Abstract
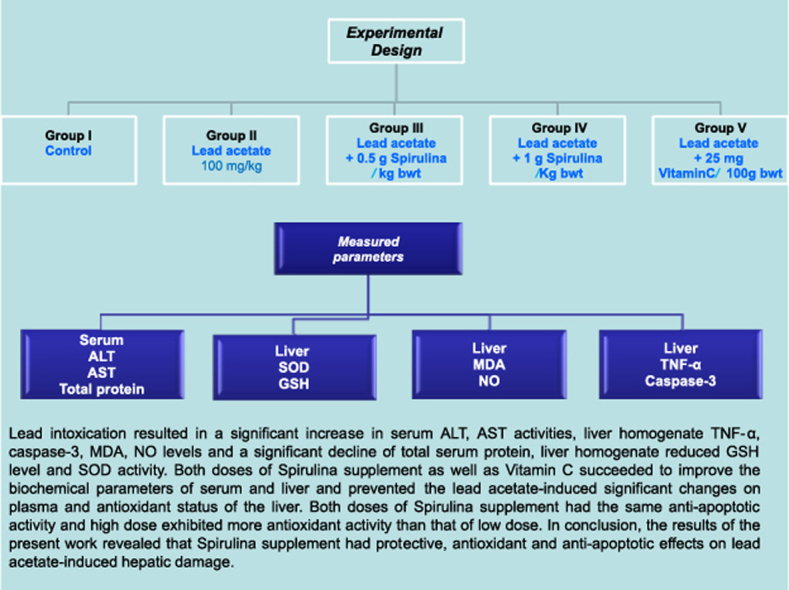
Lead is a toxic metal that induces a wide range of behavioral, biochemical and physiological effects in humans. Oxidative damage has been proposed as a possible mechanism involved in lead toxicity. The current study was carried out to evaluate the antioxidant activities of Spirulina supplement against lead acetate -induced hepatic injury in rats. Five groups of rats were used in this study, Control, Lead acetate (100 mg/kg), Lead acetate (100 mg/kg) + 0.5 g/kg Spirulina, Lead acetate (100 mg/kg) + 1 g/kg Spirulina and Lead acetate + 25 mg/100 g Vitamin C (reference drug). All experimental groups received the oral treatment by stomach tube once daily for 4 weeks. Lead intoxication resulted in a significant increase in serum alanine transaminae (ALT), aspartate transaminae (AST) activities, liver homogenate tumor necrosis factor-α (TNF-α), caspase-3, malondialdehyde (MDA), nitric oxide (NO) levels and a significant decline of total serum protein, liver homogenate reduced glutathione (GSH) level and superoxide dismutase (SOD) activity. Both doses of Spirulina supplement as well as Vitamin C succeeded to improve the biochemical parameters of serum and liver and prevented the lead acetate-induced significant changes on plasma and antioxidant status of the liver. Both doses of Spirulina supplement had the same anti-apoptotic activity and high dose exhibited more antioxidant activity than that of low dose. In conclusion, the results of the present work revealed that Spirulina supplement had protective, antioxidant and anti-apoptotic effects on lead acetate-induced hepatic damage.
Keywords: Spirulina supplement, lead acetate, anti-apoptotic, Caspase-3, Tumor necrosis factor-α
Graphical abstract
1. Introduction
Lead (Pb) is a toxic metal that induces a wide range of behavioral, biochemical and physiological effects in humans. Even though blood lead levels continue to decline over the past two decades, specific populations like infants, young children and working class are still at a higher risk.1 Childhood lead exposure is estimated to contribute to about 600 000 new cases of children developing intellectual disabilities every year. Lead exposure is estimated to account for 143 000 deaths per year with the highest burden in developing regions. About one half of the burden of disease from lead occurs in the WHO South-East Asia Region, with about one-fifth each in the WHO Western Pacific and Eastern Mediterranean Regions.2 As lead exposure tends to be sub acute, produces only subtle clinical symptoms. Chronic exposure cases are more common than acute toxicity. Lead via gastro intestinal absorption is first taken up by the red blood cells and is distributed to all vascular organs.3 Pathogenesis of lead poisoning is mainly attributed to lead-induced oxidative stress. Chronic lead exposure is known to disrupt the pro oxidant/antioxidant balance existing within the mammalian cells.4, 5 Lead is reported to cause oxidative stress by generating the release of reactive oxygen species (ROS) such as superoxide radicals, hydrogen peroxide and hydroxyl radicals and lipid peroxides. There has been increased interest among phytotherapy researchers to use medicinal plants with antioxidant activity for protection against heavy metal toxicity.6, 7, 8
Pharmaceutical drugs that depend on compounds such as colchicine and interferons are currently available for the treatment of liver cirrhosis but with either unreliable efficacies or high situations of side effects.9
A number of natural compounds produced from vegetation offer alternative healthcare options that are more effective and safe.10 Ingredients from recently found or already known plant varieties are regularly being examined on experimental animals.11 The prospective tasks and efficiency of plant extracts in liver diseases are yet to be analyzed. Spirulina Slimming Capsule, the product chooses the natural herbal product combining Spirulina platensis and Aloe extract.
