Abstract
Background: Increased thyrotropin (TSH) levels and free triiodothyronine to free thyroxine (fT3:fT4) ratios, even within the euthyroid range, have been associated with cardiometabolic risk factors in adults but are less characterized in youth. This study sought to determine relations between TSH, thyroid hormones, and cardiometabolic risk factors in euthyroid adolescents.
Methods: Data were extracted from the United States National Health and Nutrition Examination Survey, 2007–2010, for univariate and multivariate analyses of TSH, thyroid hormones, body mass index (BMI), blood pressure, lipids, and glucose metabolism. Subjects aged 12–18 years, with normal TSH and antithyroid peroxidase antibody levels, and without a history of thyroid disease, diabetes, or treatment of hypertension/dyslipidemia (n = 1167) were included. TSH and thyroid hormones were assessed for impact on BMI Z-score, systolic blood pressure (SBP) diastolic blood pressure, total cholesterol (TC), high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, apolipoprotein B, triglycerides, and glucose metabolism.
Results: Univariate analyses revealed positive linear relations between TSH and SBP, TC, fasting and two-hour glucose, and homeostasis model assessment of insulin resistance (HOMA-IR). The fT3:fT4 ratio negatively correlated with high-density lipoprotein cholesterol but positively with BMI Z-score, SBP, triglycerides, fasting and two-hour glucose, fasting insulin, and HOMA-IR. In multivariate analyses controlling for age, sex, race/ethnicity, and BMI Z-score, relations between TSH and both TC and fasting glucose remained significant, and the fT3:fT4 ratio was positively associated with fasting glucose and HOMA-IR.
Conclusions: In an unselected population of euthyroid U.S. adolescents, TSH and thyroid hormones correlate with multiple cardiometabolic risk factors, with age- and sex-independent effects on cholesterol and glucose metabolism.
Introduction
Thyroid hormone is a key regulator of metabolism. Thyrotropin (TSH) secretion is extremely sensitive to circulating levels of thyroid hormone, and is commonly used clinically as an index of thyroid hormone homeostasis (1). In defining a “normal” TSH, it is noteworthy that TSH levels can vary within an individual (2) due to the influence of factors such as circadian rhythms (3), genetic background (4), or iodine intake (5). Furthermore, the reference range within which a TSH level should be considered normal at the population level remains controversial (6,7). Increased TSH levels, even within the “euthyroid” range, have been associated with cardiometabolic risk factors such as body mass index (BMI) (8), lipid status (9,10), and blood pressure (11,12) in adults, with moderate evidence linking high-normal TSH levels with increased likelihood of metabolic syndrome and spontaneous pregnancy loss (13).
TSH is a useful screening test for an intact hypothalamic–pituitary–thyroid (HPT) axis, primarily reflecting the pituitary gland's assessment of thyroid homeostasis (1,14). Additionally, the HPT axis and serum thyroid hormones are influenced by nutritional status and energy stores, through leptin-mediated crosstalk between adipose tissue and thyrotropin-releasing hormone (TRH) (15). As a consequence, there has been increasing interest in determining if additional serum parameters of thyroid function may better inform assessments of thyroid hormone activity at the tissue level (16). Free thyroxine (fT4) has been negatively correlated with components of metabolic syndrome (17,18), while higher free triiodothyronine (fT3) has been related to increased waist circumference (19) and BMI (19,20) but lower insulin sensitivity (21). The ratio of T3 to T4 (or fT3 to fT4) has been proposed as a better estimate of tissue-specific deiodinase activity in the conversion of T4 to T3 (22). Indeed, in healthy pregnant women, an increasing fT3:fT4 ratio has been associated with decreased glucose tolerance, higher triglycerides, and higher maternal BMI, while no association between TSH and these same metabolic parameters was found (23). The relationship between higher fT3:fT4 ratios and more unfavorable metabolic phenotypes was further supported by the finding that in euthyroid healthy individuals, both higher fT3 levels and a higher fT3:fT4 ratio were directly related to BMI, waist circumference, and a less favorable lipid profile (24).
The relations between TSH, fT4, and fT3 and cardiometabolic risk factors are incompletely characterized in children and adolescents. In a retrospective chart review of children aged 2–18 years, TSH was positively correlated with BMI, triglyceride levels, fasting insulin, and insulin resistance, as estimated by the homeostatic model assessment for insulin resistance (HOMA-IR), while fT4 was negatively correlated with triglycerides (25). In addition to a positive linear association between serum TSH levels and BMI (26), TSH and fT3 have been found to be significantly higher in children and adolescents with obesity compared with healthy weight youth, without an increase in fT4 (27). TSH has been found to be positively associated not only with blood pressure (28), but also with total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and triglycerides in a population-based study of German children and adolescents, with the strongest relationship between a higher-risk lipid profile and TSH found in children who were overweight and obese (29).
It was therefore hypothesized that higher levels of TSH, even within the normal reference range, will be associated with traditional cardiometabolic risk factors, including obesity, blood pressure, and lipid and glucose metabolism in an unselected population of U.S. children and adolescents without evidence of thyroid disease. This study also sought to determine if variations in thyroid hormones (fT3 and fT4) within the normal range are associated with cardiometabolic profiles in the adolescent population.
Subjects and Methods
Study population
Data were extracted from the large cross-sectional National Health and Nutrition Examination Survey (NHANES) database, for subjects aged ≥12 years but <19 years, from 2007–2008 and 2009–2010 (two combined survey cycles, n = 20,686 subjects total, n = 9461 with thyroid function laboratory data; of these, n = 1472 subjects were adolescents meeting age criteria). Survey results have been released in two-year cycles since 1999. The last analysis of NHANES data suggests that overall, the United States has been iodine-sufficient since 2000 (30). NHANES study protocols are approved and periodically reviewed by the National Center for Health Statistics, Research Ethics Review Board. Details of the data collection and informed consent/assent process have been previously described (31). Subjects (if >16 years of age) or their guardians were asked to identify the racial/ethnic group to which they belonged. These groups were then combined into the categories of Hispanic—Mexican American, Hispanic—other Hispanic, non-Hispanic white, non-Hispanic black, or other race including multiracial.
