C.L., Rudan I., Liu L., Nair H., Theodoratou E., Bhutta Z.A. et al (2013) Global burden of childhood pneumonia and diarrhea. Lancet 381, 1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri P.A. & Phalke D.B. (2014) Beliefs and practices regarding diet in common childhood illnesses among rural caregivers. Journal of Medical Nutrition and Nutraceuticals 3, 219–221. [Google Scholar]
- Grantham‐McGregor S., Cheung Y.B., Cueto S., Glewwe P., Richter L. & Strupp B. (2007) Developmental potential in the first five years for children in developing countries. Lancet 369, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J.K. (2010) Child malnutrition. Trends and issues. Anthropologist 12 (2), 131–140. [Google Scholar]
- Gupta R.K. & Gupta R. (2000) First action management of acute diarrhea in children by rural mothers. JK Science, Journal of Medical Education and Research 2 (2), 96–98. [Google Scholar]
- Gupta N., Jain S.K., Chawla U., Hossain S. & Venkatesh S. (2007) An evaluation of diarrheal diseases and acute respiratory infections control programmes in a Delhi slum. Indian Journal of Pediatrics 74 (5), 471–476. [DOI] [PubMed] [Google Scholar]
- Hirani S.A.A. (2012) Malnutrition in young Pakistani children. A review article. Journal of Ayub Medical College 24 (2), 150–153. [PubMed] [Google Scholar]
- Hoyle B., Yunus M. & Chen L.C. (1980) Breast‐feeding and food intake among children with acute diarrheal disease. American Journal of Clinical Nutrition 33 (1), 2365–2371. [DOI] [PubMed] [Google Scholar]
- Huffman S.L. & Combest C. (1990) Role of breast‐feeding in the prevention and treatment of diarrhea. Journal of Diarrheal Disease Research 8 (3), 68–81. [PubMed] [Google Scholar]
- Jones A.D., Ickes S.B., Smith L.E., Mbuya M.N.N., Chasekwa B., Heidkamp R.A. et al (2014) World Health Organization infant and young child feeding indicators and their associations with child anthropometry: a synthesis of recent findings. Maternal and Child Nutrition 10, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasi M., Kausar P., Naz R. & Miller L.C. (1995) Treatment of diarrhea in infants by medical doctors in Balochistan, Pakistan. Journal of Diarrheal Disease Research 13 (4), 238–241. [PubMed] [Google Scholar]
- Kaur P. & Singh G. (1994) Food practices during diarrhea. Indian Journal of Public Health 38 (2), 58–61. [PubMed] [Google Scholar]
- Kaur A., Chowdhury S. & Kumar R. (1994) Mothers' beliefs and practices regarding prevention and management of diarrheal diseases. Indian Pediatrics 31 (1), 55–57. [PubMed] [Google Scholar]
- Kaushal M., Aggarwal R., Singal A., Shukla H., Kapoor S.K. & Paul V.K. (2005) Breastfeeding practices and health‐seeking behavior for neonatal sickness in a rural community. Journal of Tropical Pediatrics 51 (6), 366–376. [DOI] [PubMed] [Google Scholar]
- Kosek M., Bern C. & Guerrant R.L. (2003) The global burden of diarrheal disease, as estimated from studies published between 1992 and 2000. Bulletin of the World Health Organization 81 (3), 197–204. [PMC free article] [PubMed] [Google Scholar]
- Lutter C.K., Iannotti L., Creed‐Kanashiro H., Guyon A., Daelmans B., Robert R. et al (2013) Key principles to improve programmes and interventions in complementary feeding. Maternal and Child Nutrition 9 (2), 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik I.A., Azim S., Good M.J., Iqbal M., Nawaz M., Ashraf L. et al (1991) Feeding practices for young Pakistani children: usual diet and diet during diarrhea. Journal of Diarrheal Disease Research 9 (3), 213–218. [PubMed] [Google Scholar]
- Mangala S., Gopinath D., Narasimhamurthy N.S. & Shivaram C. (2000) Feeding practices in under‐fives during diarrhea before and after educational intervention. Indian Pediatrics 37, 312–314. [PubMed] [Google Scholar]
- Martz H. & Tomkins A.M. (1995) Nutritional management of diarrhea. Food and Nutrition Bulletin 16 (4), 349–355. [Google Scholar]
- Memon P. (2012) The crisis of poor complementary feeding in South Asia: where next? Maternal and Child Nutrition 8 (1), 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon S., Shaikh S., Kousar T. & Memon Y. (2010) Assessment of infant feeding practices at a tertiary care hospital. Journal of Pakistan Medical Association 60 (12), 1010–1015. [PubMed] [Google Scholar]
- Mishra C.P., Kumar S., Tiwari I.C. & Prasad D.N. (1990) A study on some diarrhea related practices in urban Mirzapur. Indian Journal of Public Health 34 (1), 6–10. [PubMed] [Google Scholar]
- Neumann C.G., Marquardt M. & Bwibo N.O. (2012) The impact of morbidity on food intake in rural Kenyan children. South African Journal of Clinical Nutrition 25 (3), 142–148. [Google Scholar]
- Piechulek H., Aldana J.M., Engelsmann B. & Hasan M.N. (1999) Dietary management during pregnancy, lactation and common childhood illnesses in rural Bangladesh. South‐East Asian Journal of Tropical Medicine and Public Health 30 (2), 299–306. [PubMed] [Google Scholar]
