Abstract
Sickle cell disease (SCD) is a blood disease caused by a single nucleotide substitution (T > A) in the beta globin gene on chromosome 11. The single point mutation (Glu6Val) promotes polymerization of hemoglobin S (HbS) and causes sickling of erythrocytes. Vaso-occlusive painful crises are associated with recurrent and long-term use of analgesics/opioids and hydroxyurea (HU) by people living with SCD. The present analysis offers a state-of-the-art expert review of the effectiveness of pharmacogenomics/genetics of pain management in SCD, with specific focus on HU and opioids. The literature search used the following keywords: SCD, pharmacogenomics, pharmacogenetics, pain, antalgics, opioids, morphine, and HU. The literature was scanned until March 2016, with specific inclusion of targeted landmark and background articles on SCD. Surprisingly, our review identified only a limited number of studies that addressed the genetic/genomic basis of variable responses to pain (e.g., variants in OPRM1, HMOX-1, GCH1, VEGFA COMT genes), and pharmacogenomics of antalgics and opioids (e.g., variants in OPRM1, STAT6, ABCB1, and COMT genes) in SCD. There has been greater progress made toward identifying the key genomic variants, mainly in BCL11A, HBS1L-MYB, or SAR1, which contribute to response to HU treatment. However, the complete picture on pharmacogenomic determinants of the above therapeutic phenotypes remains elusive. Strikingly, no study has been conducted in sub-Saharan Africa where majority of the patients with SCD live. This alerts the broader global life sciences community toward the existing disparities in optimal and ethical targeting of research and innovation investments for SCD specifically and precision medicine and pharmacology research broadly.
Introduction
Sickle Cell Disease (SCD) is a multisystem disease, which is associated with episode pain (chronic and acute illness) and organ damage, and commonly occurs in sub-Saharan African countries. SCD is a genetic blood disease caused by a single nucleotide substitution (T > A) in the beta globin gene on chromosome 11 (Brousseau et al., 2007). The resulting HbS leads to polymerization and precipitation of hemoglobin during deoxygenation or dehydration. This results in sickling of red blood cells, abnormal adhesion of leukocytes and platelets, inflammation, hemolysis, and hypercoagulation, which could lead to vaso-occlusive crisis and hypoxia and ultimately organ damage (Bartolucci and Galacteros, 2012).
There is a strong association between the frequency of the HbS mutation and endemicity of malaria (Charache et al., 1995; Williams et al., 2005). It is estimated that 305,800 babies are born each year with SCD worldwide with nearly 75% of the births occurring in sub-Saharan Africa (SSA) (Piel et al., 2013). However, as a result of migration, there is a reported increasing burden of SCD in other countries where it was not initially prevalent, such as South Africa (Wonkam et al., 2012), Ireland (Gibbons et al., 2015), Italy (Colombatti et al., 2013), Germany (Kunz et al., 2015; Zur, 2016), England (Pizzo et al., 2015), and France (Dzierzynski et al., 2016), with, for example, 1300–2600 affected newborns annually in France. SCD is now an accepted worldwide health problem and comparable with other major global noncommunicable diseases such as diabetes and hypertension (Weatherall and Clegg, 2008).
Despite the high incidence, there is currently no effective public health program in any SSA country focused on SCD (Rahimy et al., 2009; Tekola-Ayele and Rotimi, 2015; Wonkam et al., 2014b). As a consequence, up to 90% of infants with SCD in SSA are believed to die by the age of 5 years (Grosse et al., 2011; Makani et al., 2013). While there have been recent efforts in selected African countries to implement newborn screening (McGann et al., 2013; Rahimy et al., 2009; Tshilolo et al., 2009; Tubman et al., 2016), to use hydroxyurea (HU) more frequently (Makubi et al., 2012; Olabode and Shokunbi, 2006; Ware, 2013), and to initiate genetic studies (Cox et al., 2014; Mmbando et al., 2015; Mtatiro et al., 2014; Pule et al., 2015; Rumaney et al., 2014; Wonkam et al., 2014a, 2014b, 2014c), there is still a lack of integration and coordination of these emerging research efforts.
In sharp contrast to SSA, comprehensive clinical care programs have reduced SCD-related premature childhood deaths by 70% in high-income nations such as the United State of America (Vichinsky, 1991; Yanni et al., 2009). This evidence from the West indicates that the institution of interventions such as newborn screening and penicillin prophylaxis can reduce the horrendous disease burden in SSA (Rahimy et al., 2003). Therefore, there is a major need for research to help develop effective therapies across the life span of SCD patients in all parts of the world (Chaturvedi and DeBaun, 2016; Hamideh and Alvarez, 2013), including the incorporation of personalized medicine and pharmacogenomics.
Indeed, environmental and multiple genetic factors influence many pathophysiological aspects of SCD that contribute to a highly variable clinical expression in individual patients. Fetal hemoglobin (HbF) has emerged as a central disease modifier and genetic variants at three principal loci, BCL11A, HBS1L-MYB, and HBB cluster, which account for 10–20% of HbF variation among SCD patients in USA, Brazil, and the United Kingdom (Lettre et al., 2008; Thein and Menzel, 2009). These studies have been replicated in patients living with SCD in Tanzania and Cameroon (Makani et al., 2011; Mtatiro et al., 2014; Pule et al., 2015; Wonkam et al., 2014a). Interestingly, the expression of these modifiers is amenable to therapeutic manipulation (Bukar et al., 2013; Canver et al., 2015; Xu et al., 2011), leading to new hope for treatment routes for SCD (Orkin, 2016).
HU is the only Food and Drug Administration (FDA)-approved treatment of SCD in adults and children (Shenoy, 2011). HU is a ribonucleotide reductase inhibitor that increases the fetal hemoglobin level, a known ameliorator of the disease. Patients respond differently to HU due to genetic variations (Bockaert and Pin, 1999; Charache et al., 1995; Steinberg et al., 1997; Zimmerman et al., 2004).
Nevertheless, the common medications used by SCD patients are antalgics to manage pain. Pain in SCD is classified as acute, chronic, and mixed pain, which varies in severity (Ballas, 2015; Ballas et al., 2012; Steinberg et al., 2010). Genetic differences are suggested to be the reason for interindividual variability in pain perception and experience and variable responses to anti-inflammatory (Chou et al., 2006) and opioid drugs (Chou et al., 2006). Individuals who are homozygous for 118A>G polymorphism in the OPRM1 (a major site of action for most opioid analgesics) have more pain and need more morphine to subdue the pain (Klepstad et al., 2004). Single-nucleotide polymorphisms (SNPs) in the COMT gene affected pain sensitivity and with low COMT activity lead to increased levels of norepinephrine and epinephrine, which resulted in more pain sensitivity (Slade et al., 2007).
The aim of the present analysis was to provide an expert literature review of the effectiveness of pharmacogenomics/genetics for pain management in SCD, with specific focus on pharmacogenetics/pharmacogenomics of pain, HU, and opioids.
Methods
A comprehensive literature search was conducted by the authors covering the subject until March 2016, with specific addition of landmark and background articles on SCD published articles. We used the PubMed® (National Library of Medicine), Medline®, and Google Scholar®. Keywords included individual use or a combination of the following: “Pharmacogenomics,” “Pharmacogenetics,” “Hydroxyurea,” “Sickle Cell Disease,” “Pain,” “Painkillers,” and “Morphine” and “Opioids.” Additionally, specific expert authors' names that are active in the field of SCD and its therapeutics were also used to complement the literature searches.
