Abstract
The success of an HIV vaccine will require induction of a protective immune response in the most at-risk populations. The increased incidence of HIV infection in high-risk populations is assumed to be primarily the result of more frequent exposure to the virus or a greater inoculum of the virus; however, underlying variations in immune homeostasis may also contribute to HIV susceptibility and potentially impact vaccine responses and those required for protection. As an effective humoral immune response is likely to be a critical component of a protective HIV vaccine, we evaluated the steady-state phenotypic profile of peripheral blood B cells by flow cytometry from participants in the HIV Vaccine Trials Network (HVTN) 203 Phase 2a HIV vaccine trial considered to be at higher risk and lower risk for HIV acquisition. Overall, high-risk participants exhibited increased frequency of unswitched IgM memory and activated switched IgD−CD95+ memory B cells than low-risk participants. Most (93%) of the high-risk male participants were men who have sex with men who engaged in high-risk sexual behavior. High-risk males had a significantly increased frequency of CXCR3+ IgD−CD95+ B cells than low-risk males. These results suggest that high-risk populations have altered B cell homeostasis. The increased frequency of activated and memory B cells may suggest increased immune activation in high-risk populations, which may contribute to possible differential responses to HIV vaccine strategies.
Keywords: : HIV, B cell, IgM, CXCR3, HIV in men who have sex with men (MSM), vaccines
Introduction
A consistent outcome from HIV vaccine efficacy trials thus far is the lack of convincing efficacy in high-risk populations. The RV144 Phase 3 “Thai Trial” was a “community-risk trial” and primarily enrolled low-risk heterosexual subjects and resulted in overall vaccine efficacy of 31%. When stratified in a post hoc analysis for risk, however, efficacy was only 4% for high-risk subjects, but was 40%–47% for low- to medium-risk subjects.1 In trials targeting high-risk populations, such as VAX003, VAX004, HIV Vaccine Trials Network (HVTN) 503 (the Step Study), and HVTN 505, efficacy was not observed.2 Although increased rates or quantities of exposure to HIV may also account for lack of efficacy, immunological alterations resulting from high-risk behavior may also make vaccine efficacy more difficult to achieve. Overall, the immune profile of high-risk populations is poorly resolved, although increased immune activation has been observed in high-risk men who have sex with men (MSM)3,4 and injection drug users.5–7 Immune activation, particularly increases in activated CD4 T cells, may increase susceptibility to HIV by providing additional viral targets or facilitating rapid viral dissemination. It is becoming increasingly evident that variations in the subtle qualitative aspects of the humoral HIV vaccine response may influence its efficacy.8,9 The steady-state immune profile of a given individual reflects the context in which the vaccine response will occur, and may impact the magnitude, quality, and ultimate efficacy of a vaccine strategy.
We hypothesized that high-risk populations have altered B cell profiles, which may shed light on improved approaches to optimize immunogenicity. To address this, a pilot study to conduct steady-state B cell phenotyping was performed on samples obtained from the HVTN 203 Phase 2a trial.10 HVTN 203, a predecessor to RV144 conducted in the United States, included participants that were both at low risk (LR) and high risk (HR) for HIV infection based on their behavioral history.
Materials and Methods
Study design
Cryopreserved peripheral blood mononuclear cells (PBMCs) were obtained from participants in the HVTN protocol 203 phase 2a trial 1 year after the final immunization.10 The study enrolled healthy HIV-1-uninfected adults aged 18–60 years. Risk for HIV infection was assessed at study entry based on a standardized interview of past and current sexual and drug use behaviors. Low-risk participants were defined as either being in a mutually monogamous relationship with a known or presumed HIV seronegative partner for at least the past 6 months and no injection drug use, or having no newly acquired higher risk-associated sexually transmitted infection (i.e., excluding scabies, yeast, pediculosis pubis, bacterial vaginosis, and anogenital warts), no possibly safe or unsafe sex with a known HIV+ individual or an active injection drug user in the past 6 months, unsafe sexual activity with two or fewer partners within the past 6 months, possibly safe sexual activity with four or fewer partners within the past 6 months, and no injection drug use. High-risk participants were defined as meeting at least one of the following criteria: a newly acquired higher risk-associated sexually transmitted infection (chancroid, chlamydia, gonorrhea, syphilis, and trichomoniasis) within the past 6 months, unsafe sexual activity with three or more partners within the past 6 months, possibly safe or unsafe sex with a known HIV+ individual in the past 6 months, possibly safe sexual activity with five or more partners in the past 6 months, or any injection drug use in the past 1 year. All subjects were in good general health and HIV seronegative. All participants provided written informed consent, and each of the 10 trial sites obtained approval for the study through their local institutional review boards. In addition, this post-trial follow-up analysis of the existing samples was approved by the Research Subjects Review Board at the University of Rochester Medical Center.
