Abstract
Right atrial myxomas are rare. Its occurrence in a previously operated patient of rheumatic mitral stenosis posed clinical diagnostic challenge. We herein report a case of right atrial myxoma who had undergone mitral valve repair 20 years ago and now presented in congestive heart failure. The tumor was arising from the ostium of the coronary sinus and prolapsed into the right ventricle causing significant right ventricular inflow and outflow obstruction. Urgent repeat cardiac surgery was successfully performed to remove the tumor along with mitral valve replacement. We review the diagnostic and therapeutic problems resulting from this unusual association.
Keywords: Myxoma, Right atrium, Right ventricle, Mitral valve repair, Cardiac computerized tomography
1. Introduction
Right atrial (RA) myxoma is a rare entity. Its association with rheumatic heart disease especially in a patient previously operated for rheumatic valvular repair or replacement is rarely reported. However the coexistence of right atrial myxoma with unrelated valvular heart disease, though quite uncommon, has been reported sporadically.1, 2, 3 The presentation of this patient posed a clinical diagnostic challenge. An urgent repeat cardiac surgery was carried out to remove the right atrial tumor along with mitral valve replacement. We herein report a case of right atrial myxoma who had undergone mitral valve repair 20 years ago and now presented in congestive heart failure (CHF). We also review the diagnostic and therapeutic problems resulting from this association.
2. Case
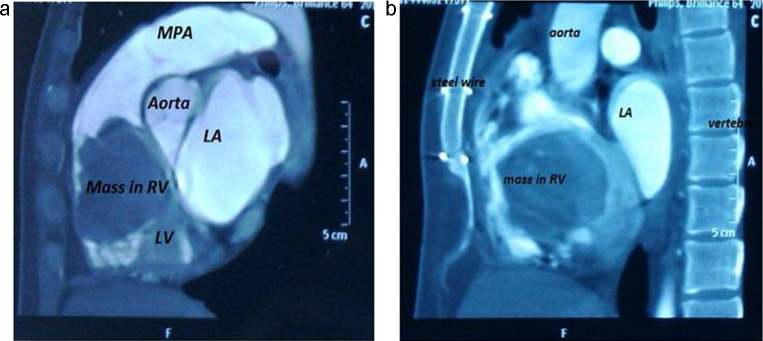
A 36 years old woman was admitted in the emergency department of our hospital with symptoms of congestive heart failure. She was a known patient of rheumatic heart disease for which she had undergone mitral valve repair surgery at the age of 16 years. She was asymptomatic for the last 20 years after her mitral valve repair. She now presented with complaints of dyspnea on exertion and palpitation since last 3 months. On admission she was grossly emaciated, weighing only 25 kg. She was dyspnoeic at rest and could not lie down in supine position. Her blood pressure was 100/70 mmHg and the pulse rate was 96/min, regular. The liver was palpable 2 cm below right costal margin, jugular veins were distended. Bilateral pedal edema was present. On auscultation the first heart sound was soft, pulmonary component of the second heart sound (P2) could not be well appreciated and there was a pan-systolic murmur (PSM) at the apex, radiating to the axilla. No opening snap, other murmurs or any added sound were heard. A chest X-ray showed no cardiomegaly or any significant pulmonary interstitial edema. A two dimensional trans-thoracic echocardiogram (2D Echo) was done which showed a large mass (76 mm × 33 mm) occupying the right atrium and right ventricle. The mass was pedunculated and its stalk was arising from the ostium of the coronary sinus (CS). The mass had heterogenic echogenicity with hypo echoic regions giving an impression of either a right atrial myxoma or a large right atrial thrombus. The mitral valve leaflets were thickened with commissural fusion, significant disease of the sub-valvular apparatus and moderate to severe mitral regurgitation. Cardiac contrast enhanced computed tomography (CECT) was performed to establish the diagnosis which revealed a large hypo attenuated, lobular, heterogeneous right atrial mass (suggestive of internal hemorrhage and infarct), prolapsing through the tricuspid valve into the right ventricle. There was no calcification demonstrated in the right atrial mass. Inferior vena cava and superior vena cava were free from the tumor (Fig. 1). There was no evidence suggestive of renal cell carcinoma on CECT abdominal sections.
