Abstract
Objective: Impaired and chronic wounds occur due to defects in one or more of the overlapping stages of healing. However, problems related to the vascular system are critical for nonhealing, and chronic wounds in humans often show the presence of fibrin cuffs/clots. We hypothesized that these clots are due to alterations in platelet function; hence, we have investigated whether alterations in platelet function are present during impaired healing.
Approach: Platelets were subjected to different agonists to determine the rate of aggregation and evaluate the molecules involved in adhesion and aggregation that could lead to faster thrombosis and potentially contribute to impaired wound healing.
Results: We show that wounding of TNFSF14/LIGHT−/− mice, which have impaired healing, leads to an enhanced response in platelet aggregation and a faster time to blood vessel occlusion (thrombosis). In addition, after wounding, platelets from these mice have increased levels of P-selectin, integrin αIIbβ3, and phosphatidylserine, molecules that contribute to platelet adhesion. They also have more extensive open canalicular system than platelets of control mice, suggesting increased surface area for interactions upon activation.
Innovation: These results show a novel function for TNFSF14/LIGHT during wound healing.
Conclusion: The absence of TNFSF14/LIGHT from the cell surface of platelets causes rapid platelet aggregation and thrombus formation that may contribute to impaired healing by reducing the ability of the blood vessels to transport nutrients and oxygen and other molecules needed for proper healing.
Keywords: : platelet aggregation, phosphatidylserine, thrombosis and occlusion, P-selectin, integrin α IIb β 3, fibrin cuffs

Manuela Martins-Green, PhD
Introduction
The occurrence of chronic wounds in humans has risen significantly in the last decade. In the United States alone, chronic wounds affect 6.5 million people and cost ∼$25B/year.1 Abnormalities associated with impaired and chronic wounds include a prolonged inflammatory phase, abnormal angiogenesis, formation of fibrin cuffs, abnormal extracellular matrix (ECM) deposition, decreased rate of re-epithelialization, and presence of biofilm-forming bacteria.2–4 Prediction of healing outcomes remains elusive due to the lack of understanding of the mechanisms involved in the etiology of impaired and chronic wounds, and often the coexisting morbidities. Furthermore, although a murine model for chronic wounds has been developed recently,5,6 mechanistic studies are still lacking, thereby hindering the advancement in the development of diagnostics and therapeutics to treat these wounds.7–9
Tissue injury causes the disruption of blood vessels and extravasation of blood constituents. Blood clotting reestablishes hemostasis and provides a provisional ECM for cell migration and proliferation. A variety of vasoactive mediators and chemotactic factors are generated by coagulation, the activated complement pathways, and by injured or activated parenchymal cells.10 This vascular injury leads to the activation of platelets as first responders to help stop hemorrhage. Platelets are major players in hemostasis and they are crucial in the pathophysiology of vascular clots and thrombosis. Moreover, platelets release molecules that interact with various factors related to inflammation, proliferation, adhesion, aggregation, and vasoconstriction.11 The α-granules of platelets contain clotting proteins such as von Willebrand factor (vWF), fibrinogen, fibronectin, and factor V. The dense granules contain adenosine diphosphate (ADP), adenosine triphosphate (ATP), and ionized calcium. Contents present in both α- and dense granules are involved in activation, aggregation, and recruitment of resting platelets to sites of injury.
The activation and aggregation of platelets occur due to their interactions with ligands on endothelial cells, leukocytes, and various ECM proteins.11 P-selectin is an adhesion molecule present in α-granules that becomes exposed on the platelet membrane upon activation. Activation of platelets is followed by adhesion and aggregation, which involves various factors, including the fibrinogen receptor integrin αIIbβ3.11 Activation-dependent conformational changes occur in integrin αIIbβ3, making it competent to bind soluble fibrinogen.12 Platelet activation also leads to the translocation of phosphatidylserine (PS) from the inner to the outer leaflet of the platelet membrane bilayer. PS has been shown to be involved in thrombin generation and hence blood coagulation.13,14 Assembly of coagulation factor complexes such as tenase and prothrombinase (which are necessary for thrombin generation), on the membrane bilayer is also dependent on PS exposure on activated platelets.15 Furthermore, procoagulant platelets display features of apoptosis, including blebbing, microvesiculation, caspase activation, and membrane contraction.14
We have previously shown that TNFSF14/LIGHT−/− mice display marked abnormalities in wound healing processes, including increased inflammation, oxidative and nitrosative stress, excessive coagulation and fibrin cuffs, vascular defects, and abnormal ECM deposition.5,16 Furthermore, we also showed that the basement membrane of the blood vessels was not continuous, the microvessels had very few associated α-smooth muscle actin-expressing periendothelial cells, and contained significant intravascular coagulation,16 resulting in immature/defective vessels. Moreover, we have recently shown that in these mice with impaired healing, platelets have enhanced aggregation as well as shorter bleeding time.6 We also found that after injury there was a significant increase in the eicosanoids 11-, 12- and 15-HETE, and the proinflammatory leukotrienes (LTD4 and LTE4) and prostaglandins (PGE2 and PGF2α)6 that are important in platelet function.