Inclusion criteria for the analysis included TSH within the assay-specific normal range (0.35–5.6 μIU/mL) and negative thyroid peroxidase antibodies (≤9.0 IU/mL). Exclusion criteria included any history of thyroid disease or diabetes mellitus (DM), or history of pharmacologic treatment for hypertension, dyslipidemia, thyroid disease, or DM. Subjects with any evidence of DM on laboratory testing (hemoglobin A1c ≥6.5%, fasting serum glucose ≥126 mg/dL, or serum glucose ≥200 mg/dL 2 h after the oral glucose tolerance test [OGTT]) were also excluded.
Measurements
Height, weight, and blood pressure were measured according to standardized protocols described in the NHANES Survey Operations Manual (available at www.cdc.gov/nchs/nhanes/survey_methods.htm). Overweight and obesity were defined as a BMI from the 85th to the 94th percentile and ≥95th percentile for age and sex, respectively. Age- and sex-specific BMI percentiles and BMI Z-scores were determined according to the 2000 CDC growth charts using a CDC SAS program (www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm). Mean systolic blood pressure (SDP) and diastolic blood pressure (DBP) were determined from an average of between one and four standardized measurements.
Laboratory assays
TSH was measured using a third-generation, two-site immunoenzymatic assay (Access 2 HYPERsensitive assay, Beckman Coulter; reference range 0.34–5.60 μIU/mL, coefficient of variation [CV] 4.4–6.5%). Thyroid peroxidase antibodies (Access TPO antibody assay, Beckman Coulter) were defined as normal if ≤9.0 IU/mL. Free and total thyroid hormones were all measured on the Beckman Coulter Access 2 platform as follows: competitive binding immunoenzymatic assays were used for fT3 (reference range 2.5–3.9 pg/mL, CV 3.5–5.6%), total T3 (TT3; reference range 87–178 ng/dL, CV 4.8–8.0%), and TT4 (reference range 6.1–12.2 μg/dL, CV 3.9–6.7%). fT4 was measured by a two-step enzyme immunoassay (Access 2 Free T4, Beckman Coulter; reference range 0.6–1.6 ng/dL, CV 2.3–9.3%). Fasting blood samples were collected for glucose, insulin, and cholesterol. An OGTT was performed after a 9 h fast, with venipuncture at baseline and 2 h after the administration of oral glucose (Trutol™), generally 75 g but adjusted for weight in those subjects weighing <94 lbs (42.7 kg). Fasting plasma glucose was measured by the hexokinase method (Roche/Hitachi Modular P Chemistry Analyzer; CV 0.8–2.6%). Insulin was measured by a two-site immunoassay (Mercodia Insulin ELISA kit; reference range 2–25 mIU/L, CV 5.5–8.8%). Apolipoprotein B was measured using the ProSpec Nephelometer (Dade Behring Diagnostics, GMBH; CV 2.0–6.2%). TC, high-density lipoprotein cholesterol (HDL-C) and triglycerides (TG) were all assayed on the Roche/Hitachi Module P chemistry analyzer: TC was measured with a single reagent, endpoint reaction method (CV 1.1–1.3%); HDL-C was measured directly with a third-generation photometric endpoint method (reportable range 3–200 mg/dL, CV 1.5–3.2%); and TG were measured using the two-reagent, endpoint reaction (CV 1.9–2.4%). For samples collected in participants of the morning survey session who had fasted for 8.5–24 h, LDL-C levels were calculated according to the Friedewald equation: LDL-C = TC – HDL-C – triglycerides/5. Estimated insulin resistance was calculated using the HOMA-IR: [fasting glucose (mg/dL) × fasting insulin (mIU/L)]/405 (32).
Defining metabolic syndrome
Subjects with metabolic syndrome were identified using the International Diabetes Federation (IDF) 2007 consensus definition of the metabolic syndrome (MetS) in children and adolescents (33). Diagnostic criteria for children aged 10 to <16 years required the presence of abdominal obesity (waist circumference ≥90th percentile, or adult cutoff if lower), in addition to at least two of the following: triglycerides ≥150 mg/dL, HDL-C <40 mg/dL, SBP ≥130 mmHg or DBP ≥85 mmHg, or fasting glucose ≥100 mg/dL (33). For subjects aged ≥16 years, the adult IDF criteria of central obesity defined as waist circumference ≥94 cm for males or ≥80 cm for females were used, plus at least two of the following: triglycerides ≥150 mg/dL, HDC-C <40 mg/dL in males or <50 mg/dL in females, SBP ≥130 mmHg or DBP ≥85 mmHg, or fasting glucose ≥100 mg/dL (33). Because of the exclusion criteria, the data set did not contain any subjects with previously diagnosed type 2 DM or hypertension.
Statistical methods
Data are reported as means ± standard deviation (SD), as distributions were verified for normality based on the large sample size and examination of the normal quantile plots. TSH was also log-transformed for analysis. Differences in mean values by sex were determined by t-tests. Univariate (Pearson correlation) and multivariate linear regression analyses were performed of associations between TSH and thyroid hormones with age, sex, race/ethnicity, BMI Z-score, SBP and DBP, fasting lipid parameters (TC, HDL-C, LDL-C, TG, and ApoB), and glucose metabolism parameters (fasting glucose, fasting insulin, 2 h glucose, and HOMA-IR), with a predetermined Bonferroni-corrected significance level of <0.001 to adjust for multiple comparisons. From the original NHANES data files, a data set that combined the 2007–2008 and 2009–2010 survey cycles and contained only subjects meeting the inclusion and exclusion criteria was constructed using SAS v9.4. All subsequent statistical analyses were performed using JMP Pro v10.0.2.
