Abstract
M2b macrophages (Mφ) play a major role in the increased susceptibility of subacutely burned patients, to sepsis stemming from enterococcal translocation. Certain opportunistic infections in severely burned mice have been controlled by murine CCL1 antisense oligodeoxynucleotide (ODN), a specific polarizer of mouse M2bMφ. In the present study, we have screened CCL1 antisense ODN, which is active against human M2bMφ. Among the 20 CCL1 antisense ODNs synthesized in our laboratory, HCA-11 was shown to be the most active polarizer for human CCL1+CD163+CD14+ cells. Burn patient CCL1+CD163+CD14+ cells (3 × 105 cells/mL) switched to quiescent CCL1−CD163−CD14+ cells within 48 h in cultures supplemented with 100 μg/mL of HCA-11. After treatment with a 25 μg/chimera dose of HCA-11, the bacterial growth was not observed in various organs of patient chimeras (γNSG mice inoculated with burn patient WBCs) infected with a lethal dose of Methicillin-resistant Staphylococcus aureus. The host antibacterial defenses against certain opportunistic pathogens should be improved in severely burned patients treated with a human CCL1 antisense ODN, HCA-11.
Introduction
Shock and respiratory failure during the first few hours to days (acute phase) of severe burn injury have been greatly reduced by the refinement of fluid resuscitation and ventilation techniques. Now, infections with opportunistic pathogens are a leading cause of morbidity and mortality in subacutely burned patients [1–3]. Plastic and heterogeneous macrophages (Mφ) readily switch from one functional phenotype to another in response to microenvironmental signals [4]. M1Mφ (IL-12+IL-10−INOS+ Mφ) are known as the main effector cells in host antibacterial innate immunities [5,6]. However, M1Mφ are not easily generated in severely burned patients whose M2Mφ (IL-12−IL-10+CD163+ Mφ) predominate [7–9], because M2Mφ inhibit the Mφ polarization from quiescent Mφ to M1Mφ.
In our studies [10–12], mouse M2bMφ polarized to quiescent Mφ after treatment with an M2bMφ polarizer, murine CCL1 antisense oligodeoxynucleotide (ODN). CCL1 has already been characterized as an essential chemokine for the maintenance of M2bMφ properties [10]. Mouse M2bMφ treated with murine CCL1 antisense ODN did not produce CCL1 (a biomarker for M2bMφ) or IL-10 (a biomarker for M2Mφ). M1Mφ were induced from the ODN-treated M2bMφ by stimulation with a TLR agonist. Furthermore, impaired host antibacterial resistance of subacutely burned mice to various opportunistic infections was greatly improved after treatment with murine CCL1 antisense ODN.
In this study, we tried to synthesize human CCL1 antisense ODN that is active against burn patient M2bMφ. In our previous studies, the effect of circulating human CCL1 antisense ODN (abbreviated as AS1) [13] on bacterial translocation and subsequent sepsis in burn patient chimeras was tested. In the results, morbidity and mortality of the chimeras treated with AS1 were slightly reduced compared to those of chimeras treated with scrambled ODN. Burn patient chimeras utilized in these experiments are γ-irradiated NOD/scid IL-2Rγnull mice (γNSG mice) inoculated with burn patient peripheral white blood cells (WBCs). In the present study, 20 human CCL1 antisense ODNs were newly synthesized and assayed for their activities to control opportunistic infections in severely burned patients. In the results, HCA-11 was shown to be the most active ODN against three different sources of human M2b monocytes (CCL1+CD163+CD14+ cells created from healthy donor peripheral blood CD14+ cells after stimulation with IL-1β/IgG or ORM1 and derived from severely burned patient peripheral blood). The antibacterial resistance of burn patient chimeras systemically infected with Methicillin-resistant Staphylococcus aureus (MRSA) was markedly improved after the administration of HCA-11. HCA-11 (human CCL1 antisense ODN No. 11) may be beneficial for the improvement of host antibacterial defenses against certain opportunistic infections in severely burned patients.
Materials and Methods
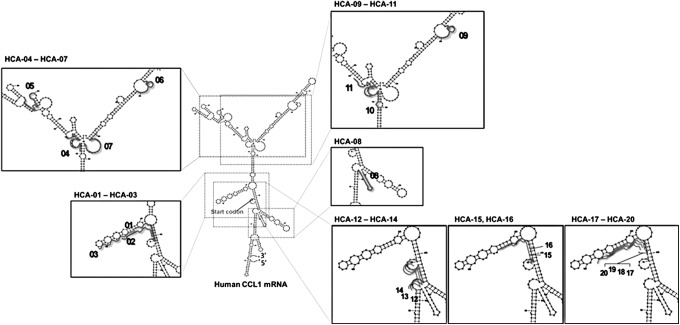
Design of antisense ODN against human CCL1 messenger RNA
The Sfold program, which is available at the website of Wadsworth Center, New York State Department of Health, was used to perform the prediction of the secondary structure of the RNA, motif searches, and GC content and binding energy calculations. In the program, the primary structure of human CCL1 messenger RNA (mRNA; NCBI Reference Sequence: NM_002981) was used as the target RNA, and the proximity of a start codon or hairpin loop structure in the mRNA was defined as the target site. We designed a total of 20 phosphorothioated antisense ODNs (HCA-01 to HCA-20) against human CCL1 mRNA (Table 1 and Fig. 1). Twelve ODNs (HCA-01 to HCA-03 and HCA-12 to HCA-20) are located at upstream and downstream regions of the start codon (Fig. 1). Eight ODNs (HCA-04 to HCA-11) are located throughout the entire region of CCL1 mRNA (Fig. 1). These ODNs were synthesized and purified by Sigma-Aldrich (St. Louis, MO). In DNA synthesis, each oligonucleotide is coupled sequentially to the growing chain in a 3′ to 5′ direction. By peak purity test using high performance liquid chromatography (HPLC), the purity of these ODNs was shown to be 80% or more. As a control for HCA-11, the scrambled sequence of HCA-11 was utilized (scrambled ODN, 5′-GAGGTAGAGTGATCACAC-3′). The synthesized ODNs were dissolved in calcium- and magnesium-free phosphate-buffered saline (PBS) and stored at −20°C until utilization in the experiments.