The goal of this study is to further determine whether the ability of the platelets of this knockout (KO) mouse to aggregate faster than control platelets is due to the presence of higher levels of molecules involved in adhesion and aggregation, whether this leads to faster thrombosis, and do they behave differently than control mice in the absence of injury/wounding. We show that, upon injury, TNFSF14/LIGHT−/− mice have enhanced aggregation of platelets in the presence of the ADP agonist and an agonist for the thromboxane A2 (TXA2) receptor (U46619), and that thrombosis is faster than the control 3 days after injury. Moreover, we show significant differences in the levels of several molecules involved in adhesion, activation, and aggregation of the TNFSF14/LIGHT−/− platelets as well as a more extensive canalicular system. Because it has been demonstrated that mouse and human platelets share similar structural features,17 our findings in this mouse model of impaired healing may provide insight into the mechanisms involved in blood vessel clotting that could be instrumental in understanding similar processes in human problematic wounds.
Clinical Problem Addressed
Impaired and chronic wounds are affecting an ever-increasing population and results in the rising cost of healthcare. The confounding number of factors that lead to the development of chronic wounds requires in-depth understanding of the mechanisms. Our data presented in this study provide evidence for the role of platelets in the development of impaired and chronic wounds. Furthermore, the results suggest a possible role of TNFSF14/LIGHT on platelets that could lead to their impaired function. Therefore, these findings could be influential in deciphering processes underlying the etiology of chronic wound development and advancement of wound therapeutics.
Materials and Methods
Dermal excision wound model
C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME), and TNFSF14/LIGHT−/− mice were a gift from Carl Ware (La Jolla Institute for Allergy and Immunology, San Diego, CA). Animals were housed at the University of California, Riverside (UCR) vivarium. All experimental protocols were approved by the UCR Institutional Animal Care and Use Committee (IACUC) and all methods were carried out according to the approved guidelines. Experiments were performed using 16-week-old mice. The procedure used was performed as previously described.18 Briefly, mice were anesthetized with a single intraperitoneal injection of ketamine (80 mg/kg body weight)/xylazine (16 mg/kg body weight). Full-thickness 7 mm punch wounds (excision of the skin and the underlying panniculus carnosus) were made on the back of the mice 24 h after nairing and shaving the hair.
Murine platelet preparation
C57BL/6 (control) or TNFSF14/LIGHT−/− mice were anesthetized and blood was collected from the ventricle in 3.8% w/v sodium citrate solution (one part sodium citrate to nine parts blood) to prevent coagulation. Platelet-rich plasma was obtained by centrifugation at room temperature. Platelets were counted with an automated hematology analyzer (Drew Scientific, Dallas, TX) and their count adjusted to 7 × 107 platelets/mL, before each experiment.
In vitro platelet aggregation assay
C57BL/6 or TNFSF14/LIGHT−/− platelets were activated with different concentrations of ADP and U46619. Platelet aggregation was measured by the turbidometric method using the model 490 aggregometer (Chrono-Log Corporation, Havertown, PA). There are no standard deviations because, to measure % aggregation increase, we needed to pool the blood of five samples given that the amount of blood we obtain from each mouse is very small.
In vivo thrombosis model
These studies were performed as described previously.19 Briefly, C57BL/6 or TNFSF14/LIGHT−/− mice were anesthetized with ketamine (200 mg/kg). Then, the left carotid artery was exposed and cleaned, and baseline carotid artery blood flow was measured with the Transonic micro-flow probe (0.5 mm; Transonic Systems, Inc., Ithaca, NY). After stabilization of blood flow, 7.5% ferric chloride (FeCl3) was applied to a filter paper disc (1-mm diameter) that was immediately placed on top of the artery for 3 min. Blood flow was continuously monitored for 30 min, or until blood flow reached stable occlusion (zero blood flow for 2 min). Data were recorded and time to vessel occlusion was calculated as the difference in time between stable occlusion and removal of the FeCl3-containing filter paper. An occlusion time of 30 min was considered the cut-off time for the purpose of statistical analysis.