Spirulina is a great source of natural protein (about 60% digestible proteins). It is a low fat, low calorie, cholesterol free source of protein with all amino acids, phyto-nutrients, antioxidants, carbohydrate, muco-polysacchrides, vitamins and trace minerals, an exclusive ingredient, prevents the digestion of dietary fat, hence reducing its absorption by the body. After sometime of consumption, body parts prone to fat accumulation (belly, arms, thighs and buttoks) could see dramatic benefits. Spirulina soft capsule promote gastrointestinal motility, remove intestinal toxins, improve constipation. Spirulina Slimming. It can reduce the absorption of lipophilic analogs by intestinal tract, control the ingestion of heat quantity, avoid the storage of fat, plug up the source of fat and burn the quondam fat by the way of heat production. It accelerate the metabolic rate of fat, which change the fat into sugar and protein, to protect the nutrition that people need and keep slimming. In addition, it has the function of hairdressing. Many people find it effective as a natural appetite suppressant. It is also known to assist blood circulation. The present study was planned to investigate the antioxidant, heptoprotective and apoptotic effects of Spirulina supplement against lead-induced hepatic injury and apoptotic changes in rats.
2. Materials & methods
Spirulina Slimming, Fat Burner Capsules 350 mg (China go2slimming LLC NO.315 Kunming Luosiwan Commercial Center Kunming, Yunnan, China 650214) was purchased from private Pharmacy, Cairo, Egypt.
2.1. Experimental design
Thirty male Wistar albino rats weighing (140–160) g were used for this study. The animals were housed in a temperature (25 ± 1 °C), humidity controlled room and a 12-h light-dark cycle. Rats were allowed free access to tap water and standard pellet diet. The institutional Animal Ethics Committee approved all experimental protocols. The animals were classified into 5 groups, each of 6 as follows:
Control group (C): Rats received distilled water.
Lead acetate-treated group (LA): Rats were orally administered lead acetate at a dose of 100 mg lead acetate/kg body weight, by stomach tube once daily for 4 weeks.
Lead acetate and 0.5 g/kg Spirulina treated group (LA + 0.5 g/kg Spirulina): Rats were orally administered lead acetate at a dose of 100 mg lead acetate/kg body weight and 0.5 g Spirulina/kg body weight by stomach tube once daily for 4 weeks.
Lead acetate and 1 g/kg Spirulina treated group (LA + 1 g/kg Spirulina): Rats were orally administered lead acetate at a dose of 100 mg lead acetate/kg body weight and 1 g Spirulina/kg body weight by stomach tube once daily for 4 weeks.
Lead acetate and 25 mg/100 g Vitamin C(reference drug) treated group (LA + 25 mg/100 g Vitamin C): Rats were orally administered 100 mg lead acetate/kg body weight and 25 mg Vitamin C/100 gm body weight by stomach tube once daily for 4 weeks.
At the end of experiment, fasting blood samples were withdrawn from the retro-orbital vein of each animal using a glass capillary tube after fasting period of 12 h. The blood samples allowed to coagulate and then centrifuged at 3000 rpm for 20 min. The separated sera were used for the estimation of serum activities of ALT and AST by using commercial kits (Quimica Clinica Aplicada, Spain). Total serum protein was evaluated using kits from Biodiagnostic, Egypt.
2.2. Preparation of liver homogenate
A portion of liver was excised, accurately weighed and homogenized in ice-saline to prepare a 10% (w/v) tissue homogenate. The homogenate was used for the determination of GSH level, SOD activity, end product of lipid perioxidation, MDA and NO levels.