Table 1.
Synthesized Antisense Oligodeoxynucleotides Screened in This Study
| ODNs | Sequence (5′ 3′) | Length | Target site |
|---|---|---|---|
| Previously described ODN (AS1) | GATGATCTGCATGTCTTC | 18 | 64–82 |
| HCA-01 | TGTGGTGATGATCTGCA | 17 | 74–90 |
| HCA-02 | GGGCTGTGGTGATGATCT | 18 | 77–94 |
| HCA-03 | AAGCACACCAGGGCTGT | 17 | 88–104 |
| HCA-04 | CATCTGGAGAAGGGTACCTG | 20 | 151–170 |
| HCA-05 | CATTGGAGCAGATGGAGCTG | 20 | 231–250 |
| HCA-06 | AAGCCATGTGGTTTCCAGAG | 20 | 386–405 |
| HCA-07 | AAGGAATGGTGTAGGGCTGG | 20 | 424–443 |
| HCA-08 | CAGAGGGTTGGGGGTTGATG | 20 | 529–548 |
| HCA-09 | GCCATGTGGTTTCCAGAG | 18 | 386–403 |
| HCA-10 | GAGAAGGGTACCTGCATG | 18 | 147–164 |
| HCA-11 | CAACATCTGGAGAAGGGT | 18 | 156–173 |
| HCA-12 | GTCTTCTGGTCTGGCTTG | 18 | 55–72 |
| HCA-13 | ATGTCTTCTGGTCTGGCT | 18 | 57–74 |
| HCA-14 | CATGTCTTCTGGTCTGGC | 18 | 58–75 |
| HCA-15 | TGTGGTGATGATCTGCAT | 18 | 73–90 |
| HCA-16 | CTGTGGTGATGATCTGCA | 18 | 74–91 |
| HCA-17 | AGGGCTGTGGTGATGATC | 18 | 78–95 |
| HCA-18 | GGGCTGTGGTGATGATC | 17 | 78–94 |
| HCA-19 | AGGGCTGTGGTGATGAT | 17 | 79–95 |
| HCA-20 | CAGGGCTGTGGTGATGAT | 18 | 79–96 |
HCA-01 to HCA-20 are phosphorothioated ODNs designed and synthesized against human CCL1 mRNA. Sequences are shown in order from 5′ to 3′. Target sites are represented as each starting-ending positions of the mRNA.
mRNA, messenger RNA; ODNs, oligodeoxynucleotides.
FIG. 1.
Binding sites of 20 synthesized ODNs. Designed position of each ODN is presented as a thick line on the predicted structure of human CCL1 mRNA. mRNA, messenger RNA; ODNs, oligodeoxynucleotides.
Reagents, bacteria, cell cultures, and mice
Human IL-10 Enzyme-linked Immunosorbent Assay (ELISA) Kits were purchased from BioLegend (San Diego, CA). Purified and biotinylated anti-human CCL1 antibodies were purchased from R&D Systems (Minneapolis, MN). Recombinant CCL1 and IL-1β were obtained from PeproTech (Rocky Hill, NJ). Human serum IgG and phosphorothioated ODNs were purchased from Sigma-Aldrich. Anti-human CD14 magnetic particles were purchased from BD Biosciences (Franklin Lakes, NJ). Macrophage-SFM, RPMI-1640 medium, and antibiotics (penicillin and streptomycin) were purchased from Gibco (Grand Island, NY). Fetal bovine serum (FBS; GE Healthcare Life Sciences, Logan, UT) was heat inactivated at 56°C for 30 min before use. Human orosomucoid 1 (ORM1; >95% of purity by sodium dodecyl sulfate–polyacrylamide gel electrophoresis) was purchased from Abcam (Cambridge, MA). MRSA (BAA-44 strain), purchased from the American Type Culture Collection (Manassas, VA), was cultured in tryptic soy broth for 12 h at 37°C for the infection experiments. The cultures were centrifuged at 2,000g for 15 min, and the bacterial pellets were suspended in PBS. The number of bacteria in each suspension was counted using a hemocytometer and adjusted to give the approximate desired inocula. To confirm the number of pathogens, the inocula were diluted and plated on tryptic soy agar. Heat-killed MRSA was prepared by heating the bacteria at 65°C for 30 min. The inactivated properties of these cells were confirmed by culturing them on agar plates. Heat-killed bacteria were stored at −80°C until needed. For cultivation of macrophages, RPMI-1640 medium supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin (complete medium) was used. After 3.5 Gy of γ-irradiation, male NOD/scid IL-2Rγnull mice (γNSG mice), purchased from The Jackson Laboratory (Bar Harbor, ME), were used to create humanized murine chimeras. γNSG mice have no functional T cells, B cells, NK cells, neutrophils, or macrophages [14]. The intravenous (i.v.)-inoculated human CD14+ cells or WBCs spread throughout the mouse body within 2 days of the cell inoculation [15]. Significant numbers of CD14+ cells are recovered from the spleen, liver, and peritoneal cavity of the chimeras until 3 weeks after the WBC inoculation [15]. Chimeras created with the WBCs isolated from burn patients reflect the antibacterial resistance shown by the WBC donor. The animal experiments performed in this study were approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch at Galveston (IACUC Approval Nos. 1005025 and 0204024).