Selection criteria
The inclusion criteria were confined to articles written in English, with major emphasis being focused on research articles and review articles describing pharmacogenomics of pain, particularly on SCD patients, and effectiveness of pharmacogenomics of drug therapies for HU and pain management. Prior knowledge of research groups working on HU, pain episodes, and SCD in Africa globally further facilitated the identification and selection of research articles. Only available full-length articles, in English, with the use of “HU,” “Painkillers,” “Morphine,” and “Opioids” were selected. In cases where multiple studies reported a similar pathway, the most recent report with the most detailed associations' studies was included. The main search was conducted, separately, by an MSc student and a PhD student (First and Second authors) in Human Genetics working on SCD (to maximize the inclusion of potentially relevant articles) and reviewed successively by a medical geneticist and a human geneticist, with expertise in SCD and pharmacogenomics (Fourth and Third authors), respectively.
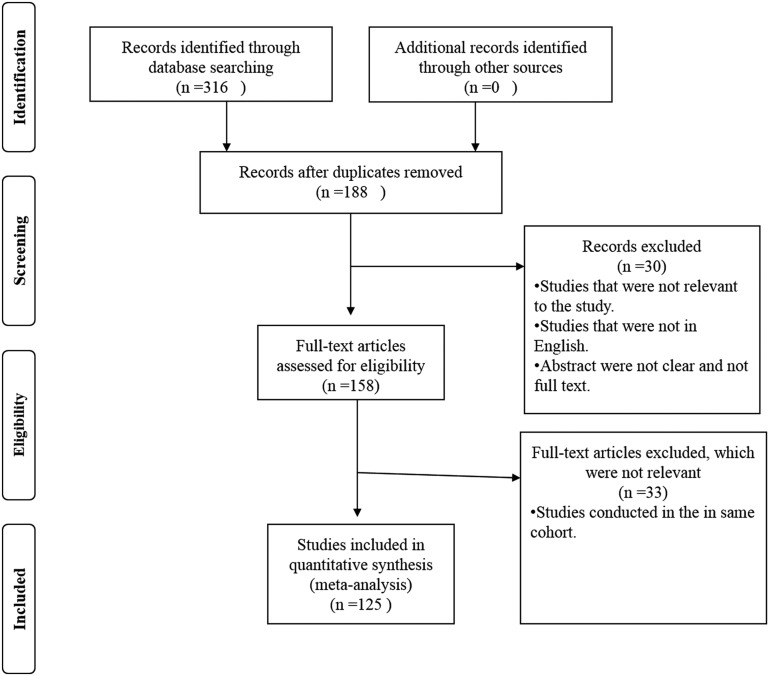
A total of 316 articles were consulted after the search from Google Scholar (of which 47 were from PubMed); exclusion criteria were performed based on the article title and its relevance to the scope of the review; additional study performed on the same cohort for the same experiment; and studies that were not clearly stated were excluded. Subsequently, 158 articles were fully retrieved and their abstract and result sections perused for further elimination, of which a final total of 125 articles were selected for inclusion in the review (Fig. 1 and Supplementary Table S1).
FIG. 1.
Flowchart of the literature review employed in the present expert review.
Data collection
Data were collected using an extraction form to summarize the following information: type of study, year of publication, patients' sample, study country, title, and author names (Supplementary Table S1).
Results
Pharmacogenomics of pain susceptibility in SCD
Acute pain acts as a protective mechanism in response to tissue injury (Ballas and Lusardi, 2005; Bergman, 2005) and can worsen and prolong to a chronic state, which results in mixed pain. Chronic pain persists longer than acute pain (Todd, 2005; Todd et al., 2006). Chronic pain can result in psychopathology disorders such as depression, anxiety, and personality disorder (Dersh et al., 2002), which is called chronic pain syndrome (Knorring, 1989). Chronic Pain in SCD has a direct impact on the quality of life of patients (Kanter and Kruse-Jarres, 2013; Platt et al., 1991; Rees et al., 2010).
A few studies have been conducted to establish the difference in pain perception and response to opioids (Stamer and Stuber, 2007). It was found that genomic variations influence both perception and vulnerability to chronic pain (Mogil, 2004; Mogil and Devor, 2004; Stamer and Stuber, 2007). Furthermore, SNPs of specific genes were associated with variable degrees of pain perception (Diatchenko et al., 2005), leading to the hypothesis that some variants were located in genes related to the inflammatory process of vaso-occlusive painful crises, resulting in nerve and tissue damage and thus the development of secondary pain (Mogil, 2004). Table 1 summarizes the selected genes that have been associated with pain susceptibility in SCD.
Table 1.
Genomic Variants that Influence Pain in Sickle Cell Disease
| Gene | SNPS | Chromosomes locus | Association | References |
|---|---|---|---|---|
| OPRM1 | rs1799971 | 6:154039662 | Pain | Joly et al. (2012); Jhun et al. (2015) |
| HMOX-1 | A(GT) VNTR | Chromosome 22 | Vaso-occlusive crises | Bean et al. (2013) |
| GCH1 | rs8007267 | 14:54912273 | Pain | Belfer et al. (2014) |
| COMMD7 | rs614803 | 18:79389574 | Painful crisis | Galarneau et al. (2013) |
| GSTM1 | GSTM1 null allele | Chromosome 1 | Severe vaso-occlusive crisis | Shiba et al. (2014) |
| MTHFR | rs1801133 (C677T) | 1:11796321 | Pain | Nishank et al. (2013) |
| FVL | rs6025 (G1691A; R506Q) | 1:169549811 | Pain | Nishank et al. (2013) |
| VEGFA | rs833068 (G398A) | 6:43774790 | Vaso-occlusive crisis | Al-Habboubi et al. (2012) |
| VEGFA | rs2010963 | 6:43770613 | Vaso-occlusive crisis | Al-Habboubi et al. (2012) |
| VEGFA | rs3025020 | 6:43781373 | Vaso-occlusive crisis | Al-Habboubi et al. (2012) |
| CYP2D6 | rs1065852 | 22:42130692 | Pain and drug metabolism | Joly et al. (2012); Jhun et al. (2015) |
| COMT | rs4633 | 22:19962712 | Pain | Joly et al. (2012); Jhun et al. (2015) |
| rs6269 | 22:19962429 | Pain | ||
| rs737865 | 22:19942598 | Pain | ||
| CPY3A | rs1057868 | 7:75985688 | Pain | Joly et al. (2012); Jhun et al. (2015) |
| UGTB7 | rs1799971 | 6:154039662 | Pain | Joly et al. (2012); Jhun et al. (2015) |
| ABCB1 | rs1045642 | 7:87509329 | Pain | Jhun et al. (2015) |
HMOX-1 codes for heme oxygenase-1, which is a rate-limiting step in the catalysis of heme. It exhibits a GT dinucleotide repeat in the promoter region, and long repeat lengths (>25 repeats) are associated with decreased activity and inducibility, and therefore higher rates of SCD patient hospitalization, but not directly associated with pain (Bean et al., 2013). It is reported that among African-Americans, a polymorphism in the GTP cyclohydrolase (GCH1) on chromosome 14 (rs8007267) is significantly associated with pain crises (Belfer et al., 2014). GCH1 catalyzes the rate-limiting step for tetrahydrobiopterin synthesis, thus variation in its gene is likely to have pathophysiological roles in pain. Acute pain has been a subject of some studies with the most relevant to SCD referring to an SNP (rs614803) located in a region about 8 kb from the COMM domain-containing protein COMMD7. This polymorphism is significantly associated with painful crises.
