Abstract
The present study was carried out to evaluate in vivo and in vitro anti-inflammatory potential of selected medicinal plants used in Indian traditional medication. The sequentially extracted plant samples as, Cissus quadrangularis, Plumbago zeylanica, Terminalia bellarica and Terminalia chebula in water, ethanol and hexane were evaluated in-vitro for COX-1 and 2 inhibitory and antioxidant activities. The in vivo anti-inflammatory activity of selected samples showing promising COX-2 inhibition was assessed using carrageenan and Phorbol Myristate Acetate (PMA) induced mice edema animal model. The results obtained reveals that most of the plants were found to inhibit COX-2 activity as compared to COX-1. It was observed that the extracts of T. bellarica (73.34 %) and T. chebula (74.81 %) showed significant COX-2 selective inhibition as compared to other samples. The ethanol extract of the selected plants demonstrated effective DPPH, OH and superoxide radical scavenging activity. In vivo anti-inflammatory study shows that, T. bellarica and T. chebulla had a significant impact on inhibition of edema formation. The cytotoxicity evaluation study of ethanolic fraction of selected medicinal plants indicates that the selected samples have no effect on cell viability. HPTLC fingerprint of flavonoids of the selected samples was also prepared as a measure of quality control. The results obtained may be useful in strengthening the standardization of the selected botanicals. Moreover the selected plants can be considered as a resource for searching novel anti-inflammatory agents possessing COX-2 inhibition.
Keywords: Cyclooxygenase, Antioxidants, Cytotoxicity, Carrageenan, PMA, Medicinal plants
Graphical abstract
1. Introduction
Cyclooxygenase also abbreviated as COX, is a prostaglandin endoperoxide synthase (E.C. 1.14.99.1) enzyme involved in the metabolism of arachidonic acid (AA) and synthesis of prostanoid including potent proinflammatory prostaglandins (PGE2, PGF2α).1, 2 In mammalian cells, COX exist in at least two isoforms COX-1 and COX-2.3, 4, 5 COX-1 is expressed constitutively in almost all cell types, including platelets and those present in stomach, kidney, vascular endothelium, forebrain and uterine epithelium and is regulated as a house keeping enzyme for various physiological functions, whereas COX-2 is inducible and expressed during tissue damage or inflammation in response to proinflammatory cytokines such as IL-1β, interferon gamma and TNF-α.6, 7, 8 A crucial proinflammatory role played by the COX has made this enzyme an attractive target for the design and development of novel anti-inflammatory agents.
Although, role of free radical in inflammatory reactions is well described. The free radicals especially, the reactive oxygen species (ROS) creates oxidative stress in the cells leading to inflammatory and infectious condition. Phagocytic cells including polymorphonuclear leukocytes (neutrophils, eosinophils) and mononuclear cells (macrophage and lymphocytes) produce excessive amount of ROS which play an important role in the host defense mechanism. Besides their defensive effects these excessively produced ROS deregulate the cellular functions causing cellular and tissue damage, which in turn augments the state of inflammation.9, 10, 11
Non steroidal anti-inflammatory drugs (NSAIDs) represent one of the most common classes of medications used world wide with an estimated usage of >30 million per day for inflammation and related disorders.12 Most of the NSAIDs are carboxylic acid containing drugs including salicylate derivatives (aspirin), carboxylic and heterocyclic acid derivatives (indomethacin), propionic acid derivatives (ibuprofen, ketoprofen, flurbiprofen) and phenyl acetic acid derivatives (diclofenac). These organic acid containing drugs act at the active site of the enzyme preventing the access of arachidonic acid (AA) to the enzyme and stop the cyclooxygenase pathway.13, 14 Unfortunately, besides the excellent anti-inflammatory potential of the NSAIDs, the severe side effects such as gastrointestinal (GI) ulceration, perforation, obstruction, and bleeding has limited the therapeutic usage of NSAIDs.15, 16 The mucosal irritation occurs due to the acidic nature of most of NSAIDs and inhibition of production of mucosal protective PGE which leads to gastric erosion.17 This is consistent with the idea that inhibition of COX-1 underlies the gastrointestinal side effects of NSAIDs and that NSAIDs selectivity toward inhibition of COX-1 over COX-2 correlates with their ability to cause gastrointestinal side effects.18, 19, 20 A recent analysis found that there is increased cardiovascular risk,21 hypertension and edema,22, 23, 24, 25 and cause nephrotoxicity,26 in patients who are at risk with COX-2 inhibitors. Searching selective COX-2 inhibitors without influencing the normal physiological functions of COX-1 has remained a major thrust area of anti-inflammatory pharmaceutical research. Nevertheless, the anti-inflammatory agents having selective COX-2 inhibition but less reactive towards COX-1 are appreciated as novel anti-inflammatory agents in the mainstream of anti-inflammatory research.27
According to World Health Organization (WHO), about three-quarters of the world population depends on traditional medicines (mainly herbs) for their healthcare. Ayurveda and Chinese medicinal systems are the most acceptable traditional system which has a considerable amount of research on pharmacognocy, chemistry, pharmacology and clinical therapeutics.28, 29 It is evident that several plants have been used in traditional ayurvedic medicine for treatment and management of distinct inflammatory disorders and wound healing activities.30 There is clear evidence addressing the importance of plant derived COX inhibitors in the management of inflammatory disorders. Baumann and coworkers were the first to report in a study that some dietary polyphenols inhibit arachidonic acid peroxidation.31 Since then several researches have reported that many dietary polyphenols possess COX-2 inhibitory or stimulatory effects.32, 33, 34 In the recent years, the use of traditional medicine information on plant has again received considerable interest. The renewed interest in medicinal plant research has focused on herbal cures among indigenous populations around the world. Nevertheless, the standardization of botanicals has remained a key issue to be addressed to the consumers and for the popularization of herbal drugs all over the world. Hence, ethanopharmacology and drug discovery using natural products has remained an important issue in the current target-rich, lead-poor scenario of pharmaceutical research.35
Taking into consideration the above facts, an attempt has been made to standardize the selected botanicals as anti-inflammatory agents using COX guided activity. The antioxidant potential and cytotoxicity profile have also been carried out to supplement the results.