Flow cytometry
PBMCs were stained similar to as previously described11,12 with anti-CD19-APC-Cy7 (SJ25C1; BD Biosciences), anti-CD20-AlexaFluor 700 (2H7; Biolegend), anti-CD3-PacificOrange (UCHT1; Invitrogen), anti-IgD-FITC (IA6-2; BD Biosciences), anti-IgG-APC (G18-145; BD Biosciences), anti-IgM-PE-Cy5 (G20-127; BD Biosciences), anti-CD27-Qdot655 (CLB-27/1; Invitrogen), anti-CD21-V450 (B-ly4; BD Biosciences), anti-HLA-DR-Qdot800 (Tü36; Invitrogen) anti-FcRL4-biotin (R&D Biosystems), and streptavidin Qdot705 (Invitrogen), anti-CD38-Qdot605 (HIT2; Invitrogen), anti-CD95-PE-Cy7 (DX2; Biolegend), anti-CD24-PE-AlexaFluor610 (SN3; Invitrogen), anti-CD183-PE (1C6/CXCR3; BD Biosciences), and Live/Dead fixable aqua dead cell stain (Invitrogen). One-to-two million total events per sample were recorded on an LSRII instrument (BD Biosciences). Staining and analysis were performed in a blinded manner, linking sample with experimental group after manual gating was completed using FlowJo software (Treestar, Inc.). Total PBMCs were gated on lymphocytes using forward scatter and side scatter. Live/Dead stain and anti-CD3 were used to exclude dead cells and T cells, respectively.
Statistical analysis
Two-tailed Mann–Whitney test was used to compare groups using Prism 5.0 software (GraphPad Software). No correction for multiple comparisons was performed because of the post hoc exploratory nature of this assessment.
Results
Study design and demographics
To compare the B cell compartment between subjects at LR and HR for HIV infection, PBMCs were analyzed from HVTN 203. HVTN 203 was a Phase 2a HIV vaccine trial conducted in the United States, and a predecessor to the RV144 HIV vaccine trial. The trial evaluated various combinations of the clade B-targeted AIDSVAX B/B HIV Envelope protein and ALVAC-vCP1452 Canarypox immunogens.10 This study enrolled both subjects at LR and HR for HIV infection based on their behavioral profiles.
To compare the steady-state global peripheral B cells between HR and LR participants, a subset of PBMC samples (n = 129 participants total) that were collected 1 year after the final immunization from all groups were utilized. This time point was selected based on sample availability and because immunization-specific effects on the global B cell compartment are expected to be minimal at such a late time.13–15 The LR group was 60% female, whereas the HR group was 82% male (Table 1). Nonheterosexual subjects comprised 38% and 78% of the LR and HR groups, respectively. Both groups were primarily white (>84%). HR participants were primarily (76%) MSM who engage in high-risk sexual behavior and, therefore, we also performed analysis focused only on male participants. Among males, of the LR and HR groups, 55% and 93% were MSM, respectively, and 76% and 100% were white, respectively. Owing to the limited number of non-MSM males in the study (n = 18), our primary focus was stratifying the participants by risk group and not by MSM status.
Table 1.
Study Demographics
| Total | Male Only | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Age | White | Black | Other | Nonhetero | Age | White | Black | Other | Nonhetero | |
| Low risk | 46 (83.6) | 33 (44.6) | 35.8 (20–52) | 67 (84.8) | 11 (13.9) | 1 (1.3) | 30 (38.0) | 34.2 (19–56) | 25 (75.8) | 7 (21.2) | 1 (3.0) | 18 (54.5) |
| High risk | 9 (17.4) | 41 (55.4) | 33.2 (19–58) | 48 (96) | 1 (2) | 1 (2) | 39 (78.0) | 36.3 (20–52) | 41 (100) | 0 (0) | 0 (0) | 38 (92.7) |
| Total | 55 (42.6) | 74 (57.4) | 34.2 (19–58) | 115 (89.1) | 12 (9.3) | 2 (1.6) | 69 (53.5) | 35.3 (19–56) | 66 (89.2) | 7 (8.3) | 1 (2.5) | 56 (75.7) |
Number of subjects (percentage).