The protein content of liver homogenates was evaluated by the method of Lowry et al12 and using bovine serum albumin as a standard. GSH level in liver tissue was measured by the method of Ellman using 5, 5′-dithiobis-(2-nitrobenzoate) at 412 nm.13
SOD activity in liver tissue was determined by the method of Marklund and Marklund14 pyrogallol (24 mmol/L) was prepared in 10 mM HC1 and kept at 4 °C Before use. Stock catalase solution (30 μmol/L) was prepared in phosphate buffer (pH 9, 0.1 M), 100 μl of the supernatant was added to Tris HC1 buffer (pH 7.8, 0.1 M) containing 25 μl pyrogallol and 10 μl catalase. The final volume was adjusted to 3 ml using the same buffer solution. Changes in the absorbance at 420 nm were recorded at 1 min interval for 3 min. Data were expressed as U/mg protein.
In liver homogenate, MDA, a stable product of lipid peroxidation was estimated by method of Ohkawa.15 In brief, 0.5 ml of homogenate was mixed with 2.5 ml of 20% trichloroacetic acid and centrifuged at 3000 rpm for 10 min. The supernatant was decanted and the precipitate was washed once with 0.05 M sulphuric acid and then 3 ml of 0.2 g/dl thiobarbituric acid reagent was added to the precipitate. The mixture was heated in a boiling water bath for 30 min. After cooling in cold water, the resulting chromogen was extracted with 4 ml of n-butyl alcohol. The organic phase was separated by centrifugation at 3000 rpm for 10 min and absorbance was recorded at wavelength of 530 nm.
NO level in liver tissues was measured by using the commercial kits supplied by Biodiagnostic, Egypt. The assay is based on the diazotization of sulfanilic acid with nitric oxide at acidic pH and subsequent coupling with N-(1-naphthyl)-ethylenediamine to yield an intensely pink colored product that was measured spectrophotometrically at 540 nm. Sodium nitrite was used as standard.
2.3. Estimation of TNF-a and caspase-3 levels
A portion of the liver was weighed (50 mg) and homogenized in 0.8 ml lysis buffer, pH 7.4. The lysate was centrifuged at 10 000 g for 15 min at 4 °C, and the supernatant was taken for estimation of the TNF-α and caspase-3 levels by a sandwich enzyme immunoassay kit (Cloud Clone Corp.) for in vitro quantitative measurement of TNF-α and caspase-3 level in rat tissue homogenates.
2.4. Statistical analysis
Results were shown as mean ± S.E. for each group. Statistical analysis was performed using SPSS 9.0 for Windows (Chicago, IL, USA). For multiple comparisons, one-way analysis of variance (ANOVA) was used. In cases where ANOVA showed significant differences, Tukey test was performed. P < 0.05 was considered to be statistically significant.
3. Results
3.1. Body weight
In the lead treated animals there was a significant reduction in the body weight (140 ± 2.9) as compared to their corresponding control animals after 4 weeks (175 ± 5), P < 0.05. While protection with 0.5 g, 1 g Spirulina as well Vitamin C showed significant increase in their body weight (160.6 ± 6), (165 ± 7) and (164 ± 5), respectively, when compared with that of lead acetate treated group, P < 0.05.
3.2. Effect of Spirulina supplement on serum ALT, AST activities and total protein
Lead toxicity caused a significant increase in serum ALT, AST activities and a significant decrease in total serum protein as compared to their corresponding controls, P < 0.05. Administration of Spirulina supplement with two doses as well as Vitamin C to lead-intoxicated rats revealed a significant decrease in serum ALT, AST activities and a significant increase in total serum protein as compared to lead treated animals, P < 0.05, (Table 1).
Table 1.
Serum total protein, ALT and AST activities in control (C), lead acetate (LA), LA + 0.5 g Spirulina/kg, LA + 1 g Spirulina/kg and 25 mg Vitamin C/100 g bwt, treated groups.
| Control | Lead acetate | Lead acetate + 0.5 g Spirulina/kg bwt | Lead acetate + 1 g Spirulina/kg bwt | Lead acetate + 25 mg vitamin C/kg bwt | |
|---|---|---|---|---|---|
| Total protein (g/dl) | 7.47 ± 0.3 | 6 ± 0.17a | 6.89 ± 0.13b | 7 ± 0.16b | 7.32 ± 0.31b |
| ALT (U/L) | 26.7 ± 0.6 | 95.8 ± 3.3a | 47.5 ± 2.1a,b,c,d | 27.6 ± 0.6b | 28.3 ± 0.46b |
| AST (U/L) | 34.8 ± 0.6 | 81.5 ± 2.6a | 65 ± 1.43a,b,c,d | 35.4 ± 0.88b | 36.9 ± 0.9b |
Results are expressed as mean ± S.E.