Flow cytometric analysis
Flow cytometric analysis was performed as described before.19 Briefly, platelets (2 × 108) were stimulated with ADP (0.0625 and 0.25 μM) or U46619 (0.5 μM) for 3 min and then fixed with 2% formaldehyde for 30 min at room temperature, followed by incubation for 30 min at room temperature with FITC-conjugated anti-P-selectin and Annexin V (Cell Signaling Technology, Inc., Danvers, MA), FITC-conjugated JON/A (Integrin αIIbβ3; EMFRET Analytics, Würzburg, Germany). Fluorescent intensities of the platelets diluted 2.5-fold with HEPES/Tyrode's buffer (pH 7.4) were measured using a BD Accuri C6 flow cytometer. Results were analyzed using CFlow Plus (BD Biosciences, Franklin Lakes, NJ). Each experiment was repeated at least three times with blood pooled from n = 6 mice.
Transmission electron microscopy
Mouse platelets were isolated and processed for transmission electron microscopy as described previously.20 Briefly, equal volumes of 0.1% glutaraldehyde in White's saline were added to the C57BL/6 or TNFSF14/LIGHT−/− platelet suspension for 15 min at 37°C. The platelets were centrifuged and incubated in ice-cold 3% glutaraldehyde in White's saline at 4°C for 1 h. After three washes, the platelets were incubated with 1% OsO4. Osmicated platelets were washed twice with water and dehydrated with a series of ethanol solutions (50%, 70%, 80%, 90%, and 100%). The platelets were rinsed twice with propylene oxide and infiltrated overnight in a 1:1 mixture of propylene oxide and Spurr's resin (4.1 g ERL-4221 [3,4 epoxycyclohexylmethyl 3,4 epoxycyclohexyl carboxylate], 5.9 g nonenyl succinic anhydride, 1.43 g DER-736 epoxy resin, and with final addition of 0.1 g 2-dimethylaminoethanol). After several washes in pure Spurr's resin, samples were embedded in Spurr's resin and polymerized at 60°C for 24 h. Polymerized blocks were sectioned (70 nm) and mounted on copper grids. Following counterstaining with uranyl acetate and lead citrate, samples were examined using FEI Tecnai 12 transmission electron microscope (FEI, Hillsboro, OR) and images were obtained with Gatan Digital micrograph software (Pleasanton, CA).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). A one-way ANOVA, followed by a pairwise comparison post-test, was conducted wherever appropriate. A 95% confidence interval was considered as statistically significant.
Results
TNFSF14/LIGHT−/− wounded mice have enhanced platelet aggregation
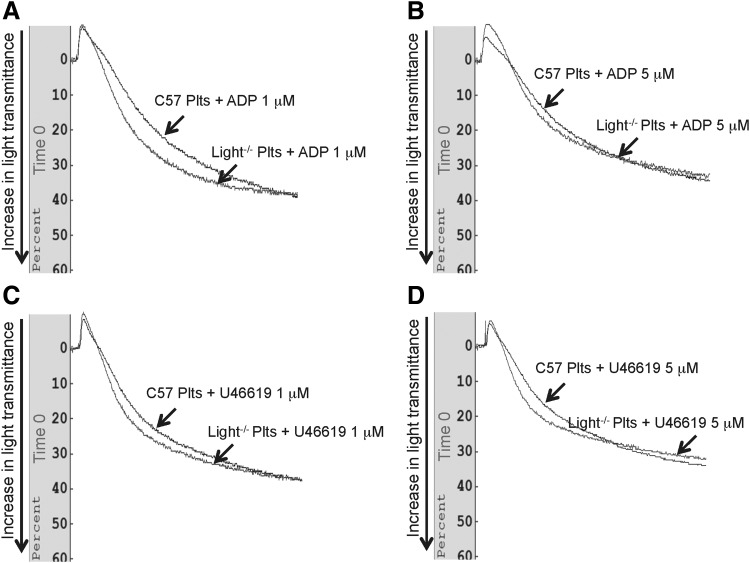
To examine platelet behavior in the TNFSF14/LIGHT−/− mice after injury, we performed platelet aggregation assays using different concentrations of the platelet aggregation agonists, ADP and U46619. We observed that platelet aggregation in the presence of ADP is increased at 3 days postwounding in the TNFSF14/LIGHT−/− mice when compared with the control C57BL/6 mice and that aggregation increased in a dose-dependent manner (Fig. 1A). Similar data were obtained when using U64419 the TXA2 mimetic (Fig. 1B). Furthermore, we examined whether the increase in platelet aggregation also occurred at a later time postwounding. Higher levels of platelet aggregation still occurred in the TNFSF14/LIGHT−/− mice at day 7 postwounding, also in a dose-dependent manner (Fig. 1C, D). However, before wounding there are no differences between the platelets in the two types of mice (Fig. 2).