Results
Study population
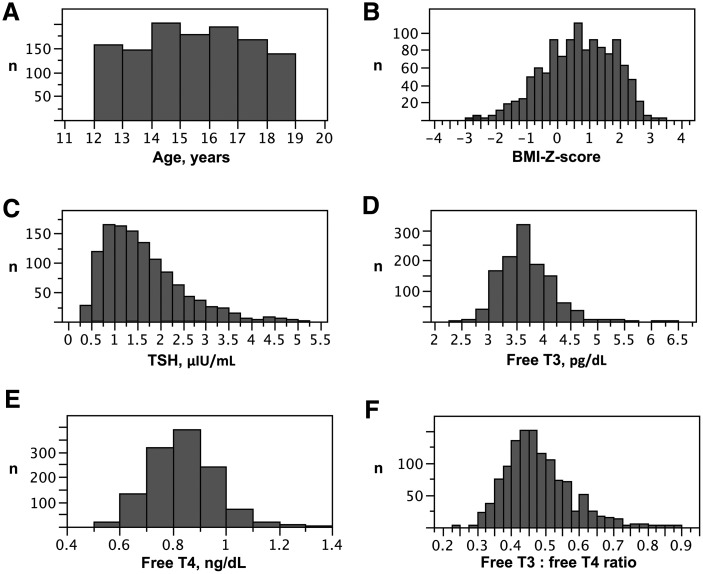
Of 20,686 samples in the combined 2007–2008 and 2009–2010 NHANES data sets, 9461 subjects had thyroid function testing results available, and 1472 of these were aged 12–18 years. Following application of the exclusion criteria, a sample of 1167 euthyroid adolescents were eligible for analysis (Mage = 15.0 ± 1.91 years; 53.0% male), with a mean TSH of 1.60 ± 0.83 μIU/mL (Fig. 1 and Table 1). Sixteen subjects had BMI data missing. Fourteen subjects (1% of all subjects) met the IDF definition (33) for metabolic syndrome in children and adolescents, with the low number likely reflective of the exclusion criteria. Females had slightly lower SBP, TSH, and fasting glucose but higher HDL-C levels compared with males. Age, BMI and BMI Z-score, diastolic blood pressure, LDL, triglycerides, ApoB, and measures of glucose metabolism did not differ by sex. Although mean fT4 did not differ between sexes, females did have significantly lower mean fT3 and TT3 levels and a lower fT3:fT4 ratio compared with males (p < 0.0001).
FIG. 1.
Distributions of age (A), body mass index (BMI) Z-score (B), thyrotropin (TSH) (C), free triiodothyronine (fT3) (D), free thyroxine (fT4) (E), and fT3:fT4 ratios (F) in 1167 euthyroid adolescents participating in the National Health and Nutrition Examination Survey 2007–2010.
Table 1.
Demographic, Anthropometric, and Metabolic Features of 1167 Euthyroid Adolescents Aged 12–18 Years, NHANES 2007–2010
| Characteristic | All subjects | Males | Females | p |
|---|---|---|---|---|
| n | 1167 | 618 (53%) | 549 (47%) | |
| Age (years) | 15.0 ± 1.9 | 15.0 ± 1.9 | 14.9 ± 1.9 | 0.4229 |
| BMI (kg/m2) | 23.8 ± 5.7 | 23.6 ± 5.7 | 24.0 ± 5.6 | 0.2220 |
| BMI Z-score (SD) | 0.66 ± 1.11 | 0.62 ± 1.18 | 0.70 ± 1.03 | 0.2504 |
| SBP (mmHg) | 108.9 ± 10.1 | 111.4 ± 10.3 | 106.0 ± 9.0* | <0.0001 |
| DBP (mmHg) | 60.0 ± 10.3 | 59.2 ± 11.0 | 61.0 ± 9.4 | 0.0029 |
| TSH (μIU/mL) | 1.60 ± 0.83 | 1.68 ± 0.85 | 1.51 ± 0.80* | 0.0003 |
| Total cholesterol (mg/dL) | 157.8 ± 28.8 | 155.2 ± 28.0 | 160.7 ± 29.4 | 0.0013 |
| HDL-cholesterol (mg/dL) | 51.2 ± 12.0 | 49.6 ± 11.7 | 53.1 ± 12.1* | <0.0001 |
| LDL-cholesterol (mg/dL) | 86.9 ± 25.0 | 85.8 ± 25.0 | 88.3 ± 25.0 | 0.2570 |
| Triglycerides (mg/dL) | 81.4 ± 45.7 | 84.2 ± 50.7 | 77.7 ± 37.9 | 0.0971 |
| Apolipoprotein B (mg/dL) | 67.9 ± 18.1 | 66.9 ± 17.7 | 69.1 ± 18.6 | 0.1537 |
| Fasting glucose (mg/dL) | 85.5 ± 10.3 | 86.9 ± 10.3 | 83.9 ± 10.0* | <0.0001 |
| Fasting insulin (μIU/mL) | 15.0 ± 12.2 | 14.6 ± 12.6 | 15.6 ± 11.6 | 0.3634 |
| 2 h glucose (mg/dL) | 97.6 ± 22.6 | 97.6 ± 22.7 | 97.6 ± 22.6 | 0.9840 |
| HOMA-IR | 3.28 ± 2.95 | 3.23 ± 2.96 | 3.35 ± 2.95 | 0.6563 |
| fT4 (ng/dL) | 0.79 ± 0.12 | 0.79 ± 0.12 | 0.79 ± 0.12 | 0.4048 |
| fT3 (pg/dL) | 3.65 ± 0.45 | 3.79 ± 0.44 | 3.50 ± 0.42* | <0.0001 |
| Total T3 (ng/dL) | 132 ± 24 | 137 ± 23 | 127 ± 25* | <0.0001 |
| fT3:fT4 ratio | 0.47 ± 0.09 | 0.49 ± 0.09 | 0.45 ± 0.09* | <0.0001 |
Data presented as means ± standard deviation. Statistically significant values are shown in bold.
p < 0.001.
NHANES, National Health and Nutrition Examination Survey; BMI, body mass index; SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure; TSH, thyrotropin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; fT4, free thyroxine; fT3, free triiodothyronine.
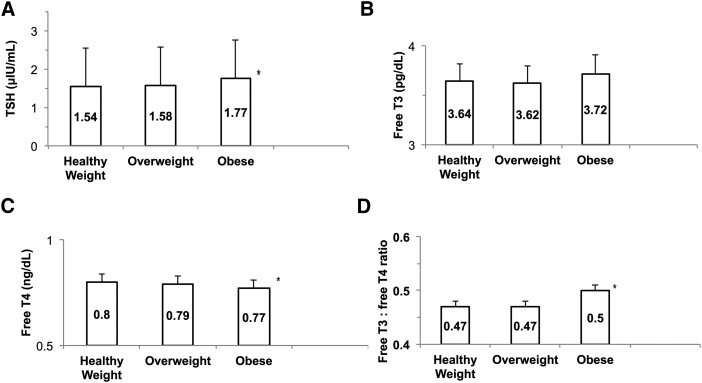
Mean TSH was significantly higher in adolescents with obesity compared with both healthy weight and overweight subjects (F-ratio 7.04, p = 0.0009; Fig. 2). Mean fT4 levels were significantly lower in obese than in healthy or overweight subjects (F-ratio 6.2172, p = 0.0019), while there were no differences in fT3 levels. Adolescents with obesity also had significantly higher fT3:fT4 ratios (F-ratio 10.75, p < 0.0001) than either healthy weight or overweight subjects. Mean BMI Z-scores differed significantly between racial/ethnic groups (F-ratio 4.28, p = 0.0019), with the highest BMI Z-scores in non-Hispanic black (0.78 ± 1.06) and Mexican American subjects (0.76 ± 1.10), while no racial/ethnic differences were found for TSH (F-ratio 2.04, p = 0.09) or the fT3:fT4 ratio (F-ratio 2.42, p = 0.0467).
