Abstract
Use of complementary and alternative medicine (CAM; 補充與替代醫學 bǔ chōng yǔ tì dài yī xué) in Parkinson disease (PD) ranged 40–70%. The objective of this study was to determine the frequency, types and factors associated with the use of CAM in Indian PD patients. PD patients, fulfilling UKPD-Society brain-bank diagnostic-criteria, attending Movement-disorders clinic of a tertiary-care teaching hospital in India from 1st May to 15th December 2012 were enrolled. Information on socio-demographic, clinical data and treatment along with factors (source of information, benefits, harms, reason for use and cost) associated with CAM use were recorded. Out of 233 consecutive PD patients, 106 (46%) used CAM. Mean ± SD age of CAM users was 56 ± 11.2 years. Among CAM users, 72% were males, with mean age-onset 49 ± 11.16 years (P = 0.042) and 73% receiving levodopa therapy (p = 0.006). Longer duration PD, higher education (graduates and above), urban residence, and fairly good perceived health were other factors seen among CAM users. Reasons for using CAM were ‘feel good factor’ (73%), 9% took CAM due to side effects from allopathic-medicines. Commonly used CAM were Ayurvedic, homeopathic medicines, and acupuncture (針灸 zhēn jiǔ) [74/106 (70%)]. Median CAM cost in Indian Rupees (INR) was 1000/month (USD16, range: 0-400USD/month in year 2012). Almost half of PD patients use CAM. Three-quarters of Indian CAM using PD patients believe that CAM is harmless, using it at a substantial cost. CAM-users are educated, young, urban dwellers, longer duration PD and receiving levodopa. Commonly used CAM was Ayurvedic, Homeopathic medicines and acupuncture.
Keywords: Parkinson's disease, Complementary and alternative medicine, CAM, Ayurveda, Acupuncture
Graphical abstract
1. Introduction
Complementary and Alternative Medicine (CAM; 補充與替代醫學 bǔ chōng yǔ tì dài yī xué) are a group of management practices that are not part of conventional western medicine.1 In US, 40% of Parkinson's disease (PD) patients use some form of CAM during the course of their illness,2 while in Korea, it is as high as 76%.3 Though using it for long periods and with other medications, most patients are not aware of possible adverse effects and potential drug interactions with their use.4 Traditionally, CAM has been used by PD patients in China, India and Amazon-region in the form of herbal preparations containing anticholinergics, levodopa and MAO-B inhibitors.5 Different forms of CAM include ingestion or, application of preparations on surface of body, activities of different severity such as yoga, meditation, dance, music and exercise. Current study was undertaken on Indian PD patients at a tertiary care teaching hospital to determine frequency, types and factors associated, along with benefits, harms and cost of CAM in PD patients.
2. Material & methods
Consecutive PD patients, fulfilling UKPD-society brain-bank clinical diagnostic criteria,6 attending Movement-disorders clinic from 1st May 2012 to 15th December 2012 were enrolled after obtaining institution's ethics committee approval and informed consent. Information on socio-demographic and clinical data; current treatment and benefits of treatment were recorded. Levodopa equivalent daily dose (LEDD) was calculated according to the conversion factors of individual anti-parkinsonian drugs.7 The LEDD calculation done as; for immediate release levodopa/carbidopa (LD) × (multiply) 1, controlled release LD/carbidopa × 0.75, Entacapone/Stalevo (LCE); LD × 0.33, Duodopa; ×1.11, Pramipexole (as salt)×100, Ropinirole;×20, Rotigotine:×30, Selegiline -Oral;×10, Selegiline-sublingual;×80, Rasagiline;×100, Amantadine;×1, Apomorphine;×10.7 Use of CAM (補充與替代醫學 bǔ chōng yǔ tì dài yī xué) atleast once during the course of disease for atleast one month was considered as CAM used. Other information collected was about the source of information about CAM, any benefit or harm observed, reason for use and cost of therapy. Perceived effect of CAM therapy was assessed using a four-graded Likert scale (worsening = 0, no improvement = 1, mild to moderate improvement = 2, substantial improvement = 3). Perceived health was recorded by a five-grade Likert scale (very bad = 0, bad = 1, fairly good = 2, good = 3, very good = 4). Patients were assisted in filling the format. UPDRS; Unified Parkinson's disease rating scale8 and modified Hoehn & Yahr (H&Y) stage9 of disease were also noted.
3. Statistical analysis
Data was analyzed using SPSS 18.0v and STATA. Descriptive statistics for all variables were obtained to characterize patients. Chi-Square, t-test and Mann–Whitney test assessed differences between CAM users and non-users with respect to socio-demographic and clinical characteristics. t-test and ANOVA were used to asses difference between CAM users and non-users. Logistic regression analysis was used to identify significance of various clinical and epidemiological factors among CAM users and non-users. P-value of <0.05 was considered statistically significant.