Global B cell phenotype
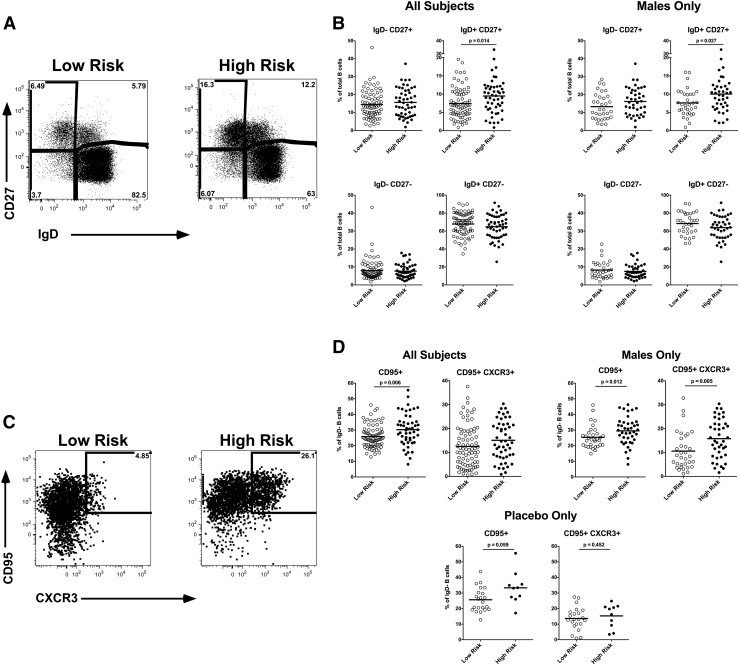
Using flow cytometry, we evaluated the frequency and phenotype of B cell subsets. Using IgD and CD27, we were able to identify the major B cell subsets including IgD+CD27− that included naive and transitional B cells, IgD+CD27+ that includes unswitched IgM memory, IgD−CD27+ the switched CD27+ memory, and the IgD−CD27− minor switched memory population (Fig. 1A). As expected, the majority of the B cells are IgD+CD27−, and IgD−CD27− B cells are a minor subset. We observed a significant increase in IgD+CD27+ memory B cells in HR, both overall (p = .014) and when evaluating male participants only (p = .027) (Fig. 1B). This IgM memory B cell subset is particularly interesting as it is thought to be important in T-independent B cell responses, including against bacterial polysaccharides, and has unique immunoglobulin repertoire features compared with classical IgD+CD27− switched memory B cells.16
FIG. 1.
Peripheral blood B cell phenotype. Peripheral blood mononuclear cell samples from subjects (n = 129) were stained and evaluated by flow cytometry. (A) Representative plots gated on live, CD3−, CD19+ total B cells. (B) Frequency of IgD/CD27 subsets. (C) Representative plots gated on IgD− CD19+ total B cells. (D) Frequency of CD95/CXCR3 subsets. Each symbol represents an individual subject. Black line represents group mean.
To further evaluate the IgD− switched memory B cells, which predominantly include IgG+ and IgA+ memory B cells,17 we assessed CD95, a marker of B cell activation,18,19 and CXCR3 (CD183) expression within this compartment (Fig. 1C). In HR overall, there was a significantly (p = .006) increased frequency of CD95+ IgD− memory B cells (Fig. 1D). This was particularly evident in the CXCR3+ CD95+ IgD− memory B cells in the HR male participants (p = .005). The functional significance of the CXCR3+ B cell subset is unclear, in T cells, CXCR3 is associated with Th1 bias, although the functions of CXCR3+ B cells remain to be determined. The observation of increased CD95+ IgD− memory B cells was even apparent, although not achieving significance (p = .059) when comparing the HR and LR participants from only the participants who received placebo. Similarly, there were no significant differences in B cell subsets when comparing placebo and vaccine recipients (data not shown), suggesting that the immunization did not contribute to the increased memory B cell subsets in the HR participants who were observed at this time point.
Despite the limited number of non-MSM males in our study, we did observe a significant trend of increased total B cells, IgD+CD27+, and IgD−CD95+CXCR3+ memory B cells when comparing MSM with non-MSM males independent of risk classification (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/aid), suggesting MSM status alone may be associated with B cell subset perturbations.