2. Materials and methods
2.1. Materials
The COX-1 & 2 (human ovine) inhibitor Screening assay kit [Catalog No. 760111] was procured from Cayman, U.S.A., MTT (3- (4, 5-dimethylthiazol-2-yl) – 2, 5- diphenyl tetrazolium bromide), DPPH (1, 1-diphenyl-2-picryl hydrazyl) were purchased from Sigma-Aldrich Co. (St. Louis MO, USA). 1–10 phenanthroline, Phenazine methosulphate (PMS), Nitroblue tetrazolium (NBT) were obtained from s.d. Fine chem. Mumbai. Nicotinamide Adenine Dinucleotide (NADH) was purchased from Spectrochem, Pvt. Ltd. Mumbai. Chang Liver cell line was requested from National Centre for Cell Science (NCCS: a National Cell Line Facility) Pune (MS), India. Medicinal plants were collected from the nearby areas of Nanded district (MS), India. All other chemicals and reagents used were of AR grade and were obtained from commercial sources.
2.2. Collection and identification of the selected medicinal plants
The selected plants Cissus quadrangularis (A-13), Plumbago zeylanica (A-15), Terminalia bellarica (A-16) and Terminalia chebulla (A-17) were collected from the nearby regions of Nanded district (MS). The plants were identified and authenticated with the help of Flora36 and Voucher specimens (A13-A17) of the plants were deposited in the herbarium center of Department of Botany, School of Life Sciences, Swami Ramanand Teerth Marathwada University, Nanded – 431 606 (MS), India. The shade dried and powdered plant samples were preserved for further experimentations.
2.3. Sequential extraction of the plant samples
The shed dried powdered plant samples (10 g) were sequentially extracted in hexane, ethanol and water up to 8 h using Soxhlet's apparatus. The extracted samples were evaporated under reduced pressure at room temperature. The dried extracts were preserved at 4 °C in refrigerator for further analysis.
2.4. HPTLC analysis
HPTLC analysis was performed using CAMAG (Germany) make instrumental thin layer chromatography. TLC plates (Merck silica gel 60 F254, 20 cm × 10 cm) were prewashed with methanol. The plate was activated in an oven at 100 °C for 10 min. Individual plant extracts of 10 μl (1 mg/ml) were spotted onto the precoated plates using a Linomat 5 application system. Rutin hydrate (5 and 10 μg/ml) was used as a marker flavonoid. The flavonoids were separated using ethyl acetate: formic acid: glacial acetic acid: water (100:11:11:27) as a mobile phase. Natural product (NP) reagent was used as a flavonoid derivatizing agent and the spots developed were visualized under CAMAG UV cabinet (366 nm) and were digitized using CAMAG photo documentation system.
2.5. COX inhibition assay
The assay was performed by using Colorimetric COX (human ovine) inhibitor Screening assay kit.37 Briefly, the reaction mixture contains, 150 μl of assay buffer, 10 μl of heme, 10 μl of enzyme (either COX-1 or COX-2), and 10 μl of plant sample (1 mg/ml). The assay utilizes the peroxidase component of the COX catalytic domain. The peroxidase activity was assayed colorimetrically by monitoring the appearance of oxidized N, N, N, N'-tetramethyl-p-phenylenediamine (TMPD) at 590 nm. Aspirin (acetylsalicylic acid, 1 mM) was used as a standard drug. The percent COX inhibition was calculated using following equation:
Where T = Absorbance of the inhibitor well at 590 nm.
C = Absorbance of the 100 % initial activity without inhibitor well at 590 nm.
2.6. DPPH radical scavenging assay
DPPH radical scavenging assay was carried out as per reported method with slight modifications.38, 39 Briefly, 1 ml of test sample (1 mg/ml) was added to equal quantity of 0.1 mM solution of DPPH in ethanol. After 20 min of incubation at room temperature, the DPPH reduction was measured by reading the absorbance at 517 nm. Ascorbic acid (1 mM) was used as reference compound.
2.7. Hydroxyl (OH) radical scavenging assay
The OH radicals scavenging activity was demonstrated with Fenton reaction.40 The reaction mixture contained, 60 μl of FeCl2 (1 mM), 90 μl of 1–10 phenanthroline (1 mM), 2.4 ml of phosphate buffer (0.2 M, pH 7.8), 150 μl of H2O2 (0.17 M) and 1.5 ml of individual plant extract (1 mg/ml). The reaction was started by adding H2O2. After 5 min incubation at room temperature, the absorbance was recorded at 560 nm. Ascorbic acid (1 mM) was used as reference compound.
2.8. Superoxide radical (SOR) scavenging assay
The SOR scavenging assay was performed by the reported method.41 Superoxide anion radicals were generated in a non-enzymatic Phenazine methosulphate – Nicotinamide Adenine Dinucleotide (PMS – NADH) system through the reaction of PMS, NADH and Oxygen. It was assayed by the reduction of Nitroblue tetrazolium (NBT). In this experiment superoxide anion was generated in 3 ml of Tris HCL buffer (100 mM, pH 7.4) containing 0.75 ml of NBT (300 μM), 0.75 ml of NADH (936 μM), and 0.3 ml of plant sample (1 mg/ml). The reaction was initiated by adding 0.75 ml of PMS (120 μM) to the mixture. After 5 min of incubation at room temperature the absorbance at 560 nm was measured in spectrophotometer. Ascorbic acid (1 mM) was used as reference compound.
2.9. MTT cytotoxicity assay
The MTT cytotoxicity assay was performed as published previously.42, 43, 44 The Chang liver cells were harvested (1.5 × 104 cells/well) and inoculated in 96 well microtiter plates. The cells were washed with phosphate buffered saline (PBS) and the cultured cells were then inoculated with and without the individual ethanolic plant extract (1 mg/ml). After 72 h incubation, the medium was aspirated followed by addition of 10 μL of MTT solution (5 mg/ml in PBS, pH 7.2) to each well and the plates were reincubated for 4 h at 37 °C. After incubation time, 100 μL of DMSO was added to the wells followed by gentle shaking to solubilize the formazan crystal for 15 min. Absorbance was read at 540 nm using Thermo make Automatic Ex-Microplate Reader (M51118170) and the % cell viability was calculated. The H2O2 (1 mM) was used as reference cytotoxic agent. The percent DPPH, OH, SOR scavenging activity and cell viability inhibition was calculated using following formula.