- Ramachandran P. & Gopalan H.S. (2009) Undernutrition and risk of infections in preschool children. Indian Journal of Medical Research 130 (11), 579–583. [PubMed] [Google Scholar]
- Rashid S.F., Hadi A., Afsana K. & Begum S.A. (2001) Acute respiratory infections in rural Bangladesh: cultural understandings, practices and the role of mothers and community health volunteers. Tropical Medicine and International Health 6 (4), 249–255. [DOI] [PubMed] [Google Scholar]
- Richard S.A., Black R.E., Gilman R.H., Guerrant R.L., Kang G., Lanata C.F. et al (2014) Catch‐up growth occurs after diarrhea in early childhood. Journal of Nutrition 144 (6), 965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha D., Akinsola A., Sharples K., Adeyemi M.O., Antonio M., Imran S. et al (2013) Health care utilization and attitudes survey: understanding diarrheal disease in rural Gambia. American Journal of Tropical Medicine and Hygiene 89 (1), 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrimshaw N.S. & Sangiovanni J.P. (1997) Synergism of nutrition, infection, and immunity: an overview. American Journal of Clinical Nutrition 66, 464S–477S. [DOI] [PubMed] [Google Scholar]
- Senarath U., Agho K.E., Akram D.‐e.‐S., Godakandage S.S.P., Hazir T., Jayawickrama H. et al (2012) Comparisons of complementary feeding indicators and associated factors in children aged 6–23 months across five South Asian countries. Maternal and Child Nutrition 8 (1), 89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M.S., Ahmad A., Khalique N., Khan I.M., Ansari M.A. & Khan Z. (2011) Do the mothers in rural Aligarh know about home based management of acute diarrhea? Biology and Medicine S3 (2), 76–80. [Google Scholar]
- Sharma K.P. & Thakur A.K. (1995) Maternal beliefs regarding diet during common childhood illnesses. Indian Pediatrics 32 (8), 909–910. [PubMed] [Google Scholar]
- Singh M.B. (1994) Maternal beliefs and practices regarding the diet and use of herbal medicines during measles and diarrhea in rural areas. Indian Pediatrics 31 (3), 340–343. [PubMed] [Google Scholar]
- Stewart C.P., Iannotti L., Dewey K.G., Michaelsen K.F. & Onyango A.W. (2013) Contextualizing complementary feeding in a broader framework for stunting prevention. Maternal and Child Nutrition 9 (S2), 27–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF (2012) Pneumonia and diarrhea. Tackling the deadliest diseases for the world's poorest children. United Nations Children's Fund, New York, New York.
- UNICEF (2015) The state of the world children. Reimagine the future: Innovation for every child. United Nation's Children's Fund, New York; 116 pp.
- UNICEF/WHO (2009) Diarrhea: why children are still dying and what can be done? United Nations Children's Fund, World Health Organization UNICEF/ WHO. New York. [Google Scholar]
- Walker S.P., Wachs T.D., Gardner J.M., Lozoff B., Wasserman G.A., Pollitt E. et al (2007). Child development. Risk factors for adverse outcomes in developing countries. Lancet 369, 145–157. [DOI] [PubMed] [Google Scholar]
- Walker C.L.F., Perin J., Aryee M.J., Boschi‐Pinto C. & Black R.E. (2012) Diarrhea incidence in low‐ and middle‐income countries in 1990 and 2010: a systematic review. BioMedCentral Public Health 12, 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C.L.F., Rudan I., Liu L., Nair H., Theodoratou E., Bhutta Z.A. et al (2013) Global burden of childhood pneumonia and diarrhea. Lancet 381 (9875), 1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2003a) The guiding principles for complementary feeding of the breastfed child. World Health Organization, Geneva, Switzerland.
- WHO (2003b) The guiding principles for complementary feeding of the non‐breastfed child. World Health Organization, Geneva, Switzerland.
- WHO (2005) Technical updates of the guidelines on the integrated management of childhood illness (IMCI). Evidence and recommendations for further adaptations. World Health Organization, Geneva, Switzerland.
- WHO (2008) Indicators for assessing infant and young child feeding practices. Part I: definitions. World Health Organization Geneva, Switzerland.
- WHO (2010) Indicators for assessing infant and young child feeding practices. Part II: measurement. World Health Organization Geneva, Switzerland.
- WHO (2013) Essential nutrition actions. Improving maternal, newborn, infant and young child health and nutrition. World Health Organization, Geneva, Switzerland. [PubMed]
- WHO/UNICEF (2003) Global strategy for infant and young child feeding. World Health Organization and United Nations Children's Fund, Geneva.
- Zeitlyn S., Rowshan R., Mahalanabis D. & Faruque A. (1993) The ethno‐physiology of digestion and diarrhea in a Bangladeshi hospital population. Journal of Diarrheal Disease Research 11 (4), 243–248. [PubMed] [Google Scholar]