Discussion
Dramatic differences in the B cell profile of HR subjects were not evident, which is consistent with all the study subjects (HR and LR) being healthy and HIV-1 negative. However, we identified significant increases in unswitched IgD+CD27+ IgM memory and switched IgD−CD95+ activated B cells in HR subjects, suggesting these nonoverlapping subsets may be more sensitive to the consequences of high-risk activity. These memory B cell subsets may be increased in HR subjects because of increased antigenic insult and subsequent antigen-specific-driven expansion, or perhaps these memory B cells are responding in an antigen-independent manner to potential increases in inflammatory cytokines, TLR ligands, or other mediators that may be elevated because of high-risk activity.
The peripheral blood IgD+CD27+ subset referred to grossly as unswitched IgM memory, based on numerous studies, is very heterogeneous and includes marginal zone-like, IgM-only, and regulatory B cell subpopulations.20–28 IgM memory develops early during an immune response, responds quickly to restimulation, is very efficient in protecting from encapsulated bacteria, and suggested to be a first line of defense against infection.16,29 IgM memory B cells are potently stimulated by neutrophils,29,30 which have previously been reported to be elevated in high-risk populations,31–33 and may represent a mechanism for the increase in IgM memory we observed in HR subjects.
CXCR3+ B cells have previously been associated with migration to inflamed tissue34–37 and may secrete interferon γ (IFNγ)38 similar to their CD4 T cell counterpart; CXCR3+ Th1 cells. CXCR3 (CD183) ligands include the IFN-induced chemokines CXCL9 (MIG) and CXCL10 (IP-10), which have been shown to be systemically increased in high-risk populations.39
Overall, there are few studies that have evaluated antibody or B cell responses to vaccines in HR subjects. However, decreased antibody responses to HPV vaccines among MSM have been reported,40,41 and decreased Hepatitis A and B vaccine responses have been reported among injection drug users.42,43 The limitations of this exploratory study include the lack of functional assessment of the B cells and correlation of B cell phenotypes with HIV-specific responses. In addition, and also outside the capacity of this study, a longitudinal assessment would be valuable to determine whether increased B cell memory subsets are a persistent or transient feature among HR subjects.
The significance of increased IgM memory and IgD−CD95+ memory B cells in HR subjects in relation to HIV prevention is likely multifaceted, at the gross level they may just be an indicator of increased immune activation, which has been associated with elevated risk of HIV transmission, additionally their increase may suggest that high-risk behavior alters B cell responses, which could extend to influencing the quality and efficacy of the humoral response to HIV infection or an HIV vaccine. Indeed, in HR subjects, who may have B cells that are already in a heightened state of activation, perhaps a fundamentally different approach to vaccine design and adjuvant selection compared with LR subjects will be required to induce protective responses.
Supplementary Material
Acknowledgments
We would like to acknowledge the HVTN 203 Study Team and the HVTN Core members, in particular Mary Gross, Nicholas Hopkinson, John Hural, and Courtney Liebi. We are grateful for the assistance of the University of Rochester Flow Cytometry Core Facility, Catherine Bunce, and extend our deepest gratitude to the participants in HVTN 203. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) U.S. Public Health Service Grant UM1 AI068614 (LOC: HIV Vaccine Trials Network) as part of the HVTN Initiatives Program (HIP). Additional funding was provided by the University of Rochester Center for AIDS Research (CFAR), an NIH-funded program (P30 AI078498), NIH/NIAID 5R0AI117787, and funding provided by the University of Rochester Department of Medicine.