Where T = Absorbance of the test sample.
C = Absorbance of the control sample.
2.10. Experimental animals
The animals used in this study were Swiss albino mice weighing between 25–30 g. They were maintained in experimental animal house at Sudhakarrao Naik Pharmacy College, Pusad (MS), India. They were kept in rat cages and fed on standard mice food (Amrut Feeds Ltd., Sangali (MS), India) and allowed free access to clean fresh water in bottles. The experimental protocols were in compliance with Institutional Animal Ethics Committee (IAEC), Sudhakarrao Naik Pharmacy College, Pusad (MS), (Proposal No. CPCSEA/IAEC/PL/09–2011).
2.11. Carrageenan-induced rat paw edema assay
The selected samples showing promising average (activity in all solvents) COX-2 selective activities were evaluated for in vivo anti-inflammatory studies using carrageenan induced rat paw edema animal model. The assay performed as described previously.45 Briefly, edema was induced on the right hind paw by subplantar injection of 20 μl carrageenan (1 % w/v) in 0.9 % saline. The extract of selected samples were prepared in 1 % w/v gum acacia and administered orally at a dose of 100 mg/kg and 250 mg/kg, 1 h before carrageenan injection. A control group received vehicle only and a standard group was treated with indomethacin (20 mg/kg, p.o.) The volume of injected and of the contra lateral paws was measured 1, 3, and 5 h after induction of inflammation, using a plethysmometer (Orchid Scientific Laboratory). The value was expressed as, the percent reduction in volume with respect to the control group of at different time intervals.
2.12. PMA induced mouse ear edema activity
According to a modified method of46 4 μg per ear of PMA, in 20 μl of acetone, was applied to both surfaces of the right ear of each mouse. The left ear (control) received the vehicle (acetone and/or DMSO, 20 μl). The selected plant extract was administered topically (50 and 100 mg per ear in DMSO) 1 h before PMA application. Two control groups were used: a control group with the application of PMA on the right ear and the reference group was treated with indomethacin (2 mg per ear in 20 μl acetone). Six hours after PMA application, mice were killed by cervical dislocation and a 6 mm diameter disc from each ear was removed with a metal punch and weighed. Ear edema was calculated by subtracting the weight of the left ear (vehicle) from the right ear (treatment), and was expressed as edema weight. Inhibition percentage was expressed as a reduction in weight with respect to the control group.
3. Results
3.1. HPTLC profiling
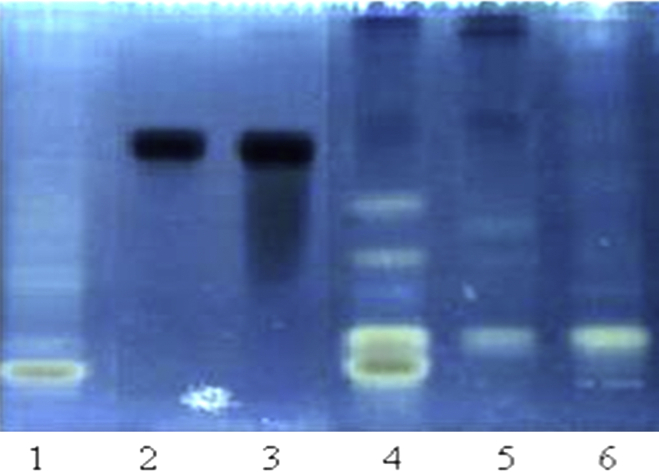
The HPTLC analysis was performed as a part of quality control of the selected plant samples. HPTLC finger print of ethanol soluble flavonoids was prepared using rutin as a marker flavonoid compound (Fig. 1.). The results of the HPTLC analysis shows the diversity of flavonoid content in T. chebulla, moreover this is the only sample containing rutin, while all other samples were devoid of rutin content.
Fig. 1.
HPTLC profile of flavonoid finger prints of ethanol extract of selected medicinal plants using Rutin as a marker compound. Track no. 1 – Plumbago zeylanica, 2 – Rutin (5 μg), 3 – Rutin (10 μg), 4 – Terminalia bellarica, 5 – Terminalia Chebulla, 6 – Cissus quadrangularis.
3.2. Effect of plant samples on COX inhibition
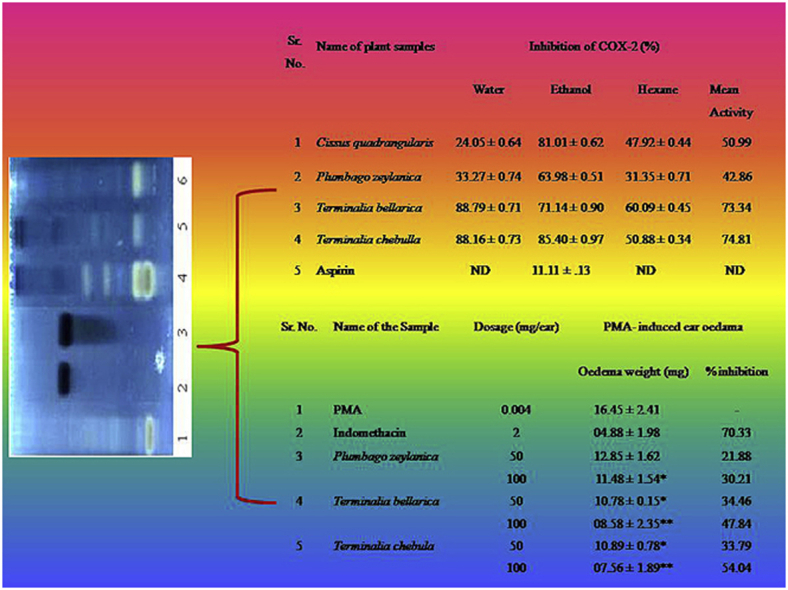
The results of the COX inhibition using different fractions of the selected plants are summarized in (Table 1, Table 2). The average COX-1 & 2 inhibition was calculated by taking the mean of COX inhibition activity in three solvent extracts. Overall it was found that the fractions of T. bellarica (mean activity COX-1, 61.83 % & COX-2, 73.34 %) and T. chebulla (mean activity COX-1, 52.82 % & COX-2, 74.38 %) were observed to be significant inhibitors of COX-1 and 2, with more selectivity towards COX-2 inhibition as compared to other plant sample. Other than Terminalia species, C. quadrangularis demonstrated considerable COX-1 selective (mean activity 53.25 %) inhibitory potential as compared to other samples. The minimum COX-1 inhibition was shown by the water extract of C. quadrangularis (28.18 %), whereas lower COX-2 inhibition was shown by the P. zeylanica (mean activity 42.86 %). A cursory look at the COX inhibition profile by the selected plants also shows that the ethanol fractions were found to be more effective COX inhibitory agents as compared to water and hexane extracts, which showed moderate or no inhibition. The results were compared with Aspirin (1 mM) showing COX-1 (08.54 ± 0.37 %) and COX-2 (11.11 ± .13 %)
Table 1.
