Abstract
Significance: Bacterial biofilms are considered to be responsible for over 80% of persistent infections, including chronic lung infections, osteomyelitis, periodontitis, endocarditis, and chronic wounds. Over 60% of chronic wounds are colonized with bacteria that reside within a biofilm. The exaggerated proteolytic environment of chronic wounds, more specifically elevated matrix metalloproteinases, is thought to be one of the possible reasons as to why chronic wounds fail to heal. However, the role of bacterial proteases within chronic wounds is not fully understood.
Recent Advances: Recent research has shown that bacterial proteases can enable colonization and facilitate bacterial immune evasion. The inhibition of bacterial proteases such as Pseudomonas aeruginosa elastase B (LasB) has resulted in the disruption of the bacterial biofilm in vitro. P. aeruginosa is thought to be a key pathogen in chronic wound infection, and therefore, the disruption of these biofilms, potentially through the targeting of P. aeruginosa bacterial proteases, is an attractive therapeutic endeavor.
Critical Issues: Disrupting biofilm formation through the inhibition of bacterial proteases may lead to the dissemination of bacteria from the biofilm, allowing planktonic cells to colonize new sites within the wound.
Future Directions: Despite a plethora of evidence supporting the role of bacterial proteases as virulence factors in infection, there remains a distinct lack of research into the effect of bacterial proteases in chronic wounds. To assess the viability of targeting bacterial proteases, future research should aim to understand the role of these proteases in a variety of chronic wound subtypes.
Keywords: : bacterial proteases, chronic wounds, infection, biofilm, Pseudomonas aeruginosa, Staphylococcus aureus

Louise Suleman, BSc, MSc, PhD
Scope and Significance
This review highlights our current understanding of bacterial proteases as facilitators of bacterial infection and immune evasion and as potential players in chronic wound pathogenesis. The therapeutic targeting of bacterial proteases and its viability as a potential treatment option in the management of chronic wounds shall be discussed.
Translational Relevance
Chronic wounds are characterized by delayed wound closure, persistent inflammation, and an amplified secretion of matrix metalloproteinases (MMPs). The colonization of microorganisms and the formation of a biofilm within a wound are thought to reduce wound closure and perpetuate inflammation. Bacterial proteases have been shown to target components of host immunity, creating a more favorable environment for the bacteria to reside. The inhibition of specific bacterial proteases may result in the disruption of biofilms and the promotion of wound closure.
Clinical Relevance
Bacterial biofilms have been strongly associated with a number of infections, including chronic lung infections, osteomyelitis, periodontitis, and healthcare-associated infections. Biofilms are of great clinical importance primarily due to their strong association with increased resistance to antimicrobial therapies, and therefore, the effective treatment of these infections poses a great challenge. The search for alternative bacterial targets for therapeutic use is underway. The use of bacterial proteases as a diagnostic marker or as a method of biofilm disruption through their inhibition could be a welcome aid in chronic wound management.
Overview
Chronic wounds, including diabetic foot ulcers, pressure ulcers, and venous leg ulcers, pose a considerable economic burden, costing the National Health Service (NHS) an estimated £2.3–£3.1 billion per year.1 These types of wounds are susceptible to colonization by numerous bacterial species (see Fig. 1). Investigation into the microbial profile of such wounds, as chronic venous leg ulcers, has revealed the most commonly isolated bacterial species to include Staphylococcus aureus (93.5%), Enterococcus faecalis (71.1%), Pseudomonas aeruginosa (52.2%), coagulase-negative Staphylococci (45.7%), Proteus species (43.1%), and anaerobic bacteria (39.1%).2 To gain a more comprehensive understanding of the bacterial species within chronic wounds, such molecular techniques as pyrosequencing, denaturing gradient gel electrophoresis (DGGE), and full ribosome shotgun sequencing have allowed the identification of Staphylococcus, Pseudomonas, Peptoniphilus, Enterobacter, Stenotrophomonas, Finegoldia, and Serratia species in diabetic foot ulcers, venous leg ulcers, and pressure ulcers.3
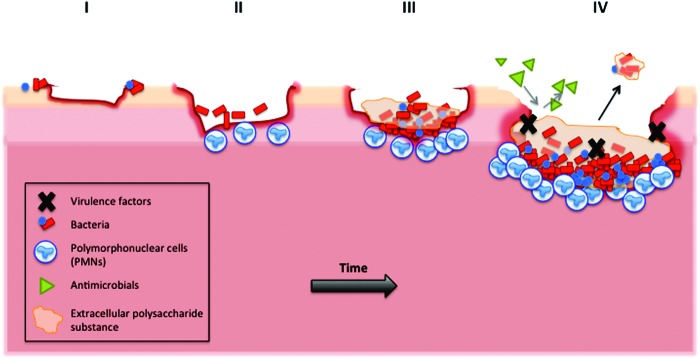
Figure 1.