Authors' Contributions
This study was conceived and experiments designed by M.C.K. and J.J.K. Experiments were performed by B.Z. and J.J.K. Data were analyzed by M.C.K and J.J.K. Reagents, materials, and analysis tools were contributed by A.F.R. J.J.K. and M.C.K wrote the article.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. : Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009;361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 2.Schiffner T, Sattentau QJ, Dorrell L: Development of prophylactic vaccines against HIV-1. Retrovirology 2013;10:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer CD, Tomassilli J, Sirignano M, et al. : Enhanced immune activation linked to endotoxemia in HIV-1 seronegative MSM. AIDS 2014;28:2162–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heiligenberg M, Lutter R, Pajkrt D, et al. : Effect of HIV and chlamydia infection on rectal inflammation and cytokine concentrations in men who have sex with men. Clin Vaccine Immunol 2013;20:1517–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomescu C, Seaton KE, Smith P, et al. : Innate activation of MDC and NK cells in high-risk HIV-1-exposed seronegative IV-drug users who share needles when compared with low-risk nonsharing IV-drug user controls. J Acquir Immune Defic Syndr 2015;68:264–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomescu C, Duh FM, Lanier MA, et al. : Increased plasmacytoid dendritic cell maturation and natural killer cell activation in HIV-1 exposed, uninfected intravenous drug users. AIDS 2010;24:2151–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piepenbrink MS, Samuel M, Carter B, Zheng B, Fucile C, Bunce C, Kiebala M, Khan AA, Thakar J, Maggriwar S, Morse D, Rosenberg AF, Haughey NJ, Valenti W, Keefer MC, Kobie JJ: Humoral dysregulation associated with increased systemic inflammation among injection heroin users. PLoS One 2016;11:e0158641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yates NL, Liao HX, Fong Y, et al. : Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 2014;6:228ra239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung AW, Kumar MP, Arnold KB, et al. : Dissecting polyclonal vaccine-induced humoral immunity against HIV using systems serology. Cell 2015;163:988–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell ND, Graham BS, Keefer MC, et al. : Phase 2 study of an HIV-1 canarypox vaccine (vCP1452) alone and in combination with rgp120: Negative results fail to trigger a phase 3 correlates trial. J Acquir Immune Defic Syndr 2007;44:203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobie JJ, Alcena DC, Zheng B, et al. : 9G4 autoreactivity is increased in HIV-infected patients and correlates with HIV broadly neutralizing serum activity. PLoS One 2012;7:e35356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobie JJ, Zheng B, Bryk P, et al. : Decreased influenza-specific B cell responses in rheumatoid arthritis patients treated with anti-tumor necrosis factor. Arthritis Res Ther 2011;13:R209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halliley JL, Kyu S, Kobie JJ, et al. : Peak frequencies of circulating human influenza-specific antibody secreting cells correlate with serum antibody response after immunization. Vaccine 2010;28:3582–3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian Y, Wei C, Eun-Hyung Lee F, et al. : Elucidation of seventeen human peripheral blood B-cell subsets and quantification of the tetanus response using a density-based method for the automated identification of cell populations in multidimensional flow cytometry data. Cytometry B Clin Cytom 2010;78 Suppl 1:S69–S82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galson JD, Truck J, Fowler A, et al. : Analysis of B cell repertoire dynamics following hepatitis B vaccination in humans, and enrichment of vaccine-specific antibody sequences. EBioMedicine 2015;2:2070–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capolunghi F, Rosado MM, Sinibaldi M, Aranburu A, Carsetti R: Why do we need IgM memory B cells? Immunol Lett 2013;152:114–120 [DOI] [PubMed] [Google Scholar]
- 17.Sanz I, Wei C, Lee FE, Anolik J: Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol 2008;20:67–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobi AM, Reiter K, Mackay M, et al. : Activated memory B cell subsets correlate with disease activity in systemic lupus erythematosus: Delineation by expression of CD27, IgD, and CD95. Arthritis Rheum 2008;58:1762–1773 [DOI] [PubMed] [Google Scholar]