FIG. 2.
Thyroid homeostasis indices by category of healthy weight, overweight (BMI from 85th to 94th percentile for age and sex), or obese (≥95th percentile for age and sex). The mean value for each category is reported in the columns. (A) TSH was significantly higher in adolescents with obesity compared with both healthy-weight and overweight subjects (F-ratio 7.04, p = 0.0009). (B) There was no difference in fT3 levels by weight category. (C) Mean fT4 levels were significantly lower in obese than in healthy-weight or overweight subjects (F-ratio 6.2172, p = 0.0019). (D) Obese adolescents had a higher fT3:fT4 ratio than healthy weight or overweight adolescents (F-ratio 7.81, p = 0.0004).
Univariate analysis
Significant positive linear correlations were observed between TSH and SBP (r = 0.11, p = 0.0004), TC (r = 0.12, p < 0.0001), fasting glucose (r = 0.16, p < 0.0001), 2 h glucose (r = 0.18, p < 0.0001), and HOMA-IR (r = 0.15, p = 0.0004). ApoB (r = 0.14, p = 0.0013) and fasting insulin (r = 0.13, p = 0.0020) trended toward a positive correlation with TSH, but did not reach predetermined criteria for statistical significance (Table 2). A negative linear relationship was found between TSH and age (r = −0.10, p = 0.0009). Univariate analysis performed with log-transformed TSH was congruent with significant correlations with all of the above variables, with the exception that HOMA-IR and logTSH (r = 0.13, p = 0.0019), which trended toward but did not reach the pre-stated significance level of <0.001.
Table 2.
Significant Univariate Correlations Between TSH, Thyroid Hormones, and Metabolic Parameters
| TSH | fT4 | fT3 | fT3:fT4 ratio | |||||
|---|---|---|---|---|---|---|---|---|
| Dependent variable | r | p | r | p | r | p | r | p |
| Age | −0.10* | 0.0009 | 0.04 | 0.2166 | −0.30* | <0.0001 | −0.23* | <0.0001 |
| BMI Z-score | 0.08 | 0.008 | −0.12* | <0.0001 | 0.03 | 0.2544 | 0.12* | <0.0001 |
| SBP | 0.11* | 0.0004 | −0.06 | 0.0335 | 0.08 | 0.0061 | 0.10* | 0.0005 |
| Total cholesterol | 0.12* | <0.0001 | −0.13* | <0.0001 | −0.09 | 0.0035 | 0.06 | 0.0401 |
| HDL cholesterol | 0.01 | 0.8473 | −0.002 | 0.9535 | −0.16* | <0.0001 | −0.11* | 0.0002 |
| Triglycerides | 0.12 | 0.0067 | −0.18* | <0.0001 | 0.17* | <0.0001 | 0.27* | <0.0001 |
| Apolipoprotein B | 0.14 | 0.0013 | −0.10 | 0.0141 | 0.001 | 0.9719 | 0.10 | 0.0259 |
| Fasting glucose | 0.16* | <0.0001 | −0.04 | 0.1823 | 0.20* | <0.0001 | 0.16* | <0.0001 |
| Fasting insulin | 0.13 | 0.0020 | −0.15* | 0.0006 | 0.12 | 0.0045 | 0.211* | <0.0001 |
| 2 h glucose | 0.18* | 0.0002 | −0.10 | 0.0405 | 0.16* | 0.0008 | 0.20* | <0.0001 |
| HOMA-IR | 0.15* | 0.0004 | −0.15* | 0.0007 | 0.14* | 0.0008 | 0.22* | <0.0001 |
p < 0.001.
With respect to free thyroid hormones, fT4 was negatively correlated with BMI Z-score (r = −0.12, p < 0.0001), TC (r = −0.12, p < 0.0001), TG (r = −0.18, p < 0.0001), fasting insulin (r = −0.15, p = 0.0006), and HOMA-IR (r = −0.15, p = 0.0007). fT3 was negatively correlated with age (r = −0.30, p < 0.0001) and HDL-C (r = −0.16, p < 0.0001), and positively correlated with TG (r = 0.17, p < 0.0001), fasting glucose (r = 0.20, p < 0.0001), 2 h glucose (r = 0.16, p = 0.0008), and HOMA-IR (r = 0.14, p = 0.0008). The fT3:fT4 ratio was negatively correlated with age (r = −0.23, p < 0.0001) and HDL-C (r = −0.11, p = 0.0002) but positively correlated with BMI Z-score (r = 0.12, p < 0.0001), SBP (r = 0.10, p = 0.0005), TG (r = 0.27, p < 0.0001), and all parameters of glucose metabolism, including fasting and 2 h glucose, fasting insulin, and HOMA-IR (p < 0.0001).
The number of subjects meeting the IDF criteria for MetS in the data set was low (n = 14), which likely reflects the exclusion criteria applied to the larger NHANES cohort. When TSH and thyroid hormone levels were compared between subjects meeting MetS diagnostic criteria and those who did not fulfill any criteria (n = 314), the fT3:fT4 ratio was found to be significantly higher in the MetS group (F-ratio 16.8, p < 0.001).
Multivariate analysis
Based on the univariate analyses of TSH and cardiometabolic risk factors, next models for multivariate analyses of TSH and the fT3:fT4 ratio were constructed. Analysis using log-transformed TSH instead of TSH did not change any of the results. Models examining TSH or the fT3:fT4 ratio as independent variables, with each of SBP, TC, ApoB, fasting and 2 h glucose, insulin, HOMA-IR, and BMI Z-score as dependent variables, were constructed, while controlling for the effects of age, sex and race/ethnicity. TSH demonstrated significant relationships with all variables after controlling for the impact of age, sex, and race/ethnicity (Table 3); age, sex, and race/ethnicity remained independent predictors of SBP, fasting glucose, and fasting insulin. Each of these models was highly significant (p < 0.0001).
Table 3.