P < 0.05 was considered to be statistically significant.
Significantly different from control group.
Significantly different from lead acetate treated group.
Significantly different from lead acetate (LA) + 1 g Spirulina/kg bwt, treated group.
Significantly different from lead acetate (LA) + and 25 mg Vitamin C/100 g bwt, treated group.
3.3. Effect of Spirulina supplement on GSH level and SOD activity in liver homogenate
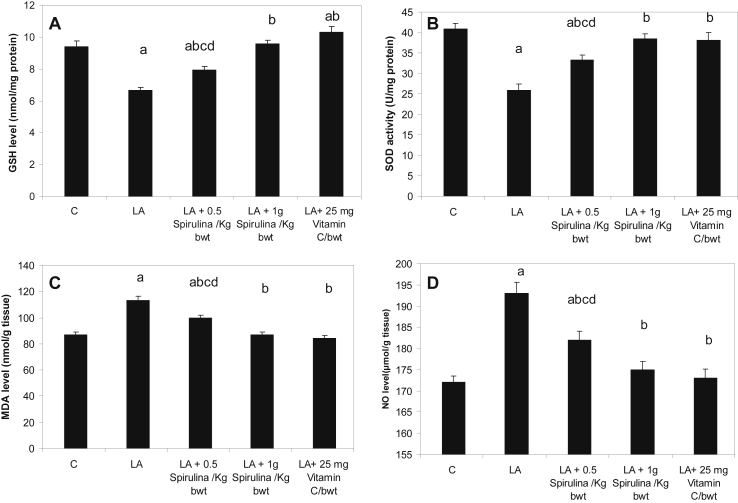
Lead toxicity caused a significant decrease in GSH level and SOD activity in liver homogenate when compared to their perspective controls, P < 0.05. Administration of Spirulina supplement with two doses as well as Vitamin C to lead-intoxicated rats revealed a significant increase in GSH level and SOD activity as compared to lead treated animals, but the higher dose showed a more significant antioxidant effect than that of lower dose, P < 0.05, Fig. 1(A) and (B).
Fig. 1.
Effect of Spirulina supplement on liver homogenate GSH (A), SOD(B), MDA (C) and NO (D) in control (C), lead acetate (LA), LA + 0.5 g Spirulina/kg, LA + 1 g Spirulina/kg and, LA + 25 mg Vitamin C/100 g bwt, treated groups. Data represent the means ± S.E.M (n = 6). a: Significantly different from control. b: Significantly different from lead acetate treated group. c: Significantly different from lead acetate (LA) + 1 g Spirulina/kg bw, treated group. d: Significantly different from lead acetate (LA) + 25 mg Vitamin C/100 g bwt, treated group.
3.4. Effect of Spirulina supplement on MDA and NO levels in liver homogenate
Lead toxicity caused a significant increase of MDA and NO levels in liver homogenate as compared to their corresponding controls, P < 0.05. Treatment of Spirulina supplement with two doses as well as Vitamin C to lead-intoxicated rats showed a significant decrease in MDA and NO levels as compared to lead treated animals, but the high dose exhibited lower significant values of MDA and NO than that of lower dose, P < 0.05, Fig. 1(C) and (D).