4. Results
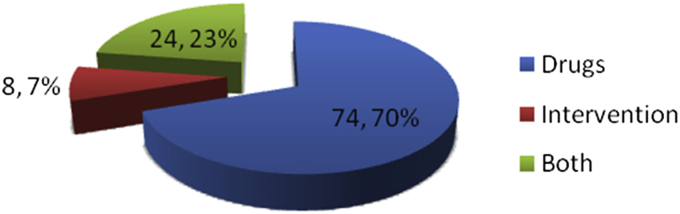
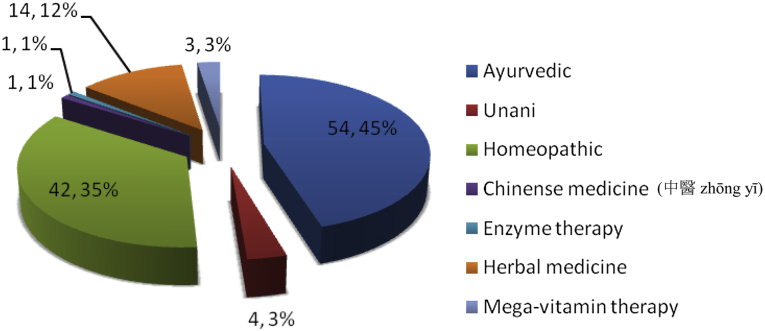
Two hundred and thirty-three PD patients (171, 73.4% men) with mean (SD) age: 57.30 (11.79) years (range 24–85 years), mean (SD) age of onset of PD symptoms: 50 (11.33) years (range 17–76) were enrolled. One hundred and six patients (46%) had used CAM (補充與替代醫學 bǔ chōng yǔ tì dài yī xué) at least once or using currently for at least one month (Table 1, Table 2). Mean ± SD age of patients who had used CAM was 56 ± 11.2 years (range 24–78). Seventy-six of 106 PD patients (72%) were males. Mean age of disease onset of PD among CAM users was younger (49 ± 11.16 years) versus non-users of CAM (52 ± 11.34) years, (p = 0.042). Mean LEDD was not significantly different in patients using CAM (787.65 ± 430.98, range 100–2500 mg) vs. those not using CAM (724.54 ± 434.97, range 30–2149 mg) (p = 0.801) (Table 2). However, significantly higher numbers of CAM users were receiving LD (77; 73%, p = 0.006) as compared to non-CAM user (n = 70; 55%). Longer duration of symptoms of PD (i.e. more than 72 months) (p = 0.001); more years of education (i.e. graduates and above) (p = 0.031), urban residence (p = 0.001), fairly good perceived health status (p = 0.001) were significant variables associated with use of CAM on bivariate as well as multivariate analysis (Table 2). Gender, PD type (tremor dominant vs. akinetic-rigid type), initial and current subjective response to LD therapy, H&Y stage of PD, distance of patients' residence from our institute showed no significant difference regarding use of CAM. Significant difference in use of CAM was observed with the higher UPDRS-motor score on bivariate analysis (P = 0.008; OR = 1.044, 95% CI: 1.01–1.08). Optimum sensitivity and specificity calculated by drawing Receiver Operating Characteristic Curve (ROC), resulted in UPDRS-motor score cut off at 24 was associated with significant use of CAM by 2.4 times (P = 0.012, OR = 2.44 95% CI = 1.20–4.97), suggesting that patients with UPDRS score higher than 24 are more likely to use CAM. Socio-economic status did not determine use of CAM. Most common source of information about CAM was friends (38%) followed by others (32%), which included promoters' advice. 66% patients reported no benefit with CAM and 10% reported harmful effect with CAM (commonest being worsening of symptoms when taken alone without allopathic medicines). Most common reason for taking CAM was “feel good” factor, a belief that CAM will improve their physical status (73%), followed by belief that CAM is harmless (9%). Twenty-nine of 106 (28%) patients took CAM alone and 15 of these 29 (50%) took it in early stage of disease before start of allopathic treatment. Nine of 106 (9%) patients took CAM with a belief that use of allopathic medicines is associated with side effects. In 74 of 106 (70%) patients, CAM included some form of oral medication/massage/special exercise or yogic postures; interventions (e.g. acupuncture, reflexology etc.) in 7% and 23% took both oral preparation as well as intervention (Fig. 1). Most common CAM oral preparation was Ayurvedic followed by homeopathic medicines (Fig. 2). Acupuncture was the most common CAM intervention (Fig. 3). Mean and median cost of CAM in Indian Rupees (INR) was 2210 (∼USD 44, calculated at rates operable in 2012) and 1000 per month (∼USD 16) respectively with range INR 0 to 25000.