Associations Between TSH or fT3:fT4 and Metabolic Parameters in Euthyroid Adolescents After Controlling for Age, Sex, and Race/Ethnicity in Multivariate Analyses
| TSH | fT3:fT4 | |||
|---|---|---|---|---|
| Dependent variable | β estimate | p | β estimate | p |
| SBP | 1.15 (0.47, 1.83)a,b,c | 0.0001 | 10.31 (3.81, 16.81)a,b,c | 0.0019 |
| Total cholesterol | 5.02 (3.03,7.01)a,b | <0.0001 | 33.99 (14.92, 53.05)a,b | 0.0005 |
| Apolipoprotein B | 3.23 (1.51, 4.95)b,c | 0.0002 | 24.39 (7.00, 41.79)a,b,c | 0.0004 |
| Fasting glucose | 1.68 (0.98, 2.38)a,b,c | <0.0001 | 13.28 (6.59,19.97)a,b,c | 0.0001 |
| Fasting insulin | 1.96 (0.82, 3.11)a,b,c | 0.0008 | 27.29 (15.84, 38.72)b,c | <0.0001 |
| 2 h glucose | 4.30 (1.87, 6.77)b | 0.0006 | 41.84 (17.54, 66.12)b | 0.0008 |
| HOMA-IR | 0.54 (0.26, 0.81)b,c | 0.0002 | 6.98 (4.20, 9.76)c | <0.0001 |
| BMI Z-score | 0.12 (0.04, 0.19)c | 0.0038 | 1.54 (0.80, 2.28)c | <0.0001 |
Reported p-values are for individual parameter estimates in each model. Significance level was set at p < 0.001. Each of the models was also highly significant overall (p < 0.0001).
Sex remained an independent predictor in the model.
Age remained an independent predictor in the model.
Race/ethnicity remained an independent predictor in the model.
To address further the well-established contributions of BMI Z-score to many of the other cardiometabolic risk factors, models assessing TSH while controlling for BMI Z-score in addition to age, sex, and race/ethnicity were analyzed. BMI Z-score was positively correlated with SBP (p < 0.0001), ApoB (p = 0.0004), fasting insulin (p < 0.0001), and HOMA-IR (p < 0.0001). Meanwhile, the fT3:fT4 ratio maintained a significant positive association with fasting glucose (p = 0.0001) and HOMA-IR (p < 0.0001). Controlling for BMI Z-score did not change the significance of these results (fasting glucose p = 0.001, and HOMA-IR p = 0.0009). Importantly, the relations between TSH and these metabolic parameters were still significant after controlling for the BMI Z-score.
Additional multivariate analyses were then performed with both TSH and the fT3:fT4 ratio as explanatory variables (Table 4). In these models, TSH was found to be a predictor for TC and fasting glucose, while the fT3:fT4 ratio was positively correlated with fasting glucose and insulin, HOMA-IR, and BMI Z-score. Subsequently, models constructed with fT3 and fT4 as independent variables, instead of the fT3:fT4 ratio, in multivariate analyses indicated significant negative correlations between fT4 and BMI Z-score (β estimate −1.04 [−1.56, −0.50], p = 0.0002) and TC (β estimate −28.28 [−41.94, −14.63], p < 0.0001), and fT3 maintained a positive relationship with fasting glucose (β estimate 2.80 [−1.37, 4.24], p = 0.0001).
Table 4.
Eight Models Constructed with TSH and the fT3:fT4 Ratio as Explanatory Variables for Metabolic Parameters in Euthyroid Adolescents After Controlling for Age, Sex, and Race/Ethnicity in Multivariate Analyses
| TSH | fT3:fT4 | |||
|---|---|---|---|---|
| Dependent variable | β estimate | p | β estimate | p |
| SBPa,b,c | 1.03 (0.35, 1.72) | 0.0032 | 9.10 (2.57, 15.62) | 0.0063 |
| Total cholesterola,b | 4.55(2.64, 6.64) | <0.0001 | 28.41 (9.35, 47.47) | 0.0035 |
| Apolipoprotein Ba,b,c | 3.31 (1.17, 4.61) | 0.0010 | 27.88 (10.58, 45.12) | 0.0016 |
| Fasting glucosea,b,c | 1.53 (0.83, 2.23) | <0.0001 | 11.44 (4.75, 18.14) | 0.0008 |
| 2 h glucoseb | 3.82 (1.38, 6.25) | 0.0022 | 36.81 (12.53,61.08) | 0.0030 |
| Fasting insulinc | 1.65(0.52, 2.79) | 0.0044 | 25.22 (13.77, 36.68) | <0.0001 |
| HOMA-IRc | 0.46 (0.18, 0.73) | 0.0012 | 6.42 (3.64, 9.19) | <0.0001 |
| BMI Z-score | 0.10 (0.02, 0.18) | 0.0155 | 1.43 (0.68, 2.17) | 0.0002 |
Significance level was set at p < 0.001. Statistically significant values are shown in bold.
Sex remained an independent predictor in the model.
Age remained an independent predictor in the model.
Race/ethnicity remained an independent predictor in the model.
Discussion
In a population-based sample of U.S. adolescents without evidence of thyroid disease, higher TSH levels within the normal reference range were found to be positively correlated with multiple well-established cardiometabolic risk factors, including BMI Z-score, SBP, TC, and estimates of insulin resistance. fT3 levels were associated with adverse metabolic risk factors, as they were positively associated with TG and worsened glucose metabolism and negatively associated with HDL-C. In contrast, fT4 levels were negatively associated with BMI Z-score, TG, and HOMA-IR. Of note, in univariate analyses, the fT3:fT4 ratio was found to have significant correlations with more of the assessed lipid and glucose variables (specifically BMI Z-score, SBP, HDL-C, TG, fasting glucose and insulin, 2 h glucose, and HOMA-IR) than fT4, fT3, or TSH alone. The finding that the fT3:fT4 ratio is higher in the subset of subjects with MetS than in subjects not meeting any of the IDF MetS criteria supports that the fT3:fT4 ratio may be a useful marker for higher cardiometabolic risk. Multivariate models controlling for sex, age, and BMI Z-score revealed that higher TSH predicts both higher TC and fasting glucose. Additionally, higher TSH levels trended toward significance for predicting higher ApoB and HOMA-IR. Addition of the fT3:fT4 ratio to multivariate analyses indicated that this ratio is related to indexes of carbohydrate metabolism, specifically fasting glucose and HOMA-IR.