Preparation of CCL1+CD163+CD14+ cells
Three different sources of CCL1+CD163+CD14+ cells were utilized in this study as follows: (1) CCL1+CD163+CD14+ cells (IL-1β/IgG-CCL1+CD163+CD14+ cells) were generated from healthy donor peripheral blood CD14+ cells (3 × 105 cells/mL), magnetically isolated from the peripheral blood mononuclear cells (PBMCs) [16], after 3-day cultivation with immobilized human IgG (10 μg/100 μL/well) and 20 ng/mL of IL-1β. The purity of CD14+ cells harvested was routinely more than 97% [17]. In this procedure, more than 72% of cells switched to CCL1+CD163+CD14+ cells. (2) CCL1+CD163+CD14+ cells (ORM-CCL1+CD163+CD14+ cells) were induced from healthy donor peripheral blood CD14+ cells (5 × 105 cells/mL) after stimulation with 1 mg/mL of orosomucoid 1 (human alpha-1 acid glycoprotein, ORM1) [18]. ORM1 has been described as an inducer of M2Mφ [19–25], which plays a major role in the increased susceptibility of these patients to opportunistic infections. In our previous study [18], we demonstrated that ORM1 is active in CD14+ cell polarization to the M2b phenotype. In this procedure, more than 70% of cells switched to CCL1+CD163+CD14+ cells. (3) CCL1+CD163+CD14+ cells were isolated from PBMCs of three severely burned patients (three male, 6–18 years old, 59%–70% total body surface area (TBSA), 3 − 5 weeks after burn injury) (patient-CCL1+CD163+CD14+ cells) admitted to the Shriners Hospitals for Children at Galveston. The human studies were approved by the Institutional Review Board of the University of Texas Medical Branch (IRB approved No. 12–179). For blood sampling from children, written parental informed consent was obtained. Ethical approval was obtained from the Ethics and Scientific Committee of the University of Texas Medical Branch.
In vitro Mφ polarization assay
IL-1β/IgG-CCL1+CD163+CD14+ cells (3 × 105 cells/mL) were cultured in complete medium supplemented with 0.16–100 μg/mL of synthesized CCL1 antisense ODNs. Two days after cultivation, the cells were washed with media and recultured with fresh media for 24 h. Harvested culture fluids were assayed for CCL1 (a biomarker of M2bMφ) by ELISA. The viability of cells was not influenced by these doses of the ODNs. ORM-CCL1+CD163+CD14+ cells treated with 100 μg/mL of HCA-11 were cultured with 105 heat-killed MRSA in fresh complete medium for 24 h. Culture fluids were harvested and assayed for IL-12 by ELISA. Patient-CCL1+CD163+CD14+ cells (1 × 106 cells/mL) were cultured with 100 μg/mL of HCA-11 for 2 days. Then, the cells were washed with PBS and recultured with fresh complete medium for 24 h. Harvested culture fluids were assayed for CCL1 and IL-10.
Humanized murine chimeras and the ODN treatment
Two different sources of human cells, ORM-CCL1+CD163+CD14+ cells and patient WBCs, were used for the preparation of humanized murine chimeras. ORM-CCL1+CD163+CD14+ cell chimeras and patient chimeras were created in γNSG mice after i.v. inoculation with ORM-CCL1+CD163+CD14+ cells (5 × 106 cells/mouse) and burn patient (one male, two female, 6–8 years old, 31%–65% TBSA, 3–4 weeks after burn injury) WBCs (5 × 106 cells/mouse). γNSG mice inoculated with healthy donor CD14+ cells or healthy donor WBCs served as control groups (healthy chimeras). The chimeras were subcutaneous (s.c.) treated with HCA-11 (25 μg/chimera) twice a day for 2 days. Two days after the cell inoculation, these chimeras were i.v. infected with 105 CFU/chimera of MRSA. This amount of the pathogen corresponds to <0.005 LD50 in chimeras created with healthy donor WBCs. The severity of infection in the chimeras was evaluated by bacterial growth in liver, spleen, and kidneys. To measure the quantity of bacteria in the organs, the specimens were weighed and homogenized in 3 mL of PBS using a THQ Tissue Homogenizer (OMNI International, Kennesaw, GA). A serial 10-fold dilution of the homogenates was plated onto blood agar plates and incubated for 24 h at 37°C. The colonies were counted, and the number of bacteria per gram organ was calculated.
Statistical analysis
Infection experiments were statistically analyzed by Student's t-test for groups treated with the ODN and groups treated with PBS. The results were considered significant if the probability was <0.05.