Fig. 1.
Contrast enhanced ECG-gated cardiac CT, sagittal multiplanar reconstruction image shows a large predominantly low density mass occupying most of right ventricle with extension into right atrium. Final diagnosis was giant myxoma. RA: right atrium, LA: left atrium, RV: right ventricle, LV: left ventricle, MPA: main pulmonary artery, Steel wire: sternal steel wire from previous surgery.
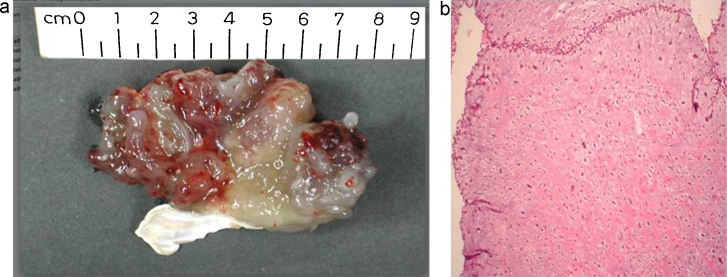
She was taken up for urgent surgery to remove the tumor and replace the mitral valve. The heart was exposed through a repeat median sternotomy. Cardiopulmonary bypass was established by canulating ascending aorta for arterial return and superior vena cava and right femoral vein for venous drainage. Right femoral vein cannulation in place of inferior vena cava was done to avoid any manipulation in the vicinity of the friable mass (to avoid possible tumor fragmentation and embolization). The patient was cooled to 32 °C and after applying aortic cross clamp, antegrade cold blood cardioplegia was delivered in the aortic root. The right atrium was opened through an incision 1 cm parallel to the atrio-ventricular groove after tightening the loops around vena cavae. There was a large lobulated friable mass in the right atrium which was arising from the ostium of the CS and protruding into right ventricle through the tricuspid valve. The tumor mass was excised in toto and base of the stalk was coagulated with diathermy (Fig. 2a). The mitral valve was replaced through the trans- right atrial, trans-septal route with a size 29 SJM (St. Jude Medical, Minneapolis, MN, USA) mechanical prosthesis. The tricuspid valve was tested and found to be competent. The operation was completed uneventfully. The patient made a successful recovery and she was discharged from the hospital on 4th post-operative day. Pathologic examination showed a large globular lobulated mass measuring 7 cm × 5 cm × 3 cm, which was congested, and displayed multiple areas of hemorrhage on cut section. Histopathological examination revealed the classical features of large stellate cells with vacuolated cytoplasm in the myxoid background which is pathognomonic of myxoma (Fig. 2b). The patient is doing well 5 years after surgery with normal prosthetic valve function and there is no evidence of any recurrence of the tumor.
Fig. 2.
(a) Showing resected surgical specimen or RA myxoma in total with partial resection of attaching stalk. (b) Histopathology slide showing sparsely cellular, stellate shaped large cells with vacuolated cytoplasm and myxoid background pathognomonic myxoma.
3. Discussion
Myxomas are the commonest intracardiac tumors. Atrial myxomas account for 35–50% of primary cardiac tumors.4 Approximately 20% of myxomas arise from the right atrium. Right atrial myxomas are typically solitary pedunculated tumors that arise from the fossa ovalis or base of interatrial septum but they can also arise from the atrial wall, and the appendage.5, 6 Myxomas arising from tricuspid and Eustachian valves have also been reported.7, 8 This patient had a right atrial myxoma arising from the ostium of the CS which is unusual and not mentioned in the recent reviews of patients with intracardiac myxoma.9, 10 The tumor occupied most of the right atrial and right ventricular cavity causing significant right ventricular inflow and outflow obstruction. Giant right atrial myxoma occupying most of the right ventricular cavity causing both right ventricular inflow and outflow obstruction is rare. Association of rheumatic heart disease and right atrial tumor is reported uncommonly in literature.1, 2, 3 Mazuz et al. reported left atrial myxoma and concomitant rheumatic mitral stenosis in one patient.1 Pores et al. described a single case of right atrial tumor associated with rheumatic mitral valve disease which obscured the presence of the tumor.2 In our patient it was presumed clinically that she was suffering from recurrence of rheumatic heart disease as she was treated earlier for rheumatic mitral valve stenosis by surgical mitral valve repair. She was admitted for anti-failure treatment and further management.