Schematic representation the development of a biofilm within a wound. (I) Bacteria, either present on the skin or contamination from an external source, reversibly attach to areas of slough or necrotic tissue. (II) Bacteria on the surface of the wound proliferate and become irreversibly attached through the use of bacterial appendages that anchor the bacteria to the tissue. (III) Colonization occurs when attached bacteria proliferate and produce extracellular polymeric substances (EPS), which is thought to protect the bacteria from external disruption. (IV) The mature biofilm, surrounded in EPS, is resistant to the use of antimicrobials. The release of virulence factors, including bacterial proteases, helps protect from a host immune response. Parts of the mature biofilm can break away from the main biofilm, a process known as dispersal. Ultimately, the dispersal of bacteria from the biofilm can lead to dissemination, whereby these bacteria attach and colonize new sites, perpetuating infection. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
There is ever-emerging evidence to suggest that the bacterial species within a wound reside within a biofilm. James and colleagues examined the presence of biofilms in both acute and chronic wounds, using scanning electron microscopy. They discovered a significant difference in the presence of biofilms between chronic and acute wounds, with 60% of chronic wounds containing a biofilm compared to just 6% in acute wounds (p < 0.001). Peptide nucleic acid-based fluorescence in situ hybridization has been used to determine the structural organization of bacteria within a chronic wound, ultimately showing the aggregation of bacteria into microcolonies within an alginate matrix, with very few planktonic cells present.4 Biofilms are a major health concern, primarily due an increased recalcitrance to antimicrobial therapies compared to bacteria within a planktonic state.5 According to the National Institute of Health (United States), over 80% of persistent microbial infections within the body involve biofilms. Prevalent examples of biofilm-associated infections include chronic lung infections, periodontitis, endocarditis and osteomyelitis.6–10 Bjarnsholt et al. hypothesized that the failure of a chronic wound to heal is due to the presence of P. aeruginosa biofilms.11 Indeed, both in vitro and in vivo studies have demonstrated the deleterious effects of P. aeruginosa on wound closure.12–14 The secretion of proteases from many species of bacteria is an essential process for bacterial growth and virulence. Therefore it is important to consider the impact of secreted bacterial proteases within biofilm-infected chronic wounds and whether targeting these proteases as a means of therapeutic intervention may be a fruitful venture.
Discussion
Bacterial proteases
A brief introduction
Bacterial proteases embody a large and diverse group of proteases that are ubiquitously produced by all microorganisms, possessing a variety of physiological and biochemical functions.15 The intracellular expression and extracellular secretion of proteases in both Gram-positive and Gram-negative bacteria are fundamental contributors to infection through the turnover of unfolded proteins in the host environment and the proteolysis of regulatory proteins upon environmental stimuli. Not dissimilar to mammalian proteases, bacterial proteases can be categorized into serine-, metallo-, cysteine-, and aspartic proteases. The synthesis of bacterial proteases begins within the cell and is an inactive proenzyme form, which becomes activated following extracellular autocatalytic cleavage.16 Secreted extracellular proteases from bacterial species can act as toxins or virulence factors, and some simply play a role in the degradation of proteins (see Table 1).
Table 1.
Major secreted extracellular bacterial proteases of Pseudomonas aeruginosa and Staphylococcus aureus, their substrate specificities, and associated biological processes
| Organism | Bacterial Protease | MEROPS Family | Protease Class | Substrate | Associated Process | References |
|---|---|---|---|---|---|---|
| P. aeruginosa | Elastase A (LasA) | M23 | Metallo- | Fibrinogen, elastin | ECM destruction | 16,52 |
| Elastase B (Las B) | M4 | Metallo- | Elastin, collagen III, collagen IV, MMP-1/MMP-9 (proenzyme), elastase B | ECM destruction, MMP proteolysis, autoproteolytic processing | 16,53 | |
| Alkaline protease (AprA) | M10 | Metallo- | Fibrinogen, gelatin, casein, hemoglobin, cytokines | Complement inactivation, host immune evasion | 16,54,55 | |
| Protease IV | S1 | Serine- | Plasminogen, fibrinogen, complement protein C3 | Complement inactivation | 16,56 | |
| S. aureus | Aureolysin | M4 | Metallo- | Plasminogen, complement protein C3 | Complement inactivation | 16,23,57 |
| Staphopain A (ScpA) | C47 | Cysteine- | Elastin | ECM destruction | 16 | |
| Staphopain B (SspB) | C47 | Cysteine- | Fibrinogen, fibronectin, elastin | ECM destruction, complement inactivation | 16,57 | |
| Staphylococcal serine protease (SspA) | S1 | Serine- | Actin, collagenase, IgG1 heavy chain, serum albumin, vimentin, casein | Host immune evasion, ECM degradation | 16,57,58 |
ECM, extracellular matrix; MMP, matrix metalloproteinase.