Table 1.
Demographic profile of PD patients included in the study.
| Variables | Levels | Value n (%) |
|---|---|---|
| Gender | Males | 171 (73.4) |
| Females | 62 (26.6) | |
| Age; years. Mean ± SDa (range) | 57.30 ± 11.79 (24–85) | |
| Age of PD onset; years. Mean ± SD (range) | 50 ± 11.33 (17–76) | |
| Duration of PD; months. Mean ± SD (range) | 86 ± 63.82 (5–384) | |
| Type of PD | Tremor dominant | 129 (55.4) |
| Akinetic – rigid dominant | 104 (44.6) | |
| H and Y Stage;b n (%) | 1.0 | 17 (10.9) |
| 1.5 | 12 (7.7) | |
| 2.0 | 54 (34.6) | |
| 2.5 | 40 (25.6) | |
| 3.0 | 23 (14.7) | |
| 4.0 | 10 (6.4) | |
| UPDRS-total;c mean ± SD (range) | 24.63 ± 11.60 (4–60) | |
| LEDD (mg);d mean ± SD (range) | 753.5 ± 434.30 (100–2500) | |
| Antiparkinsonian drugs; n (%) | LD/CDe | 147 (63.1) |
| Syncapone (LD/CD/Entacom)f | 53 (22.7) | |
| Rasagiline | 179 (76.8) | |
| Pramipexole | 108 (46.4) | |
| Roperinole | 75 (32.2) | |
| Amantadine | 109 (46.8) |
Standard deviation.
Modified Hoehn & Yahr stage.
Unified Parkinson's disease rating scale.
Levodopa equivalent daily dose.
Levodopa/Carbidopa.
Levodopa + Carbidopa + Entacapone.
Table 2.
Factors associated CAM; Bivariate & multivariate analysis.
| Variables | Levels | CAM (n = 106; 46%) | No CAM (n = 127; 54%) | Bivariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|---|---|---|
| p-value | ORb | 95% CIc | p-value | OR | 95% CI | ||||
| Age in years, mean ± SDa (range) | 56 ± 11.2 (24–78) | 58 ± 12 (33–85) | 0.276 | ||||||
| Gender Males, n (%) | 76 (72) | 95 (75) | 0.285 | 0.853 | 0.48–1.53 | ||||
| Age onset PD in years, mean ± SD (range) | 49 ± 11.16 (17–76) | 52 ± 11.34 (29–76) | 0.042 | 0.976 | 0.95–0.99 | 0.048 | 0.968 | 0.94–1.00 | |
| Duration PD, months; n (%) | <36 | 19 (18) | 38 (30) | 0.001 | 1 | 0.003 | 1 | ||
| 36–72 | 16 (15) | 44 (35) | 0.727 | 0.33–1.61 | 0.913 | 0.37–2.24 | |||
| >72 | 71 (58) | 45 (35) | 3.156 | 1.62–6.14 | 3.081 | 1.48–6.43 | |||
| PD type | Tremor | 56 (53) | 73 (58) | 0.477 | 0.828 | 0.49–1.39 | |||
| Akinetic-rigid | 50 (48) | 54 (43) | 1.00 | ||||||
| dH & Y Stage, mean ± SD (range) | 2.35 ± 0.79 (1–4) | 2.18 ± 0.68 (1–4) | 0.156 | 0.120 | −0.65–0.40 | ||||
| eUPDRS-III mean ± SD (range) | 28 ± 11.73 (11–60) | 22 ± 11.0 (4–59) | 0.008 | 1.044 | 1.01–1.08 | ||||
| gLD/CD prescribed | Yes | 77 (73) | 70 (55) | 0.006 | 2.162 | 1.25–3.75 | |||
| No | 29 (27) | 57 (45) | |||||||
| LD Dose; mg/day in-group, n (%) | <250 | 14 (20) | 18 (23) | 0.801 | |||||
| 250–400 | 30 (43) | 27 (35) | |||||||
| 400–600 | 13 (19) | 15 (20) | |||||||
| >600 | 13 (19) | 17 (22) | |||||||
| fLEDD; mean ± SD (range) | 787.7 ± 431 (100–2500) | 724.5 ± 435 (30–2149) | 0.174 | ||||||
| Initial LD response | >70% | 63 (59) | 63 (50) | 0.532 | |||||
| 25-69% | 22 (21) | 37 (29) | |||||||
| <25% | 8 (8) | 7 (6) | |||||||
| No response | 5 (5) | 5 (3) | |||||||