Effect of different fractions of selected medicinal plants on COX-1 activity.
| Sr. No. | Name of plant samples | Inhibition of COX-1 (%) |
|||
|---|---|---|---|---|---|
| Water | Ethanol | Hexane | Mean activity | ||
| 1 | Cissus quadrangularis | 28.18 ± 0.70 | 84.94 ± 0.99 | 46.64 ± 0.69 | 53.25 |
| 2 | Plumbago zeylanica | NR | 41.48 ± 0.62 | 36.25 ± 0.54 | 25.91 |
| 3 | Terminalia bellarica | 75.16 ± 0.41 | 62.24 ± 0.79 | 48.09 ± 0.56 | 61.83 |
| 4 | Terminalia chebulla | 72.16 ± 0.93 | 86.32 ± 1.18 | NR | 52.82 |
| 5 | Aspirin | ND 08.54 ± 0.37 ND | ND | ||
Results summarized are the mean values of n = 3 ± S.D., NR – no reaction under experimental condition, ND – not determined.
Table 2.
Effect of different fractions of selected medicinal plants on COX-2 activity.
| Sr.No. | Name of plant samples | Inhibition of COX-2 (%) |
|||
|---|---|---|---|---|---|
| Water | Ethanol | Hexane | Mean activity | ||
| 1 | Cissus quadrangularis | 24.05 ± 0.64 | 81.01 ± 0.62 | 47.92 ± 0.44 | 50.99 |
| 2 | Plumbago zeylanica | 33.27 ± 0.74 | 63.98 ± 0.51 | 31.35 ± 0.71 | 42.86 |
| 3 | Terminalia bellarica | 88.79 ± 0.71 | 71.14 ± 0.90 | 60.09 ± 0.45 | 73.34 |
| 4 | Terminalia chebulla | 88.16 ± 0.73 | 85.40 ± 0.97 | 50.88 ± 0.34 | 74.81 |
| 5 | Aspirin | ND 11.11 ± .13 ND | ND | ||
Results summarized are the mean values of n = 3 ± S.D., ND – not determined.
3.3. DPPH radical scavenging activity
The results are summarized in Table 3. It was observed that the water and ethanol soluble contents of selected plants (1 mg/ml) were found to be potent DPPH reducing agents. The maximum DPPH radical scavenging activity was observed in ethanolic extract of T. bellarica (85.89 ± 1.82 %) while the minimum activity was observed in ethanolic extract of C. quadrangularis (8.99 ± 1.65 %). All other samples showed DPPH reducing activity in the range of 5.37–84.19 % as compared with ascorbic acid (81.27 ± 0.87 %).
Table 3.
Antioxidant activity of selected medicinal plants.
| Sr. No. | Name of the plants | Plant extracts | % Radical scavenging activity |
||
|---|---|---|---|---|---|
| DPPH | OH | SOR | |||
| 1 | Cissus quadrangularis | (W) | 16.15 ± 1.32 | 86.47 ± 0.77 | 31.39 ± 1.18 |
| (E) | 8.99 ± 1.65 | 57.60 ± 0.85 | 03.81 ± 0.80 | ||
| (H) | 5.37 ± 1.06 | 52.73 ± 0.79 | 00.79 ± 0.51 | ||
| 2 | Plumbago zeylanica | (W) | 52.61 ± 1.20 | 78.28 ± 0.56 | 14.60 ± 0.76 |
| (E) | 75.44 ± 0.77 | 96.59 ± 0.58 | 57.21 ± 0.88 | ||
| (H) | NR | 62.89 ± 0.94 | NR | ||
| 3 | Terminalia bellarica | (W) | 82.22 ± 1.39 | NR | 34.55 ± 0.91 |
| (E) | 85.89 ± 1.82 | 67.32 ± 1.22 | 22.21 ± 1.22 | ||
| (H) | NR | 88.39 ± 0.95 | NR | ||
| 4 | Terminalia chebula | (W) | 84.02 ± 1.22 | NR | 27.48 ± 0.92 |
| (E) | 84.19 ± 3.36 | 38.05 ± 1.22 | 25.55 ± 0.78 | ||
| (H) | NR | 91.65 ± 1.11 | NR | ||
| 5 | AA | (E) | 81.27 ± 0.87 | 2.63 ± 0.73 | 52.95 ± 0.83 |
Results presented here are the mean value of n = 3; ± S.D., NR = No reaction under experimental condition, (W) = Water, (E) = Ethanol, (H) = Hexane, AA = Ascorbic acid.
3.4. OH radical scavenging activity
The profile of OH radical scavenging activity of selected medicinal plants is shown in Table 3. Except water extract of T. bellarica and T. chebulla, all samples are found to be promising OH radical scavengers. The ethanol extract of P. zeylanica (96.59 ± 0.58 %) possess maximum activity while ethanol extract of T. chebulla (38.05 ± 0.77 %) showed poor OH radical scavenging ability as compared to ascorbic acid (2.63 ± 0.73 %).
3.5. SOR scavenging assay
The results obtained are shown in Table 3. The water and ethanolic extract of all selected plants showed promising SOR scavenging activity as compared to hexane extract. The high SOR scavenging activity was found in ethanolic extract of P. zeylanica (57.21 %) while the lower SOR was reported in hexane extract of C. quadrangularis (0.79 %.). From the tested samples it was observed that the SOR scavenging activity was recorded in the range of 3.81–34.55 %. The results were compared with ascorbic acid (52.95 ± 0.83 %).