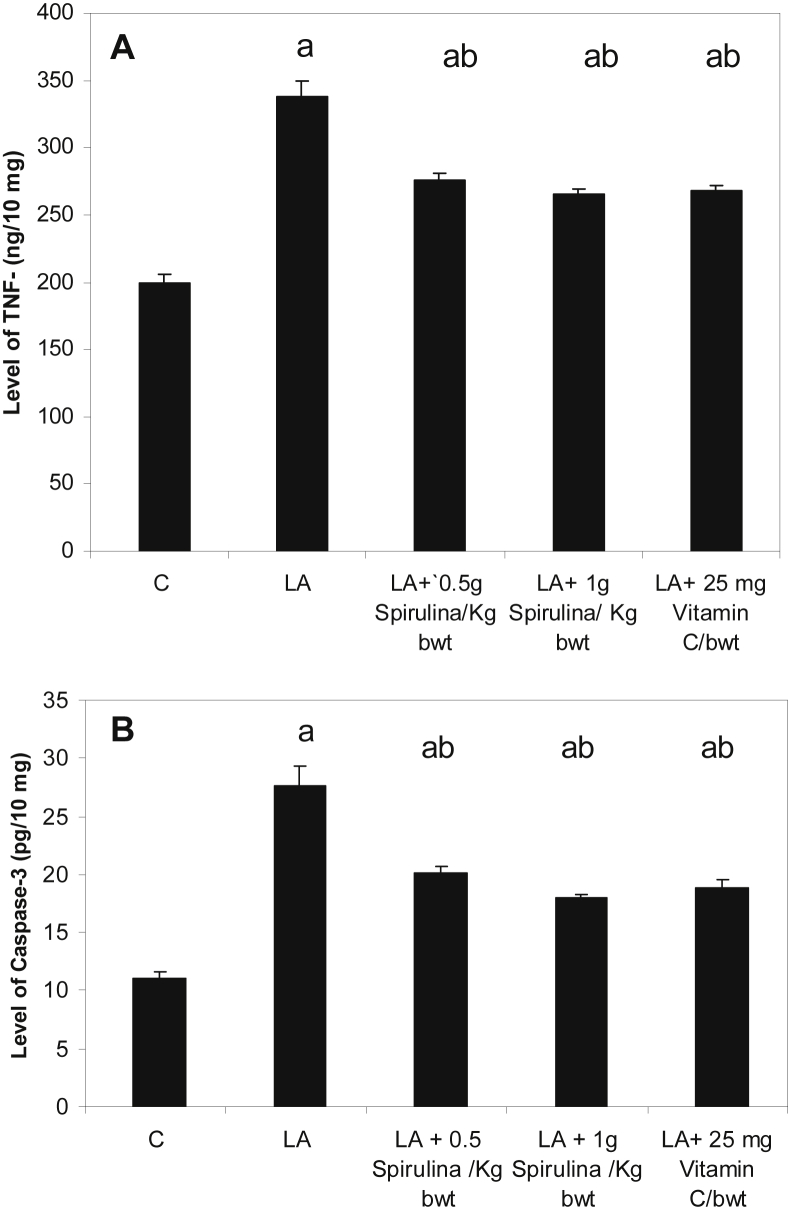
3.5. Effect of Spirulina supplement on TNF-α and caspase-3 levels in liver homogenate
Lead toxicity caused a significant increase of TNF-α and caspase-3 levels in liver homogenate when compared to their corresponding controls, P < 0.05. Treatment of Spirulina supplement with two doses as well as Vitamin C to lead-intoxicated rats revealed a significant decrease in TNF-α and caspase-3 levels as compared to lead treated animals, P < 0.05, Fig. 2(A) and (B).
Fig. 2.
Effect of Spirulina supplement on liver homogenate TNF- α (A) and Caspase-3 (B) level in control (C), lead acetate (LA), LA + 0.5 g Spirulina/kg, LA + 1 g Spirulina/kg and, LA + 25 mg Vitamin C/100 g bwt, treated groups. Data represent the mean ± S.E.M (n = 6). a: Significantly different from control. b: Significantly different from lead acetate treated group.
4. Discussion
The liver is considered one of the target organs affected by lead toxicity owing to its storage in the liver after lead exposure. Also, the liver being one of the major organs involved in the storage, biotransformation and detoxification of toxic substances, is of interest in heavy metal poisoning.16 The present study was planned to investigate the antioxidant, anti-apoptotic and heptoprotective effects of Spirulina supplement against lead-induced hepatic injury in rats.
The activities of ALT and AST are indicators of hepatotoxicity.17 In the present study, there was a significant increase of ALT and AST activities in lead treated group as compared to the control group. These results showed that the exposure to lead affects hepatic tissue, which is consistent with other reports.18
Lead is a heavy metal that produces oxidative damage in the liver by enhancing lipid peroxidation.19 Lead toxicity leads to free radical damage by two separate, although related, pathways: (1) the generation of reactive oxygen species (ROS), including hydroperoxides, singlet oxygen, and hydrogen peroxides, evaluated by MDA levels as the final products of lipid peroxidation, and (2) the direct depletion of antioxidant reserves.20 In the present study, there was significant increase of MDA levels and a significant decrease of GSH levels in lead-intoxicated rats. Our results are in agreement with other previous studies.21, 22 Also, in the present work, there was a significant decrease in SOD activity in lead-intoxicated rats. These results were in agreement with that of Ponce-Canchihuamán et al.23 The presence of increased MDA level observed in the current study was also due to decreased SOD activity, an indicator of oxidative stress. The possible explanation could be related to the proposed role of GSH in the active excretion of lead through bile by binding to the thiol group of GSH and then being excreted. A decrease in GSH levels could lead to oxidative stress and a consequent increase in MDA.6 Among therapeutics for liver diseases, protective drugs such as antioxidants have attracted more and more attention and proton radical-scavenging action is well known as an important mechanism of antioxidation. In our work, the treatment of lead-intoxicated rats with Spirulina supplement revealed a significant increase in GSH level, SOD activity, decrease in MDA and NO levels as compared to lead-intoxicated rats indicating its antioxidant activity.