| Cannot qualify | 8 (8) | 15 (12) | |||||||
| Current LD response | >70% | 45 (43) | 51 (40) | 0.736 | |||||
| 25–69% | 34 (32) | 44 (35) | |||||||
| <25% | 12 (11) | 11 (9) | |||||||
| No response | 7 (7) | 6 (5) | |||||||
| Cannot qualify | 8 (8) | 15 (12) | |||||||
| Perceived health, n (%) | Quite bad | 7 (7) | 1 (1) | 0.001 | <0.001 | 1 | |||
| Bad | 19 (14) | 16 (13) | 0.424 | 0.390 | 0.04–3.91 | ||||
| Fairly good | 62 (59) | 59 (47) | 0.199 | 0.234 | 0.03–2.15 | ||||
| Good | 18 (17) | 51 (41) | 0.017 | 0.004 | 0.01–0.61 | ||||
| Education | Illiterate | 4 (3.8) | 11 (8.7) | 0.002 | 1 | 0.006 | 1 | ||
| <X std. | 13 (12) | 36 (28) | 0.992 | 0.993 | 0.27–3.67 | 0.985 | 1.014 | 0.24–4.25 | |
| X/XII std. | 31 (29) | 38 (30) | 0.201 | 2.243 | 0.65–7.74 | 0.315 | 0.205 | 0.52–7.76 | |
| >Graduate | 58 (55) | 44 (33) | 0.031 | 3.798 | 1.13–12.75 | 0.040 | 0.049 | 1.07–15.35 | |
| Distance from hospital, hKm; n (%) | <100 | 62 (58) | 83 (65) | 0.254 | |||||
| 100–200 | 15 (14) | 9 (7) | |||||||
| 200–500 | 10 (9) | 16 (13) | |||||||
| >500 | 19 (18) | 19 (15) | |||||||
| Location, n(%) | Rural | 5 (4.7) | 24 (19) | 0.001 | 1.00 | 0.011 | 1.00 | ||
| Urban | 101 (95) | 103 (81) | 0.002 | 4.707 | 1.73–12.82 | 4.119 | 1.38–12.29 | ||
Bold values are the values which are statistically significant.
Standard deviation.
Odds ratio.
confidence interval.
modified Hoehn & Yahr stage.
Unified Parkinson's disease rating scale.
Levodopa equivalent daily dose.
Levodopa/Carbidopa.
Kilometer.
Fig. 1.
CAM (補充與替代醫學 bǔ chōng yǔ tì dài yī xué) modalities (n, percentage of CAM modalities).
Fig. 2.
CAM* drugs (n, percentage of CAM modalities). * Complementary and Alternative Medicines.
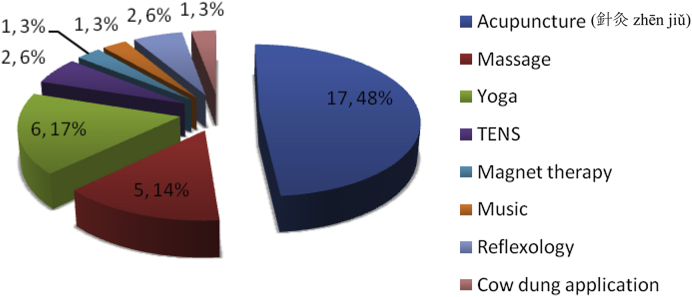
Fig. 3.
CAM* interventions (n, percentage of CAM modalities). * Complementary and Alternative Medicines.
5. Discussion
Our study shows that CAM (補充與替代醫學 bǔ chōng yǔ tì dài yī xué) is quite a common modality of treatment among PD patients attending our Movement Disorders clinics. About half (46%) of our Indian PD patients report using it, which is similar to that observed by other authors,2, 3, 10, 11, 12 being higher than reported in USA and lower than reported in Korea. India being the seat of origin of Ayurveda, it was our belief that use of CAM would be higher in India. Also, since per capita income and consequently paying capacity of Indians is lower than that in the western countries, we believed that CAM being less expensive and easily available would be practiced by more Indian PD patients as compared to PD patients in USA and European countries. However this systematic study showed results contrary to our belief. Our own results in Indian PD patients showed that, more educated, those residing in urban setting, individuals who have better access to information, more knowledge, higher paying capacity and higher education level use CAM more often.