3.6. MTT cytotoxicity assay
It was observed that none of the plant sample showed significant cytotoxic effect on normal Chang liver cell viability at 1 mg/ml concentration. The observed and calculated percent inhibition of cell viability of C. quadrangularis is (−3.08 ± 0.41), P. zeylanica (−0.32 ± 0.17), T. bellarica (−3.20 ± 0.63), and T. chebulla (−1.81 ± 0.57). The H2O2 (1 mM) was used as a standard cytotoxic (3.13 ± 0.50 %) agent for comparison purpose.
3.7. Profile of carrageenan induced anti-inflammatory activity
The results of the oral administration of P. zeylanica, T. bellarica and T. chebulla plant extracts showed promising anti-inflammatory activity by reducing the carrageenan induced mice paw edema volume (Table 4). In the present studies carrageenan induced 69, 86, and 99 % edema formation at 1, 3 and 5 h respectively, compared with 0 h readings in control group. It was observed that at a higher dose of 250 mg/kg, T. bellarica (32.85 %) and T. chebulla (34.28 %) showed significant decrease in edema volume after 1 h. Moreover, at the same dose of T. bellarica (22.77 %) and T. chebulla (20.80 %) considerable reduction in the edema volume was observed, however only 10.34 and 17.34 % reduction in edema volume was observed in respective samples after 3 h. Similar trend of results of edema volume reduction was observed with a dose of 100 mg/kg. The results were compared with standard anti-inflammatory drug such as Indomethacin (20 mg/kg) which showed effective inhibition (51.48 %) at 5 h. One Way ANOVA for multiple comparison test followed by dunnet test were performed to assign the level of significance.
Table 4.
Summary of carrageenan induced anti-inflammatory activity of selected plants.
| Sr. No. | Name of the samples | Dosage (mg/kg) | Percent inhibition of edema volume at different time intervals (hrs.) |
||
|---|---|---|---|---|---|
| 1 | 3 | 5 | |||
| 1 | Control | – | 69.24 ± 0.010 | 86.07 ± 0.010 | 99.93 ± 0.011 |
| 2 | Indomethacin | 20 | 22.85 ± 0.108 | 26.43 ± 0.128∗ | 51.48 ± 0.098∗∗ |
| 3 | Plumbago zeylanica | 100 | 21.54 ± 0.142 | 17.98 ± 1.012 | 15.59 ± 1.521 |
| 250 | 23.48 ± 1.860 | 18.56 ± 0.793 | 13.98 ± 0.154 | ||
| 3 | Terminalia bellarica | 100 | 27.14 ± 0.016 | 10.34 ± 0.012 | 13.86 ± 0.018 |
| 250 | 32.85 ± 0.013∗ | 10.34 ± 0.020 | 22.77 ± 0.010∗ | ||
| 4 | Terminalia chebulla | 100 | 25.71 ± 0.010 | 08.05 ± 0.014 | 07.92 ± 0.010 |
| 250 | 34.28 ± 0.016∗∗ | 17.24 ± 0.010 | 20.80 ± 0.010∗ | ||
* (P < 0.05), ∗∗ (P < 0.01) indicates significant decrease in the paw edema volume compared to control value for respective time interval (One Way ANOVA for multiple comparison test followed by dunnet test).
3.8. Oedama induced by PMA in mouse ear activity
It can be seen in Table 5 that the selected plant samples inhibit PMS indued inflammation in mouse ear. The extracts of T. chebulla (54.06 %) and T. bellerica (47.84 %) shows significant (P < 0.05) inhibitory results as compare to P. zeylanica (30.21 %) at the higer dose (100 mg/ear). Indomethacin was used as reference compound also possess an excellent inhibitory activity.
Table 5.
Effect of plant samples on edema induced by PMS in mouse ear.
| Sr. No. | Name of the sample | Dosage (mg/ear) | PMA- induced ear edema |
|
|---|---|---|---|---|
| edema weight (mg) | % Inhibition | |||
| 1 | PMA | 0.004 | 16.45 ± 2.41 | – |
| 2 | Indomethacin | 2 | 04.88 ± 1.98 | 70.33 |
| 3 | Plumbago zeylanica | 50 | 12.85 ± 1.62 | 21.88 |
| 100 | 11.48 ± 1.54* | 30.21 | ||
| 4 | Terminalia bellarica | 50 | 10.78 ± 0.15* | 34.46 |
| 100 | 08.58 ± 2.35** | 47.84 | ||
| 5 | Terminalia chebula | 50 | 10.89 ± 0.78* | 33.79 |
| 100 | 07.56 ± 1.89** | 54.04 | ||
* (P < 0.05), ∗∗ (P < 0.01) statistical significance compared with PMA groups (One Way ANOVA for multiple comparison test followed by dunnet test).
4. Discussion
Developing novel, effective and safe anti-inflammatory agents has remained a major thrust area in the main stream of ‘finding alternatives to NSAID's. Anti-inflammatory agents possessing selective COX-2 inhibition and showing no or negligible effect on COX-1 activity are more appreciated as safe drugs as these agents have minimum gastrointestinal side effects. Natural product, especially medicinal plants and drug discovery has remained a very successful combination for the inventorization of new therapeutic agents. The main intention of the present study was to perform the COX activity guided standardization of selected medicinal plants with focus on antioxidant and cytotoxicity profile. Variety of phytochemicals like flavonoids, terpenoids, alkaloids and saponins has been described to possess significant anti-inflammatory activity. Several studies proved that naturally occurring coumarins47 and flavonoids48 act as dual inhibitors of cyclooxygenase and 5-lipoxygenase activities. The Indian spice turmeric, (Curcuma longa L.) possessing curcumin (and synthetic analogs) have established reputation as an anti-inflammatory agent by inhibiting COX-1 and COX-2.49 Flavonoids inhibit biosynthesis of prostaglandins (the end products of the COX and lipoxygenase pathways), which acts as a secondary messengers and are involved in various immunologic responses.50 Inhibition of these enzymes provides the mechanism by which flavonoids inhibit inflammatory disorders.51
Few years back, highly effective class of novel anti-inflammatory drugs such as Celecoxib, Rofecoxib, and Valdecoxib etc. were introduced in the pharmaceutical market but unfortunately most of them were withdrawn from the market on account of their serious cardio functioning side effects, especially in high sensitive patients like pregnant women, new born children, elderly people etc.15, 16 Worldwide, there is an increasing concern in finding new anti-inflammatory remedies not only having improved therapeutic index but also harmless. The results of the COX inhibition studies focus the importance of selected botanicals as an important resource for the isolation and identification of new COX-2 selective anti-inflammatory agents. The medicinal plants such as T. bellarica and T. chebulla are one of the major constituents of a popular ayurvedic formulation ‘Triphala’, prescribed by most of the traditional healthcare practitioners as well as by clinical physicians in India and many Asian countries.52 According to the traditional Indian medicinal system, especially in ayurveda, ‘Triphala’ strengthens and activates different tissues of the body, prevents ageing and promotes health. It is also reputed for immunomodulatory properties which improves body's defense system.53 In recent years there are several reports in the literature which suggest that ‘Triphala’ possesses antimutagentic, radioprotecting and antioxidant activity.54, 55, 56
A detailed review of literature reveals that the selected plants have not been tested yet for their ability to manage inflammation by selective inhibition of COX enzyme cascade, although they are widely used in the management of diverse inflammatory disorders. The study undertaken evidently demonstrates first time, their thriving activity for COX inhibition along with their in vivo anti-inflammatory, antioxidant and cytotoxic activities.