In order to evaluate the hepatic inflammation and apoptosis, TNF- α and caspase-3 were measured. Lead, in the present study, caused marked increase in hepatic TNF-α level, which were consistent with previous report.24 On the other hand, increased oxidative stress caused disturbances in mitochondrial membrane permeability, causing leakage of free radicals and cytochrome-c from the mitochondria to the cytosol. Once cytochrome-c is released into the cytosol, it binds to another protein, Apaf-1, and promotes activation of the caspase cascade, leading to cell death.25 In the present study, Spirulina treatment significantly reduced the elevated levels of hepatic TNF-α and caspase-3 observed in the lead treated group an effect that might be attributed to reduction in oxidative stress. Treatment of lead-intoxicated animals with two doses of Spirulina supplement (0.5 and 1 g/Kg body weight) showed anti-inflammatory, anti-apoptotic and anti-oxidant activities, while the antioxidant activity was more pronounced in the dose of 1 g/Kg body weight.
One of the components of Spirulina supplement is Spirulina platensis, is blue - green algae due to the presence of both chlorophyll (green) and phycocyanin (blue) pigments in its cellular structure. Chlorophyll (green) helps cleanse the body of toxins, phycocyanin (blue) supports protein absorption and healthy immune function and beta carotene (orange) encourages antioxidant activity. Spirulina contains all eight essential amino acids, making it a complete protein, plus B-complex vitamins, beta carotene, vitamin E, carotenoids, manganese, zinc, copper, iron, gamma linoleic acid (GLA) and calcium.26
In a previous work conducted by Kepekçi et al27 the author investigated the protective effects of the biomass of Spirulina platensis with low amounts of phenolics (SP1) and with high amounts of phenolics (SP2) against CCl4-induced acute hepatotoxicity in rats. The author concluded that showed that SP2 was more potent than SP1 in protecting the liver from toxic injury of CCl4 and preserving the hepatocyte ultrastructure and suggested that the probable superiority of SP2 in hepatoprotective activity in rats might be due to the following effects: stabilizing the hepatocyte membrane by preventing lipid peroxidation, ameliorating the activities of the antioxidant enzymes, inhibition of the inflammation and the radical scavenging activity. These effects might be attributed to the increased amounts of phycocyanine and phenolic compounds and the antioxidant capacity as confirmed by three different antioxidant activity tests. Probably, SP2 restored the liver injuries by scavenging the free radicals generated by CCl4 and preventing infIammation more effectively. The increased phycocyanine content of SP2 may also be partly involved in the inflammatory processes. It has been recently reported that phycocyanine could block inflammatory infiltration through its anti-inflammatory activities.27
Also, in our study, Spirulina succeeded to induce an improvement in body weight and the biochemical parameters. Several reports have indicated that Spirulina has antioxidant effects28, 29, 30 due to its higher content of some macro- and micronutrients including high quality protein, iron, gamma-linolenic fatty acid, carotenoids, vitamins B1 and B231 β-carotene, α-tocopherol and phycocyanin.32 The phycocyanin has been considered the predominant compound in the antioxidant activity of the Spirulina.33, 34
The other component of Spirulina supplement is Aloe extract. In another work by Chandan et al35 the author studied hepatoprotective potential of Aloe barbadensis against carbon tetrachloride induced hepatotoxicity. The author reported that the possible mechanism of hepatoprotective action of Aloe barbadensis extract may be due to its antioxidant activity as indicated by protection against increased lipid peroxidation and maintained glutathione contents.
5. Conclusion
This study has demonstrated that exposure to lead could have generated oxidative stress which resulted in lipid peroxidation in the liver associated with the reduction in the antioxidant status. Spirulina supplement co-treatment resulted in the prevention of the lead induced damages.