Skin colonization and infection
There is emerging evidence to support the role of bacterial proteases in the attachment and penetration of skin. The Gram-positive anaerobe Finegoldia magna, a commensal microorganism commonly associated with skin microbiota, secretes two virulence factors that facilitate the attachment and penetration of the epidermal and dermal layers of the skin. In vitro studies have shown that the F. magna adhesion protein, Finegoldia magna adhesion factor binds with the keratinocyte marker galectin-7, while the extracellular serine protease SufA degrades collagen-IV, a major protein of the basement membrane, and collagen-V.17 S. aureus staphylokinase (Sak), has been associated with the activation of plasminogen into plasmin, which in turn degrades fibrin clots and components of the extracellular matrix and activates latent MMPs.18 Later studies by Kwiecinski et al. demonstrated the ability of transgenic S. aureus strains with high Sak expression (LS-1spa-sak) and moderate Sak expression (LS-1sak) to penetrate keratinocyte monolayers, fibrin clots, and reconstituted basal membranes in vitro.19 Furthermore, immunocompromised wild-type and human plasminogen transgenic mice subcutaneously injected with S. aureus LS-1spa-sak and LS-1EP (no Sak expression) showed comparable systemic infection, demonstrating that the activation of plasminogen by Sak does not cause the spread of infection. However, the bacterial-driven degradation of essential components of the basement membrane does not go unnoticed. The bacterial proteases P. aeruginosa elastase B (Las B), P. aeruginosa alkaline protease (AprA), S. aureus aureolysin, and S. aureus staphylococcal serine protease (SspA) were shown to cleave the C-terminal laminin G-domain-like modules of laminin α chains, a major glycoprotein of the basement membrane. However the cleavage of these laminin modules resulted in biologically active peptide fragments, which were shown to have antimicrobial properties and induce wound closure through the increase in keratinocyte migration and proliferation.20
Bacterial proteases and host immunity
In order for a pathogen to successfully invade the host and cause persistent infection, the pathogen must be able to evade host immune responses. Extracellular bacterial proteases have displayed the ability to evade host immune responses and target immune cell mediators. The first line of defense against invading microorganisms involves the infiltration of neutrophils and monocytes, which are able to effectively engulf pathogens. Despite this, the S. aureus cysteine protease staphopain B (SspB) is able to enhance its pathogenesis through the cleavage of CD11b, an essential component in phagocytosis, ultimately leading to phagocyte cell death through necrosis and apoptosis.21 S. aureus cysteine proteases have also been implicated in the cleavage of a pulmonary surfactant protein A, which has been linked to a reduction in innate immune responses against lung infection such as neutrophil-driven phagocytosis.22
Bacteria can also use secreted proteases to degrade components of the complement system. Laarman et al. showed that the S. aureus metalloprotease aureolysin could effectively prevent complement-mediated phagocytosis through the cleavage of the C3 protein complex, facilitating immune evasion.23 Furthermore, P. aeruginosa elastase can inactivate components of the complement system and complement-derived phagocytic factors.24 P. aeruginosa is a prominent example of a microorganism that can successfully evade host immune responses. Early studies by Horvat and Parmely demonstrated the inhibitory effect of P. aeruginosa AprA on T-cell-derived interferon-γ (IFN- γ), reducing the antiviral capacity and immunomodulatory activity of IFN- γ.25 P. aeruginosa LasB can effectively inactivate host antimicrobial peptides (AMPs), more specifically AMP LL-37, an important component of host innate immunity.26 Specific bacterial proteases can also interfere with the host's biological communication networks through the cleavage or inactivation of host growth factors and cytokines.27,28
Impact of bacterial proteases in wound healing
Currently, there is no available research that investigates the impact of bacterial proteases on wound closure. Kirker et al. tested the effects of the conditioned medium of chronic wound-derived S. aureus in planktonic and biofilm form, on the in vitro wound closure of human epidermal keratinocytes, which resulted in a significant reduction in keratinocyte wound closure, decreased cell viability, and increased apoptosis.13 Furthermore, later studies by the same research group showed that the presence of methicillin-resistant S. aureus (MRSA) planktonic-conditioned medium not only caused a significant reduction in human dermal fibroblast wound closure in vitro but also induced the release of a number of proinflammatory cytokines, including interleukin-6 (IL-6) and IL-8, growth factors, including vascular endothelial growth factor and transforming growth factor- beta, and the MMPs, MMP-1, and MMP-3.12 Importantly, the treatment of human dermal fibroblasts with the MRSA biofilm-conditioned medium caused similar effects in fibroblast wound closure, however, there was a more prominent release of tumor necrosis factor-α. This simple, yet informative study emphasizes the differential effects of the soluble products of bacteria in planktonic and biofilm forms on host responses.