Plethora of literature has accumulated in the past 15 years linking the role of free radical species (superoxide, hydroxyl radicals, nitric oxide, peroxynitrite, and the free radical-derived product hydrogen peroxide) in initiating and modulating inflammatory disorders. In support of this contention, several reports have shown that administration of scavengers of free radicals or free radical products to intact animals blunts or prevents reductions in muscle-specific force generation in animal models of systemic inflammation.57, 58 There is a strong need for effective antioxidants from natural sources as alternatives to synthetic antioxidant in order to prevent the free radicals implicated diseases which can have serious effects on the cardiovascular system, either through lipid peroxidation or vasoconstriction.59 The extracts and essential oils of many plants have been investigated for their antioxidant activity.60, 61, 62 The polyphenolic compounds are secondary plant metabolites found in numerous plant species, these polyphenolic compounds have been reported to play key antioxidant roles. The phytochemicals especially the flavonoids have been extensively studied for their antioxidant activities using the mechanism of delocalization of the single electron of the radical. The plants studied in the present investigation demonstrated considerable free radical scavenging activity, which could be supplementary for the amelioration of inflammatory reactions.
Authenticity of the plant drugs and safety are the key issues in the standardization of the botanicals. Nevertheless these issues need to be addressed to the end users for their satisfaction and for the popularization of the drugs from plant origin. The non toxic nature of the selected plants addresses the safety issue of these botanicals on health grounds.
In conclusion the results of the present study may strengthen the process of standardization of botanicals containing the selected plants as one of the ingredients. In many instances, the actual compound/s isolated from the plants may not serve as the drug, but leads to the development of potential novel therapeutic agents. With the rapid identification of new molecules from plant resources having significant anti-inflammatory effects are proving to be important agents in the mainstream of anti-inflammatory drug discovery marathon.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgment
The study was supported by University Grant Commission (UGC), New Delhi (India), F. No. 33–157/2007 (SR). Authors are thankful to the Director, School of Life Sciences, S.R.T.M. University, Nanded for providing necessary laboratory facilities to carry out present work.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Limongelli V., Bonomi M., Marinelli L. Molecular basis of cyclooxygenase enzymes (COXs) selective inhibition. Proc Nat Acad Sci. 2010;107:5411–5416. doi: 10.1073/pnas.0913377107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao H., Yu R., Choi Y. Discovery of cyclooxygenase inhibitors from medicinal plants used to treat inflammation. Pharmacol Res. 2010;61:519–524. doi: 10.1016/j.phrs.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang I.K., Yi S.S., Yoo K.Y. Effects of treadmill exercise on cyclooxygenase-2 in the hippocampus in type 2 diabetic rats: correlation with the neuroblasts. Brain Res. 2010;1341:84–92. doi: 10.1016/j.brainres.2010.02.057. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y.J., Quilley J. Fenofibrate treatment of diabetic rats reduces nitrosative stress, renal cyclooxygenase-2 expression, and enhanced renal prostaglandin release. J Pharmacol Exp Ther. 2008;324:658–663. doi: 10.1124/jpet.107.129197. [DOI] [PubMed] [Google Scholar]
- 5.Jianhua F., Eliana L., Gregor F. Cardiac remodeling hinders activation of cyclooxygenase-2, diminishing protection by delayed pharmacological preconditioning: role of HIF1α and CREB. Cardiovasc Res. 2008;78:98–107. doi: 10.1093/cvr/cvn016. [DOI] [PubMed] [Google Scholar]
- 6.Scott D.L., Wolfe F., Huizinga T.W. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 7.Lubberts E. Th17 cytokines and arthritis. Semin Immunopathol. 2010;32:43–53. doi: 10.1007/s00281-009-0189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabat R. IL-10 family of cytokines. Cytokine Growth Factor Rev. 2010;21:315–324. doi: 10.1016/j.cytogfr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Aitken S.L., Karcher E.L., Rezamand P. Evaluation of antioxidant and proinflammatory gene expression in bovine mammary tissue during the periparturient period. J Dairy Sci. 2009;92:589–598. doi: 10.3168/jds.2008-1551. [DOI] [PubMed] [Google Scholar]
- 10.Bishop A. Role of oxygen in wound healing. J Wound Care. 2008;17:399–402. doi: 10.12968/jowc.2008.17.9.30937. [DOI] [PubMed] [Google Scholar]
- 11.Schafer F.Q., Quian S.Y., Buettner G.R. Iron and free radical oxidations in cell membranes. Cell Mol Bio. 2000;46:657–662. [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace J.L., Ferraz J.G. New pharmacologic therapies in gastrointestinal disease. Gastroenterol Clin North Am. 2010;39:709–720. doi: 10.1016/j.gtc.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Marnett L.J. The COXIB experience: a look in the rearview mirror. Annu Rev Pharmacol Toxicol. 2009;49:265–290. doi: 10.1146/annurev.pharmtox.011008.145638. [DOI] [PubMed] [Google Scholar]