The protective effects of Spirulina supplement may be due to the radical scavenging activity of its components. Consequently, higher dose of Spirulina supplement could be useful in the preventive treatment of lead toxicity. Further extensive studies are needed to use Spirulina as a economic therapy in lead poisoning and to assess the safety of Spirulina in children.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Godwin H.A. The biological chemistry of lead. Curr Opin Chem Biol. 2001;5:223–227. doi: 10.1016/s1367-5931(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 2.WHO Lead poisoning and health, Fact sheet N°379 Reviewed October 2014.
- 3.Rahman S., Sultana S. Chemopreventive activity of glycyrrhizin on lead acetate mediated hepatic oxidative stress and its hyperproliferative activity inWistar rats. Chem Biol Interact. 2006;160:61–69. doi: 10.1016/j.cbi.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Bolin C.M., Basha R., Cox D. Exposure to lead and the developmental origin of oxidative DNA damage in the aging brain. FASEB J. 2006;20:788–790. doi: 10.1096/fj.05-5091fje. [DOI] [PubMed] [Google Scholar]
- 5.Hsu C.P., Guo L.Y. Antioxidant nutrients and lead toxicity. Toxicol. 2002;180:33–44. doi: 10.1016/s0300-483x(02)00380-3. [DOI] [PubMed] [Google Scholar]
- 6.Senapati S.K., Dey S., Dwivedi S.K., Swarup D. Effect of garlic (Allium sativum L.) extract on tissue lead levels in rats. J Ethnopharmacol. 2001;76:229–232. doi: 10.1016/s0378-8741(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 7.Singh M.K., Dwivedi S., Yadav S.S., Sharma P., Khattri S. Arsenic induced hepatic toxicity and its attenuation by fruit extract of Emblica officinalis (Amla) in mice. Indian J Clin Biochem. 2014;29:29–37. doi: 10.1007/s12291-013-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu P.C., Liu M.Y., Hsu C.C., Chen L.Y., Leon G.Y. Lead exposure causes generation of reactive oxygen species and functional impairment in rat sperm. Toxicol. 1997;122:133–143. doi: 10.1016/s0300-483x(97)00090-5. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y., Li G., Han C., Sun L., Zhao R., Cui S. Protective effect of Hippophae rhamnoides L. juice on lead induced neurotoxicity in mice. Biol Pharm Bull. 2005;28:490–494. doi: 10.1248/bpb.28.490. [DOI] [PubMed] [Google Scholar]
- 10.Raison C.L., Demetrashvili M., Capuron L., Miller A.H. Neuropsychiatric adverse effects of interferon-α: recognition and management. CNS Drugs. 2005;19:105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang H., Sung S.H., Kim Y.C. Two new hepatoprotective stilbene glycosides from acer mono leaves. J Nat Prod. 2005;68:101–103. doi: 10.1021/np0497907. [DOI] [PubMed] [Google Scholar]
- 12.Wang N., Li P., Wang Y. Hepatoprotective effect of Hypericum japonicum extract and its fractions. J Ethnopharmacol. 2008;116:1–6. doi: 10.1016/j.jep.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 13.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. J Biol Chem. 1951;193:265. (The original method) [PubMed] [Google Scholar]
- 14.Ellman G.L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;74:214–216. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 15.Marklund S.L., Marklund G. Involvement of the superoxide anion radical in the autooxidation of pyrogallol and convenient assay for superoxide dismutase. Eur J Biopchem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 16.Ohkawa H., Ohishi N., Yagi K. Assay of lipid peroxide in animal tissue by thiobarbituric acid reaction. Ann Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 17.Herman D.S., Geraldine M., T V. Influence of minerals on lead-induced alterations in liver function in rats exposed to long-term lead exposure. J Hazard Mater. 2009;166:1410–1414. doi: 10.1016/j.jhazmat.2008.12.070. [DOI] [PubMed] [Google Scholar]
- 18.Yang R.Z., Park S., Reagan W.J. Alanine aminotransferase isoenzymes: molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology. 2009;49:598–607. doi: 10.1002/hep.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Can S., Bağci C., Ozaslan M. Occupational lead exposure effect on liver functions and biochemical parameters. Acta Physiol Hung. 2008;95:395–403. doi: 10.1556/APhysiol.95.2008.4.6. [DOI] [PubMed] [Google Scholar]