Figure 1.
Platelet aggregation is increased during impaired healing. Platelet aggregation was determined using an aggregometer and the agonists ADP and U46619. (A) Platelet aggregation 3 days postwounding was significantly enhanced in the TNFSF14/LIGHT−/− mice and the aggregation occurred in an ADP dose-dependent manner. (B) Similar effects were seen when the platelets were stimulated with the agonist U46619. (C, D) At day 7 postwounding, there was enhanced platelet aggregation in TNFSF14/LIGHT−/− mice when both ADP and U46619 were used as the agonist for activation. We pooled the blood from five mice because this assay requires a significant amount of blood. Therefore, in total n = 5 for each assay. TNFSF14, tumor necrosis factor superfamily 14. ADP, adenosine diphosphate.
Figure 2.
Platelets of unwounded mice show no difference in time to aggregation. Platelet aggregation was determined using an aggregometer and the agonists (A, B) ADP and (C, D) U46619. No difference in time to aggregation was seen. For each assay we pooled the blood from five mice.
Thrombogenesis is enhanced in wounded TNFSF14/LIGHT−/− mice
To determine whether thrombus formation, evaluated by occlusion time, was affected in the TNFSF14/LIGHT−/− mice, we used a FeCl3-induced carotid artery injury thrombosis model (Fig. 3). No significant differences in occlusion time were observed between unwounded TNFSF14/LIGHT−/− and control (C57BL/6) mice. However, at day 3 postwounding, the time to occlusion in TNFSF14/LIGHT−/− mice was significantly decreased in comparison to control C57BL/6 mice. Complete occlusion of the vessel wall after FeCl3 treatment of the KO mouse occurred in about 4 min, whereas wounded control mice took more than 22 min. At day 7 postwounding, the occlusion time in TNFSF14/LIGHT−/− wounded mice continued to be low, but because of variability in the occlusion time for the control C67BL/6 mice, the difference in time to occlusion between the two strains of mice was no longer statistically different.
Figure 3.
Occlusion time is shortened during impaired healing. C57BL/6 (control) and TNFSF14/LIGHT−/− mice were anesthetized and the FeCl3-induced carotid artery injury model was performed as discussed in Materials and Methods section. Each point represents the occlusion time of a single animal. Occlusion time postwounding in TNFSF14/LIGHT−/− mice was significantly reduced compared with the C57BL/6 wounded mice at day 3 postwounding (n = 5).
Activated TNFSF14/LIGHT−/− platelets have enhanced P-selectin levels
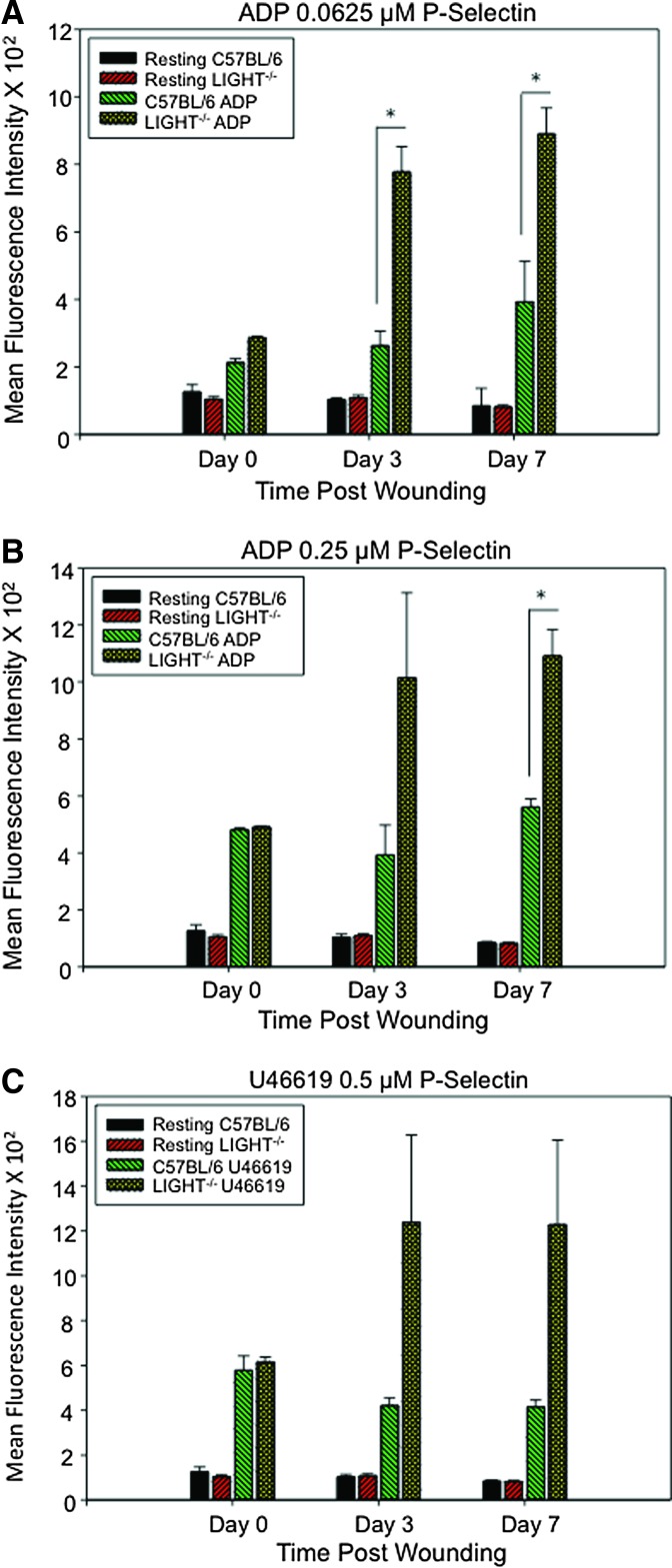