COMMD7 modulates many proteins and is associated with NF-kappa-B complex, suppressing its transcriptional activity (Galarneau et al., 2013). Investigations among Egyptians reported the GSTM1 null allele to be significantly associated with increased risk of severe vaso-occlusive crises (Shiba et al., 2014). GSTM1 is located on chromosome 1 and catalyzes the addition of glutathione on molecules to increase the antioxidant status, while the GSTM1 null refers to deletion of this gene. A higher incidence of pain was observed among SCD patients who were carriers of the methylenetetrahydrofolate reductase (MTHFR; C677T) polymorphism as well as Factor V Leiden (FVL; G191A) polymorphism (Nishank et al., 2013). The vascular endothelial growth factor gene (VEGFA) has several mutations of which three, rs2010963, rs833068, and rs3025020, have been associated with vaso-occlusive crisis when inherited in a homozygous state (Al-Habboubi et al., 2012).
Morphine metabolism
Morphine is a member of the opioid family and is mostly used because it is globally available and shows successful clinical efficacy (Adegbola, 2009). Morphine is derived from codeine through the action of CYP2D6-catalyzed demethylation. Through the actions of UDP-glucuronosyltransferases, 2B7 and 1A1 (UGT2B7 and UGT1A1), morphine is converted to morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G) through glucuronic acid conjugation. Ultimately, the glucuronidated morphine is effluxed by transporters such as ABCB1, ABCC2, ABCC3, and SLC01B1. M6G is responsible for analgesia contribution by binding to μ-opioid receptor; there are arguments about the role of M6G in analgesia that results from morphine (Höllt, 2002; Murthy et al., 2002; Osborne et al., 1990; Smith et al., 1990). M3G has small pull force for opioid receptors (Smith et al., 1990) and it might be responsible for the excitatory effect of morphine (Smith et al., 1990). Blood plasma concentration of morphine and its metabolites is a function of morphine dose and renal clearance, which might be affected by genetic variations, and it is therefore anticipated that variants in the above genes could be associated with variable response to the drug treatment in patients living with SCD. This is supported by evidence from a population study, indicating that the allele variation in genes that are involved in morphine mechanism might regulate the response of opioid analgesic (Lotsch and Geisslinger, 2006).
Genetic variations and morphine metabolism
Patients respond differently to drugs due to variations in genes coding for metabolizing enzymes (Table 2). UGT2B7 (rs7438135), OPRM1 (rs1799971), and ABCB1 (rs1045642) influence the pharmacokinetic and pharmacodynamic measurements and affect the clinical effectiveness of morphine (Adegbola, 2009). COMT (rs4633) is not directly involved in the metabolism, but can improve the productivity of morphine. This can occur by influencing μ-opioid receptors and its concentration in different areas of the brain by affecting the neuronal activity; with reduction in COMT activity then resulting in sensitivity to pain and morphine (Bockaert and Pin, 1999; Bohn et al., 1999; Kraus et al., 2001; Loh et al., 1998; Matthes et al., 1996; Meineke et al., 2002; Rakvåg et al., 2005; Weinshilboum and Raymond, 1977; Zubieta et al., 2003). Individuals who have the lowest COMT activity (met/met variant) have higher sensory and higher effective rates of pain, as well as a more effective state, as the met/met variant reduces the ability to activate the μ-opioid receptor system (Zubieta et al., 2003). This also causes upregulation of the opioid receptors and low concentrations of morphine are required to produce sufficient analgesia to ease the pain (Rakvåg et al., 2005).
Table 2.
Genetic Variants Associated with Morphine Metabolism
| Gene | SNPS | Chromosome locus | Effect of variant allele | References |
|---|---|---|---|---|
| UGT2B7 | rs7438135 | 4:69095621 | Drug metabolism | Höllt (2002); Duguay et al. (2004) |
| OPRM1 | rs1799971 | 6:154039662 | Pain and mediates analgesic effect of morphine. | Lötsch et al. (2002); Zubieta et al. (2003); Jhun et al. (2015) |
| ARRB2 | rs1045280 | 17:4719343 | Drug metabolism | Ross et al. (2005) |
| STAT6 | rs167769 | 12:57109992 | Drug metabolism | Ross et al. (2005); Jhun et al. (2015) |
| rs841718 | 12:57099213 | Drug metabolism | ||
| rs3024971 | 12:57099944 | Drug metabolism | ||
| COMT | rs4633 | 22:19962712 | Drug metabolism and pain | Zubieta et al. (2003); Jhun et al. (2015) |
| ABCB1 | rs1045642 | 7:87509329 | Responsible for analgesia | Meineke et al. (2002); Jhun et al. (2015) |
ABCB1, which is also known as the MDR1 transporter gene (Weinshilboum and Raymond, 1977), contributes to the variability in morphine metabolism to produce analgesia by moving the efflux of morphine and M6G across the blood–brain barrier (Darbari et al., 2008). OPRM1 is the major site of action for most opioid analgesics, including morphine (Adegbola, 2009; Beyer et al., 2004; Lotsch and Geisslinger, 2006). This gene is responsible for both pain response and opioid addiction (Adegbola, 2009; Compton et al., 2003). Each individual has different responses to morphine due to polymorphisms in OPRM1, which affect the functioning and expression of the binding site (Adegbola, 2009; Chou et al., 2006; Klepstad et al., 2004; Lotsch and Geisslinger, 2006; Mantione et al., 2005; Stamer and Stuber, 2007); and OPRM1 has two SNPS; A118G and C17T, with A118 being the one that is a commonly identified SNP (Adegbola, 2009; Bond et al., 1998). There is therefore an urgent need to explore the knowledge on pharmacogenomics on morphine metabolism among the population of people affected by SCD.
Pharmacogenomics of HU
HU is the only available treatment for induction of HbF in patients living with SCD that has been approved by both the FDA in 1998 and by the European Medicines Agency in 2007. It was also mentioned as an effective treatment for both adult and children with SCD by the National Institutes of Health (Officer of Medical Applications of Research) (NIH-OMAR) and the Agency of Healthcare Research and Quality (AHRQ) (Herrick, 2000; Loh et al., 1998; Weatherall et al., 2005). HU is an oral, S-phase-specific cytotoxic, antimetabolic, and antineoplastic drug treatment. It is a strong inhibitor of a universal enzyme called ribonucleotide reductase (Elford, 1968; Modell and Darlison, 2008). In 1984, the first clinical application of HU in hemoglobinopathies successfully demonstrated a swift and vivid increase in HbF concentration within immature red blood cells called reticulocytes (Platt et al., 1984).
Besides increasing HbF, HU also plays an important clinical role by increasing the concentration of hemoglobin and simultaneously decreasing white blood cells, absolute neutrophil count, absolute reticulocyte count, and platelets (Charache et al., 1992; de Montalembert et al., 2006; Kinney et al., 1999; Thornburg et al., 2009; Zimmerman et al., 2004). Treatment of HU is associated with a decrease in the frequency of pain episodes, acute chest syndrome, hospitalization, and the need for a blood transfusion (Charache et al., 1995).
The reduction of the clinical phenotype results in increase of efficiency in survival rates and life expectancy among SCD patients (Nagel et al., 1985; Voskaridou et al., 2010; Zago et al., 2000). It may also provide protection against cerebrovascular disease (Zimmerman et al., 2007), long-term drug safety, capacity to prevent organ damage, and reduced morbidity and mortality in school-age children (Kinney et al., 1999), toddlers (Hankins et al., 2005; Thornburg et al., 2009), and infants (Alvarez et al., 2012). HU also helps with related complications of SCD such as stroke prevention, priapism, and pulmonary hypertension (DeBaun, 2014). Maximum tolerated dose for various phenotypes was observed to be different for patients using HU, showing that patients respond differently to HU (Charache et al., 1992; Heeney and Ware, 2008; Ware et al., 2011).