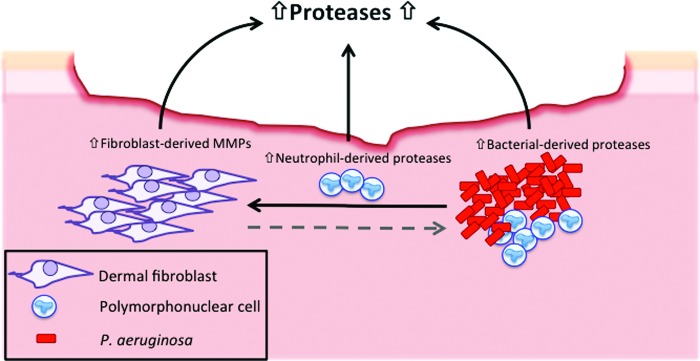
Elevated protease levels within chronic wounds, more specifically matrix metalloproteases (MMPs), have been well-documented.29–31 The secretion of bacterial proteases within a wound may contribute to the exaggerated proteolytic environment of chronic wounds through the activation of MMPs (see Fig. 2). P. aeruginosa proteases have been shown to cleave and activate host MMPs in a model of corneal infection.32 In addition, a mouse model of corneal infection immunized against P. aeruginosa AprA resulted in the reduction of host proteases, including MMP-2 and MMP-9.33 This study highlighted the importance of P. aeruginosa AprA in the activation of host MMPs and the excessive tissue destruction evident in corneal infections.
Figure 2.
Schematic representation of the potential contribution of bacterial proteases to the proteolytic environment of chronic wounds. The presence of bacterial biofilms, particularly Pseudomonas aeruginosa, may contribute to the excessive production of proteases in chronic wound pathology, through the release of extracellular bacterial proteases. Furthermore, the presence of a P. aeruginosa biofilm within the wound may induce the release of matrix metalloproteinases (MMPs) from resident dermal fibroblasts. It is unknown whether fibroblast-derived MMPs effect the production of extracellular bacterial-derived proteases (indicated by dashed grey arrow). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Bacterial proteases as diagnostic markers in chronic wounds
Bacterial proteases are often secreted into the surrounding environment and therefore present an opportunity to utilize these proteases as diagnostic markers. There is a small amount of evidence to suggest that chronic wound-derived P. aeruginosa secrete bacterial proteases. One of the first research articles to acknowledge protease-producing bacteria isolated from chronic wounds was published in 2001, whereby P. aeruginosa from chronic leg ulcers showed varying levels of P. aeruginosa elastase, AprA, and an unidentified 100 kDa protease.34 Wysocki et al. then went on to identify 18 different chronic wound-derived bacterial species (10 Gram-positive and 8 Gram-negative) that displayed proteolytic activity. The proteolytic activity of these bacterial isolates was not found to be consistent in any of these species after repeated isolation.35 However, it is important to note that in this study, no quantitative data were presented to determine the varying levels of protease production between species. Another study by Wildeboer et al. sought to determine a correlation between the protease activity and bacterial load in chronic wounds using fluorescent-labeled peptide substrates. Although there was no correlation with most species identified in the wound and protease activity, the signal detection of two substrates strongly correlated with P. aeruginosa numbers.36 This study highlights a potential use of these substrates as the basis for a diagnostic tool in the identification of P. aeruginosa colonization in chronic wounds. Zdzalik et al. aimed to identify a link between specific S. aureus-derived extracellular proteases and various types of infection through the investigation of prevalent S. aureus extracellular protease genes derived from cases of wound infection, pneumonia, sepsis, cystic fibrosis, skin infection, and bone infection to name a few.37 In this study, the authors did not determine any correlation in gene expression patterns with specific types of infection, however, most of the S. aureus proteases investigated were expressed and secreted during the course of infection.
Detection of bacterial proteases
The proteolytic activity of extracellular secreted proteases can be detected by the use of substrates such as collagen, gelatin, and casein, using a variety of techniques. Probably, the most common and basic method to assess the proteolytic activity in a laboratory setting is the incorporation of these substrates into microbiological agar, whereby the presence of extracellular proteases results in a zone of clearance in the agar. The use of substrates conjugated to an azo dye such as azocasein or azocoll provide a more quantitative approach, in which the presence of proteases within a sample will cleave the substrate, releasing the conjugated dye. This can then be detected by using a spectrophotometer. However, this methodology lacks specificity; therefore complex biological samples with both host- and bacterial-derived proteases will be detected. A more specific method of bacterial protease detection is the use of fluorescent- or colorimetric-labeled peptide probes as demonstrated by Wildeboer et al. in the detection of P. aeruginosa proteases.36 The use of specific peptide substrates allows the measurement of both qualitative and quantitative data. Despite the excellent specificity of commercially available peptide substrates, complex clinical samples consisting of numerous proteases from both host and microorganisms can still result in nonspecific proteolytic cleavage, creating concern for their potential use as diagnostic tools.