Release of granule content is the earliest event in the platelet activation process. The results described above show that the platelets in wounded TNFSF14/LIGHT−/− are hyperactive. Therefore, we conducted platelet responsiveness studies to different agonists by measuring the levels of P-selectin using flow cytometry. P-selectin levels in activated platelets of unwounded mice were not significantly different in TNFSF14/LIGHT−/− and C57BL/6 mice (Fig. 4A). However, by day 3 postwounding, TNFSF14/LIGHT−/− platelets displayed significantly higher levels of P-selectin than those of C57BL/6 mice, a difference that continued for day 7 wounds. The ADP increased the P-selectin levels in a dose-dependent manner (Fig. 4B) and similar results were found using U46619 (0.5 μm), the mimetic for the TXA2 receptor (Fig. 4C).
Figure 4.
Surface P-selectin levels are enhanced during impaired wounding. Surface P-selectin levels on the platelet plasma membrane after platelet activation were measured using FACS. (A, B) Platelet activation using 0.0625 μM ADP led to significant increases in P-selectin on the platelet membrane in TNFSF14/LIGHT−/− mice when compared with C57BL/6 at both day 3 and 7 postwounding. An overall increase in P-selectin was observed at day 3 in TNFSF14/LIGHT−/− mice and the levels were significantly higher at day 7 post wounding in TNFSF14/LIGHT−/− mice when compared with C57BL/6 mice. (C) Agonist U46619 0.5 μM caused similar effects on P-selectin levels in TNFSF14/LIGHT−/− wounds after wounding when compared with C57BL/6 wounded mice (n = 5). *p < 0.05, ** p < 0.01, ***p < 0.001. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Integrin αIIbβ3 levels are higher in activated platelets from wounded TNFSF14/LIGHT−/−
It is well documented that integrin αIIbβ3 plays a critical role in platelet aggregation in response to physiological agonists and that it mediates thrombus formation.21 To determine whether the enhanced platelet aggregation from wounded TNFSF14/LIGHT−/− mice is associated with an increase in the levels of integrin αIIbβ3, we performed fluorescence-activated cell sorting analysis with an antibody specific to this integrin. We show that, upon activation with ADP (0.062 μm and 0.25 μm), the platelets collected from unwounded TNFSF14/LIGHT−/− and C57BL/6 mice showed equal levels of integrin αIIbβ3 on the plasma membrane. However, upon wounding, the levels of integrin αIIbβ3 on the platelet membrane in the TNFSF14/LIGHT−/− mice at both days 3 and 7 were significantly higher than those of C57BL/6 platelets (Fig. 5A, B). We also found that the levels of integrin αIIbβ3 in the wounded TNFSF14/LIGHT−/− mice platelets were significantly enhanced with the agonist for the TXA2 receptor U46619 (Fig. 5C). However, there was no significant difference on the levels of integrin αIIbβ3 between days 3 and 7 postwounding.
Figure 5.