Genetic variation in HU treatment response
Induced HbF levels range from 10% to greater than 30% (Kinney et al., 1999; Zimmerman et al., 2004) among patients with SCD, highlighting the variation in response to HU. This is due to pharmacogenomic interactions (Steinberg et al., 2003). Previous studies have shown that haplotypes in the HBB gene cluster that are associated with SCD could possibly affect the clinical response to HU, likely refereed by their genetically determined effect on the HbF level (Adekile, 2011; Friedrisch et al., 2008). XMNL-HHBG2 (rs7482144) is associated with high level of HbF in response to HU drug treatment in both SCD and β-thalassemia individuals (Alebouyeh et al., 2004; Dixit et al., 2005; Yavarian et al., 2004). Research provides some evidences that the effect of HU on HbF level could act through other HbF-promoting loci such as BCL11A (Ware et al., 2011). BCL11A is central to the fetal switch. It is coexpressed with SOX-6 as well as directly interacting and co-occupying the β-globin loci. It also has an association with the Mi-2/nucleosome remodeling and deacetylase (NuRD) complex for long-range reconformation of the β-globin cluster for the transcriptional silencing of γ-globin (Xu et al., 2010).
Besides BCL11A, from DNA structural alteration to sequence modification, the secretion-associated and ras-related protein (SAR-1) has been shown to play a significant role in γ-globin regulation (Zhu et al., 2014) and three SNPs in the SAR-1a promoter sequence have been associated with HbF level in the peripheral blood of SCD patients on HU (Kumkhaek et al., 2008). In addition, in relation to HU responses, it was reported that 17 SNPs are associated with HbF and 20 SNPs with response to HU (Solovieff et al., 2010). It was shown that the absence of KLF10 (rs3191333) was found to be significantly associated with induction of HbF level in β-thalassemia intermedia compared with the majority patients with β-thalassemia and healthy individuals (Borg et al., 2012). Additional variants, which have been less consistently associated with HU-induced HbF level, are summarized in Table 3.
Table 3.
Genomic Variants Associated with Hydroxyurea-Induced HbF Level
| Gene | SNPs | Chromosome: locus | References |
|---|---|---|---|
| HBB | rs7482144 | 11:5254939 | Friedrisch et al. (2008); Adekile (2011) |
| BCL11A | rs1427407 | 2:60490908 | Ware et al. (2011); Ware (2013); Friedrisch et al. (2008); Adekile (2011) |
| rs4671393 | 2:60491212 | ||
| rs7606173 | 3:60493111 | ||
| rs7557939 | 2:60494212 | ||
| rs1186868 | 2:61764103 | ||
| ARG1/2 | rs2295644 | 14:67599842 | Friedrisch et al. (2008); Adekile (2011) |
| rs17599586 | 6:131583579 | ||
| rs28384513 | 6:135055071 | ||
| HBS1L-MYB | rs9399137 | 6:135097880 | Friedrisch et al. (2008); Adekile (2011) |
| SAR1 | rs2310991 | 3:142444839 | Kumkhaek et al. (2008); Zhu et al. (2014) |
| rs4282891 | 10:70171890 | ||
| rs76901216 | 10:70170313 | ||
| SALL2 | rs61743453 | 14:21523209 | Sheehan et al. (2013) |
| FLT1 | rs2182008 | 13:28412924 | Ma et al. (2007) |
| rs8002446 | 13:28423263 | ||
| rs9319428 | 13:28399484 | ||
| rs3751395 | 13:28384818 | ||
| rs2387634 | 13:28416291 | ||
| TOX | rs826729 | 8:58826354 | Ma et al. (2007) |
| rs765587 | 8:58878344 | ||
| rs9693712 | 8:59034864 | ||
| rs172652 | 8:59045582 | ||
| rs380620 | 8:59069973 | ||
| rs2693430 | 8:58812489 | ||
| rs12155519 | 8:58936271 | ||
| ARG2 | rs10483801 | 14:67650289 | Ma et al. (2007) |
| rs10483802 | 14:67650704 | ||
| NOS1 | rs816361 | 12:117217326 | Ma et al. (2007) |
| rs7977109 | 12:117292535 | ||
| rs7309163 | 12:117291469 | ||
| NOS2A | rs1137933 | 17:27778906 | Ma et al. (2007) |
| rs944725 | 17:27782545 | ||
| MAP3K5 | rs9376230 | 6:136781227 | Ma et al. (2007) |
| rs9483947 | 6:136784262 | ||
| PDE7B | rs11154849 | 6:136032167 | Ma et al. (2007) |
| rs9376173 | 6:136038308 | ||
| rs1480642 | 6:136178390 | ||
| rs487278 | 6:136180690 | ||
| HAO2 | rs10494225 | 1:119375480 | Ma et al. (2007) |
| KLF10 | rs3191333 | 8:102649991 | Borg et al. (2012) |
SNP, single-nucleotide polymorphism.
Discussion
There are emerging data summarized in the present article that indicate that genetic differences in SCD individuals influence the sensitivity to pain (Table 1). There are also a few studies indicating variability in analgesic response that is produced by morphine treatment with some considerable overlap with variants in genes also associated with pain sensitivity (Table 2). However, there are limited data on genetic interindividual variants and responses to morphine treatment in SCD that is directly associated with morphine metabolism. Surprisingly, there are very few data on pharmacogenetics of antalgics and analgesics and specifically opioids used in managing SCD and no data from SSA. Understanding the pharmacogenomics of pain medication in SCD could potentially improve personalized medicine and explore new routes for therapeutic intervention.
Besides antalgics and analgesics, HU drug treatment, which is prescribed for SCD patients, has produced successful results in both children and adults by decreasing pain, blood transfusions, and hospitalization. There is more consistent evidence of the association between several SNPs and HbF levels in response to HU treatment (Table 3). Again, none of the studies were conducted in SSA where the disease burden is highest, further supporting a call for action if the wide use of HU is to be implemented in Africa. Fortunately, there are emerging clinical data from multiple sites on the implementation of HU in Africa in an effort to close this gap, and they have taken the opportunity to perform association studies that could hopefully provide new insight into pharmacogenomics of HU in SCD.
Expert Commentary
Vaso-occlusive painful crises are the main clinical events of SCD and are associated with recurrent and long-term use of antalgics/opioids and HU. The present article has provided evidence of the scarcity of studies investigating the variable response to pain in SCD patients. More consistent studies have addressed the various mechanisms to understand genomic variation affecting the response to HU, but the full understanding of the variable HU-mediated HbF production among individuals affected by SCD remains elusive. Therefore, more research is needed to understand their various mechanisms and pharmacogenomics of both painkiller/opioids and HU to improve the management of people living with SCD.
Five-Year View
The global burden of SCD is anticipated to increase due to an increase in the life expectancy of people living with SCD in the West as well as in Africa. This is due to the emerging implementation of newborn screening and the use of HU treatment and the global migration that is associated with the increase in SCD incidence in countries where this condition was not initially prevalent. The improvement in the treatment of SCD will continue to contribute toward an increase in the global burden of the disease as well as dependency on chronic medications. Therefore, it is expected that most patients living with SCD will have access to pain and HU treatment worldwide, including in SSA. Thus, it could be anticipated in the coming years to observe more global interest in the field of pharmacogenetics of SCD, especially for antalgics and HU.
