Abstract
Rationale: In cystic fibrosis, abnormal glucose tolerance is associated with decreased lung function and worsened outcomes. Translational evidence indicates that abnormal glucose tolerance may begin in early life.
Objectives: To determine whether very young children with cystic fibrosis have increased abnormal glucose tolerance prevalence compared with control subjects. The secondary objective was to compare area under the curve for glucose and insulin in children with cystic fibrosis with control subjects.
Methods: This is a prospective multicenter study in children ages 3 months to 5 years with and without cystic fibrosis.
Measurements and Main Results: Oral glucose tolerance testing with glucose, insulin, and C-peptide was sampled at 0, 10, 30, 60, 90, and 120 minutes. Twenty-three children with cystic fibrosis and nine control subjects had complete data. All control subjects had normal glucose tolerance. Nine of 23 subjects with cystic fibrosis had abnormal glucose tolerance (39%; P = 0.03). Of those, two met criteria for cystic fibrosis–related diabetes, two indeterminate glycemia, and six impaired glucose tolerance. Children with cystic fibrosis failed to exhibit the normal increase in area under the curve insulin with age observed in control subjects (P < 0.01), despite increased area under the curve glucose (P = 0.02).
Conclusions: Abnormal glucose tolerance is notably prevalent among young children with cystic fibrosis. Children with cystic fibrosis lack the normal increase in insulin secretion that occurs in early childhood despite increased glucose. These findings demonstrate that glycemic abnormalities begin very early in cystic fibrosis, possibly because of insufficient insulin secretion.
Keywords: cystic fibrosis, cystic fibrosis–related diabetes mellitus, infants, children, abnormal glucose tolerance
At a Glance Commentary
Scientific Knowledge on the Subject
In those with cystic fibrosis (CF), the presence of abnormal glucose metabolism is associated with more severe lung function decline and excess morbidity and mortality. The historic assumption that abnormal glucose metabolism in CF does not start until adolescence or later has been challenged by recent findings that abnormal glucose regulation is present at birth in ferret and pig models of CF. This study was designed to determine whether infants and very young children with CF exhibit abnormal glucose tolerance.
What This Study Adds to the Field
This study is a prospective, controlled assessment of abnormal glucose tolerance in children with CF 3 months to 6 years of age. Abnormal glucose metabolism was present in 39% of the children with CF studied. Even though glucose levels increased with age in children with CF, insulin secretion did not increase with age in children with CF in the normal manner seen in control subjects. These results indicate that the pathophysiology of CF-related diabetes begins at a very young age in humans with CF and is potentially linked to age-related differences in insulin secretion.
Cystic fibrosis (CF) is a severe, autosomal-recessive disease of high prevalence caused by mutations of the cystic fibrosis transmembrane conductance regulator (CFTR) gene. CF-related diabetes (CFRD) is an important and prevalent comorbidity of CF. In some pediatric diabetes practices, CFRD now represents the second largest group of patients after children with type 1 diabetes mellitus (1).
CFRD is a distinct form of diabetes with unique pathophysiology and clinical characteristics differing from type 1 and type 2 diabetes mellitus. Importantly, CFRD increases risk of pulmonary death in CF (2). Even mild hyperglycemia is detrimental and clinical decline begins before overt diabetes, during the time when only abnormal glucose tolerance (AGT) is present (3, 4).
In youth 10–20 years of age with CF, AGT is associated with decreased lung function and worsened nutritional status (4, 5). Even glucose elevation at only intermediate time points during oral glucose tolerance testing (OGTT) has a strong negative association with pulmonary function (6). Among children with CF, available data indicate AGT is highly prevalent (up to 40%) in those who are 6–10 years of age (7). Alarmingly, AGT in 6–10 year olds strongly predicts early onset CFRD (7).
There is a paucity of data regarding the prevalence of AGT among children with CF younger than 6 years of age (8). However, glucose tolerance has been studied in animal models of CF at very young ages, including CFTR null and deltaF508 pigs and CFTR null ferrets. CFTR null ferrets and pigs exhibit abnormal glycemia and abnormal endocrine pancreas function as early as the neonatal period (9, 10). Importantly, this indicates glucose abnormalities in humans may also begin very early in infancy or early childhood.