Surface platelet integrin αIIbβ3 levels are increased in impaired healing. Integrin αIIbβ3 levels resulting from platelet activation were determined by flow cytometry. Integrin αIIbβ3 levels were significantly increased in TNFSF14/LIGHT−/− mice by (A) 0.0625 μM ADP, (B) 0.25 μM ADP, and (C) 0.5 μM U46619. Significant increases in receptor levels were observed in TNFSF14/LIGHT−/− mice at day 3 and 7 postwounding. No difference was seen in αIIbβ3 levels in activated platelets from unwounded mice of both strains (day 0) (n = 5). *p < 0.05, **p < 0.01, ***p < 0.001. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
PS levels are higher in platelets from wounded TNFSF14/LIGHT−/−
Exposure of negatively charged PS to the outer leaflet of the plasma membrane bilayer occurs in activated platelets and is followed by increased platelet procoagulant activity.22 Therefore, we determined whether the platelets from wounded TNFSF14/LIGHT−/− mice had enhanced PS levels upon agonist activation. Platelets from unwounded TNFSF14/LIGHT−/− and C57BL6/7 mice, when activated using ADP, show no significant difference in flipping of PS to the outer leaflet of the membrane (Fig. 6A, B; day 0). However, platelets from wounded TNFSF14/LIGHT−/− mice show an augmented presence of PS on the outer leaflet of the plasma membrane compared with platelets from C57BL/6 mice (Fig. 6A, B; days 3 and 7). Moreover, U46619 activation of platelets also caused significant increases in PS exposure on TNFSF14/LIGHT−/− wounded mice platelets in comparison to controls (Fig. 6C; days 3 and 7).
Figure 6.
Wounded TNFSF14/LIGHT−/− mice platelets exhibit increased PS on the outer leaflet of the membrane. Exposure of PS on the surface of platelets in the resting and activated state was estimated using FACS. The translocation of PS onto the outer leaflet of the platelet plasma membrane was significantly increased in TNFSF14/LIGHT−/− mice by (A) 0.0625 μM ADP, (B) 0.25 μM ADP, and (C) 0.5 μM U46619 agonist activation of platelets. The significant increase in translocation of PS onto the outer leaflet of the platelet plasma membrane from wounded TNFSF14/LIGHT−/− when compared with wounded C57BL/6 mice, supports our results of increase in aggregation of platelets in TNFSF14/LIGHT−/− mice (n = 5). PS, phosphatidylserine. *p < 0.05, **p < 0.01, ***p < 0.001. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Platelets from wounded TNFSF14/LIGHT−/− mice have a more extensive open canalicular system
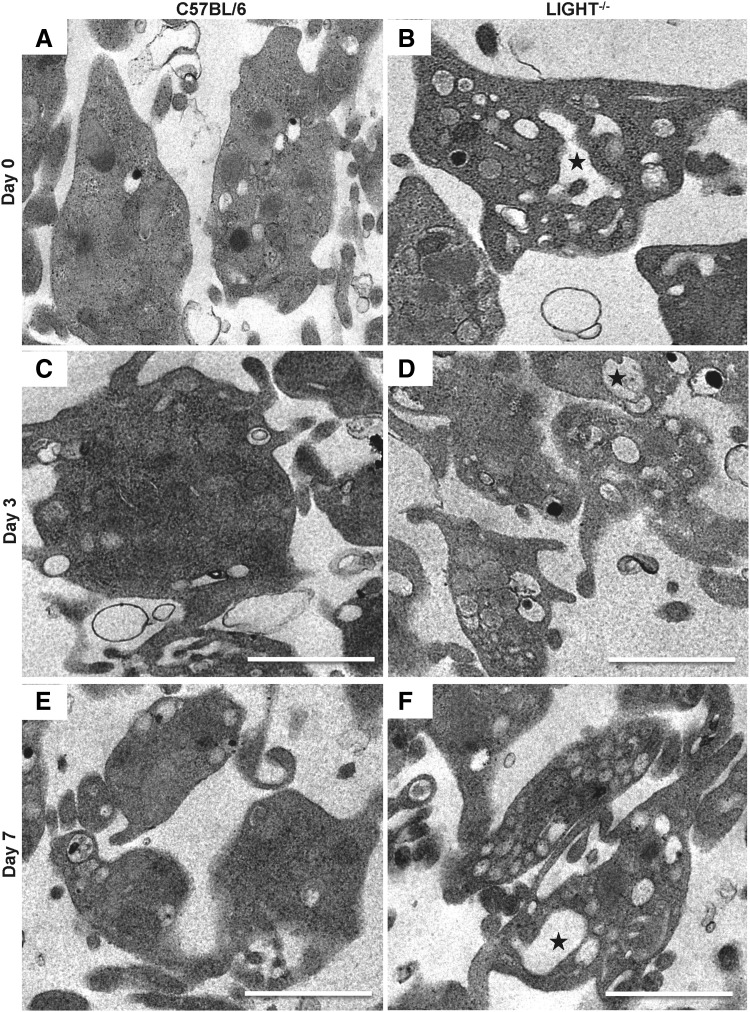
Because we observed a significant increase in the levels of aggregation and of prothrombotic markers after activation, we used transmission electron microscopy to determine whether the resting platelets showed differences in their structural integrity. We found that while the overall structure of the platelets was intact in both the C57BL/6 and TNFSF14/LIGHT−/− mice, the presence of a dilated open canalicular system (OCS) was more extensive in TNFSF14/LIGHT−/− platelets (Fig. 7A–F).