It is expected that future studies will also give potential explanations regarding the regulatory mechanism level of these drugs and associated gene expression, which could reveal additional pathways to explore novel therapeutic interventions that could maximize benefits while avoiding side effects. As the level of science advances, it is also suspected that there will be more medications that will be developed that are outside HbF induction, for example, to induce stress hematopoiesis, endothelial nitric oxide release, the reduction of leucocyte counts, the reduction of red blood cell adhesion to the endothelium, the reduction in inflammation processes, or medication aiming to reduce blood viscosity to name a few.
There are also topics of SCD pharmacogenomics in need of more research that have not been discussed in the current article, such as those related to recurrent blood transfusions and associated immunogenic issues, and chronic use of antibio-prophylaxy with penicillin in SCD. More research on pharmacogenomics in various aspects of treatment of SCD will result, hopefully, in a complete profile and possible algorithm that could be usable for a successful personalized medicine in SCD.
Key Issues
• Vaso-occlusive painful crises are associated with the recurrent pain and long-term use of antalgics/opioid by people living with SCD. Surprisingly, the present article has provided evidence of limited number of studies to understand the variable responses to pain and pharmacogenomics of antalgics and opioids in people living with SCD.
• There has been great progress made toward understanding and identifying key genomic variants in BCL11A, HBS1L-MYB, or SAR1 that predispose the response to the HU treatment; however, the complete picture remains elusive.
• The global burden of SCD is anticipated to increase due to increase of the life expectancy of patients in the West, emerging implementation of newborn screening and the use of HU treatment in Africa, and the global migrations. Therefore, it could be anticipated to see, in the coming years, more global interest in the field of pharmacogenetics of SCD.
• Strikingly, no study has been conducted in SSA where majority of the patients with SCD live. This alerts the broader global life sciences community toward the existing disparities in optimal and ethical targeting of research and innovation investments for SCD specifically and precision medicine and pharmacology research broadly.
Supplementary Material
Abbreviations Used
- FDA
Food and Drug Administration
- HbS
hemoglobin sickle
- HU
hydroxyurea
- SCD
sickle cell disease
- SNP
single-nucleotide polymorphism
- SSA
sub-Saharan Africa
Acknowledgments
The student's bursary was funded by the National Research Foundation (NRF) and the National Health Laboratory Services (NHLS), South Africa, to K.M.; A.W. is funded by the NIH, USA, and Grant number 1U01HG007459-01. The funders had no role in study design, data collection, and analysis; decision to publish; or preparation of the manuscript.
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Adegbola MA. (2009). Can heterogeneity of chronic sickle-cell disease pain be explained by genomics? A literature review. Biol Res Nurs 11, 81–97 [DOI] [PubMed] [Google Scholar]
- Adekile AD. (2011). Limitations of Hb F as a phenotypic modifier in sickle cell disease: Study of Kuwaiti Arab patients. Hemoglobin 35, 607–617 [DOI] [PubMed] [Google Scholar]
- Alebouyeh M, Moussavi F, Haddad-Deylami H, and Vossough P. (2004). Hydroxyurea in the treatment of major β-thalassemia and importance of genetic screening. Ann Hematol 83, 430–433 [DOI] [PubMed] [Google Scholar]
- Al-Habboubi HH, Mahdi N, Abu-Hijleh TM, Abu-Hijleh FM, Sater MS, and Almawi WY. (2012). The relation of vascular endothelial growth factor (VEGF) gene polymorphisms on VEGF levels and the risk of vasoocclusive crisis in sickle cell disease. Eur J Haematol 89, 403–409 [DOI] [PubMed] [Google Scholar]
- Alvarez O, Miller ST, Wang WC, et al. (2012). Effect of hydroxyurea treatment on renal function parameters: Results from the multi-center placebo-controlled BABY HUG clinical trial for infants with sickle cell anemia. Pediatr Blood Cancer 59, 668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas SK. (2015). Sickle Cell Pain. Lippincott: Williams and Wilkins [Google Scholar]
- Ballas SK, and Lusardi M. (2005). Hospital readmission for adult acute sickle cell painful episodes: Frequency, etiology, and prognostic significance. Am J Hematol 79, 17–25 [DOI] [PubMed] [Google Scholar]
- Ballas SK, Gupta K, and Adams-Graves P. (2012). Sickle cell pain: A critical reappraisal. Blood 120, 3647–3656 [DOI] [PubMed] [Google Scholar]
- Bartolucci P, and Galacteros F. (2012). Clinical management of adult sickle-cell disease. Curr Opin Hematol 19, 149–155 [DOI] [PubMed] [Google Scholar]
- Bean CJ, Boulet SL, Yang G, et al. (2013). Acute chest syndrome is associated with single nucleotide polymorphism-defined beta globin cluster haplotype in children with sickle cell anaemia. Br J Haematol 163, 268–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfer I, Youngblood V, Darbari DS, et al. (2014). A GCH1 haplotype confers sex-specific susceptibility to pain crises and altered endothelial function in adults with sickle cell anemia. Am J Hematol 89, 187–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman S. (2005). Psychosocial aspects of chronic widespread pain and fibromyalgia. Disabil Rehabil 27, 675–683 [DOI] [PubMed] [Google Scholar]
- Beyer A, Koch T, Schröder H, Schulz S, and Höllt V. (2004). Effect of the A118G polymorphism on binding affinity, potency and agonist-mediated endocytosis, desensitization, and resensitization of the human mu-opioid receptor. J Neurochem 89, 553–560 [DOI] [PubMed] [Google Scholar]
- Bockaert J, and Pin JP. (1999). Molecular tinkering of G protein-coupled receptors: An evolutionary success. EMBO J 18, 1723–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, and Lin FT. (1999). Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286, 2495–2498 [DOI] [PubMed] [Google Scholar]
- Bond C, Laforge KS, Tian M, et al. (1998). Single-nucleotide polymorphism in the human mu opioid receptor gene alters ß-endorphin binding and activity: Possible implications for opiate addiction. Proc Natl Acad Sci USA 95, 9608–9613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg J, Phylactides M, Bartsakoulia M, et al. (2012). KLF10 gene expression is associated with high fetal hemoglobin levels and with response to hydroxyurea treatment in β-hemoglobinnopathy patients. Pharmacogenomics 13, 1487–1500 [DOI] [PubMed] [Google Scholar]
- Bukar AA, Abjah UAM, Kagu MB, et al. (2013). Seroprevalence of parvovirus B19 and its clinical effect among anaemic SCA patients in Northeastern Nigeria. Am J Sci Ind Res 4, 195–200 [Google Scholar]
- Brousseau DC, McCarver DG, Drendel AL, et al. (2007). The effect of CYP2D6 polymorphisms on the response to pain treatment for paediatric sickle cell pain crisis. J Pediatr 150, 623–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canver MC, Smith EC, Sher F, et al. (2015). BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature 527, 192–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charache S, Dover GJ, Moore RD. et al. (1992). Hydroxyurea: Effects on hemoglobin F production in patients with sickle cell anemia. Blood 79, 2555–2565 [PubMed] [Google Scholar]