Existing and potential treatment strategies and their effectiveness against bacterial proteases
Wound dressings
The discovery of elevated levels of MMPs within chronic wounds sparked the production of wound dressings comprising superabsorptive polymers that act to effectively regulate the overproduction of proteases residing in wound exudate.38 Likewise, the incorporation of collagen-I substrates into wound dressings has been shown to effectively sequester not only the mammalian gelatinases MMP-2 and MMP-9 but also Clostridium histolyticum bacterial collagenase.39
Photodynamic therapy
Photodynamic therapy (PDT) is the application of a photoactive dye followed by irradiation, which leads to cell death in the presence of oxygen. The application of PDT has been used in the treatment of cancerous skin lesions and cancerous tumors of the head, neck, lung, and esophagus.40 The concept of PDT to treat nonhealing chronic wounds and eradicate bacterial biofilms has gained much attention.41 Interestingly, an in vitro study by Kömerik et al. demonstrated the effectiveness of PDT against P. aeruginosa proteases, whereby there was a significant reduction in P. aeruginosa proteases following exposure to red light in the presence of toluidine blue.42 PDT may be a viable option in the treatment of biofilm-infected wounds through microbial cell death, reduction in bacterial proteases, and the promotion of wound closure.43,44
Bacterial proteases as therapeutic targets
The developing prevalence of antibiotic-resistant microorganisms, particularly in the context of healthcare-associated infections and their management, has propelled research into the discovery of new, effective treatment strategies and novel antimicrobials. The therapeutic targeting of proteases by pharmacologically attractive compounds has been successfully used in the treatment of many diseases, including hypertension, human immunodeficiency virus, and hepatitis C virus (HCV). For instance, pharmacologically approved serine protease inhibitor boceprevir (Victrelis; Merck) reversibly binds to and inhibits the HCV nonstructural 3 active site, preventing viral replication and thus sustaining the virologic response in patients with previously untreated, chronic HCV infection.45,46 Despite this, the pharmacological targeting of bacterial proteases in the context of bacterial infection has not been fully exploited.
Current research in biofilm-infected wounds
Many of the secreted bacterial proteases are involved in bacterial virulence or growth, and therefore, the inhibition of these proteases may disrupt biofilm formation or increase biofilm susceptibility to antimicrobials.47 Indeed, P. aeruginosa proteases have been shown to regulate biofilm formation, and therefore, the inhibition of these proteases in vitro has resulted in the disruption of the biofilm. P. aeruginosa LasB has been investigated as a target of protease inhibition. A novel and potent inhibitor of LasB, N-mercaptoacetyl-Phe-Tyr-amide, has been developed and shown to reduce P. aeruginosa biofilm growth, and when combined with additional antimicrobials, such as ciprofloxacin and gentamicin, can fully eradicate the biofilm in vitro.48 Similarly, the deletion of LasB in P. aeruginosa PA01, referred to as a Lasb deletion mutant strain, has been shown to exhibit decreased bacterial attachment and microcolony formation. However, microcolony formation in the LasB deletion mutants was restored following exogenous rhamnolipid supplementation, therefore it was hypothesized that LasB may promote biofilm formation through rhamnolipid-mediated regulation.49
The inhibition of other bacterial proteases, however, may not necessarily result in the disruption of the biofilm. Research by Loughran et al. identified that S. aureus aureolysin and, to a lesser extent, the proteases staphopain A and SspB actually promote the dispersal of S. aureus biofilms.50
Summary
While there is a clear role for bacterial proteases in the mediation of infection, the investigation of these proteases within chronic wounds has been somewhat marginalized. Research into the extracellular secreted proteases of P. aeruginosa, more specifically LasB, has revealed a regulatory role of this protease in biofilm development and therefore making LasB an attractive target not only for diagnostic purposes but also as an antibacterial target. Like many of the antibiofilm strategies that have been employed in medicine, the disruption of the bacterial biofilm simply leads to its dispersal, therefore allowing planktonic bacteria to colonize other sites. Therefore, it is important to consider the use of antimicrobials in addition to biofilm disruption to discourage new colonization sites.
While the specific targeting of bacterial proteases associated with key pathogens such as P. aeruginosa may help weaken bacterial virulence, it is imperative that more research into the detection of these proteases in a variety of chronic wound types is performed. Indeed, no two chronic wounds are the same, and, in the context of microbial burden, the variability of microbial profiles in varying wound types has been demonstrated.3,51 Therefore, in addition to the varying conditions of wounds, factors such as a nutrient availability and the interactions between multiple species of microorganisms may alter specific protease production. Nevertheless, the use of bacterial proteases to control infection processes will provide interesting research in the field of microbiology and chronic wounds.
Take-Home Messages.
• Bacteria secrete proteases that facilitate the infection process, through skin penetration and host immune evasion, creating a more favorable environment for bacteria to reside.
• As many bacterial proteases are secreted into the surrounding environment, these proteases become an ideal candidate for the creation of a diagnostic tool or an antimicrobial target.