In response to these findings, we conducted this small prospective study to interrogate oral glucose tolerance in children with CF at 3 months to 5 years of age. Our primary hypothesis was that children with CF would exhibit an increased risk of abnormal glucose intolerance as compared with control children. Secondary hypotheses were that glucose area under the curve (AUC) would be higher and insulin AUC would be lower in these young children with CF as compared with control subjects.
Some of the results of this study have been previously reported in the form of an abstract (11).
Methods
Children were prospectively recruited from the CF centers at the University of Iowa and the University of Minnesota. Control subjects were recruited from friends and family members of children with CF who did not themselves have CF, e-mails, and advertisements. The protocol was approved by the institutional review boards of both institutions. At least one parent provided verbal and written consent for each child. Inclusion criteria were greater than or equal to 3 months of age and less than 6 years of age. Exclusion criteria were preexisting diagnosis of diabetes. subjects with CF were excluded if they did not have a firm diagnosis of CF defined as two known disease-causing CFTR alleles and/or diagnostic sweat chloride testing.
Length of fasting was weight-based. Children less than or equal to 10 kg fasted 4 hours, those greater than 10 kg and less than 15 kg fasted 6 hours, and greater than or equal to 15 kg fasted 8 hours. The child was weighed and measured and medication and illness history obtained. Body mass index (BMI) and BMI z score or weight for length and weight-for-length z score (if the child was <2 yr of age) were calculated. Both BMI z score and weight-for-length z score were calculated via web-based calculators (Children’s Hospital of Philadelphia, http://stokes.chop.edu/web/zscore/ and Medscape, http://reference.medscape.com/calculator/infant-weight-length-percentile, respectively).
A peripheral intravenous (IV) catheter was placed and baseline venous blood sample was drawn. The child then drank 1.75 g/kg glucose of oral glucose solution (Trutol 100; Thermo Fisher Scientific, Middletown, VA). Additional samples were obtained at 10, 30, 60, 90, and 120 minutes after administration of the glucose solution. The medical record was extracted to verify the child’s medications, major medical conditions, pancreatic sufficiency status, gene mutations, and stool elastase.
Plasma glucose was measured using a glucose analyzer (Analox Instruments, Lunenburg MA). Plasma insulin and C-peptide were measured using a Human Metabolic Hormone Magnetic Bead Panel (EMD Millipore Cooperation, Billerica, MA).
Mixed effect modeling was used to account for repeated measures on AUCs of glucose, insulin, and C-peptide, using the R package lme4. The Satterthwaite approximation implemented in the R package lmerTest was used to compute the P values. For baseline continuous descriptive variables mean and SD were calculated and significance was tested using Student’s t test for normally distributed variables and Wilcoxon rank sum test for variables with a nonnormal distribution. Categorical variables were tested using Fisher exact test. Significance level was set at P less than 0.05. AUC was calculated by the trapezoidal method for the values of interest (glucose, insulin, and C-peptide).
Based on established clinical practice in CFRD (12), three mutually exclusive patterns of AGT were used. Impaired glucose tolerance (IGT) was defined as a fasting blood glucose less than 126 mg/dl (7 mmol/L) and a 120-minute blood glucose value greater than or equal to 140 mg/dl (7.8 mmol/L), but less than 200 mg/dl (11.1 mmol/L). Indeterminate glycemia (INDET) was defined as a fasting blood glucose less than 126 mg/dl (7 mmol/L) and a 120-minute blood glucose value of less than 140 mg/dl (7.8 mmol/L), but at least one intermediate blood glucose value of greater than or equal to 200 mg/dl (11.1 mmol/L). CFRD was defined as fasting blood glucose greater than or equal to 126 mg/dl (7 mmol/L) and/or a 120-minute blood glucose value greater than or equal to 200 mg/dl (11.1 mmol/L). For the purposes of this study, AGT was defined as the presence of IGT, INDET, or CFRD. Normal glucose tolerance (NGT) was defined as the absence of AGT.
Results
Population
Twenty-seven subjects with CF attended at least one study visit. One CF subject had no samples available because of IV failure. Three additional subjects with CF lacked samples from intermediate time points. Therefore, 23 unique subjects with CF had full data available. Ten were from the University of Minnesota and 13 from the University of Iowa. Eleven control children attended at least one study visit. Two had no samples available because of IV failure and nine had full data available. Nine of the subjects with CF and one control subject attended multiple study visits. Two subjects with CF had five visits, one had three visits, and six had two visits; however, two of those had incomplete data at one visit. The one control subject seen multiple times attended four visits. Altogether there were a total of 55 visits, 49 with complete data.