The results of these analyses are consistent with the findings of a similar survey conducted in a euthyroid U.S. adult population, which demonstrated positive correlations between BMI and both TSH and fT3 but not between BMI and fT4 (20). Of note, in the adult population study, no assessment of insulin resistance or fT3:fT4 ratio was performed. Overall, the most well-established relationships between thyroid status within the normal range and cardiometabolic risk factors are between TSH and BMI, blood pressure, and lipids, as noted in a meta-analysis by Taylor et al. (13), which included mainly cross-sectional studies in adults. With respect to relations between TSH and BMI in children, a very large population-based study of >12,000 German children and adolescents aged 3–17 years indicated that TSH and BMI are positively correlated (26).
Furthermore, in children and adolescents, TSH and blood pressure have been shown to be positively related in a cross-sectional study that included subjects with overt hyper- and hypothyroidism (28). A retrospective analysis of a large community-based pediatric clinic population in Jerusalem, which included some subjects with subclinical hypothyroidism, found positive correlations between BMI and TSH, fT3, and fT3:fT4 ratios. Conversely, fT4 demonstrated a negative relation with BMI (27). Although the relations between thyroid hormones and BMI were significant only in children with obesity, higher TSH correlated with higher fT3 (but not fT4) levels, even in healthy-weight children (27). A retrospective review of U.S. children and adolescents confirmed an association with higher TSH values at or slightly above the normal reference range with obesity; the free thyroid hormone data were not sufficient for analysis (34).
With respect to lipid status, a positive relationship between TSH and TC, and a positive but nonsignificant trend between TSH and TG were found. These results are in contrast to a retrospective study of euthyroid children aged 2–18 years, which reported a positive correlation with only TG (25). Of note, the reasons for performing TSH testing in this population were not described, and TSH testing is not routinely recommended as part of well-child care. However, the present findings are consistent with the results of another population-based study of unselected subjects showing that TSH and TG were positively associated with TSH (29).
With respect to thyroid hormone levels and insulin sensitivity, an inverse relationship between insulin sensitivity and TSH levels was previously observed, even after adjusting for age and BMI in a small cohort (n = 36) of euthyroid obese adolescents undergoing OGTT. This difference was significant only in males (who also had higher T3 levels) (35). However, in a study of 100 Mexican adolescents with risk factors for type 2 DM (obesity, overweight, or type 2 DM in a first-degree relative) and no overtly abnormal TSH or thyroid hormone levels, fT4 correlated negatively with HOMA-IR, while no relation between TSH and HOMA-IR was demonstrated (36). An intensive inpatient two-month weight-loss program for children and adolescents indicated that the change in TSH with weight loss predicted reductions in HOMA-IR after controlling for change in body weight, age, and sex, while the changes in fT3 and fT4 did not (37). Sex-related differences were observed in TSH, fT3 levels, and the fT3:fT4 ratios in this NHANES adolescent cohort, but since data on sexual maturity rating (Tanner Staging) were not recorded, it was not possible to assess the impact of pubertal progression on thyroid hormone levels directly, for which studies are conflicting (38,39). The variability in these results highlights the need for further prospective studies elucidating the relations between thyroid hormones, TSH, and weight fluctuations in children and adolescents at various stages of pubertal development.
Given the role of thyroid hormones in the regulation of energy expenditure and thermogenesis (40), and the increasingly well-established relations between subtle alterations in TSH, thyroid hormone levels, and cardiometabolic risk factors (13,17–21,23,24,28,29,35), there has been interest in assessing the effects of weight loss on thyroid hormone levels. In a study of adult overweight or obese otherwise healthy subjects undergoing a controlled lifestyle modification program, modest weight loss (6.5 ± 1.0%) resulted in reductions in TT3 without significant changes in TSH or fT4. The TT3:fT4 ratio also declined significantly in those who lost >5% body weight (41). In children and adolescents with obesity enrolled in an intensive two-month inpatient weight-loss program, changes in TSH correlated with changes in fasting insulin and HOMA-IR. With weight loss, a significant decrease in TSH and fT3 (but again not fT4) was noted (37). In German children undergoing a longer-term (one year) outpatient lifestyle modification program, followed by an additional year of follow-up, those with a decrease in BMI Z-score of >0.5 during the intervention had higher TSH and fT3 at baseline than those with less weight loss. TSH and fT3 both declined significantly with weight loss, with greater reductions in thyroid hormone levels associated with greater weight loss (42). However, children who experienced a greater decline in TSH and fT3 during the lifestyle intervention period also experienced a greater BMI rebound over the year following the end of the intervention (42).
These findings suggest that TSH and fT3 concentrations may be predictive not only of the changes in weight that may occur during weight loss interventions, but also of weight regain if lifestyle modifications are not maintained. As TSH receptors are present on adipocytes, and receptors for leptin have been identified on the TRH-secreting neurons of the paraventricular nucleus (15), evidence points to a bidirectional communication between adipose tissue and the HPT axis that must be overcome to counteract the reductions in energy expenditure typically associated with weight loss. Because decreases in fT3 with weight loss are associated with decreases in resting energy expenditure in both adults and children (43,44), consideration of T3 levels may help to identify further a group of individuals who may require more intensive or prolonged modifications to achieve sustained weight loss.
The strengths of this study include the large number of subjects included in this population-based, unselected sample of U.S. children and adolescents. In most of the retrospective series that have examined the relations between TSH, thyroid hormones, and body composition, the sampling of thyroid tests was performed for clinical reasons. Hence, a confounder effect cannot be ruled out (25,27,29). The present assessment of an unselected U.S. population (by default devoid of specific clinical indications for testing) minimizes the risk of selection bias. While other published analyses of population databases have not addressed any potential racial or ethnic differences (10,11,17–19,21,23–25,28,29), this NHANES cohort consisted of participants who predominantly self-identified as non-Hispanic white, non-Hispanic black, Mexican-American, or other Hispanic. The lack of any race/ethnicity differences in TSH and thyroid hormones suggests that with respect to race/ethnicity, other factors such as BMI (which did show ethnicity differences in the analysis) still play a greater role than the thyroid axis in determining overall cardiometabolic risk.
The limitations of this study include its cross-sectional design, which prevents the establishment of causality. Additionally, the lack of data on pubertal status (Tanner staging) of study subjects may represent a confounder, since there is a significant reduction in insulin resistance with progression to the later stages of puberty (45). However, it is noteworthy that even after controlling for age, which could be considered as a rough approximation of pubertal status, TSH remained significantly related to fasting glucose. For the fT3:fT4 ratio, significant relationships remained for both fasting glucose and HOMA-IR.