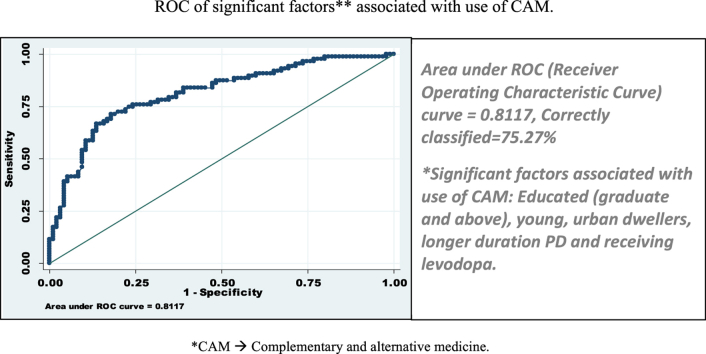
Variables in disease profile, which showed association with CAM use, were younger age of symptom onset, longer disease duration, higher baseline UPDRS motor score, fairly good perceived subjective health and use of l-dopa. Socio-demographic factors associated with CAM use were higher education status (more than graduation) and urban living. These all variables showed overall predictor accuracy of use of CAM as 75.27% (or factors were correctly classified in 75.27%) excluded patients having higher baseline UPDRS motor score, fairly good perceived health and using l-dopa (Fig. 4). In earlier studies, use of complementary therapy for PD correlated significantly with younger age of onset of symptoms.2, 13 Longer disease duration was also associated with higher CAM use, similar observation was made in previous studies.3, 12 Another study from Singapore comprising of 159 PD patients, observed rate of starting CAM was 1.2/100 person months. At 3 years after the diagnosis of PD, 48% of participants were using CAM, at 5 years 62% and by 10 years 75% had started using CAM. Median time of starting CAM in PD was 38 months (95% CI: 25–51).11 Relationship with duration of disease and CAM use has not been observed by many authors.2, 11 PD patients with higher mean UPDRS motor score, suggesting severe/advanced disease was more often associated with CAM use in our study. This observation is supported by previous study, which showed 2.5 times greater risk for CAM use among participants with higher baseline motor score (P = 0.031; 95% CI: 1.1–5.8).11 However, in that study the cut off UPDRS was 16, while among Indian PD patients it was much higher, that is 24. UPDRS-motor score of 24 or above was associated with 2.44 times higher risk of use of CAM, suggesting that Indian PD patients probably can endure more disability before they seek CAM. In our cohort, there was one patient with UPDRS of 37 who was not taking any treatment at all, further supporting our belief. We did not find CAM use having any relation with other parameter of disease severity such as H&Y stage as observed by other authors.2, 3, 10 We did not find any gender preference for use of CAM, although other authors12, 14 observed females used CAM more often than males.
Fig. 4.
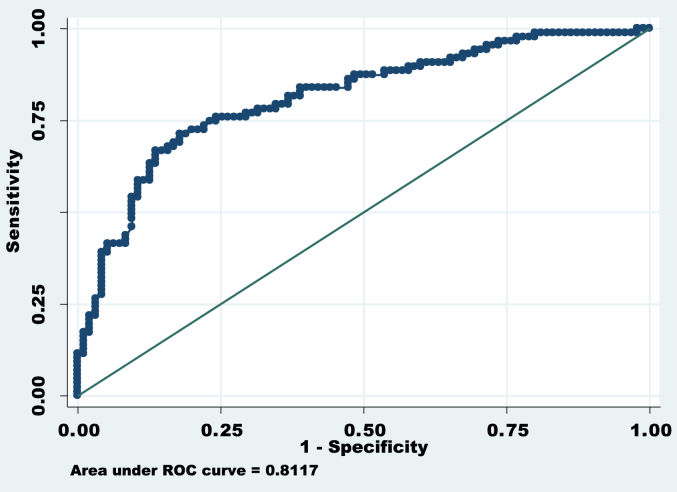
ROC of significant factors*. Area under ROC (Receiver Operating Characteristic Curve) curve = 0.8117, Correctly classified = 75.27%. * Factors included for ROC curve are: Age of onset, duration of PD, education status, residence of patients (urban or rural).
Interestingly our study showed that PD patients who were prescribed l-dopa used CAM more often than those who were not prescribed l-dopa. Probable reason for this is that those in whom l-dopa was started had more advanced disease requiring higher dopa-agonist drugs dose/l-dopa to treat symptoms of PD. Though use of l-dopa was associated with higher risk of CAM use, its dose did not influence use of CAM. This observation was at variance with other studies.3, 12 Mean LEDD did not differ between CAM users and non-users in our study. Generally, Indian patients receive lower mean LD dose and LEDD as compared to various other cohorts.3, 12 This may be because Indian PD patients have lower body weight or they are cautious about side effects of drugs and are reluctant to increase drug dose.