• Some bacterial proteases play a regulatory role in biofilm formation, and in vitro studies have concluded that the inhibition of specific bacterial proteases, such as P. aeruginosa LasB, can lead to the disruption of the biofilm.
• The disruption of biofilm may lead to dissemination and colonization of new sites within the wound, and therefore, the inhibition of specific bacterial proteases must be approached with caution.
• Further research into the role of bacterial proteases in the pathogenesis of chronic wounds should be considered.
Abbreviations and Acronyms
- AprA
alkaline protease
- AMP
antimicrobial peptide
- DGGE
denaturing gradient gel electrophoresis
- ECM
extracellular matrix
- HCV
hepatitis C virus
- IFN-γ
interferon-gamma
- IL-6
interleukin-6
- LasA
elastase A
- LasB
elastase B
- MRSA
methicillin-resistant Staphylococcus aureus
- MMPs
matrix metalloproteinases
- PDT
photodynamic therapy
- ScpA
staphopain A
- SspA
staphylococcal serine protease
- SspB
staphopain B
- Sak
staphylokinase
Acknowledgments and Funding Sources
No funding sources were obtained for the writing of this review.
Author Disclosure and Ghostwriting
The author has no conflicts of interest. The content of this article was expressly written by the author listed. No ghostwriters were used to write this article.
About the Authors
Louise Suleman, PhD, received her BSc in Biomedical Sciences from Lancaster University in 2010, followed by the Institute of Biomedical Science (IBMS) Certificate of Competence as a Practitioner in Biomedical science. In 2011, she completed an MSc in Biomedical Science (by research) at Lancaster University, studying the cellular mediators responsible for fibrosis in Crohn's disease. Since then, she has completed a PhD at the University of Liverpool in 2015, researching bacterial proteases, biofilms, chronic wounds, and wound healing in both human and equine models.
References
- 1.Posnett J, Franks PJ. The burden of chronic wounds in the UK. Nurs Times 2008;104:44. [PubMed] [Google Scholar]
- 2.Gjødsbøl K, Christensen JJ, Karlsmark T, Jørgensen B, Klein BM, Krogfelt KA. Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J 2006;3:225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowd SE, Sun Y, Secor PR, et al. . Survey of bacterial diversity in chronic wounds using pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiol 2008;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirketerp-Møller K, Jensen PØ, Fazli M, et al. . Distribution, organization, and ecology of bacteria in chronic wounds. J Clin Microbiol 2008;46:2717–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 2002;15:167–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg E. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 2000;407:762–764 [DOI] [PubMed] [Google Scholar]
- 7.Distel JW, Hatton JF, Gillespie MJ. Biofilm formation in medicated root canals. J Endod 2002;28:689–693 [DOI] [PubMed] [Google Scholar]
- 8.Gristina AG, Oga M, Webb LX, Hobgood CD. Adherent bacterial colonization in the pathogenesis of osteomyelitis. Science 1985;228:990–993 [DOI] [PubMed] [Google Scholar]
- 9.Mohamed JA, Huang W, Nallapareddy SR, Teng F, Murray BE. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect Immun 2004;72:3658–3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Høiby N, Ciofu O, Bjarnsholt T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol 2010;5:1663–1674 [DOI] [PubMed] [Google Scholar]
- 11.Bjarnsholt T, Kirketerp-Møller K, Jensen PØ, et al. . Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen 2008;16:2–10 [DOI] [PubMed] [Google Scholar]
- 12.Kirker KR, James GA, Fleckman P, Olerud JE, Stewart PS. Differential effects of planktonic and biofilm MRSA on human fibroblasts. Wound Repair Regen 2012;20:253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirker KR, Secor PR, James GA, Fleckman P, Olerud JE, Stewart PS. Loss of viability and induction of apoptosis in human keratinocytes exposed to Staphylococcus aureus biofilms in vitro. Wound Repair Regen 2009;17:690–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurjala AN, Geringer MR, Seth AK, et al. . Development of a novel, highly quantitative in vivo model for the study of biofilm-impaired cutaneous wound healing. Wound Repair Regen 2011;19:400–410 [DOI] [PubMed] [Google Scholar]
- 15.Schaechter M. Encyclopedia of Microbiology. Oxford, UK: Academic Press; 2009 [Google Scholar]