Echocardiography, computed tomography, and magnetic resonance imaging are important non-invasive diagnostic tools. The diagnosis of myxoma is usually made by two dimensional echocardiography whereas trans esophageal echocardiography provides accurate information in respect of size, exact location, morphological characteristic and the point of attachment.6 After the initial screening echocardiography was done in this patient, the two important differential diagnoses were large atrial thrombus and giant atrial myxoma. Echocardiography features differentiating atrial thrombus from atrial myxomas have been described. Thrombi are often irregular, laminated, and immobile with a broad base attached to the posterior atrial wall usually associated with valvular stenosis whereas myxomas are characterized by mobility and presence of stalk.2 On CECT atrial myxoma and thrombi can sometimes be differentiated by their distinguishing features of size, origin, shape, mobility and prolapse. Attenuation coefficient and/or the presence of calcification are not useful discriminating features.11, 12 The presence of a stalk and mobility favors atrial myxoma. Magnetic resonance imaging (MRI) is more sensitive than Echocardiography or CECT for differentiating atrial myxoma from atrial thrombus.11, 12 The clinical features of myxomas are determined by their location, size and mobility. Most patients present with one or more of the triad of embolism, intra-cardiac obstruction and constitutional symptoms.13 Right atrial myxomas can present with a variety of signs and symptoms of right ventricular failure, tricuspid stenosis, constrictive pericarditis or pulmonary embolism.13, 14 Sometimes they may present with a syncopal attack or sudden cardiac death due to tricuspid valve obstruction. Peculiar location and size of the tumor may pose specific surgical problems. One of the important concerns is tumor embolization (fragmentation due to manipulation) and pulmonary thromboembolism during venous cannulation (inferior vena cava). To minimize this complication we avoided direct cannulation of the inferior vena cava for venous return during cardiopulmonary bypass.
Tumor recurrence is a real problem and to prevent it, radical full thickness excision of the tissue is desirable along with the tumor pedicle which is easily performed when the tumor arises from the free atrial wall or fossa ovalis. Unfortunately full thickness excision was not possible in our case as the tumor was arising from the ostium of CS. The tumor pedicle was excised partially and base of the stalk was desiccated using bipolar electrocoagulation diathermy. Periodic non-invasive monitoring of such patient is mandatory for detection of recurrence. Surgical removal of the tumor along with full thickness excision of the point of attachment is usually curative in non-familial group of myxomas. Additional surgery for tricuspid valve and mitral valve may be required as large tumors may make the valves incompetent. Though the annulus of the tricuspid valve was dilated, there was good coaptation of the leaflets. There was no tricuspid regurgitation in this patient despite the valve being compromised by the bulk of the tumor. The mitral valve was severely diseased due to recurrence of rheumatic activity; hence it was replaced with a mechanical prosthesis. Regular follow-up with screening echocardiography is mandatory for recurrences especially in cases where full thickness excision was not possible. The patient is doing well five years after surgery with a normally functioning prosthetic valve and without any recurrence of the tumor.
4. Conclusion
We report a rare coexistence of a giant right atrial myxoma and rheumatic heart disease, mimicking recurrence of mitral valve disease in a previously operated patient for rheumatic mitral valve stenosis. The tumor was occupying most of the right ventricular cavity causing significant inflow and outflow obstruction. There were mainly two differential diagnoses namely a large atrial thrombus and a giant atrial myxoma after the initial screening echocardiography. Surgery followed by histopathological examination confirmed the final diagnosis. She had a smooth post-operative recovery and is currently leading a normal life 5 years after redo-surgery. Follow up echocardiography at 5 years showed no recurrence of tumor, trivial tricuspid valve regurgitation, normal prosthetic valve and biventricular function.