In conclusion, the present observations in a large unselected population of U.S. adolescents demonstrate that thyroid hormone homeostasis, and indexes of peripheral metabolism of thyroid hormone, have an important predictive role in cardiometabolic risk determination. Future longitudinal studies would provide a better assessment of whether these relations persist into adulthood, or if the subtle differences in thyroid hormone levels may be indicators of a cohort of adolescents who may benefit from earlier and more intensive interventions aimed at improving lifetime cardiometabolic risk.
Acknowledgments
An NIH grant (K23HD053742) was awarded to E.P.W.
Author Disclosure Statement
F.S.C. has previously consulted for Akrimax Pharmaceuticals. T.N.L. and E.P.W. have nothing to declare.
References
- 1.Klee GG, Hay ID. 1997. Biochemical testing of thyroid function. Endocrinol Metab Clin North Am 26:763–775 [DOI] [PubMed] [Google Scholar]
- 2.Andersen S, Pedersen KM, Bruun NH, Laurberg P. 2002. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab 87:1068–1072 [DOI] [PubMed] [Google Scholar]
- 3.Philippe J, Dibner C. 2015. Thyroid circadian timing: roles in physiology and thyroid malignancies. J Biol Rhythms 30:76–83 [DOI] [PubMed] [Google Scholar]
- 4.Hansen PS, Brix TH, Sorensen TI, Kyvik KO, Hegedus L. 2004. Major genetic influence on the regulation of the pituitary–thyroid axis: a study of healthy Danish twins. J Clin Endocrinol Metab 89:1181–1187 [DOI] [PubMed] [Google Scholar]
- 5.Ristic-Medic D, Dullemeijer C, Tepsic J, Petrovic-Oggiano G, Popovic T, Arsic A, Glibetic M, Souverein OW, Collings R, Cavelaars A, de Groot L, van't Veer P, Gurinovic M. 2014. Systematic review using meta-analyses to estimate dose–response relationships between iodine intake and biomarkers of iodine status in different population groups. Nutr Rev 72:143–161 [DOI] [PubMed] [Google Scholar]
- 6.Surks MI, Goswami G, Daniels GH. 2005. The thyrotropin reference range should remain unchanged. J Clin Endocrinol Metab 90:5489–5496 [DOI] [PubMed] [Google Scholar]
- 7.Wartofsky L, Dickey RA. 2005. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab 90:5483–5488 [DOI] [PubMed] [Google Scholar]
- 8.Asvold BO, Bjoro T, Vatten LJ. 2009. Association of serum TSH with high body mass differs between smokers and never-smokers. J Clin Endocrinol Metab 94:5023–5027 [DOI] [PubMed] [Google Scholar]
- 9.Meisinger C, Ittermann T, Tiller D, Agger C, Nauck M, Schipf S, Wallaschofski H, Jorgensen T, Linneberg A, Thiery J, Kluttig A, Greiser KH, Werdan K, Burkhardt K, Volzke H. 2014. Sex-specific associations between thyrotropin and serum lipid profiles. Thyroid 24:424–432 [DOI] [PubMed] [Google Scholar]
- 10.Asvold BO, Vatten LJ, Nilsen TI, Bjoro T. 2007. The association between TSH within the reference range and serum lipid concentrations in a population-based study. The HUNT Study. Eur J Endocrinol 156:181–186 [DOI] [PubMed] [Google Scholar]
- 11.Asvold BO, Bjoro T, Nilsen TI, Vatten LJ. 2007. Association between blood pressure and serum thyroid-stimulating hormone concentration within the reference range: a population-based study. J Clin Endocrinol Metab 92:841–845 [DOI] [PubMed] [Google Scholar]
- 12.Ittermann T, Tiller D, Meisinger C, Agger C, Nauck M, Rettig R, Hofman A, Jorgensen T, Linneberg A, Witteman JC, Franco OH, Greiser KH, Werdan K, Doring A, Kluttig A, Stricker BH, Volzke H. 2013. High serum thyrotropin levels are associated with current but not with incident hypertension. Thyroid 23:955–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor PN, Razvi S, Pearce SH, Dayan CM. 2013. Clinical review: a review of the clinical consequences of variation in thyroid function within the reference range. J Clin Endocrinol Metab 98:3562–3571 [DOI] [PubMed] [Google Scholar]
- 14.Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA, American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults 2012. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid 22:1200–1235 [DOI] [PubMed] [Google Scholar]
- 15.Santini F, Marzullo P, Rotondi M, Ceccarini G, Pagano L, Ippolito S, Chiovato L, Biondi B. 2014. Mechanisms in endocrinology: the crosstalk between thyroid gland and adipose tissue: signal integration in health and disease. Eur J Endocrinol 171:R137–152 [DOI] [PubMed] [Google Scholar]
- 16.Abdalla SM, Bianco AC. 2014. Defending plasma T3 is a biological priority. Clin Endocrinol (Oxf) 81:633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehran L, Amouzegar A, Tohidi M, Moayedi M, Azizi F. 2014. Serum free thyroxine concentration is associated with metabolic syndrome in euthyroid subjects. Thyroid 24:1566–1574 [DOI] [PubMed] [Google Scholar]
- 18.Roos A, Bakker SJ, Links TP, Gans RO, Wolffenbuttel BH. 2007. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab 92:491–496 [DOI] [PubMed] [Google Scholar]
- 19.De Pergola G, Ciampolillo A, Paolotti S, Trerotoli P, Giorgino R. 2007. Free triiodothyronine and thyroid stimulating hormone are directly associated with waist circumference, independently of insulin resistance, metabolic parameters and blood pressure in overweight and obese women. Clin Endocrinol (Oxf) 67:265–269 [DOI] [PubMed] [Google Scholar]
- 20.Kitahara CM, Platz EA, Ladenson PW, Mondul AM, Menke A, Berrington de Gonzalez A. 2012. Body fatness and markers of thyroid function among U.S. men and women. PLoS One 7:e34979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roef G, Lapauw B, Goemaere S, Zmierczak HG, Toye K, Kaufman JM, Taes Y. 2012. Body composition and metabolic parameters are associated with variation in thyroid hormone levels among euthyroid young men. Eur J Endocrinol 167:719–726 [DOI] [PubMed] [Google Scholar]