- 16.Rawlings ND, Barrett AJ, Bateman A. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res 2012;40:D343–D350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy EC, Mörgelin M, Reinhardt DP, Olin AI, Björck L, Frick IM. Identification of molecular mechanisms used by Finegoldia magna to penetrate and colonize human skin. Mol Microbiol 2014;94:403–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lijnen H, Van Hoef B, De Cock F, et al. . On the mechanism of fibrin-specific plasminogen activation by staphylokinase. J Biol Chem 1991;266:11826–11832 [PubMed] [Google Scholar]
- 19.Kwiecinski J, Jacobsson G, Karlsson M, et al. . Staphylokinase promotes the establishment of Staphylococcus aureus skin infections while decreasing disease severity. J Infect Dis 2013;208:990–999 [DOI] [PubMed] [Google Scholar]
- 20.Senyürek I, Kempf WE, Klein G, et al. . Processing of laminin α chains generates peptides involved in wound healing and host defense. J Innate Immun 2014;6:467–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smagur J, Guzik K, Magiera L, et al. . A new pathway of staphylococcal pathogenesis: apoptosis-like death induced by Staphopain B in human neutrophils and monocytes. J Innate Immun 2009;1:98–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kantyka T, Pyrc K, Gruca M, et al. . Staphylococcus aureus proteases degrade lung surfactant protein A potentially impairing innate immunity of the lung. J Innate Immun 2013;5:251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laarman AJ, Ruyken M, Malone CL, van Strijp JA, Horswill AR, Rooijakkers SH. Staphylococcus aureus metalloprotease aureolysin cleaves complement C3 to mediate immune evasion. J Immunol 2011;186:6445–6453 [DOI] [PubMed] [Google Scholar]
- 24.Schultz DR, Miller KD. Elastase of Pseudomonas aeruginosa: inactivation of complement components and complement-derived chemotactic and phagocytic factors. Infect Immun 1974;10:128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horvat RT, Parmely MJ. Pseudomonas aeruginosa alkaline protease degrades human gamma interferon and inhibits its bioactivity. Infect Immun 1988;56:2925–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidtchen A, Frick IM, Andersson E, Tapper H, Björck L. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol Microbiol 2002;46:157–168 [DOI] [PubMed] [Google Scholar]
- 27.Matheson NR, Potempa J, Travis J. Interaction of a novel form of Pseudomonas aeruginosa alkaline protease (aeruginolysin) with interleukin-6 and interleukin-8. Biol Chem 2006;387:911–915 [DOI] [PubMed] [Google Scholar]
- 28.Leidal KG, Munson KL, Johnson MC, Denning GM. Metalloproteases from Pseudomonas aeruginosa degrade human RANTES, MCP-1, and ENA-78. J Interferon Cytokine Res 2003;23:307–318 [DOI] [PubMed] [Google Scholar]
- 29.Nwomeh BC, Liang H-X, Cohen IK, Yager DR. MMP-8 is the predominant collagenase in healing wounds and nonhealing ulcers. J Surg Res 1999;81:189–195 [DOI] [PubMed] [Google Scholar]
- 30.Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol 1993;101:64–68 [DOI] [PubMed] [Google Scholar]
- 31.Yager DR, Zhang L-Y, Liang H-X, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol 1996;107:743–748 [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto K, Shams N, Hanninen LA, Kenyon KR. Cleavage and activation of corneal matrix metalloproteases by Pseudomonas aeruginosa proteases. Invest Ophthalmol Vis Sci 1993;34:1945–1953 [PubMed] [Google Scholar]
- 33.Kernacki K, Fridman R, Hazlett L, Lande M, Berk R. In vivo characterization of host and bacterial protease expression during Pseudomonas aeruginosa corneal infections in naive and immunized mice. Curr Eye Res 1997;16:289–297 [DOI] [PubMed] [Google Scholar]
- 34.Schmidtchen A, Wolff H, Hansson C. Differential proteinase expression by Pseudomonas aeruginosa derived from chronic leg ulcers. Acta Derm Venereol 2001;81:406–409 [DOI] [PubMed] [Google Scholar]
- 35.Wysocki AB, Bhalla-Regev SK, Tierno PM, Stevens-Riley M, Wiygul R-C. Proteolytic activity by multiple bacterial species isolated from chronic venous leg ulcers degrades matrix substrates. Biol Res Nurs 2012;15:407–415 [DOI] [PubMed] [Google Scholar]
- 36.Wildeboer D, Hill KE, Jeganathan F, et al. . Specific protease activity indicates the degree of Pseudomonas aeruginosa infection in chronic infected wounds. Eur J Clin Microbiol Infect Dis 2012;31:2183–2189 [DOI] [PubMed] [Google Scholar]
- 37.Zdzalik M, Karim AY, Wolski K, et al. . Prevalence of genes encoding extracellular proteases in Staphylococcus aureus–important targets triggering immune response in vivo. FEMS Immunol Med Microbiol 2012;66:220–229 [DOI] [PubMed] [Google Scholar]