- Charache S, Terrin ML, Moore RD, et al. (1995). Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med 332, 1317–1322 [DOI] [PubMed] [Google Scholar]
- Chaturvedi S, and DeBaun MR. (2016). Evolution of sickle cell disease from a life-threatening disease of children to a chronic disease of adults: The last 40 years. Am J Hematol 91, 5–14 [DOI] [PubMed] [Google Scholar]
- Chou W, Wang C, Liu P, Liu C, Tseng C, and Jawan B. (2006). Human opioid receptor A118G polymorphism affects intravenous patient-controlled analgesia morphine consumption after total abdominal hysterectomy. Anesthesiology 105, 334–337 [DOI] [PubMed] [Google Scholar]
- Colombatti R, Perrotta S, Samperi P, et al. (2013). Organizing national responses for rare blood disorders: The Italian experience with sickle cell disease in childhood. Orphanet J Rare Dis 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton P, Geschwind DH, and Alarcon M. (2003). Association between human μ-opioid receptor gene polymorphism, pain tolerance, and opioid addiction. Am J Med Genet B Neuropsychiatr Genet 121, 76–82 [DOI] [PubMed] [Google Scholar]
- Cox SE, Makani J, Soka D, et al. (2014). Haptoglobin, alpha-thalassaemia and glucose-6-phosphate dehydrogenase polymorphisms and risk of abnormal transcranial Doppler among patients with sickle cell anaemia in Tanzania. Br J Haematol 165, 699–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbari DS, Minniti CP, Rana S, and van den Anker J. (2008). Pharmacogenetics of morphine: Potential implications in sickle cell disease. Am J Hematol 83, 233–236 [DOI] [PubMed] [Google Scholar]
- de Montalembert M, Brousse V, Elie C, et al. (2006). Long-term hydroxyurea treatment in children with sickle cell disease: Tolerance and clinical outcomes. Haematologica 91, 125–128 [PubMed] [Google Scholar]
- DeBaun MR. (2014). Hydroxyurea therapy contributes to infertility in adult men with sickle cell disease: A review. Expert Rev Hematol 7, 767–773 [DOI] [PubMed] [Google Scholar]
- Dersh J, Polatin PB, and Gatchel RJ. (2002). Chronic pain and psychopathology: Research findings and theoretical considerations. Psychosom Med 64, 773–786 [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Slade GD, Nackley AG, et al. (2005). Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Gen 14, 135–143 [DOI] [PubMed] [Google Scholar]
- Dixit A, Chatterjee T, Mishra P, et al. (2005). Hydroxyurea in thalassemia intermedia—A promising therapy. Ann Hematol 84, 441–446 [DOI] [PubMed] [Google Scholar]
- Duguay Y, Baar C, Skorpen F, and Guillemette C. (2004). A novel functional polymorphism in the uridine diphosphate-glucuronosyltransferase 2B7 promoter with significant impact on promoter activity. Clin Pharmacol Ther 75, 223–233 [DOI] [PubMed] [Google Scholar]
- Dzierzynski N, Stojanovic KS, Georgin-Lavialle S, and Lionnet F. (2016). Enjeux et difficultés de la relation entre soignants et patients drépanocytaires au cours de la crise douloureuse aiguë. Rev Med Interne 37, 111–116 [DOI] [PubMed] [Google Scholar]
- Elford HL. (1968). Effect of hydroxyurea on ribonucleotide reductase. Biochem Biophys Res Commun 33, 129–135 [DOI] [PubMed] [Google Scholar]
- Friedrisch JR, Prá D, Maluf SW, et al. (2008). DNA damage in blood leukocytes of individuals with sickle cell disease treated with hydroxyurea. Mutat Res Genet Toxicol Environ Mutagen 649, 213–220 [DOI] [PubMed] [Google Scholar]
- Galarneau G, Coady S, Garrett ME, et al. (2013). Gene-centric association study of acute chest syndrome and painful crisis in sickle cell disease patients. Blood 122, 434–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons C, Geoghegan R, Conroy H, et al. (2015). Sickle cell disease: Time for a targeted neonatal screening programme. Ir Med J 8, 43–45 [PubMed] [Google Scholar]
- Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, and Williams TN. (2011). Sickle cell disease in Africa: A neglected cause of early childhood mortality. Am J Prev Med 41, 398–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamideh D, and Alvarez O. (2013). Sickle cell disease related mortality in the United States (1999–2009). Pediatr Blood Cancer 60, 1482–1486 [DOI] [PubMed] [Google Scholar]
- Hankins JS, Ware RE, Rogers ZR, et al. (2005). Long-term hydroxyurea therapy for infants with sickle cell anemia: The HUSOFT extension study. Blood 106, 2269–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeney MM, and Ware RE. (2008). Hydroxyurea for children with sickle cell disease. Pediatr Clin North Am 55, 483–501 [DOI] [PubMed] [Google Scholar]
- Herrick JB. (2000). Peculiar elongated and sickle-shaped red blood corpuscles in a case of severe anemia. Yale J Biol Med 74, 179–184 [PMC free article] [PubMed] [Google Scholar]
- Höllt V. (2002). A polymorphism (A118G) in the μ-opioid receptor gene affects the response to morphine-6-glucuronide in humans. Pharmacogenet Genomics 12, 1–2 [DOI] [PubMed] [Google Scholar]
- Jhun EH, Yao Y, He Y, et al. (2015). Prevalence of pain-related single nucleotide polymorphisms in patients of African origin with sickle cell disease. Pharmacogenomics 16, 1795–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly P, Gagnieu MC, Bardel C, et al. (2012). Genotypic screening of the main opiate‐related polymorphisms in a cohort of 139 sickle cell disease patients. Am J Hematol 87, 534–536 [DOI] [PubMed] [Google Scholar]
- Kanter J, and Kruse-Jarres R. (2013). Management of sickle cell disease from childhood through adulthood. Blood Rev 27, 279–287 [DOI] [PubMed] [Google Scholar]
- Kinney TR, Helms RW, O'Branski EE, et al. (1999). Safety of hydroxyurea in children with sickle cell anemia: Results of the HUG-KIDS study, a phase I/II trial. Pediatric Hydroxyurea Group. Blood 94, 1550–1554 [PubMed] [Google Scholar]
- Klepstad P, Rakvåg T, Kaasa S, et al. (2004). The 118 A> G polymorphism in the human μ-opioid receptor gene may increase morphine requirements in patients with pain caused by malignant disease. Acta Anaesthesiol Scand 48, 1232–1239 [DOI] [PubMed] [Google Scholar]
- Knorring LV. (1989). The pathogenesis of chronic pain syndromes. Nord Psykiatr Tidsskr 43, 35–41 [Google Scholar]
- Kraus J, Borner C, Giannini E, et al. (2001). Regulation of mu-opioid receptor gene transcription by interleukin-4 and influence of an allelic variation within a STAT6 transcription factor binding site. J Biol Chem 276, 43901–43908 [DOI] [PubMed] [Google Scholar]
- Kumkhaek C, James G, Taylor VI, et al. (2008). Fetal haemoglobin response to hydroxycarbamide treatment and sar1a promoter polymorphisms in sickle cell anaemia. Br J Haematol 141, 254–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz JB, Awad S, Happich M, et al. (2015). Significant prevalence of sickle cell disease in Southwest Germany: Results from a birth cohort study indicate the necessity for newborn screening. Ann Hematol 95, 397–402 [DOI] [PubMed] [Google Scholar]
- Lettre G, Sankaran VG, Bezerra MA, et al. (2008). DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci U S A 105, 11869–11874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh HH, Liu H, Cavalli A, Yang W, Chen Y, and Wei L. (1998). μ Opioid receptor knockout in mice: Effects on ligand-induced analgesia and morphine lethality. Mol Brain Res 54, 321–326 [DOI] [PubMed] [Google Scholar]