- 22.Nicoloff JT, Lum SM, Spencer CA, Morris R. 1984. Peripheral autoregulation of thyroxine to triiodothyronine conversion in man. Horm Metab Res Suppl 14:74–79 [PubMed] [Google Scholar]
- 23.Bassols J, Prats-Puig A, Soriano-Rodriguez P, Garcia-Gonzalez MM, Reid J, Martinez-Pascual M, Mateos-Comeron F, de Zegher F, Ibanez L, Lopez-Bermejo A. 2011. Lower free thyroxin associates with a less favorable metabolic phenotype in healthy pregnant women. J Clin Endocrinol Metab 96:3717–3723 [DOI] [PubMed] [Google Scholar]
- 24.Roef GL, Rietzschel ER, Van Daele CM, Taes YE, De Buyzere ML, Gillebert TC, Kaufman JM. 2014. Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid 24:223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nader NS, Bahn RS, Johnson MD, Weaver AL, Singh R, Kumar S. 2010. Relationships between thyroid function and lipid status or insulin resistance in a pediatric population. Thyroid 20:1333–1339 [DOI] [PubMed] [Google Scholar]
- 26.Ittermann T, Thamm M, Schipf S, John U, Rettig R, Volzke H. 2013. Relationship of smoking and/or passive exposure to tobacco smoke on the association between serum thyrotropin and body mass index in large groups of adolescents and children. Thyroid 23:262–268 [DOI] [PubMed] [Google Scholar]
- 27.Karavani G, Strich D, Edri S, Gillis D. 2014. Increases in thyrotropin within the near-normal range are associated with increased triiodothyronine but not increased thyroxine in the pediatric age group. J Clin Endocrinol Metab 99:E1471–1475 [DOI] [PubMed] [Google Scholar]
- 28.Ittermann T, Thamm M, Wallaschofski H, Rettig R, Volzke H. 2012. Serum thyroid-stimulating hormone levels are associated with blood pressure in children and adolescents. J Clin Endocrinol Metab 97:828–834 [DOI] [PubMed] [Google Scholar]
- 29.Witte T, Ittermann T, Thamm M, Riblet NB, Volzke H. 2015. Association between serum thyroid-stimulating hormone levels and serum lipids in children and adolescents: a population-based study of german youth. J Clin Endocrinol Metab 100:2090–2097 [DOI] [PubMed] [Google Scholar]
- 30.Caldwell KL, Makhmudov A, Ely E, Jones RL, Wang RY. 2011. Iodine status of the U.S. population, National Health and Nutrition Examination Survey, 2005–2006 and 2007–2008. Thyroid 21:419–427 [DOI] [PubMed] [Google Scholar]
- 31.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. 2013. National health and nutrition examination survey: plan and operations, 1999–2010. National Center for Health Statistics. Vital Health Stat 1 56:1–37 [PubMed] [Google Scholar]
- 32.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- 33.Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, Wong G, Bennett P, Shaw J, Caprio S, Group IDFC. 2007. The metabolic syndrome in children and adolescents—an IDF consensus report. Pediatr Diabetes 8:299–306 [DOI] [PubMed] [Google Scholar]
- 34.Dekelbab BH, Abou Ouf HA, Jain I. 2010. Prevalence of elevated thyroid-stimulating hormone levels in obese children and adolescents. Endocr Pract 16:187–190 [DOI] [PubMed] [Google Scholar]
- 35.Javed A, Balagopal PB, Vella A, Fischer PR, Piccinini F, Dalla Man C, Cobelli C, Giesler PD, Laugen JM, Kumar S. 2015. Association between thyrotropin levels and insulin sensitivity in euthyroid obese adolescents. Thyroid 25:478–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garduno-Garcia Jde J, Camarillo Romero E, Loe Ochoa A, Romero-Figueroa S, Huitron Bravo G, Torres Garcia R, Montenegro-Morales P, Mendieta-Zeron H. 2015. Thyroid function is associated with insulin resistance markers in healthy adolescents with risk factors to develop diabetes. Diabetol Metab Syndr 7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aeberli I, Jung A, Murer SB, Wildhaber J, Wildhaber-Brooks J, Knopfli BH, Zimmermann MB. 2010. During rapid weight loss in obese children, reductions in TSH predict improvements in insulin sensitivity independent of changes in body weight or fat. J Clin Endocrinol Metab 95:5412–5418 [DOI] [PubMed] [Google Scholar]
- 38.Kaloumenou I, Duntas LH, Alevizaki M, Mantzou E, Chiotis D, Mengreli C, Papassotiriou I, Mastorakos G, Dacou-Voutetakis C. 2010. Gender, age, puberty, and BMI related changes of TSH and thyroid hormones in schoolchildren living in a long-standing iodine replete area. Horm Metab Res 42:285–289 [DOI] [PubMed] [Google Scholar]
- 39.Elmlinger MW, Kuhnel W, Lambrecht HG, Ranke MB. 2001. Reference intervals from birth to adulthood for serum thyroxine (T4), triiodothyronine (T3), free T3, free T4, thyroxine binding globulin (TBG) and thyrotropin (TSH). Clin Chem Lab Med 39:973–979 [DOI] [PubMed] [Google Scholar]
- 40.Mullur R, Liu YY, Brent GA. 2014. Thyroid hormone regulation of metabolism. Physiol Rev 94:355–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agnihothri RV, Courville AB, Linderman JD, Smith S, Brychta R, Remaley A, Chen KY, Simchowitz L, Celi FS. 2014. Moderate weight loss is sufficient to affect thyroid hormone homeostasis and inhibit its peripheral conversion. Thyroid 24:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolters B, Lass N, Reinehr T. 2013. TSH and free triiodothyronine concentrations are associated with weight loss in a lifestyle intervention and weight regain afterwards in obese children. Eur J Endocrinol 168:323–329 [DOI] [PubMed] [Google Scholar]
- 43.Cavallo E, Armellini F, Zamboni M, Vicentini R, Milani MP, Bosello O. 1990. Resting metabolic rate, body composition and thyroid hormones. Short term effects of very low calorie diet. Horm Metab Res 22:632–635 [DOI] [PubMed] [Google Scholar]
- 44.Kiortsis DN, Durack I, Turpin G. 1999. Effects of a low-calorie diet on resting metabolic rate and serum tri-iodothyronine levels in obese children. Eur J Pediatr 158:446–450 [DOI] [PubMed] [Google Scholar]
- 45.Goran MI, Gower BA. 2001. Longitudinal study on pubertal insulin resistance. Diabetes 50:2444–2450 [DOI] [PubMed] [Google Scholar]