Baseline subject characteristics are presented in Table 1. The mean BMI of the subjects with CF was lower than the control subjects (15.9 ± 0.79 vs. 17.5 ± 2.0; P = 0.04), as was the mean weight z score (−0.47 ± 0.83 vs. 0.38 ± 1.00; P = 0.02). No other measures differed significantly between the groups (Table 1). The difference in percentage of African American subjects (29% vs. 0%) represents the typical ethnic distribution of CF. All but one child with CF had a clinical diagnosis of pancreatic insufficiency (see Table E1 in the online supplement). Fecal elastase values were unavailable for eight subjects. One subject who was clinically classified as pancreatic insufficient had a fecal elastase value of 222 μg/g at 1 month of age. However, at the time of study he was treated with pancreatic enzymes and known to have steatorrhea when untreated. One subject had history of duodenal atresia post surgical repair, and one subject had history of meconium ileus (see Table E1). CFTR genotype of each subject is reported in Table E1. All were clinically classified as disease-causing mutations.
Table 1.
Baseline Characteristics at First Visit
| CF | Control | Total | P Value* | |
|---|---|---|---|---|
| Total, n | 27 | 11 | 38 | |
| Female, n (%) | 12 (44) | 3 (27) | 15 (39) | 0.47 |
| African American, n (%) | 0 (0) | 2 (18) | 2 (5) | 0.08 |
| Hispanic, n (%) | 1 (4) | 0 (0) | 1 (3) | 1.0 |
| Age, yr, mean ± SD | 2.64 ± 1.77 | 2.76 ± 1.78 | 2.67 ± 1.75 | 0.82 |
| Height, cm, mean ± SD | 88 ± 17 | 90 ± 17 | 89 ± 17 | 0.53 |
| Height z score | −0.21 ± 0.93 | 0.065 ± 1.01 | −0.17 ± 0.94 | 1.00 |
| Weight, kg, mean ± SD | 12.8 ± 4.67 | 14.3 ± 5.3 | 13.2 ± 4.8 | 0.48 |
| Weight z score | −0.47 ± 0.83 | 0.38 ± 1.00 | −0.27 ± 1.02 | 0.02 |
| Weight for length, kg/cm, mean ± SD | 40.17 ± 22.44 | 69.66 ± 23.72 | 48.43 ± 26.07 | 0.02 |
| Weight-for-length z score† | −0.20 ± 0.94 | 0.51 ± 0.77 | 0.019 ± 0.94 | 0.05 |
| BMI, kg/m2, mean ± SD | 15.9 ± 0.79 | 17.5 ± 2.0 | 16.26 ± 1.61 | 0.04 |
| BMI z score, mean ± SD‡ | 0.02 ± 0.92 | 0.35 ± 1.35 | 0.1 ± 1.03 | 0.81 |
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis.
P values given are for CF versus control.
Weight-for-length z score could not be calculated for heights >109 cm.
BMI z score is only calculated for subjects 2 years of age or older because it cannot be calculated for younger children.
Primary Outcome: AGT
At their first visit, 9 of the 23 subjects with CF with full data available met criteria for AGT (P = 0.03 compared with control subject). One had INDET, six had IGT, and two met criteria for CFRD. None of the control subjects had AGT. The rate of AGT was not different by center (University of Iowa vs. University of Minnesota; P = 0.11). In subjects who had multiple visits, three additional subjects with CF subsequently developed IGT (P = 0.01 for having exhibited AGT at any visit CF compared with control subject).
Comparing the subjects with CF with AGT at any point with subjects with CF who remained NGT, there was no significant relationship between the presence of AGT and sex, BMI, BMI z score, weight for length, weight-for-length z score, CFTR mutation class, or AUC insulin (all P ≥ 0.2). As expected, AUC glucose did trend higher in subjects with CF with AGT (P = 0.06). CFTR genotype and fecal elastase values for each subject are presented in association with their glucose tolerance category in Table E1.
Apparent Association between AGT and Age in CF
The prevalence of AGT demonstrated a unimodal relationship with age. When subjects with CF were divided into roughly equal age quintiles, AGT occurred in all age quintiles. There was an increase in the frequency of AGT with age until the second oldest quintile, followed by a marked decline among the oldest quintile (Figure 1B). This trend in AGT frequency was mirrored by a similar unimodal trend in glucose AUC among subjects with CF peaking at 3.4 years of age, whereas this pattern was not observed in control children (Figure 1A).