Figure 7.
Resting TNFSF14/LIGHT−/− mice platelets show increased OCS. Resting C57BL/6 (A, C, E) and TNFSF14/LIGHT−/− (B, D, F) platelet structure was assessed by using transmission electron microscopy. Increased presence of a dilated OCS was observed only in resting platelets from TNFSF14/LIGHT−/− mice (stars in B, D, F), suggesting increased surface area for interaction upon activation and granule release. OCS, open canalicular system.
Discussion
The role of platelet function in impaired wound healing has not been extensively studied. In the present study, we provide the first evidence of the presence of hyperactive platelets and problems in thrombosis in a model of impaired healing, the TNFSF14/LIGHT−/− mouse. Platelets from these wounded KO mice have higher aggregation response when treated with agonist than do the wounded control mice. They also have higher levels of P-selectin, integrin αIIbβ3, and PS on the plasma membrane after activation than do the wounded control C57BL/6 mice. Resting platelets obtained from TNFSF14/LIGHT−/− mice have a large OCS when compared with control resting platelets. The enlarged presence of OCS in resting platelets in the TNFSF14/LIGHT−/− mice suggests availability of extensive surface for release of α-granules and other platelet components, including provision for increased area for surface interaction after activation.
The significantly higher levels of P-selectin on activated platelets from wounded TNFSF14/LIGHT−/− mice suggest an increase in adhesion and formation of platelet–leukocyte complexes. Furthermore, high levels of P-selectin have been associated with increasing size and stability of platelet aggregates.23 This suggests that the formation of clots in blood vessels in various tissues of wounded TNFSF14/LIGHT−/− mice16 is a result of abnormal platelet behavior. Moreover, these aggregates could play a critical role in creating a hypoxic environment that can further contribute to impaired wounds.
Elevated levels of integrin αIIbβ3 detected on activated platelets of wounded TNFSF14/LIGHT−/− mice indicate that they have a significantly high risk of formation of atherosclerotic plaques.24 Indeed, we observed that some of these mice died and, when analyzed, had very large numbers of clots in vessels of the heart (unpublished results). The increased levels of integrin αIIbβ3 could also explain its increased availability for binding to fibrinogen, where fibrinogen and vWF compete (fibrinogen outcompetes vWF) to bind to integrin αIIbβ3.25 Furthermore, integrin αIIbβ3-fibrinogen interactions help in the firm bridging of platelets with the endothelium causing thrombus formation, initially stabilized by the presence of P-selectin, which was also highly present in TNFSF14/LIGHT−/− mice platelets. These observations suggest critical interactions facilitating the development of larger platelet aggregates.23,26 Moreover, increase in PS in wounded TNFSF14/LIGHT−/− mice indicates a higher degree of thrombin generation and coagulation observed in the blood of these mice. In addition, increase in platelet integrin αIIbβ3 that supports the binding of activated platelets to the fibrin network could also increase PS exposure on the platelet surface.27
An important structure of the platelet membrane is the presence of invaginations known as OCS that provide the capability to increase the surface to volume ratio of the membrane upon activation of platelets. The OCS provides the vehicle for external microenvironmental components to interact with the interior of the platelet, while the platelet contents, including the granules, exit through the OCS upon activation.28 The presence of bigger and more dilated OCS suggests the availability of more surface-to-volume interaction of the TNFSF14/LIGHT−/− platelets with the surrounding microenvironment. Furthermore, the presence of integrin αIIbβ3 on the surface of OCS before activation of the platelets, may explain the significant increase in the exposure of this integrin in activated TNFSF14/LIGHT−/− platelets.29
Although there have been a few studies showing that TNFSF14/LIGHT plays a role in platelet biology, they have been primarily focused on the release of this protein from the platelets. Indeed, these studies have shown that when TNFSF14/LIGHT is released in high levels from the platelets, it goes into circulation and induces a vigorous inflammatory response that affects both endothelial cells and inflammatory cells alike, resulting in a variety of diseases/disorders.30–34 It has also been proposed that, upon activation, platelets release superoxide anions and that the availability of superoxide anions leads to greater metabolism of ADP, enhancing further recruitment and activation of platelets.35 Furthermore, collagen-induced platelet activation in wounds can produce high amounts of H2O2 that could stimulate platelet aggregation pathways.36 We recently showed that TNFSF14/LIGHT−/− mice have high levels of oxidative stress early in wound healing with increased presence of H2O2 and isoprostanes,18 suggesting the enhancement of the platelet activation we now report here. Moreover, reactive oxygen species frequently observed in impaired and chronic wounds have increasingly been shown to be major players in the activation of platelets and to cause thrombus formation.37,38