Better subjective perception of health status among CAM users was observed in our study. This is in difference from observation of other authors. One study from Sweden, although not included PD patients, reported that chronically ill patients used CAM more often.15 In another study patients who perceived poor subjective health status, used CAM more often.12 We believe our observation can possibly be explained as follows: people, who have positive attitude towards life, perceive their health as “good” even though they may be having more advanced/severe disease and try all modalities that may be available for improving their status further, including CAM. We can only contemplate on this aspect, since we did not study personality trait of our patients. Higher use of CAM among PD patients with higher education and urban living possibly supports the hypothesis that availability of information/knowledge through magazines, Internet/television and easy access to these are reasons for higher use in these patients. In our study, PD patients with higher education level i.e. graduation or more were more likely to use CAM, which is an observation similar to other studies.2, 12, 13 In a Swedish population-based study on patients with cardiovascular disease, 30.5% reported CAM use in the preceding 2 weeks observed CAM use more frequently in persons with higher education,16 supporting our observation. Similarly, patients living in urban set-up use CAM more often because they have access and competency to utilize all possible sources of therapy. According to a telephone survey in USA, 43% of general population had used CAM during the previous year.17 A survey done on 1001 random sample in Sweden found 49% of population surveyed were CAM users, as compared to 34% from Norway and 45% in Denmark.14
Socioeconomic status did not determine use of CAM among PD patients in our study, which is not in agreement with observation of other.2, 13 Possibly, in India many different types of CAM are easily available at affordable cost. Paucity of Neurologists and low ratio of neurologist to general population results in non availability of neurologists to a large number of population, forcing most PD patients to seek other options, which may be easily available.
Most common source of information was friends followed by others, which included promoters' advice as seen on television and Internet. This suggests availability of information to PD patients with higher education and living in urban setup. Majority of CAM users did not obtain any benefit from it (66%) while 10% additionally reported harms with CAM use (most common being worsening of symptoms when taken alone without allopathic medicines), possibly because of progression of symptoms due to progressive nature of disease. However we cannot rule out the benefits or harms of CAM on the basis of this cross sectional study. Large randomized trial (well designed and well defined homogenous intervention of CAM) is required to see the benefits or harms of CAM on PD patients. Most common reason for taking CAM was feel good factor (73%), a belief that CAM will improve their physical status followed by belief that CAM is harmless (9%). ‘Feel good factor’ is a widespread feeling of well-being and financial security.18 Many patients considered ‘Feel good factor’ as a feeling of general well being that makes people feel happy and positive about their lives, and that the intervention will do them good. Twenty-nine of 106 (28%) patients used CAM alone and 15 of these 29 (50%) took it before start of allopathic treatment, while 9 of 106 (9%) took CAM due to side effects from allopathic medicines. It is often believed that CAM including Ayurvedic medicines etc. does not have any side effects; therefore many patients try these without informing their physicians.
Seventy percent of CAM included some form of drugs (Fig. 1); like Ayurvedic (majority) and homeopathic medicine. Other forms of CAM drugs used are as mentioned in Fig. 2, few could identify these Ayurvedic medicine as powder of seeds of Macuna pruriens commercially available in India, ‘Ashwagandha’, ‘panchkarma’, ‘Brahmi’, ‘Jeeva mala’, Kampavatras', ‘Mochpak’, ‘Swarasta rist’, ‘Til oil’, ‘Nutralite’. Powder of dried seeds of Macuna pruriens is a natural sources of levodopa, Macuna Pruriens and ‘Vicia faba’ have been studied in small randomized trials but neither has been tested formally against conventional levodopa in randomized blinded trials to show its equivalence, superiority/inferiority or comparative study of side effects.19, 20 ‘Ashwagandha’ (herb; Withania somnifera, family Solanaceae) Sitoindosides, Withaferin-A and acyl-steryl-glucosides in Ashwagandha and ‘Brahmi’ an herb from Bacopa Monnieri family: (scrophulariaceae, water hyssop.) have been used in PD and other neurodegenerative disorders.21, 22 ‘Panchkarma’; consisting of five types of therapeutic measures/activities.23 ‘Til oil’ or Sesame seed oil is one of the common edible oil in India and in other Asian countries; a study has evaluated its neuroprotective effect in PD mice model and shows a good neuroprotective and anti-inflammatory benefit.24 Another modality is herbal medicines such as ‘Aloevera juice’ (Ayurvedic Miracle Plant), ‘Wheat grass’, ‘Amla (India gooseberry) juice’, ‘Balwach’, ‘Sumanta’. Aloe Vera Barbadenis, is used for therapy in formulations as external application or oral use ‘Aloe vera’ leaves contain 96% water and also Vitamins A, B, C, E, calcium, amino acids etc. ‘Amla juice’ or Indian gooseberry (Phyllanthus emblica or Emblica officinalis (油柑 Yóu Gān)) is rich in vitamin C and is extensively used in Ayurveda. One unusual CAM was oral consumption of placental cord blood reported by one PD patient. Among interventions (Fig. 1, Fig. 3), acupuncture (48%) was the most commonly utilized and other reported interventions were ‘Yoga’, ‘Massage’, ‘Mud application’ over whole body, Transcutaneous electric nerve stimulation’ (TENS), ‘Magnet therapy’, ‘Cow dung’ application over whole body, foot reflexology and music therapy. Twenty-one percent of PD patients took both CAM drugs and interventions.