Figure 1.
Percent of population with cystic fibrosis (CF) with abnormal glucose tolerance (AGT). (A) Area under the curve (AUC) glucose values for CF and control subjects plotted against age at the time of measurement. All control subjects had normal glucose tolerance (NGT). Subjects with CF with AGT are marked by a diamond. The two subjects with CF with two repeat AGT measures are marked by * and †. The dotted curved line shows the local regression curve fitting for the CF population. (B) The CF population was separated by age quintiles with the percent of each age group with AGT shown by the open circle. The number of children in each age group is shown in parentheses within the bar. %AGT was calculated as the percentage of subjects at a given age range demonstrating AGT. Individuals who received more than one oral glucose tolerance testing (OGTT) during an age range contributed to %AGT if they had at least one abnormal test. (C) Heat map of glucose and insulin measurements for children with CF and without CF with hierarchical cluster of measurements on the left of the heat map. Darker red indicates higher values and darker blue indicates lower values. The age of the child in years and subject number are listed underneath the heat map with the indicated age ranges shown in B. Twenty-four subjects contributed data to the heat map; subject number 4 was missing only one time point from the OGTT. However, subject 4 was not included in other analyses because AGT status could not be completely determined and AUC could only be approximated owing to the missing data point. The red asterisks below the age values on the heat map mark those children who were categorized as AGT during that visit. Fasting insulin and glucose levels are from time 0 before the child received the glucose beverage. Insulin and glucose AUC represent the AUC from the 0-to-120-minute time points of the OGTT. Peak glucose is the maximal glucose value that occurred during OGTT. Glucose 120 is the glucose value at the 120-minute time point. (D) Box-and-whisker plot of glucose AUC for subjects with CF and without CF at the indicated age ranges. (E) Box-and-whisker plot of insulin AUC for subjects with CF and without CF at the indicated age ranges. (F) Box-and-whisker plot of C-peptide AUC for subjects with CF and without CF at the indicated age ranges. Red circles in D–F indicate those subjects classified as AGT. Significant differences by Mann Whitney U test are shown with brackets for comparisons between age groups within a single-subject classification and between-subject classifications of a single age group (*P < 0.05; **P < 0.01; ***P < 0.001).
Secondary Outcome: Glucose, Insulin, and C-Peptide AUC
AUC glucose was higher in subjects with CF than control subjects (16,567 ± 1,059 vs. 14,696 ± 1,523; P = 0.02) and increased with age in subjects with CF but not in control subjects (P = 0.002). In subjects with CF AUC glucose was higher in 3-to-5-year-old children compared with younger children (P = 0.006), but this difference was not seen in control subjects (P = 0.39) (Figure 1D).
AUC insulin and C-peptide increased strikingly in 3-to-5-year-old control children compared with younger control children (P = 0.002 and P = 0.02, respectively) (Figures 1E and 1F), but exhibited only small albeit statistically significant increases in 3-to-5-year-old children with CF compared with younger children with CF (insulin, P = 0.008; C-peptide, P = 0.004) (Figures 1C, 1E, and 1F). Similarly, when AUC insulin was analyzed as a continuous variable, insulin increased with age to a greater extent in control children compared with children with CF (P = 0.03 for interaction). Similarly, C-peptide also increased with age to a greater extent in control subjects compared with children with CF (P = 0.04). Neither AUC glucose, insulin, nor C-peptide correlated with sex or race in a statistically significant manner (all P > 0.2). CFTR mutation class (I-V) was associated with AUC insulin only (P = 0.04). However, when separated into subjects with at least one class IV-V mutation versus subjects with all mutations of class I-III, there was no significant difference in AUC insulin (P = 0.07).