Results
Polarization of CCL1+CD163+CD14+ cells by newly synthesized ODNs
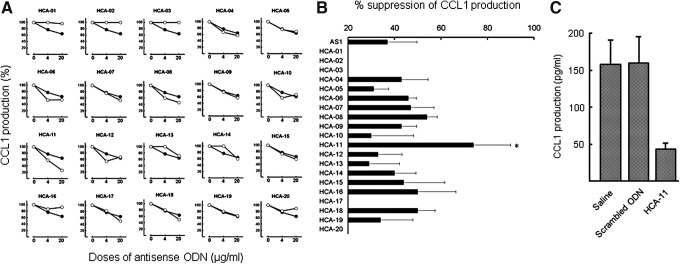
M2bMφ (CCL1+CD163+CD14+ cells) play a major role in the increased susceptibility of subacutely burned patients to sepsis. CCL1 is an essential chemokine for the maintenance of M2bMφ properties [10]. Opportunistic infections in severely burned mice have been controlled by murine CCL1 antisense ODN, a specific polarizer of murine M2bMφ. In this study, we synthesized various human CCL1 antisense ODNs, (Fig. 1) which are active against human M2bMφ. IL-1β/IgG-CCL1+CD163+CD14+ cells (3 × 105 cells/mL) were treated with 4 or 20 μg/mL of AS1 [13] or the same dose of 20 newly synthesized CCL1 antisense ODNs. As a control, the cells were treated with the same volume of saline. Twenty-four hours after cultivation, culture fluids were harvested and CCL1 production was measured by ELISA. In the results, 70% or more of CCL1 production was reduced in IL-1β/IgG-CCL1+CD163+CD14+ cell cultures supplemented with 20 μg/mL of HCA-11, whereas AS1 suppressed CCL1 production by 37% in the same manner (Fig. 2A, B). The CCL1 production by IL-1β/IgG-CCL1+CD163+CD14+ cells was not significantly inhibited by the other ODNs (Fig. 2B). Similar to the control, CCL1 was produced by IL-1β/IgG-CCL1+CD163+CD14+ cells in cultures supplemented with 20 μg/mL of scrambled ODN (Fig. 2C). Dose–response inhibitory effects of HCA-11 on the CCL1 production by IL-1β/IgG-CCL1+CD163+CD14+ cells were examined next. As shown in Fig. 3, about 60% of CCL1 production was suppressed in cultures of IL-1β/IgG-CCL1+CD163+CD14+ cells treated with 4 μg/mL of HCA-11, compared to 20% suppression by the same dose of AS1. Almost 90% inhibition of the CCL1 production was shown in IL-1β/IgG-CCL1+CD163+CD14+ cell cultures supplemented with 100 μg/mL of HCA-11. In addition, the CCL1 production by IL-1β/IgG-CCL1+CD163+CD14+ cells was not influenced by various doses of scrambled ODN (Fig. 3A). Consistently, CCL1 mRNA, derived from IL-1β/IgG-CCL1+CD163+CD14+ cells cultured with 20 μg/mL of HCA-11 for 2 days, was reduced, but not from the cells cultured with same dose of scrambled ODN (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/nat). These results indicate that HCA-11 is inhibitory on the CCL1 production by IL-1β/IgG-CCL1+CD163+CD14+ cells.
FIG. 2.
CCL1 production by CCL1+CD163+CD14+ cells treated with synthesized ODNs. IL-1β/IgG-CCL1+CD163+CD14+ cells (3 × 105 cells/mL) were cultured with the ODNs for 2 days. Cells were harvested and recultured with fresh medium for 24 h. Culture fluids were harvested and assayed for CCL1 by ELISA. (A) CCL1 production in IL-1β/IgG-CCL1+CD163+CD14+ cell cultures supplemented with 4 or 20 μg/mL of the ODNs. ○, synthesized ODN; ●, AS1. (B) Summary in the results of (A) experiments. Data are shown as mean ± SD. *P < 0.05 compared with AS1. (C) CCL1 production by IL-1β/IgG-CCL1+CD163+CD14+ cells treated with 20 μg/mL of HCA-11 or scrambled ODN. ELISA, enzyme-linked immunosorbent assay; SD, standard deviation.
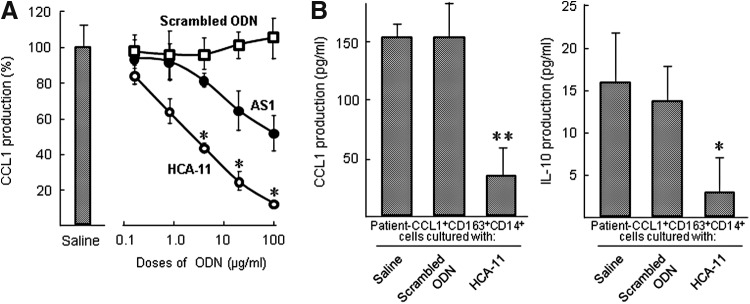
FIG. 3.
Effects of various doses of HCA-11 on the CCL1 production by CCL1+CD163+CD14+ cells. (A) IL-1β/IgG-CCL1+CD163+CD14+ cells (3 × 105 cells/mL) were cultured with 0.16–100 μg/mL of AS1, HCA-11, or scrambled ODN for 2 days, respectively. The same cell population cultured with complete medium alone served as a control. Cells were harvested and recultured with fresh medium for 24 h. Culture fluids obtained were assayed for CCL1. Data are shown as mean ± SD. *P < 0.05 compared with AS1. (B) Patient-CCL1+CD163+CD14+ cells (1 × 106 cells/mL), isolated from three burn patients, were cultured with 100 μg/mL of HCA-11 or scrambled ODN for 2 days. Cells were harvested and recultured with fresh medium for 24 h. Culture fluids obtained were measured for CCL1 and IL-10. Data are shown as mean ± SD. *P < 0.05, **P < 0.01 compared with control.