- 14.Inotai A., Hanko B., Meszaro A. Trends in the non-steroidal anti-inflammatory drug market in six central-eastern european countries based on retail information. Pharmacoepidemiol Drug Saf. 2010;19:183–190. doi: 10.1002/pds.1893. [DOI] [PubMed] [Google Scholar]
- 15.Vonkeman H.E., van de Laar M.A. Nonsteroidal anti-inflammatory drugs: adverse effects and their prevention. Semin Arthritis Rheum. 2010;39:294–312. doi: 10.1016/j.semarthrit.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Garcia Rodriguez L.A., Tacconelli S., Patrignani P. Role of dose potency in the prediction of risk of myocardial infarction associated with nonsteroidal anti-inflammatory drugs in the general population. J Am Coll Cardiol. 2008;52:1628–1636. doi: 10.1016/j.jacc.2008.08.041. [DOI] [PubMed] [Google Scholar]
- 17.John L.W., Linda V. NSAID-induced gastrointestinal damage and the design of GI-sparing NSAIDs. Curr Opin Invest Drugs. 2008;9:1151–1156. [PubMed] [Google Scholar]
- 18.Laine L., Connors L., Griffin M., Curtis S., Kaur A., Cannon C. Prescription rates of protective co-therapy for NSAID users at high GI risk and results of attempts to improve adherence to guidelines. Aliment Pharmacol Ther. 2009;30:767–774. doi: 10.1111/j.1365-2036.2009.04090.x. [DOI] [PubMed] [Google Scholar]
- 19.Chan F. The David Y. Graham lecture: use of nonsteroidal anti-inflammatory drugs in a cox-2 restricted environment. Am J Gastroenterol. 2008;103:221–227. doi: 10.1111/j.1572-0241.2007.01545.x. [DOI] [PubMed] [Google Scholar]
- 20.Yeomans N., Lanas A., Labenz J. Efficacy of esomeprazole (20 mg once daily) for reducing the risk of gastroduodenal ulcers associated with continuous use of low dose aspirin. Am J Gastroenterol. 2008;103:2465–2473. doi: 10.1111/j.1572-0241.2008.01995.x. [DOI] [PubMed] [Google Scholar]
- 21.Rosenson R.S. Future role for selective phospholipase A2 inhibitors in the prevention of atherosclerotic cardiovascular disease. Cardiovas Drugs Ther. 2009;23:93–101. doi: 10.1007/s10557-008-6148-1. [DOI] [PubMed] [Google Scholar]
- 22.Inger L.M., van de Laar Mart AFJ., Harald E.V. Non-steroidal anti-inflammatory drugs: an overview of cardiovascular risks. Pharmaceuticals. 2010;3:2146–2162. doi: 10.3390/ph3072146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walter H.H. Nonsteroidal anti-inflammatory drugs and the kidney. Pharmaceuticals. 2010;3:2291–2321. doi: 10.3390/ph3072291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bannwarth B. Safety of the nonselective NSAID nabumetone: focus on gastrointestinal tolerability. Drug Saf. 2008;31:485–503. doi: 10.2165/00002018-200831060-00004. [DOI] [PubMed] [Google Scholar]
- 25.Ragia H., Mohamed A., Madiha A. Cardiorenal effects of Newer NSAIDs (Celecoxib) versus classic NSAIDs (Ibuprofen) in patients with arthritis. J Toxicol. 2011 doi: 10.1155/2011/862153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harirforoosh S., Jamali F. Renal adverse effects of nonsteroidal anti-inflammatory drugs. Expert Opin Drug Saf. 2009;8:669–681. doi: 10.1517/14740330903311023. [DOI] [PubMed] [Google Scholar]
- 27.Bandgar B.P., Kinkar S.N., Chavan H.V., Jalde S.S., Shaikh R.U., Gacche R.N. Synthesis and biological evaluation of asymmetric indole curcumin analogues as potential anti-inflammatory and antioxidant agents. J Enz Inhib Med Chem. 2014;29:7–11. doi: 10.3109/14756366.2012.743536. [DOI] [PubMed] [Google Scholar]
- 28.Kiranjot S., Kunwarjeet P. Indigenous use of medicinal plants for health care. Ethno Med. 2010;4:145–148. [Google Scholar]
- 29.Ayannar M., Ignacimuthu S. Ethnobotanical survey of medicinal plants commonly used by the Kani tribals in Tirunelveli hills of Western Ghats, India. J Ethnopharmacol. 2011;134:851–864. doi: 10.1016/j.jep.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Gacche R.N., Shaikh R.U., Pund M.M., Deshmukh R.R. Cyclooxygenase inhibitory, cytotoxicity and free radical scavenging activities of selected medicinal plants used in indian traditional medicine. Pharmacog J. 2011;1:57–64. [Google Scholar]
- 31.Viji V., Helen A. Inhibition of lipoxygenases and cyclooxygenase-2 enzymes by extracts isolated from Bacopa monniera (L.) Wettst. J Ethnopharmacol. 2008;118:305–311. doi: 10.1016/j.jep.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Jing W., Xinyi C., Li H., Li L., Hongfei L., Wei L. Effects of tea polyphenols on the expression of Nf-κb, cox-2 and surviving in Lewis lung carcinoma xenografts in c57bl/6 mice. Chin J Lung Cancer. 2012;15:271–276. doi: 10.3779/j.issn.1009-3419.2012.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patwardhan, Vaidya B., Ashok D.B. Natural products drug discovery: accelerating the clinical candidate development using reverse pharmacology approaches. Indian J Exp Biol. 2010;48:220–227. [PubMed] [Google Scholar]
- 34.Palmieri D., Pane B., Barisione C. Resveratrol counteracts systemic and local inflammation involved in early abdominal aortic aneurysm development. J Surg Res. 2011;171:e237–e246. doi: 10.1016/j.jss.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 35.Selvum C., Jachak S. Cyclooxygenase inhibitory bioflavonoid from the seeds of Semecarpus anacardium. J Ethanopharmacol. 2004;95:209–212. doi: 10.1016/j.jep.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 36.Naik V.N. vol I & II. Amrut Prakashan; Aurangabad (MS): 1998. Flora of Marathwada. [Google Scholar]