- Lotsch J, Sharke C, Grosch S, et al. (2002). The polymorphism A118G of the human mu-opioid receptor gene decreases the pupil constrictory effect of morphine-6-glucuronide but not that of morphine. Pharmacogenetics 12, 3–9 [DOI] [PubMed] [Google Scholar]
- Lotsch J, and Geisslinger G. (2006). Current evidence for a genetic modulation of the response to analgesics. Pain 121, 1–5 [DOI] [PubMed] [Google Scholar]
- Ma Q, Wyszynski DF, Farrell JJ, et al. (2007). Fetal hemoglobin in sickle cell anaemia: Genetic determinants of response to hydroxyurea. Pharmacogenomics J 7, 386–394 [DOI] [PubMed] [Google Scholar]
- Makani J, Menzel S, Nkya S, et al. (2011). Genetics of fetal hemoglobin in Tanzanian and British patients with sickle cell anemia. Blood 117, 1390–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makani J, Ofori-Acquah SF, Nnodu O, Wonkam A, and Ohene-Frempong K. (2013). Sickle cell disease: New opportunities and challenges in Africa. Sci World J 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makubi A, Soka D, and Makani J. (2012). Moyamoya disease, a rare cause of recurrent strokes in an African sickle cell child: Does hydroxyurea have a role in this context? Int J Child Health Nutr 1, 82–85 [Google Scholar]
- Mantione KJ, Goumon Y, Esch T, and Stefano GB. (2005). Morphine 6beta glucuronide: Fortuitous morphine metabolite or preferred peripheral regulatory opiate? Med Sci Monitr 11, 43–46 [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, et al. (1996). Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ-opioid-receptor gene. Nature 383, 819–823 [DOI] [PubMed] [Google Scholar]
- McGann PT, Ferris MG, Ramamurthy U, et al. (2013). A prospective newborn screening and treatment program for sickle cell anemia in Luanda, Angola. Am J Hematol 88, 984–989 [DOI] [PubMed] [Google Scholar]
- Meineke I, Freudenthaler S, Hofmann U, et al. (2002). Pharmacokinetic modelling of morphine, morphine-3-glucuronide and morphine-6-glucuronide in plasma and cerebrospinal fluid of neurosurgical patients after short-term infusion of morphine. Br J Clin Pharmacol 54, 592–603 [DOI] [PubMed] [Google Scholar]
- Mmbando BP, Mgaya J, Cox SE, et al. (2015). Negative epistasis between sickle and foetal haemoglobin suggests a reduction in protection against malaria. PLoS One 10, e0125929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modell B, and Darlison M. (2008). Global epidemiology of haemoglobin disorders and derived service indicators. Bull WHO 86, 480–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS. (2004). Complex trait genetics of pain in the laboratory mouse. Pain Res Manag 28, 123–150 [Google Scholar]
- Mogil JS, and Devor M. (2004). Introduction to pain genetics. Pain Res Manag 28, 1–20 [Google Scholar]
- Mtatiro SN, Singh T, Rooks H., et al. (2014). Genome wide association study of fetal hemoglbin in sickle cell anemia in Tanzania. PLoS One 9, e111464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy BP, Pollack GM, and Brouwer KL. (2002). Contribution of morphine-6-glucuronide to antinociception following Intravenous Administration of Morphine to Healthy Volunteers. J Clin Pharmacol 42, 569–576 [DOI] [PubMed] [Google Scholar]
- Nagel RL, Fabry ME, Pagnier J, et al. (1985). Hematologically and genetically distinct forms of sickle cell anemia in Africa: The Senegal type and the Benin type. N Engl J Med 312, 880–884 [DOI] [PubMed] [Google Scholar]
- Nishank SS, Singh MPSS, and Yadav R. (2013). Clinical impact of factor V Leiden, prothrombin G20210A, and MTHFR C677T mutations among sickle cell disease patients of Central India. Eur J Haematol 91, 462–466 [DOI] [PubMed] [Google Scholar]
- Olabode JO, and Shokunbi WA. (2006). Types of crises in sickle cell disease patients presenting at the haematology day care unit (HDCU), University College Hospital (UCH), Ibadan. West Afr J Med 25, 284–288 [PubMed] [Google Scholar]
- Orkin SH. (2016). Recent advances in globin research using genome-wide association studies and gene editing. Ann NY Acad Sci 1368, 5–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne R, Joel S, Trew D, and Slevin M. (1990). Morphine and metabolite behavior after different routes of morphine administration: Demonstration of the importance of the active metabolite morphine-6-glucuronide. Clin Pharmacol Ther 47, 12–19 [DOI] [PubMed] [Google Scholar]
- Piel FB, Patil AP, Howes RE, et al. (2013). Global epidemiology of sickle haemoglobin in neonates: A contemporary geostatistical model-based map and population estimates. Lancet 381, 142–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo E, Laverty AA, Phekoo KJ, et al. (2015). A retrospective analysis of the cost of hospitalizations for sickle cell disease with crisis in England, 2010/11. J Public Health (Oxf) 37, 529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt OS, Orkin SH, Dover G, Beardsley GP, Miller B, and Nathan DG. (1984). Hydroxyurea enhances fetal hemoglobin production in sickle cell anemia. J Clin Invest 74, 652–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt OS, Thorington BD, Brambilla DJ, et al. (1991). Pain in sickle cell disease: Rates and risk factors. N Engl J Med 325, 11–16 [DOI] [PubMed] [Google Scholar]
- Pule GD, Mowla S, Novitzky N, Wiysonge CS, and Wonkam A. (2015). A systematic review of known mechanisms of hydroxyurea-induced fetal hemoglobin for treatment of sickle cell disease. Expert Rev Hematol 8, 669–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimy MC, Gangbo A, Ahouignan G, et al. (2003). Effect of a comprehensive clinical care program on disease course in severely ill children with sickle cell anemia in a sub-Saharan African setting. Blood 102, 834–838 [DOI] [PubMed] [Google Scholar]
- Rahimy MC, Gangbo A, Ahouignan G, and Alihonou E. (2009). Newborn screening for sickle cell disease in the Republic of Benin. J Clin Pathol 62, 46–48 [DOI] [PubMed] [Google Scholar]
- Rakvåg TT, Klepstad P, Baar C, et al. (2005). The Val158Met polymorphism of the human catechol-O-methyltransferase (COMT) gene may influence morphine requirements in cancer pain patients. Pain 116, 73–78 [DOI] [PubMed] [Google Scholar]
- Rees DC, Williams TN, and Gladwin MT. (2010). Sickle-cell disease. Lancet 376, 2018–2031 [DOI] [PubMed] [Google Scholar]
- Ross JR, Rutter D, Welsh K, et al. (2005). Clinical response to morphine in cancer patients and genetic variation in candidate genes. Pharmacogenomics J 5, 324–336 [DOI] [PubMed] [Google Scholar]
- Rumaney MB, Bitoungui VJN, Vorster AA, et al. (2014). The co-inheritance of alpha-thalassemia and sickle cell anemia is associated with better hematological indices and lower consultations rate in Cameroonian patients and could improve their survival. PLoS One 9, e100516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan VA, Luo Z, Flanagan JM, et al. (2013). Genetic modifiers of sickle cell anaemia in the BABY HUG cohort: Influence on laboratory and clinical phenotypes. Am J Hematol 88, 571–576 [DOI] [PubMed] [Google Scholar]
- Shenoy S. (2011). Hematopoietic stem cell transplantation for sickle cell disease: Current practice and emerging trends. Hematology ASH Educ 2011, 273–279 [DOI] [PubMed] [Google Scholar]