- 20.El-Nekeety A.A., El-Kady A.A., Soliman M.S., Hassan N.S., Abdel-Wahhab M.A. Protective effect of Aquilegia vulgaris (L.) against lead acetate-induced oxidative stress in rats. Food Chem Toxicol. 2009;47:2209–2215. doi: 10.1016/j.fct.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Newairy A.S., Abdou H.M. Protective role of flax lignans against lead acetate induced oxidative damage and hyperlipidemia in rats. Food Chem Toxicol. 2009;47:813–818. doi: 10.1016/j.fct.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Reglero M.M., Taggart M.A., Monsalve-González L., Mateo R. Heavy metal exposure in large game from a lead mining area: effects on oxidative stress and fatty acid composition in liver. Environ Pollut. 2009;157:1388–1395. doi: 10.1016/j.envpol.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 23.Upasani C.D., Balaraman R. Protective effect of Spirulina on lead induced deleterious changes in the lipid peroxidation and endogenous antioxidants in rats. Phytother Res. 2003;17:330–334. doi: 10.1002/ptr.1135. [DOI] [PubMed] [Google Scholar]
- 24.Ponce-Canchihuamán J.C., Pérez-Méndez O., Hernández-Muñoz R., Torres-Durán P.V., Juárez-Oropeza M.A. Protective effects of Spirulina maxima on hyperlipidemia and oxidative-stress induced by lead acetate in the liver and kidney. Lipids Health Dis. 2010;9:35–41. doi: 10.1186/1476-511X-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu M.Y., Cheng Y.J., Chen C.K., Yang B.C. Co exposure of lead- and lipopolysaccharide-induced liver injury in rats: involvement of nitric oxide-initiated oxidative stress and TNF-alpha. Shock. 2005;23:360–364. doi: 10.1097/01.shk.0000158116.77328.1d. [DOI] [PubMed] [Google Scholar]
- 26.Holly T.A., Drincic A., Byun Y. Caspase inhibition reduces myocyte cell death induced by myocardial ischemia and reperfusion in vivo. J Mol Cell Cardiol. 1999;31:1709–1715. doi: 10.1006/jmcc.1999.1006. [DOI] [PubMed] [Google Scholar]
- 27.Karadeniz A., Yildirim A., Simsek N., Kalkan Y., Celebi F. Spirulina platensis protects against gentamicin-induced nephrotoxicity in rats. Phytother Res. 2008;22:1506–1510. doi: 10.1002/ptr.2522. [DOI] [PubMed] [Google Scholar]
- 28.Kepekçi R.A., Polat S., Çelik A., Bayat N., Saygideger S.D. Protective effect of Spirulina platensis enriched in phenolic compounds against hepatotoxicity induced by CCl4. Food Chem. 2013;141:1972–1979. doi: 10.1016/j.foodchem.2013.04.107. [DOI] [PubMed] [Google Scholar]
- 29.Park H.J., Lee Y.J., Ryu H.K., Kim M.H., Chung H.W., Kim W.Y. A randomized double-blind, placebo-controlled study to establish the effects of spirulina in elderly Koreans. Ann Nutr Metab. 2008;52:322–328. doi: 10.1159/000151486. [DOI] [PubMed] [Google Scholar]
- 30.Baños G., Pérez-Torres I., El Hafidi M. Medicinal agents in the metabolic syndrome. Cardiovasc Hematol Agents Med Chem. 2008;6:237–252. doi: 10.2174/187152508785909465. [DOI] [PubMed] [Google Scholar]
- 31.Thaakur S.R., Jyothi B. Effect of Spirulina maxima on the haloperidol induced tardive dyskinesia and oxidative stress in rats. J Neural Transm. 2007;114:1217–1225. doi: 10.1007/s00702-007-0744-2. [DOI] [PubMed] [Google Scholar]
- 32.Mazo V.K., Gmoshinskiĭ I.V., Zilova I.S. Microalgae Spirulina in human nutrition. Vopr Pitan. 2004;73:45–53. [PubMed] [Google Scholar]
- 33.Khan Z., Bhadouria P., Bisen P.S. Nutritional and therapeutic potential of Spirulina. Curr Phram Biotechnol. 2005;6:373–379. doi: 10.2174/138920105774370607. [DOI] [PubMed] [Google Scholar]
- 34.Wang L., Pan B., Sheng J., Xu J., Hu Q. Antioxidant activity of Spirulina platensis extracts by supercritical carbon dioxide extraction. Food Chem. 2007;105:36–41. [Google Scholar]
- 35.Chandan B.K., Saxena A.K., Shukla S. Hepatoprotective potential of Aloe barbadensis Mill. against carbon tetrachloride induced hepatotoxicity. J Ethnopharmacol. 2007;111:560–566. doi: 10.1016/j.jep.2007.01.008. [DOI] [PubMed] [Google Scholar]