Subjects with Change in NGT/AGT Status
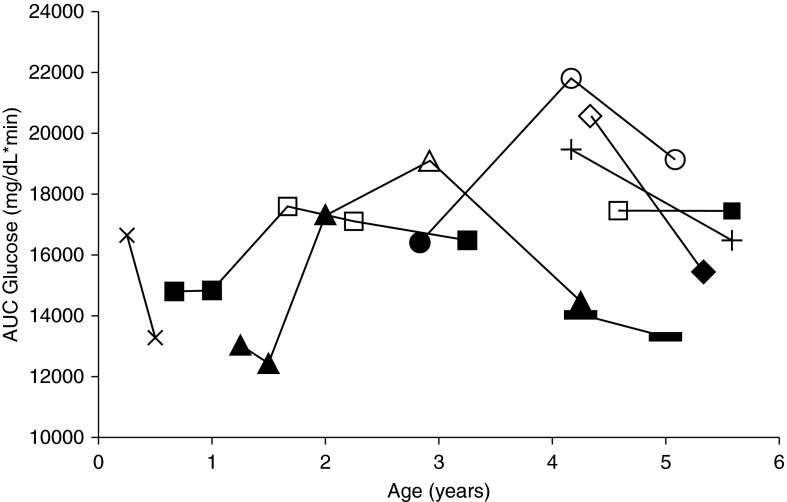
Waxing and waning of OGTT categories is well documented in older children and adults with CF. Of the eight subjects with CF who had multiple visits, six had a change in their glucose tolerance status during the study (Figure 2). Of these, three were NGT at their first visit. One was NGT at 8 months, NGT at 12 months, and then IGT at 18 months, and IGT at 2 years, but NGT again at 3 years. The second was NGT at 12 months, 18 months, and 2 years, IGT at 3 years, and NGT again at 4 years. A third was NGT at just less than 3 years and INDET at 4 and 5 years. Three older children with CF were classified as AGT at their initial visit. One was INDET at 4 years and NGT at 5 years, the second met criteria for CFRD at 4 years but was NGT at 5 years. The third was IGT at 4 years and NGT at 5 years. Two subjects with CF who had more than one visit remained NGT for the entire duration of the study. One was NGT both at 4 and 5 years of age and the other at 3 months and 6 months. None of the control subjects were AGT at any point in the study.
Figure 2.
Area under the curve (AUC) glucose by age in children with cystic fibrosis with multiple visits. This figure represents the children with cystic fibrosis who had multiple study visits. Points graphed are the AUC glucose at a given study visit by exact age at the time of the study visits. Points associated with normal glucose tolerance status are solid; those associated with abnormal glucose tolerance (AGT) status are open. Points relating to a single subject are connected with lines between the points. Six of the subjects with multiple visits had change in AGT status, and two subjects with multiple visits were AGT for all visits. All control subjects had normal glucose tolerance for the duration of the study and are not included in the figure.
Adverse Effects of OGTT
There were no adverse events secondary to OGTT. None of the subjects experienced hypoglycemia. Some children did not have successful IV placement, as noted previously.
Discussion
CFRD is a common significant complication of CF. It is increasingly prevalent with age and affects a significant proportion of the CF population (13), with milder abnormalities of glucose tolerance being even more common (5). Although CFRD has been described since the 1950s (14), only recently has it become a major focus of CF care. The pathophysiology of this disease is currently incompletely understood (reviewed in Reference 15) hampering rational treatment and prevention efforts. Historically, CFRD was believed to be caused by endocrine pancreas damage collateral to exocrine pancreas destruction. Although this is an important component of the problem, it is now recognized that CFRD pathophysiology is more complicated, with evidence that type 2 susceptibility genes play a role (16), that insulin secretory defects predate exocrine pancreatic destruction (17) and may be related to the CFTR channel defect itself (18, 19), and that CFRD occurs despite relative sparing of β-cell mass (9, 10). In ferret and pig models abnormalities of glucose tolerance and insulin secretion are striking even in young animals, indicating pathology begins in very early life.
This study of modest power shows that glucose tolerance abnormalities are present in a nontrivial number of very young children with CF. Nearly 40% of the children with CF age 0–5 years in our study met clinical criteria for AGT, with 9% meeting criteria for previously unrecognized CFRD. Because this is not a population study, it is not possible to know conclusively whether 40% of the general childhood CF population has AGT; nonetheless, these numbers are striking. The long-term significance of this finding is unclear, but it is important to note that among children 6–10 years of age, AGT on OGTT predicts a more rapid progression to CFRD at a younger age (7). Moreover, AGT in older children is associated with rapid lung function decline (6, 20) and increased mortality (21, 22). Whether AGT detected at a very young age will predict higher morbidity and mortality remains to be determined.
Normally, children experience increased insulin levels with age, thought to be caused by increases in growth hormone levels, a transition that occurs around 2 years of age (23) and induces relative insulin resistance (24). Although our control children demonstrated this normal trend, it was significantly attenuated in the children with CF.