Polarization of patient-CCL1+CD163+CD14+ cells treated with HCA-11
CCL1+CD163+CD14+ cells (1 × 106 cells/mL) were freshly isolated from peripheral blood of three burned patients and cultured with 100 μg/mL of HCA-11 or scrambled ODN for 2 days. These CD14+ cells were then washed and recultured with complete media for 24 h. Culture fluids were harvested and CCL1 and IL-10 productions were measured by ELISA. In the results, patient-CCL1+CD163+CD14+ cells produced 140–169 pg/mL of CCL1 in their culture fluids (Fig. 3B), while 78.9% of the CCL1 production by patient-CCL1+CD163+CD14+ cells was reduced in the cultures supplemented with 100 μg/mL of HCA-11. Similarly, the IL-10 production by patient-CCL1+CD163+CD14+ cells was reduced (81.3% reduction) in the cultures supplemented with the ODN (Fig. 3B). Both CCL1 and IL-10 were produced by patient-CCL1+CD163+CD14+ cells in cultures supplemented with 100 μg/mL of scrambled ODN (Fig. 3B). These results indicate that the CCL1 production by patient-CCL1+CD163+CD14+ cells is clearly suppressed by HCA-11.
M1 CD14+ cells switched from patient-CCL1+CD163+CD14+ cells
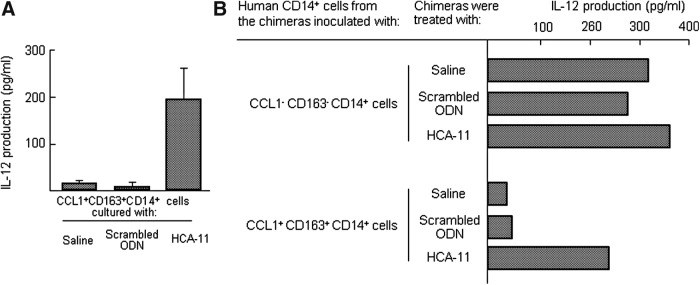
ORM1 has been described as an inducer of M2Mφ [19–25]. In our previous study [18], ORM1 specifically polarize CD14+ cell polarization to the M2b phenotype. In this study, ORM1-induced M2Mφ have been utilized for the evaluation of Mφ-reverting activity of HCA-11. Appearance of M1Mφ in M2Mφ cultures supplemented with HCA-11 was confirmed by IL-12 produced in the cultures. ORM-CCL1+CD163+CD14+ cells (5 × 105 cells/mL) were treated with HCA-11 or scrambled ODN for 2 days. These cells were washed with fresh media and recultured with 105 heat-killed MRSA in fresh medium for 24 h. Obtained culture fluids were assayed for IL-12 by ELISA. IL-12 was shown to be produced in ORM-CCL1+CD163+CD14+ cell cultures supplemented with HCA-11, but IL-12 was not produced in the cultures supplemented with scrambled ODN (Fig. 4A). In addition, the chimeras created with ORM-CCL1+CD163+CD14+ cells (5 × 106 cells/chimera) (ORM-CCL1+CD163+CD14+ cell chimera) were s.c. treated with 25 μg/chimera of HCA-11 or scrambled ODN twice a day for 2 days after the cell inoculation. Human CD14+ cells, isolated from the spleen of these chimeras 2 days after i.v. stimulation with 107 heat-killed MRSA, were assayed for their ability to produce IL-12. In the results, IL-12 was not produced in cultures of splenic CD14+ cells from chimeras treated with saline or scrambled ODN, while IL-12 was produced by splenic CD14+ cells isolated from chimeras treated with HCA-11 (Fig. 4B). These results indicate that CCL1+CD163+CD14+ cells treated with HCA-11 switched to IL −12+CD163−CD14+ cells.
FIG. 4.
CCL1+CD163+CD14+ cells treated with HCA-11 switched to IL12+CCL1−CD163−CD14+ cells. (A) CCL1+CD163+CD14+ cells (5 × 105 cells/mL), induced from healthy donor peripheral blood CD14+ cells after stimulation with ORM1, were cultured with HCA-11 or scrambled ODN for 48 h. The same cell preparations cultured with complete medium supplemented with saline alone served as control. Both groups of cells were washed with fresh media and recultured with 105 heat-killed MRSA. Culture fluids obtained 24 h after recultivation were assayed for IL-12 by ELISA. (B) The chimeras created with CCL1−CD163−CD14+ cells or CCL1+CD163+CD14+ cells (5 × 106 cells/chimera) were s.c. treated with HCA-11 (25 μg/chimera), scrambled ODN (25 μg/chimera), or saline (0.2 mL) twice a day for 2 days after the cell inoculation; CCL1−CD163−CD14+ cells were obtained from healthy donor peripheral blood and CCL1+CD163+CD14+ cells were prepared from CD14+ cells after stimulation with ORM1. Human CD14+ cells (1 × 106 cells/mL), isolated from the spleen of chimeras 2 days after i.v. stimulation with 107 heat-killed MRSA, were assayed for their ability to produce IL-12. i.v., intravenous; MRSA, Methicillin-resistant Staphylococcus aureus; s.c, subcutaneous.