- 38.Wiegand C, Abel M, Ruth P, Hipler U. Superabsorbent polymer-containing wound dressings have a beneficial effect on wound healing by reducing PMN elastase concentration and inhibiting microbial growth. J Mater Sci Mater Med 2011;22:2583–2590 [DOI] [PubMed] [Google Scholar]
- 39.Metzmacher I, Ruth P, Abel M, Friess W. In vitro binding of matrix metalloproteinase-2 (MMP-2), MMP-9, and bacterial collagenase on collagenous wound dressings. Wound Repair Regen 2007;15:549–555 [DOI] [PubMed] [Google Scholar]
- 40.Lui H, Anderson R. Photodynamic therapy in dermatology: shedding a different light on skin disease. Arch Dermatol 1992;128:1631. [PubMed] [Google Scholar]
- 41.Percival SL, Suleman L, Francolini I, Donelli G. The effectiveness of photodynamic therapy on planktonic cells and biofilms and its role in wound healing. Future Microbiol 2014;9:1083–1094 [DOI] [PubMed] [Google Scholar]
- 42.Kömerik N, Wilson M, Poole S. The effect of photodynamic action on two virulence factors of gram-negative bacteria. Photochem Photobiol 2000;72:676–680 [DOI] [PubMed] [Google Scholar]
- 43.Vecchio D, Dai T, Huang L, Fantetti L, Roncucci G, Hamblin MR. Antimicrobial photodynamic therapy with RLP068 kills methicillin-resistant Staphylococcus aureus and improves wound healing in a mouse model of infected skin abrasion PDT with RLP068/Cl in infected mouse skin abrasion. J Biophotonics 2013;6:733–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Motta S, Monti M. Photodynamic therapy—a promising treatment option for autoimmune skin ulcers: a case report. Photochem Photobiol Sci 2007;6:1150–1151 [DOI] [PubMed] [Google Scholar]
- 45.Venkatraman S. Discovery of boceprevir, a direct-acting NS3/4A protease inhibitor for treatment of chronic hepatitis C infections. Trends Pharmacol Sci 2012;33:289–294 [DOI] [PubMed] [Google Scholar]
- 46.Poordad F, McCone J, Jr., Bacon BR, et al. . Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011;364:1195–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaman WE, Hays J, Endtz H, Bikker F. Bacterial proteases: targets for diagnostics and therapy. Eur J Clin Microbiol Infect Dis 2014;33:1081–1087 [DOI] [PubMed] [Google Scholar]
- 48.Cathcart GR, Quinn D, Greer B, et al. . Novel inhibitors of the Pseudomonas aeruginosa virulence factor LasB. A potential therapeutic approach for the attenuation of virulence mechanisms in pseudomonal infection. Antimicrob Agents Chemother 2011;55:2670–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu H, He X, Xie W, et al. . Elastase LasB of Pseudomonas aeruginosa promotes biofilm formation partly through rhamnolipid-mediated regulation. Can J Microbiol 2014;60:227–235 [DOI] [PubMed] [Google Scholar]
- 50.Loughran AJ, Atwood DN, Anthony AC, et al. . Impact of individual extracellular proteases on Staphylococcus aureus biofilm formation in diverse clinical isolates and their isogenic sarA mutants. Microbiologyopen 2014;3:897–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith DM, Snow DE, Rees E, et al. . Evaluation of the bacterial diversity of pressure ulcers using bTEFAP pyrosequencing. BMC Med Genomics 2010;3:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morihara K, Tsuzuki H, Oka T, Inoue H, Ebata M. Pseudomonas aeruginosa elastase isolation, crystallization, and preliminary characterization. J Biol Chem 1965;240:3295–3304 [PubMed] [Google Scholar]
- 53.Okamoto T, Akaike T, Suga M, et al. . Activation of human matrix metalloproteinases by various bacterial proteinases. J Biol Chem 1997;272:6059–6066 [DOI] [PubMed] [Google Scholar]
- 54.Barrett AJ, Woessner JF, Rawlings ND. Handbook of Proteolytic Enzymes. London, UK: Elsevier, 2012 [Google Scholar]
- 55.Parmely M, Gale A, Clabaugh M, Horvat R, Zhou W-W. Proteolytic inactivation of cytokines by Pseudomonas aeruginosa. Infect Immun 1990;58:3009–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Engel LS, Hill JM, Caballero AR, Green LC, O'Callaghan RJ. Protease IV, a unique extracellular protease and virulence factor from Pseudomonas aeruginosa. J Biol Chem 1998;273:16792–16797 [DOI] [PubMed] [Google Scholar]
- 57.Massimi I, Park E, Rice K, Müller-Esterl W, Sauder D, McGavin MJ. Identification of a novel maturation mechanism and restricted substrate specificity for the SspB cysteine protease of Staphylococcus aureus. J Biol Chem 2002;277:41770–41777 [DOI] [PubMed] [Google Scholar]
- 58.Ryan MH, Petrone D, Nemeth JF, Barnathan E, Björck L, Jordan RE. Proteolysis of purified IgGs by human and bacterial enzymes in vitro and the detection of specific proteolytic fragments of endogenous IgG in rheumatoid synovial fluid. Mol Immunol 2008;45:1837–1846 [DOI] [PubMed] [Google Scholar]