Our study was not designed to determine the reasons insulin levels failed to increase in children with CF; however, we can speculate. One possibility could be that insulin requirements may be lower in children with CF compared with control subjects as a result of malnutrition, perturbed growth hormone axis, and/or high metabolic demands that are insulin-independent (25, 26). Such factors may underlie the low insulin levels in children with CF with NGT. However, the high prevalence of AGT and increased glucose levels in subjects with CF in our cohort suggests that lower insulin levels are not a benign physiologic phenomenon. The children with AGT had no higher insulin levels than control subjects and only a modest increase in insulin compared with NGT children with CF despite higher glucose levels, implying that these children lack the ability to appropriately increase insulin secretion in the face of increased demand. Therefore, deficiency in insulin secretion is likely the primary cause of AGT in these children, similar to older children and adults with CF (3, 27, 28). This may be coupled with increased insulin clearance, which would explain some of the difference found between measures of insulin and C-peptide and is described in others with CF (29).
Interestingly, our data suggests an age-dependent predilection to AGT among young children with CF, with increasing rates with increasing age. The apparent improvement in glucose tolerance at age 5 may be an artifact of small numbers, but it potentially may be representative of a real phenomenon, given that similar patterns have previously been reported for exocrine pancreatic function over the first 5 years of age in children with CF (30). Also, waxing and waning of endocrine function on OGTT is commonly described in older subjects with CF (31), in whom abnormal values on at least one occasion are predictive of negative clinical outcomes even in those with subsequent normal values (3, 13). We continue to follow this cohort longitudinally to gather more data.
Our study has strengths and limitations. The major strength of our study was the inclusion of very young children (<6 years old), an age range that is underrepresented in the literature, but informative regarding the pathophysiology of this disease. We are limited by the small sample size, especially control subjects. However, despite the low sample size, control children demonstrated very little variability in glucose and insulin values compared with children with CF. Furthermore, the rate of glucose abnormalities found in our CF population was in the range we reported previously (7). Most of the control subjects in our study were siblings of children with CF, and as such many are likely CF carriers. However, CF carrier status is not known to affect glycemia.
In summary, our data show that nearly 40% of very young children with CF exhibit AGT with poor insulin responsiveness. These abnormalities wax and wane with age, and seem to be related to inadequate insulin secretion. Our work suggests that further investigations in young children and animal models are likely to be informative in regards to the pathophysiology of CFRD. We are hopeful that future studies will uncover short- and long-term clinical implications of AGT in young children with CF.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Gretchen Cress at the University of Iowa, Iowa City, Iowa, for her help with study management.
Footnotes
Supported by the National Institutes of Health (NIH) grants NIDDK R24 DK96518 (J.F.E., A.W.N., and A.M.), R01 DK097820 (A.U. and A.W.N.), and P30 DK054759 (J.F.E.) and by the Fraternal Order of Eagles Diabetes Research Center Faculty Scholar Award (A.W.N.). The Institute for Clinical and Translational Science at the University of Iowa is supported by the NIH Clinical and Translational Science Award (CTSA) program, grant U54TR001356. The Clinical and Translational Science Institute at the University of Minnesota is supported through the NIH CTSA program, grant UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Contributions: K.L.O. initially conceived and designed the study with A.M., with design refinements by A.W.N., J.F.E., A.U., Y.Y., and X.S. Data acquisition was performed by K.L.O., A.M., Y.Y., and X.S. Data analysis and interpretation was performed by K.L.O., Y.Y., K.W., J.F.E., and A.W.N. The manuscript was drafted by K.L.O. and Y.Y. and critically revised by K.L.O., A.W.N., K.W., X.S., A.U., A.M., and J.F.E. All authors gave final approval of the version to be published. Literature review was performed by K.L.O., A.M., J.F.E., and A.W.N.