Anti-MRSA resistance of chimeras treated with HCA-11
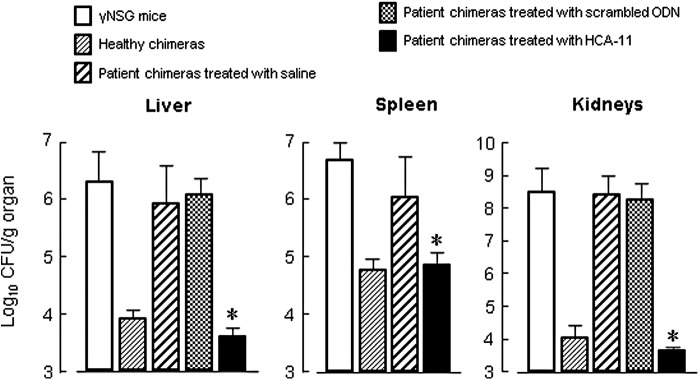
WBCs, freshly isolated from peripheral blood of five healthy donors and three burned patients, were inoculated to γNSG mice (5 × 106 cells/mouse) (abbreviated as healthy chimera and patient chimera). Then, these chimeras were i.v. infected with MRSA (105 CFU/chimera) 2 days after the WBC inoculation. Three days after the infection, the numbers of bacteria in the organs of these chimeras were measured by a standard colony counting method. The pathogens did not grow in liver, spleen, and kidneys of healthy chimeras, while the bacteria grew severely in the organs of patient chimeras. At this time, the bacterial growth was shown to be minimal in patient chimeras treated with HCA-11 (25 μg/chimera, twice a day for 2 days) (Fig. 5), whereas the bacterial growth in patient chimeras treated with scrambled ODN was similar to saline-treated group. The bacterial growth in organs was shown to be similar in healthy chimeras and patient chimeras treated with HCA-11, suggesting that impaired host defense of burn patient WBCs against the bacterial infection was completely restored. These results indicate that host antibacterial resistance of patient chimeras to a lethal dose of MRSA infection is controlled by CCL1 antisense ODN No. 11 (HCA-11).
FIG. 5.
Anti-MRSA resistance of patient chimeras treated with HCA-11. Chimeras were created in γNSG mice after i.v. inoculation with 5 × 106 cells/mouse healthy donor WBCs (healthy donors No. 1 − 5, healthy chimeras) or burn patient WBCs (burn patients No. 4 − 6, patient chimeras). Patient chimeras were treated with HCA-11 (25 μg/chimera, s.c.), scrambled ODN (25 μg/chimera, s.c.), or saline (0.2 mL, s.c.) twice a day for 2 days. Two days after the cell inoculation, these chimeras were infected with MRSA (105 CFU/chimera, i.v.). Bacterial growth in the liver, spleen, and kidneys of chimeras was determined 3 days after the infection. Data are shown as mean ± SD. *P < 0.05 compared with chimera treated with saline.
Discussion
In previous studies [10], M2bMφ have been shown to be CCL1-producing cells. This cytokine has been identified as an essential chemokine for the maintenance of cellular properties of M2bMφ. Thus, M2bMφ easily polarize to the quiescent Mφ phenotype in CCL1-depleted circumstances. Therefore, CCL1+CD163+CD14+ cells treated with CCL1 antisense ODN are able to convert to IL-12+CCL1−CD163−CD14+ cells under stimulation with M1Mφ inducers [10]. In this study, we synthesized 20 human CCL1 antisense ODNs. CCL1 production by three different sources of CCL1+CD163+CD14+ cells (IL-1β/IgG-CCL1+CD163+CD14+ cells, ORM-CCL1+CD163+CD14+ cells, and patient-CCL1+CD163+CD14+ cells) was greatly suppressed by CCL1 antisense ODN No. 11. However, the CCL1 production by CCL1+CD163+CD14+ cells was not significantly suppressed by the other 19 ODNs. CCL1+CD163+CD14+ cells treated with HCA-11 switched to IL-12+CCL1−CD163−CD14+ cells in response to antigen stimulation. Patient chimeras utilized in the experiments were γNSG mice inoculated with subacutely burned patient WBCs. Patient chimeras treated with HCA-11 were shown to be resistant against MRSA systemic infections. A majority of peripheral blood CD14+ cells of subacutely burned patients have been previously demonstrated to be the M2b phenotype. CD163 is well known to be a marker for M2a and M2c macrophages. In addition, M2b macrophages have been shown to express CD163 [26,27]. In our previous studies [12,28], human M2b monocytes expressed CD163. In addition, CD163 expression in human monocytes is upregulated by IL-10 [29]. These facts indicate that CD163 is one of the biomarkers for M2b monocytes/macrophages, as well as M2a and M2c subtypes.
Antisense ODN binds to RNA to modulate the function of the targeted RNA. Different types of antisense mechanisms are classified as RNase H-dependent cleavage mechanisms or occupancy-only mechanisms. Oligonucleotides that work through an RNase H-dependent cleavage mechanism are the most common compounds to have progressed to clinical trial. In this study, HCA-11 was designed to bind to position 156–173 of CCL1 mRNA, a core structural part of this mRNA. In fact, CCL1 mRNA level was reduced in IL-1β/IgG-CCL1+CD163+CD14+ cells after treatment with 100 μg/mL of HCA-11, compared with that in the same cells treated with saline (Supplementary Fig. S1). These observations suggest that HCA-11 may suppress CCL1 production through RNase H-dependent cleavage of CCL1 mRNA.