- 19.Adlowitz DG, Barnard J, Biear JN, et al. : Expansion of activated peripheral blood memory B cells in rheumatoid arthritis, impact of B cell depletion therapy, and biomarkers of response. PLoS One 2015;10:e0128269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerutti A, Cols M, Puga I: Marginal zone B cells: Virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol 2013;13:118–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiLillo DJ, Matsushita T, Tedder TF: B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci 2010;1183:38–57 [DOI] [PubMed] [Google Scholar]
- 22.Reynaud CA, Descatoire M, Dogan I, Huetz F, Weller S, Weill JC: IgM memory B cells: A mouse/human paradox. Cell Mol Life Sci 2012;69:1625–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weill JC, Weller S, Reynaud CA: Human marginal zone B cells. Annu Rev Immunol 2009;27:267–285 [DOI] [PubMed] [Google Scholar]
- 24.Pillai S, Cariappa A: The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol 2009;9:767–777 [DOI] [PubMed] [Google Scholar]
- 25.Avrameas S, Selmi C: Natural autoantibodies in the physiology and pathophysiology of the immune system. J Autoimmun 2013;41:46–49 [DOI] [PubMed] [Google Scholar]
- 26.Baumgarth N: Innate-like B cells and their rules of engagement. Adv Exp Med Biol 2013;785:57–66 [DOI] [PubMed] [Google Scholar]
- 27.Good-Jacobson KL, Tarlinton DM: Multiple routes to B-cell memory. Int Immunol 2012;24:403–408 [DOI] [PubMed] [Google Scholar]
- 28.Tangye SG, Good KL: Human IgM+CD27+ B cells: Memory B cells or “memory” B cells? J Immunol 2007;179:13–19 [DOI] [PubMed] [Google Scholar]
- 29.Seifert M, Przekopowitz M, Taudien S, et al. : Functional capacities of human IgM memory B cells in early inflammatory responses and secondary germinal center reactions. Proc Natl Acad Sci U S A 2015;112:E546–E555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puga I, Cols M, Barra CM, et al. : B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol 2012;13:170–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levinson P, Kaul R, Kimani J, et al. : Levels of innate immune factors in genital fluids: Association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. AIDS 2009;23:309–317 [DOI] [PubMed] [Google Scholar]
- 32.Ho JL, He S, Hu A, et al. : Neutrophils from human immunodeficiency virus (HIV)-seronegative donors induce HIV replication from HIV-infected patients' mononuclear cells and cell lines: An in vitro model of HIV transmission facilitated by Chlamydia trachomatis. J Exp Med 1995;181:1493–1505 [PMC free article] [PubMed] [Google Scholar]
- 33.Arnold KB, Burgener A, Birse K, et al. : Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol 2016;9:194–205 [DOI] [PubMed] [Google Scholar]
- 34.Corcione A, Ferlito F, Gattorno M, et al. : Phenotypic and functional characterization of switch memory B cells from patients with oligoarticular juvenile idiopathic arthritis. Arthritis Res Ther 2009;11:R150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu RX, Wei Y, Zeng QH, et al. : Chemokine (C-X-C motif) receptor 3-positive B cells link interleukin-17 inflammation to protumorigenic macrophage polarization in human hepatocellular carcinoma. Hepatology 2015;62:1779–1790 [DOI] [PubMed] [Google Scholar]
- 36.Schierloh P, Landoni V, Balboa L, et al. : Human pleural B-cells regulate IFN-gamma production by local T-cells and NK cells in a Mycobacterium tuberculosis-induced delayed hypersensitivity reaction. Clin Sci (Lond) 2014;127:391–403 [DOI] [PubMed] [Google Scholar]
- 37.Mizuochi T, Ito M, Saito K, et al. : Possible recruitment of peripheral blood CXCR3+ CD27+ CD19+ B cells to the liver of chronic hepatitis C patients. J Interferon Cytokine Res 2010;30:243–252 [DOI] [PubMed] [Google Scholar]
- 38.Harris DP, Goodrich S, Gerth AJ, Peng SL, Lund FE: Regulation of IFN-gamma production by B effector 1 cells: Essential roles for T-bet and the IFN-gamma receptor. J Immunol 2005;174:6781–6790 [DOI] [PubMed] [Google Scholar]
- 39.Lajoie J, Poudrier J, Massinga Loembe M, et al. : Chemokine expression patterns in the systemic and genital tract compartments are associated with HIV-1 infection in women from Benin. J Clin Immunol 2010;30:90–98 [DOI] [PubMed] [Google Scholar]
- 40.Castellsague X, Giuliano AR, Goldstone S, et al. : Immunogenicity and safety of the 9-valent HPV vaccine in men. Vaccine 2015;33:6892–6901 [DOI] [PubMed] [Google Scholar]
- 41.Hillman RJ, Giuliano AR, Palefsky JM, et al. : Immunogenicity of the quadrivalent human papillomavirus (type 6/11/16/18) vaccine in males 16 to 26 years old. Clin Vaccine Immunol 2012;19:261–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamath GR, Shah DP, Hwang LY: Immune response to hepatitis B vaccination in drug using populations: A systematic review and meta-regression analysis. Vaccine 2014;32:2265–2274 [DOI] [PubMed] [Google Scholar]
- 43.Baral S, Sherman SG, Millson P, Beyrer C: Vaccine immunogenicity in injecting drug users: A systematic review. Lancet Infect Dis 2007;7:667–674 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.