- 37.Maurias M. Resveratrol analogues as selective cyclooxygenase-2 inhibitors: synthesis and structure activity relationship. Bioorg Med Chem. 2004;12:5571–5578. doi: 10.1016/j.bmc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Padmanabhan P., Jangle S.N. Evaluation of DPPH radical scavenging activity and reducing power of four selected medicinal plants and their combinations. Int J Pharma Sci Drug Res. 2012;4:143–146. [Google Scholar]
- 39.Chaturvedi P., Khare K.B., Kwape T.E., Moses M. Antioxidant properties of two edible mushrooms, Agaricus bisporus and Pleurotus ostreatus grown in Botswana. Mushroom Res. 2011;20:1–6. [Google Scholar]
- 40.Sawant O., Kadam J., Ghosh R. In vitro free radical scavenging and antioxidant activity of Adiantum lunulactum. J Herb Med Toxicol. 2009;3:39–44. [Google Scholar]
- 41.Umamaheswari M., Chatterjee T.K. In vitro antioxidant activities of the fractions of Coccinia grandis L. leaf extract. Afr J Trad Compl Alt Med. 2008;5:61–73. [PMC free article] [PubMed] [Google Scholar]
- 42.Sini K.R., Haribabu Y., Sajith M.S., Surya S.K. In-vitro Cytotoxic activity of Orthosiphon thymiflorus (Roth.) sleensen leaf extract against dalton lymphoma ascites cell line. J Chem Pharma Res. 2012;4:917–921. [Google Scholar]
- 43.Ummuhan S.H., Akito N., Iclal S. Antioxidant and cytotoxic effects of Moltkia aurea boiss. Rec Nat Prod. 2012;6:62–66. [Google Scholar]
- 44.Gacche R.N., Shaikh R.U., Pund M.M. In vitro evaluation of anticancer and antimicrobial activity of selected medicinal plants from Ayurveda. Asian J Trad Med. 2011;6:1–7. [Google Scholar]
- 45.Ayoola G.A., Alpanika G.A., Awabajo F.O. Anti-inflammatory properties of the fruits of Allanblanckia floribunda olive (guttiferae) Bot Res Int. 2009;2:21–26. [Google Scholar]
- 46.Griswold D.E., Martin L.D., Badger A.M., Breton J., Chabot-Fletcher M. Evaluation of the cutaneous anti-inflammatory activity of azapiranes. Inflamm Res. 1998;47:56–61. doi: 10.1007/s000110050270. [DOI] [PubMed] [Google Scholar]
- 47.Yasser A.S., Nabil H.O. Anti-inflammatory new coumarin from the Ammi majus L. Org Med Chem Lett. 2012;2:1–4. doi: 10.1186/2191-2858-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selvum C., Jachak S.M., Bhutani K.K. Cyclooxygenase inhibitory flavonoids from the Stem Bark of Semecarpus anacardium Linn. Phytoth Res. 2004;18:582–584. doi: 10.1002/ptr.1492. [DOI] [PubMed] [Google Scholar]
- 49.Julie S.J. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Alt Med Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- 50.Min H.P., Ching S.L., Chi T.H. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010;1:15–31. doi: 10.1039/c0fo00103a. [DOI] [PubMed] [Google Scholar]
- 51.Ram H.N., Sriwastava N.K., Makhija I.K., Shreedhara C.S. Anti-inflammatory activity of Ajmodadi Churna extract against acute inflammation in rats. J Ayurveda Integr Med. 2012;3:33–37. doi: 10.4103/0975-9476.93946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rege N.N., Thatte U.M., Dahanukar S.A. Adaptogenic properties of six rasayana herbs used in Ayurvedic medicine. Phytoth Res. 1999;13:275–291. doi: 10.1002/(SICI)1099-1573(199906)13:4<275::AID-PTR510>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 53.Srikumar R., Parthasarathy N.J., Devi R.S. Immunomodulatory activity of triphala on neutrophil functions. Biol Pharma Bull. 2005;28:1398–1403. doi: 10.1248/bpb.28.1398. [DOI] [PubMed] [Google Scholar]
- 54.Jagetia G.C., Baliga M.S., Malagi K.J., Sethukumar K.M. The evaluation of the radioprotective effect of triphala (an ayurvedic rejuvenating drug) in the mice exposed to gammaradiation. Phytomedicine. 2002;9:99–108. doi: 10.1078/0944-7113-00095. [DOI] [PubMed] [Google Scholar]
- 55.Kaur S., Arora S., Kaur K., Kumar S. The in vitro antimutagenic activity of triphala an Indian herbal drug. Food & Chem Toxicol. 2002;40:527–534. doi: 10.1016/s0278-6915(01)00101-6. [DOI] [PubMed] [Google Scholar]
- 56.Vani T., Rajani M., Sarkar S., Shishoo C.J. Antioxidant properties of the ayurvedic formulation triphala and its constituents. Inter J Pharmacog. 1997;35:313–317. [Google Scholar]
- 57.Fujimura N., Sumita S., Narimatsu E. Alteration in diaphragmatic contractility during septic peritonitis in rats: effect of polyethylene glycol absorbed superoxide dismutase. Crit Care Med. 2000;28:2406–2414. doi: 10.1097/00003246-200007000-00036. [DOI] [PubMed] [Google Scholar]
- 58.Young C.N., Koepke J.I., Terlecky L.J., Borkin M.S., Boyd M.S., Terlecky S.R. Reactive oxygen species in tumour necrosis factor-α-activated primary human keratinocytes: implications for psoriasis and inflammatory skin disease. J Invest Dermatol. 2008;128:2606–2614. doi: 10.1038/jid.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lachance P.A., Nakat Z., Jeong W.S. Antioxidants: an integrative approach. Nutrition. 2001;17:835–838. doi: 10.1016/s0899-9007(01)00636-0. [DOI] [PubMed] [Google Scholar]
- 60.Amakura Y., Umino Y., Tsuji S. Constituents and their antioxidative effects in eucalyptus leaf extract used as a natural food additive. Food Chem. 2002;77:47–56. [Google Scholar]
- 61.Aruoma O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J Ame Oil Chem Soc. 1998;75:199–212. doi: 10.1007/s11746-998-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yehia A.T.A., Alzowahi F.A.M., Kadam T.A., Shaikh R.U. In vitro evaluation of antimicrobial and antioxidant activity of Dragon's blood tree (Dracaena cinnabari Balf.f.) of Socotra Island (Yemen) J Coast Life Med. 2013;1:111–117. [Google Scholar]