Conflicts of interest
The authors have none to declare.
Acknowledgement
Our sincere thanks to Dr. Narendra Krishnani, Professor, Dept. of Pathology, Dr. Zaffar Neyaz, Associate Professor, Dept. of Radiology, Sanjay Gandhi Post graduate institute of Medical sciences, Lucknow, for their valuable input in the patient management.
References
- 1.Mazuz M., Pandian N., Kerber R. Left atrial myxoma and unrelated mitral valve disease. J Am Coll Cardiol. 1983;1(4):1170–1173. doi: 10.1016/s0735-1097(83)80123-5. [DOI] [PubMed] [Google Scholar]
- 2.Pores I.H., Abel R.M., Gray L., Jacobs G.P. Giant right atrial myxoma with rheumatic mitral valve disease. Angiology. 1984;35(5):313–319. doi: 10.1177/000331978403500508. [DOI] [PubMed] [Google Scholar]
- 3.Seagle R.L., Nomeir A.M., Watts L.E. Left atrial myxoma associated with rheumatic mitral stenosis. Clin Cardiol. 1984;7(6):370–372. doi: 10.1002/clc.4960070609. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell M.C., Boitnott J.K., Kaufman S., Cameron J.L., Maddrey W.C. Budd-Chiari syndrome: etiology, diagnosis and management. Medicine (Baltimore) 1982;61(4):199–218. doi: 10.1097/00005792-198207000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Burakovsky V.I., Tuckerman G.I., Kossatch G.A., Golossovskaya M.A., Javorskaya L.A. Surgical treatment of cardiac myxomas. J Thorac Cardiovasc Surg. 1988;96(5):800–805. [PubMed] [Google Scholar]
- 6.Reynen K. Cardiac myxomas. N Engl J Med. 1995;333(24):1610–1617. doi: 10.1056/NEJM199512143332407. [DOI] [PubMed] [Google Scholar]
- 7.Kuroda H., Nitta K., Ashida Y., Hara Y., Ishiguro S., Mori T. Right atrial myxoma originating from the tricuspid valve. J Thorac Cardiovasc Surg. 1995;109(6):1249–1250. doi: 10.1016/S0022-5223(95)70211-3. [DOI] [PubMed] [Google Scholar]
- 8.Teoh K.H., Mulji A., Tomlinson C.W., Lobo F.V. Right atrial myxoma originating from the Eustachian valve. Can J Cardiol. 1993;9(5):441–443. [PubMed] [Google Scholar]
- 9.Chitwood W.R., Jr. Cardiac neoplasms: current diagnosis, pathology, and therapy. J Card Surg. 1988;3(2):119–154. doi: 10.1111/j.1540-8191.1988.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 10.Silverman N.A. Primary cardiac tumors. Ann Surg. 1980;191(2):127–138. doi: 10.1097/00000658-198002000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araoz P.A., Mulvagh S.L., Tazelaar H.D., Julsrud P.R., Breen J.F. CT and MR imaging of benign primary cardiac neoplasms with echocardiographic correlation. Radiographics. 2000;20(5):1303–1319. doi: 10.1148/radiographics.20.5.g00se121303. [DOI] [PubMed] [Google Scholar]
- 12.Scheffel H., Baumueller S., Stolzmann P. Atrial myxomas and thrombi: comparison of imaging features on CT. AJR Am J Roentgenol. 2009;192(3):639–645. doi: 10.2214/AJR.08.1694. [DOI] [PubMed] [Google Scholar]
- 13.Wold L.E., Lie J.T. Cardiac myxoma. A clinicopathological profile. Am J Pathol. 1980;101(1):219–240. [PMC free article] [PubMed] [Google Scholar]
- 14.Emanuel R.W., Lloyd W.E. Right atrial myxoma mistaken for constrictive pericarditis. Br Heart J. 1962;24(6):796–800. doi: 10.1136/hrt.24.6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]