Mean and median cost of CAM per month was INR 2211 (∼USD36, in 2012) and 1000 (∼USD16) per month respectively (range INR 0–25000), whereas the mean ± SD per capita income per month of our study cohort was INR 12,202.70 ± 9073 (range INR 1200–50000). According to Indian government sources per capita income of an Indian at current price was INR 53,331/year in 2011–12 which makes per capita income per month as INR 4,444.25.25 It appears our study population had higher per capita income per month in comparison to average Indian. Our PD cohort used almost 20–40% of their monthly income on CAM. It is to be noted that for most Indian patients treatment cost is not reimbursed and must be borne by the individual.
There are many gray areas in CAM; lack of randomized double blind trials published in peer reviewed journals, as well as heterogeneity of products and practices. Due to relatively high prevalence of CAM use in our study and in others, physicians should routinely enquire patients about CAM, as its use could increase risk of unwanted side effects, drug interactions, contamination of CAM with other agents, or trauma inflicted by inexperienced CAM practitioners. Once informed, physicians should respect patient's beliefs, perspectives, and cultural practices, and be open-minded about evaluating CAM treatment together with them. This raises ethical and professional problems as physician has a responsibility to ensure that patients' management is safe and in agreement with scientific tenets.26 To assure effect of CAM, it is important to have objective means of rating patients' state before and after treatment.27 Thus, there is a need for evidence-based effectiveness and safety information about CAM therapies, together with respect for patients' beliefs and choices.
6. Limitations
Concerning the functional outcome after use of CAM (補充與替代醫學 bǔ chōng yǔ tì dài yī xué) is not observed using objective scales and also we did not follow up patients for a significant longer duration to assess any form of benefits or harms related to CAM. Also there is large diversity of CAM therapeutic interventions in this study, and not clearly defined. Also this cross sectional survey was not designed to specifically study the prevalence of use of Ayurveda but all the complementary and alternative medicines in Indian PD patients. This is the limitation of the study that various subtypes of Ayurveda treatment have not been studied. Duration of the CAM treatment has not been investigated. It needs a well-designed randomized study with well-defined homogenous intervention of CAM.
7. Conclusion
Forty-six percent of Indian PD patients attending a tertiary care teaching hospital in India use CAM (補充與替代醫學 bǔ chōng yǔ tì dài yī xué). Most commonly used CAM is Ayurveda therapy. Early age at symptom onset, longer duration of illness, urban residence, higher education status, fairly good perceived subjective health, higher disease severity (UPDRS-motor score), and levodopa use, are associated with higher use of CAM. However, levodopa dose and LEDD did not influence use of CAM. Many PD patients reported no benefits with CAM in this cross sectional study; however, it needs to be confirmed in large well-designed randomized trials. Median expense of CAM in Indian Rupees is 1000 per month (∼USD 16 in year 2012) with range of INR 0–25000; which is approximately 0–25% of per capita income. Knowledge about use of CAM will help to understand any drug related side effects or drug inter-actions in the patients.
Conflict of interest
None.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Pearson N.J., Chesney M.A. The CAM Education Program of the National Center for Complementary and Alternative Medicine: an overview. Acad Med J Assoc Am Med Coll. 2007;82:921–926. doi: 10.1097/ACM.0b013e31814a5014. [DOI] [PubMed] [Google Scholar]
- 2.Ferry P., Johnson M., Wallis P. Use of complementary therapies and non-prescribed medication in patients with Parkinson's disease. Postgrad Med J. 2002;78:612–614. doi: 10.1136/pmj.78.924.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim S.R., Lee T.Y., Kim M.S., Lee M.C., Chung S.J. Use of complementary and alternative medicine by Korean patients with Parkinson's disease. Clin Neurol Neurosurg. 2009;111:156–160. doi: 10.1016/j.clineuro.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Bush T.M., Rayburn K.S., Holloway S.W. Adverse interactions between herbal and dietary substances and prescription medications: a clinical survey. Altern Ther Health Med. 2007;13:30–35. [PubMed] [Google Scholar]
- 5.Manyam B.V., Sánchez-Ramos J.R. Traditional and complementary therapies in Parkinson's disease. Adv Neurol. 1999;80:565–574. [PubMed] [Google Scholar]
- 6.Hughes A.J., Daniel S.E., Kilford L., Lees A.J. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomlinson C.L., Stowe R., Patel S., Rick C., Gray R., Clarke C.E. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord Off J Mov Disord Soc. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 8.http://www.mdvu.org/library/ratingscales/pd/updrs.pdf.