- Shiba HF, El-Ghamrawy MK, Shaheen IAE, Ali RAE, and Mousa SM. (2014). Glutathione S-transferase gene polymorphisms (GSTM1, GSTT1, and GSTP1) in Egyptian pediatric patients with sickle cell disease. Pediatr Dev Pathol 17, 265–270 [DOI] [PubMed] [Google Scholar]
- Slade GD, Diatchenko L, Bhalang K, et al. (2007). Influence of psychological factors on risk of temporomandibular disorders. J Dent Res 86, 1120–1125 [DOI] [PubMed] [Google Scholar]
- Smith MT, Watt JA, and Cramond T. (1990). Morphine-3-glucuronide-a potent antagonist of morphine analgesia. Life Sci J 47, 579–585 [DOI] [PubMed] [Google Scholar]
- Solovieff N, Milton JN. and Steinberg MH. (2010). Fetal hemaglobin in sickle cell anemia: Genome-wide associated studies suggest a regulatory region in the 5′ olfactory receptor gene cluster. Blood 4, 1815–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer UM, and Stuber F. (2007). Genetic factors in pain and its treatment. Curr Opin Anaesthesiol 20, 478–484 [DOI] [PubMed] [Google Scholar]
- Steinberg MH, Barton F, Castro O, et al. (2003). Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: Risks and benefits up to 9 years of treatment. JAMA 289, 1645–1651 [DOI] [PubMed] [Google Scholar]
- Steinberg MH, Lu ZH, Barton FB, Terrin ML, Charache S, and Dover GJ. (1997). Fetal hemoglobin in sickle cell anemia: Determinants of response to hydroxyurea. Multicenter Study of Hydroxyurea. Blood 89,1078–1088 [PubMed] [Google Scholar]
- Steinberg MH, McCarthy WF, Castro OP, et al. (2010). The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17.5 year follow-up. Am J Hematol 85, 403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekola-Ayele F, and Rotimi CN. (2015). Translational genomics in low- and middle-income countries: Opportunities and challenges. Public Health Genomics 18, 242–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein SL, and Menzel S. (2009). Discovering the genetics underlying foetal haemoglobin production in adults. Br J Haematol 145, 455–467 [DOI] [PubMed] [Google Scholar]
- Thornburg CD, Dixon N, Burgett S, et al. (2009). A pilot study of hydroxyurea to prevent chronic organ damage in young children with sickle cell anemia. Pediatr Blood Cancer 52, 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd KH. (2005). Chronic Pain and aberrant drug-related behavior in the emergency department. J Law Med Ethics 33, 761–769 [DOI] [PubMed] [Google Scholar]
- Todd KH, Green C, Bonham VL, Haywood C, and Ivy E. (2006). Sickle cell disease related pain: Crisis and conflict. J Pain 7, 453–458 [DOI] [PubMed] [Google Scholar]
- Tshilolo L, Aissi LM, Lukusa D, et al. (2009). Neonatal screening for sickle cell anaemia in the Democratic Republic of the Congo: Experience from a pioneer project on 31 204 newborns. J Clin Pathol 62, 35–38 [DOI] [PubMed] [Google Scholar]
- Tubman VN, Marshall R, Jallah W, et al. (2016). Newborn screening for sickle cell disease in Liberia: A Pilot Study. Pediatr Blood Cancer 63, 671–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichinsky EP. (1991). Comprehensive care in sickle cell disease: Its impact on morbidity and mortality. Sem Hematol 28, 220–226 [PubMed] [Google Scholar]
- Voskaridou E, Christoulas D, Bilalis A, et al. (2010). The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: Results of a 17-year, single-center trial (LaSHS). Blood 115, 2354–2363 [DOI] [PubMed] [Google Scholar]
- Ware RE. (2013). Is sickle cell anemia a neglected tropical disease? PLoS Negl Trop Dis 7, e2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware RE, Despotovic JM, Mortier NA, et al. (2011). Pharmacokinetics, pharmacodynamics, and pharmacogenetics of hydroxyurea treatment for children with sickle cell anemia. Blood 118, 4985–4991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherall DJ, and Clegg JB. (2008). The Thalassaemia Syndromes, 4th ed. John Wiley & Sons, New Jersey, USA [Google Scholar]
- Weatherall D, Hofman K, Rodgers G, Ruffin J, and Hrynkow S. (2005). A case for developing North-South partnerships for research in sickle cell disease. Blood 105, 921–923 [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, and Raymond FA. (1977). Inheritance of low erythrocyte catechol-o-methyltransferase activity in man. Am J Hum Genet 29, 125–135 [PMC free article] [PubMed] [Google Scholar]
- Williams TN, Mwangi TW, Wambua S, et al. (2005). Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. Int J Infect Dis 192, 178–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonkam A, Bitoungui VJN, Vorster AA, et al. (2014a). Association of variants at BCL11A and HBS1L-MYB with hemoglobin F and hospitalization rates among sickle cell patients in Cameroon. PLoS One 9, e92506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonkam A, Mba CZ, Mbanya D, Ngogang J, Ramesar R, and Angwafo FF. (2014b). Psychosocial stressors of sickle cell disease on adult patients in Cameroon. J Genet Couns 23, 948–956 [DOI] [PubMed] [Google Scholar]
- Wonkam A, Ponde C, Nicholson N, Fieggen K, Ramesar R, and Davidson A. (2012). The burden of sickle cell disease in Cape Town. SAMJ 102, 753–754 [DOI] [PubMed] [Google Scholar]
- Wonkam A, Rumaney MB, Ngo Bitoungui VJ, Vorster AA, Ramesar R, and Ngogang J. (2014c). Coinheritance of sickle cell anemia and α-thalassemia delays disease onset and could improve survival in cameroonian's patients (Sub-Saharan Africa). Am J Hematol 89, 664–665 [DOI] [PubMed] [Google Scholar]
- Xu J, Peng C, Sankaran VG, et al. (2011). Correction of sickle cell disease in adult mice by interference with fetal hemoglobin silencing. Science 334, 993–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Sankaran VG, Ni M, et al. (2010). Transcriptional silencing of {gamma}-globin by BCL11A involves long-range interactions and cooperation with SOX6. Genes Dev 24, 783–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanni E, Grosse SD, Yang Q, and Olney RS. (2009). Trends in pediatric sickle cell disease-related mortality in the United States, 1983–2002. J Pediatr 154, 541–545 [DOI] [PubMed] [Google Scholar]
- Yavarian M, Karimi M, Bakker E, Harteveld CL, and Giordano PC. (2004). Response to hydroxyurea treatment in Iranian transfusion-dependent beta-thalassemia patients. Haematologica 89, 1172–1178 [PubMed] [Google Scholar]
- Zago M, Silva W, Dalle B, et al. (2000). Atypical beta(s) haplotypes are generated by diverse genetic mechanisms. Am J Hematol 63, 79–84 [DOI] [PubMed] [Google Scholar]
- Zhu J, Chin K, Aerbajinai W, Kumkhaek C, Li H, and Rodgers GP. (2014). Hydroxyurea-inducible SAR1 gene acts through the Gialpha/JNK/Jun pathway to regulate gamma-globin expression. Blood 124, 1146–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman SA, Schultz WH, Burgett S, Mortier NA, and Ware RE. (2007). Hydroxyurea therapy lowers transcranial Doppler flow velocities in children with sickle cell anemia. Blood 110, 1043–1047 [DOI] [PubMed] [Google Scholar]
- Zimmerman SA, Schultz WH, Davis JS, et al. (2004). Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood 103, 2039–2045 [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Smith YR, et al. (2003). COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science 299, 1240–1243 [DOI] [PubMed] [Google Scholar]
- Zur B. (2016). Increase in genetically determined anemia as a result of migration in Germany. Der Internist 57, 444–445 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.