Mφ are highly plastic and assume their functional phenotypes dependent on microenvironmental signals [4]. M1Mφ have been identified as primary effector cells on the host antibacterial innate immunities [5,6]. However, M1Mφ are not easily generated in immunosuppressed hosts (such as severely burned patients) with a predominance of M2bMφ. M2Mφ inhibit the Mφ conversion from quiescent Mφ to M1Mφ [7–9]. Therefore, hosts who are carriers of M2Mφ are greatly susceptible to various opportunistic infections [7–9]. M2Mφ are composed of three different subtypes: M2a (CCL17+LIGHT−CD209+ Mφ), M2b (CCL1+LIGHT+CD209− Mφ), and M2c (CXCL13+LIGHT−CD209+ Mφ) [30–33]. M2aMφ switch to quiescent Mφ with the disappearance of IL-4 from their environment [34,35]. Thus, their phenotypic properties are unstable. In contrast, M2bMφ have poor plasticity and live longer in severely burned hosts. Host antibacterial defenses are severely suppressed as long as M2bMφ are present.
In our recent studies [28], CCL1+CD163+CD14+ cells predominated in peripheral blood of alcoholics. These cells contributed to the increased susceptibility of alcoholics to pneumonia and gut bacteria-associated sepsis. We also demonstrated that M2bMφ were predominantly isolated from the bacterial translocation sites [mesenteric lymph nodes (MLNs) and lamina propria (LP)] of chronic alcohol-consuming (CAC) mice, and M1Mφ were not induced by bacterial antigens in cultures of F4/80+ cells derived from MLNs and LP of CAC mice [12]. The increased susceptibility of CAC mice to bacterial pneumonia and sepsis stemming from enterococcal translocation was improved to a level shown in normal mice after treatment with CCL1 antisense ODN. These studies suggest the possibility that some opportunistic infections are controllable by HCA-11 treatment in patients whose M2bMφ predominate.
Supplementary Material
Acknowledgments
This work was supported by grants from Shriners Hospitals for Children (no. 85230) and NIH (U01 AI107355).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Morrison VA. (2001). Infections in the immunocompromised host. In: Management of Antimicrobials in Infectious Diseases. Mainous AG, III, Pomeroy C, eds. Humana Press, Totowa, NJ, pp. 315–330 [Google Scholar]
- 2.Hunsicker LM, Heggers JP. and Patel JA. (2001). Infections in burn patients. In: Clinical Management of Infections in Immunocompromised Infants and Children. Patrick CC, ed. Lippincott Williams & Wilkins, Philadelphia, pp. 327–349 [Google Scholar]
- 3.Davey V. (2002). Immunocompromised persons and emerging infectious diseases. In: Emerging Infectious Diseases. Lashley FR, Durham JD, eds. Springer Publishing, New York, pp. 303–324 [Google Scholar]
- 4.Sica A. and Mantovani A. (2012). Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122:787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon S. (2003). Alternative activation of macrophages. Nat Rev Immunol 3:23–35 [DOI] [PubMed] [Google Scholar]
- 6.Mosser DM. (2003). The many faces of macrophage activation. J Leukoc Biol 73:209–212 [DOI] [PubMed] [Google Scholar]
- 7.Katakura T, Miyazaki M, Kobayashi M, Herndon DN. and Suzuki F. (2004). CCL17 and IL-10 as effectors that enable alternatively activated macrophages to inhibit the generation of classically activated macrophages. J Immunol 172:1407–1413 [DOI] [PubMed] [Google Scholar]
- 8.Katakura T, Yoshida T, Kobayashi M, Herndon DN. and Suzuki F. (2005). Immunological control of methicillin-resistant Staphylococcus aureus (MRSA) infection in an immunodeficient murine model of thermal injuries. Clin Exp Immunol 142:419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi M, Jeschke MG, Shigematsu K, Asai A, Yoshida S, Herndon DN. and Suzuki F. (2010). M2b monocytes predominated in peripheral blood of severely burned patients. J Immunol 185:7174–7179 [DOI] [PubMed] [Google Scholar]
- 10.Asai A, Nakamura K, Kobayashi M, Herndon DN. and Suzuki F. (2012). CCL1 released from M2b macrophages is essentially required for the maintenance of their properties. J Leukoc Biol 92:859–867 [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi M, Nakamura K, Cornforth M. and Suzuki F. (2012). Role of M2b macrophages in the acceleration of bacterial translocation and subsequent sepsis in mice exposed to whole body [137Cs] γ-irradiation. J Immunol 189:296–303 [DOI] [PubMed] [Google Scholar]
- 12.Ohama H, Asai A, Ito I, Suzuki S, Kobayashi M, Higuchi K. and Suzuki F. (2014). M2b macrophage elimination and improved resistance of mice with chronic alcohol consumption to opportunistic infections. Am J Pathol 185:420–431 [DOI] [PubMed] [Google Scholar]
- 13.Haque NS, Zhang X, French DL, Li J, Poon M, Fallon JT, Gabel BR, Taubman MB, Koschinsky M. and Harpel PC. (2000). CC chemokine I-309 is the principal monocyte chemoattractant induced by apolipoprotein(a) in human vascular endothelial cells. Circulation 102:786–792 [DOI] [PubMed] [Google Scholar]