- 9.Goetz C.G., Poewe W., Rascol O. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord Off J Mov Disord Soc. 2004;19:1020–1028. doi: 10.1002/mds.20213. [DOI] [PubMed] [Google Scholar]
- 10.Rajendran P.R., Thompson R.E., Reich S.G. The use of alternative therapies by patients with Parkinson's disease. Neurology. 2001;57:790–794. doi: 10.1212/wnl.57.5.790. [DOI] [PubMed] [Google Scholar]
- 11.Tan L.C.S., Lau P.-N., Jamora R.D.G., Chan E.S.Y. Use of complementary therapies in patients with Parkinson's disease in Singapore. Mov Disord Off J Mov Disord Soc. 2006;21:86–89. doi: 10.1002/mds.20662. [DOI] [PubMed] [Google Scholar]
- 12.Lökk J., Nilsson M. Frequency, type and factors associated with the use of complementary and alternative medicine in patients with Parkinson's disease at a neurological outpatient clinic. Park Relat Disord. 2010;16:540–544. doi: 10.1016/j.parkreldis.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Ryan M., Johnson M.S. Use of alternative medications in patients with neurologic disorders. Ann Pharmacother. 2002;36:1540–1545. doi: 10.1345/aph.1A455. [DOI] [PubMed] [Google Scholar]
- 14.Hanssen B., Grimsgaard S., Launsø L., Fønnebø V., Falkenberg T., Rasmussen N.K. Use of complementary and alternative medicine in the Scandinavian countries. Scand J Prim Health Care. 2005;23:57–62. doi: 10.1080/02813430510018419. [DOI] [PubMed] [Google Scholar]
- 15.Al-Windi A. Determinants of complementary alternative medicine (CAM) use. Complement Ther Med. 2004;12:99–111. doi: 10.1016/j.ctim.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson M., Trehn G., Asplund K. Use of complementary and alternative medicine remedies in Sweden. A population-based longitudinal study within the northern Sweden MONICA Project. Multinational Monitoring of trends and determinants of cardiovascular disease. J Intern Med. 2001;250:225–233. doi: 10.1046/j.1365-2796.2001.00882.x. [DOI] [PubMed] [Google Scholar]
- 17.Eisenberg D.M., Davis R.B., Ettner S.L. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA J Am Med Assoc. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 18.http://www.oxforddictionaries.com/definition/english/feel-good-factor?q=feel-good+factor.
- 19.Katzenschlager R., Evans A., Manson A. Mucuna pruriens in Parkinson's disease: a double blind clinical and pharmacological study. J Neurol Neurosurg Psychiatry. 2004;75:1672–1677. doi: 10.1136/jnnp.2003.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabey J.M., Vered Y., Shabtai H., Graff E., Korczyn A.D. Improvement of parkinsonian features correlate with high plasma levodopa values after broad bean (Vicia faba) consumption. J Neurol Neurosurg Psychiatry. 1992;55:725–727. doi: 10.1136/jnnp.55.8.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calabrese C., Gregory W.L., Leo M., Kraemer D., Bone K., Oken B. Effects of a standardized Bacopa monnieri extract on cognitive performance, anxiety, and depression in the elderly: a randomized, double-blind, Placebo-controlled trial. J Altern Complement Med. 2008;14:707–713. doi: 10.1089/acm.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh H.K., Dhawan B.N. Neuropsychopharmacological effects of the ayurvedic nootropic Bacopa monneri Linn. (Bramhi) Indian J Pharmacol. 1997;29:359–365. [Google Scholar]
- 23.Conboy L., Edshteyn I., Garivaltis H. Ayurveda and Panchakarma: measuring the effects of a holistic health intervention. ScientificWorldJournal. 2009;9:272–280. doi: 10.1100/tsw.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad S., Khan M.B., Hoda M.N. Neuroprotective effect of sesame seed oil in 6-hydroxydopamine induced neurotoxicity in mice model: cellular, biochemical and neurochemical evidence. Neurochem Res. 2012;37:516–526. doi: 10.1007/s11064-011-0638-4. [DOI] [PubMed] [Google Scholar]
- 25.http://indiabudget.nic.in/es2011-12/estat1.pdf.
- 26.Lynöe N. Ethical and professional aspects of the practice of alternative medicine. Scand J Soc Med. 1992;20:217–225. doi: 10.1177/140349489202000406. [DOI] [PubMed] [Google Scholar]
- 27.Henneberg A. Additional therapies in Parkinson's disease patients: useful tools for the improvement of the quality of life or senseless loss of resources? J Neurol. 1998;245(suppl 1):S23–S27. doi: 10.1007/PL00007734. [DOI] [PubMed] [Google Scholar]