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201512-2518OC on July 22, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gregory JW. What are the main research findings during the last 5 years that have changed my clinical practice in diabetes medicine? Arch Dis Child. 2012;97:436–439. doi: 10.1136/adc.2011.300710. [DOI] [PubMed] [Google Scholar]
- 2.Chamnan P, Shine BSF, Haworth CS, Bilton D, Adler AI. Diabetes as a determinant of mortality in cystic fibrosis. Diabetes Care. 2010;33:311–316. doi: 10.2337/dc09-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milla CE, Billings J, Moran A. Diabetes is associated with dramatically decreased survival in female but not male subjects with cystic fibrosis. Diabetes Care. 2005;28:2141–2144. doi: 10.2337/diacare.28.9.2141. [DOI] [PubMed] [Google Scholar]
- 4.Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med. 2000;162:891–895. doi: 10.1164/ajrccm.162.3.9904075. [DOI] [PubMed] [Google Scholar]
- 5.Moran A, Doherty L, Wang X, Thomas W. Abnormal glucose metabolism in cystic fibrosis. J Pediatr. 1998;133:10–17. doi: 10.1016/s0022-3476(98)70171-4. [DOI] [PubMed] [Google Scholar]
- 6.Brodsky J, Dougherty S, Makani R, Rubenstein RC, Kelly A. Elevation of 1-hour plasma glucose during oral glucose tolerance testing is associated with worse pulmonary function in cystic fibrosis. Diabetes Care. 2011;34:292–295. doi: 10.2337/dc10-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ode KL, Frohnert B, Laguna T, Phillips J, Holme B, Regelmann W, Thomas W, Moran A. Oral glucose tolerance testing in children with cystic fibrosis. Pediatr Diabetes. 2010;11:487–492. doi: 10.1111/j.1399-5448.2009.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mozzillo E, Raia V, Fattorusso V, Falco M, Sepe A, De Gregorio F, Nugnes R, Valerio G, Franzese A. Glucose derangements in very young children with cystic fibrosis and pancreatic insufficiency. Diabetes Care. 2012;35:e78. doi: 10.2337/dc12-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uc A, Olivier AK, Griffin MA, Meyerholz DK, Yao J, Abu-El-Haija M, Buchanan KM, Vanegas Calderón OG, Abu-El-Haija M, Pezzulo AA, et al. Glycaemic regulation and insulin secretion are abnormal in cystic fibrosis pigs despite sparing of islet cell mass. Clin Sci (Lond) 2015;128:131–142. doi: 10.1042/CS20140059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olivier AK, Yi Y, Sun X, Sui H, Liang B, Hu S, Xie W, Fisher JT, Keiser NW, Lei D, et al. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest. 2012;122:3755–3768. doi: 10.1172/JCI60610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ode KL, Yi Y, Laguna T, Moran A, Engelhardt JE. Glucose tolerance in infants and young children with cystic fibrosis: poster session abstracts. Pediatr Pulmonol. 2014;49:S216–S456. [Google Scholar]
- 12.Moran A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G, Robinson KA, Sabadosa KA, Stecenko A, Slovis B CFRD Guidelines Committee. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33:2697–2708. doi: 10.2337/dc10-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care. 2009;32:1626–1631. doi: 10.2337/dc09-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caldwell DM, McNamara DH. Fibrocystic disease of the pancreas and diabetes in an adult with unusual pulmonary manifestations. Calif Med. 1958;89:280–284. [PMC free article] [PubMed] [Google Scholar]
- 15.Ode KL, Moran A. New insights into cystic fibrosis-related diabetes in children. Lancet Diabetes Endocrinol. 2013;1:52–58. doi: 10.1016/S2213-8587(13)70015-9. [DOI] [PubMed] [Google Scholar]
- 16.Blackman SM, Hsu S, Ritter SE, Naughton KM, Wright FA, Drumm ML, Knowles MR, Cutting GR. A susceptibility gene for type 2 diabetes confers substantial risk for diabetes complicating cystic fibrosis. Diabetologia. 2009;52:1858–1865. doi: 10.1007/s00125-009-1436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handwerger S, Roth J, Gorden P, Di Sant’ Agnese P, Carpenter DF, Peter G. Glucose intolerance in cystic fibrosis. N Engl J Med. 1969;281:451–461. doi: 10.1056/NEJM196908282810901. [DOI] [PubMed] [Google Scholar]