- 14.Yoshida S, Lee JO, Nakamura K, Suzuki S, Hendon DN, Kobayashi M. and Suzuki. F. (2014). Effect of glycyrrhizin on pseudomonal skin infections in human-mouse chimeras. PLoS One 9:e83747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, et al. (2002). NOD/SCID/Rγnull mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100:3175–3182 [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi M, Takahashi H, Sanford AP, Herndon DN, Pollard RB. and Suzuki F. (2002). An increase in the susceptibility of burned patients to infectious complications due to impaired production of macrophage inflammatory protein 1 alpha. J Immunol 169:4460–4466 [DOI] [PubMed] [Google Scholar]
- 17.Sironi M, Martinez FO, D'Ambrosio D, Gattorno M, Polentarutti N, Locati M, Gregorio A, Iellem A, Cassatella MA, et al. (2006). Differential regulation of chemokine production by Fcgamma receptor engagement in human monocytes: association of CCL1 with a distinct form of M2 monocyte activation (M2b, Type 2). J Leukoc Biol 80:342–349 [DOI] [PubMed] [Google Scholar]
- 18.Nakamura K, Ito I, Kobayashi M, Herndon DN. and Suzuki F. (2015). Orosomucoid 1 drives opportunistic infections through the polarization of monocytes to the M2b phenotype. Cytokine 73:8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibata Y, Tamura K. and Ishida N. (1983). In vivo analysis of the suppressive effects of immunosuppressive acidic protein, a type of alpha 1-acid glycoprotein, in connection with its high level in tumor-bearing mice. Cancer Res 43:2889–2896 [PubMed] [Google Scholar]
- 20.Bories PN, Guenounou M, Féger J, Kodari E, Agneray J. and Durand G. (1987). Human alpha 1-acid glycoprotein-exposed macrophages release interleukin 1 inhibitory activity. Biochem Biophys Res Commun 147:710–715 [DOI] [PubMed] [Google Scholar]
- 21.Majima T, Otsuji K, Nagatomi R. and Konno T. (1995). Polysaccharide (ANK-102) from Polianthes tuberosa cells deteriorates the resistance of mice to Listeria monocytogenes infection. Immunopharmacol Immunotoxicol 17:59–68 [DOI] [PubMed] [Google Scholar]
- 22.Fu YX, Watson GA, Kasahara M. and Lopez DM. (1994). The role of tumor-derived cytokines on the immune system of mice bearing a mammary adenocarcinoma. I. Induction of regulatory macrophages in normal mice by the in vivo administration of rGM-CSF. J Immunol 46:783–789 [PubMed] [Google Scholar]
- 23.Komori H, Watanabe H, Shuto T, Kodama A, Maeda H, Watanabe K, et al. (2012). α1-Acid glycoprotein up-regulates CD163 via TLR4/CD14 protein pathway: possible protection against hemolysis-induced oxidative stress. J Biol Chem 287:30688–30700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi M, Katakura T, Shimoda M, Roberts NJ, Jr, Herndon DN. and Suzuki F. (2003). α1-Acid glycoprotein (AGP) stimulates resident macrophages to generate alternatively activated macrophages (AAMu). Part I. Biological properties of AGP-induced AAMu. FASEB J 17:C160 [Google Scholar]
- 25.Takano S, Sami S, Majima T. and Ishida N. (1986). Low molecular weight immunosuppressive factors found in elevated amounts in cancer ascetic fluids of mice. 2. 1-Methyladenosine isolated from cancer ascitic fluids enhances Listeria infection in mice. J Immunopharmacol 8:59–73 [DOI] [PubMed] [Google Scholar]
- 26.David S. and Kroner A. (2011). Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12:388–399 [DOI] [PubMed] [Google Scholar]
- 27.Gea-Sorlí S, Guillamat R, Serrano-Mollar A. and Closa D. (2011). Activation of lung macrophage subpopulations in experimental acute pancreatitis. J Pathol 223:417–424 [DOI] [PubMed] [Google Scholar]
- 28.Tsuchimoto Y, Asai A, Tsuda Y, Ito I, Nishiguchi T, Garcia MC, Suzuki S, Kobayashi M, Higuchi K. and Suzuki F. (2015). M2b monocytes provoke bacterial pneumonia and gut bacteria-associated sepsis in alcoholics. J Immunol 195:5169–7517 [DOI] [PubMed] [Google Scholar]
- 29.Sulahian TH, Högger P, Wahner AE, Wardwell K, Goulding NJ, Sorg C, Droste A, Stehling M, Wallace PK, Morganelli PM. and Guyre PM. (2000). Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine 12:1312–1321 [DOI] [PubMed] [Google Scholar]
- 30.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A. and Locati M. (2004). The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686 [DOI] [PubMed] [Google Scholar]
- 31.Edwards JP, Zhang X, Frauwirth KA. and Mosser DM. (2006). Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol 80:1298–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrante CJ. and Leibovich SJ. (2012). Regulation of macrophage polarization and wound healing. Adv Wound Care (New Rochelle) 1:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q, Ni H, Lan L, Wei X, Xiang R. and Wang Y. (2010). Fra-1 protooncogene regulates IL-6 expression in macrophages and promotes the generation of M2d macrophages. Cell Res 20:701–712 [DOI] [PubMed] [Google Scholar]
- 34.Vergadi E, Vaporidi K, Theodorakis EE, Doxaki C, Lagoudaki E, Ieronymaki E, Alexaki VI, Helms M, Kondili E, et al. (2014). Akt2 deficiency protects from acute lung injury via alternative macrophage activation and miR-146a induction in mice. J. Immunol 192:394–406 [DOI] [PubMed] [Google Scholar]
- 35.Das A, Ganesh K, Khanna S, Sen CK. and Roy S. (2014). Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immunol 192:1120–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.