- 18.Bellin MD, Laguna T, Leschyshyn J, Regelmann W, Dunitz J, Billings J, Moran A. Insulin secretion improves in cystic fibrosis following ivacaftor correction of CFTR: a small pilot study. Pediatr Diabetes. 2013;14:417–421. doi: 10.1111/pedi.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes D, Jr, McCoy KS, Sheikh SI. Resolution of cystic fibrosis-related diabetes with ivacaftor therapy. Am J Respir Crit Care Med. 2014;190:590–591. doi: 10.1164/rccm.201405-0882LE. [DOI] [PubMed] [Google Scholar]
- 20.Alicandro G, Battezzati PM, Battezzati A, Speziali C, Claut L, Motta V, Loi S, Colombo C. Insulin secretion, nutritional status and respiratory function in cystic fibrosis patients with normal glucose tolerance. Clin Nutr. 2012;31:118–123. doi: 10.1016/j.clnu.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Bismuth E, Laborde K, Taupin P, Velho G, Ribault V, Jennane F, Grasset E, Sermet I, de Blic J, Lenoir G, et al. Glucose tolerance and insulin secretion, morbidity, and death in patients with cystic fibrosis J Pediatr 2008152540–545.e1 [DOI] [PubMed] [Google Scholar]
- 22.Lewis C, Blackman SM, Nelson A, Oberdorfer E, Wells D, Dunitz J, Thomas W, Moran A. Diabetes-related mortality in adults with cystic fibrosis. Role of genotype and sex. Am J Respir Crit Care Med. 2015;191:194–200. doi: 10.1164/rccm.201403-0576OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogilvy-Stuart AL. Growth hormone deficiency (GHD) from birth to 2 years of age: diagnostic specifics of GHD during the early phase of life. Horm Res. 2003;60:2–9. doi: 10.1159/000071219. [DOI] [PubMed] [Google Scholar]
- 24.Takano A, Haruta T, Iwata M, Usui I, Uno T, Kawahara J, Ueno E, Sasaoka T, Kobayashi M. Growth hormone induces cellular insulin resistance by uncoupling phosphatidylinositol 3-kinase and its downstream signals in 3T3-L1 adipocytes. Diabetes. 2001;50:1891–1900. doi: 10.2337/diabetes.50.8.1891. [DOI] [PubMed] [Google Scholar]
- 25.Laursen EM, Lanng S, Rasmussen MH, Koch C, Skakkebaek NE, Müller J. Normal spontaneous and stimulated GH levels despite decreased IGF-I concentrations in cystic fibrosis patients. Eur J Endocrinol. 1999;140:315–321. doi: 10.1530/eje.0.1400315. [DOI] [PubMed] [Google Scholar]
- 26.Darmaun D, Hayes V, Schaeffer D, Welch S, Mauras N. Effects of glutamine and recombinant human growth hormone on protein metabolism in prepubertal children with cystic fibrosis. J Clin Endocrinol Metab. 2004;89:1146–1152. doi: 10.1210/jc.2003-031409. [DOI] [PubMed] [Google Scholar]
- 27.Battezzati A, Mari A, Zazzeron L, Alicandro G, Claut L, Battezzati PM, Colombo C. Identification of insulin secretory defects and insulin resistance during oral glucose tolerance test in a cohort of cystic fibrosis patients. Eur J Endocrinol. 2011;165:69–76. doi: 10.1530/EJE-10-1003. [DOI] [PubMed] [Google Scholar]
- 28.Costa M, Potvin S, Hammana I, Malet A, Berthiaume Y, Jeanneret A, Lavoie A, Lévesque R, Perrier J, Poisson D, et al. Increased glucose excursion in cystic fibrosis and its association with a worse clinical status. J Cyst Fibros. 2007;6:376–383. doi: 10.1016/j.jcf.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Battezzati A, Bedogni G, Zazzeron L, Mari A, Maria Battezzati P, Alicandro G, Bertoli S, Colombo C. Age- and sex-dependent distribution of OGTT-related variables in a population of cystic fibrosis patients. J Clin Endocrinol Metab. 2015;100:2963–2971. doi: 10.1210/jc.2015-1512. [DOI] [PubMed] [Google Scholar]
- 30.O’Sullivan BP, Baker D, Leung KG, Reed G, Baker SS, Borowitz D. Evolution of pancreatic function during the first year in infants with cystic fibrosis. J Pediatr. 2013;162:808–812, e1. doi: 10.1016/j.jpeds.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Sterescu AE, Rhodes B, Jackson R, Dupuis A, Hanna A, Wilson DC, Tullis E, Pencharz PB. Natural history of glucose intolerance in patients with cystic fibrosis: ten-year prospective observation program. J Pediatr. 2010;156:613–617. doi: 10.1016/j.jpeds.2009.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.