In conclusion, this work shows a novel function for TNFSF14/LIGHT during wound healing; in the absence of this molecule from the cell surface, platelets show higher levels of aggregation, potentially contributing to rapid thrombosis during the early stages of healing, which might contribute to impaired healing by reducing the ability of the blood vessels to transport nutrients and oxygen as well as other serum molecules needed for proper healing of the wound tissue. Furthermore, this environment can lead to cell death, contributing to impaired healing. These findings also suggest a role for TNFSF14/LIGHT molecule in controlling platelet function during human wound healing. Because chronic venous stasis ulcers in humans have an underlying state of platelet activation similar to those seen in TNFSF14/LIGHT−/− mice, we speculate that dysregulation of platelet function in these chronic wounds could be due to decreased levels or absence of TNFSF14/LIGHT on the surface of platelets.
Innovation
Advancement in therapeutics to treat impaired wounds has been hindered due to lack of understanding of mechanisms behind chronic wound etiology. Upon injury, reestablishment of hemostasis occurs through activation of platelets, the first responders that are also crucial in the pathophysiology of vascular clots and thrombosis. However, their role in impaired wounds is not well understood. Higher levels of aggregation observed in platelets of injured LIGHT−/− mice might explain their part in establishing an environment conducive to improper healing. Our results, for the first time, suggest that dysregulated platelet function could be due to abnormal expression of TNFSF14/LIGHT on the surface of platelets.
Limitations of the System
Because the TNFSF14/LIGHT KO mouse has a global and not platelet-specific deletion of the gene, our FeCl3 thrombosis model could also have contributions from the endothelial cells in the vasculature and other blood components. Furthermore, the aggregation response observed in the platelets requires pooling of the blood from several mice making it difficult to analyze platelets from individual animals to better understand the variability.
Key Findings.
• Enhanced response in platelet aggregation and faster time to blood vessel occlusion (thrombosis) was observed upon wounding in the TNFSF14/LIGHT−/− mouse model of impaired healing.
• Platelets from wounded TNFSF14/LIGHT−/− mice have increased levels of P-selectin, integrin αIIbβ3, and PS.
• TNFSF14/LIGHT−/− platelets have more extensive OCS than platelets of control mice.
Abbreviations and Acronyms
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- ECM
extracellular matrix
- FeCl3
ferric chloride
- HETE
hydroxyeicosatetraenoic acid
- KO
knockout
- LIGHT
lymphotoxin-like inducible protein that competes with Glycoprotein D for binding herpes virus entry mediator on T cells
- LT
leukotrienes
- OCS
open canalicular system
- OsO4
osmium tetroxide
- PG
prostaglandins
- PS
phosphatidylserine
- TNFSF14
tumor necrosis factor superfamily 14
- TX
thromboxane
- UCR
University of California, Riverside
- vWF
von Willebrand factor
Authors’ Contributions
S.D., Z.A.K., F.K., and M.M.G. conceived and designed the study. S.D. and Z.A.K. performed the experiments. S.D., Z.A.K., F.K., and M.M.G. discussed the results and contributed to the data interpretation. S.D. and M.M.G. wrote the article and Z.A.K. and F.K. edited the article. All authors reviewed and revised the semi-final version of the article.
Acknowledgments
The authors thank Raquelle Alamat and Anthony Castro for help with the nairing of the mice. They also thank Mathias Rommelfanger from the Central Facility for Advanced Microscopy and Microanalysis at the University of California, Riverside for help in sectioning the tissues and use of the Transmission Electron Microscope.
Author Disclosure and Ghostwriting
The authors have no conflicts of interest to declare. There are no ghostwriter contributions in this work.
About the Authors
Sandeep Dhall, PhD, is currently a Scientist (Research & Development) at Osiris Therapeutics, Inc. He obtained his PhD in Bioengineering, followed by Postdoctoral training in Cell Biology and Neuroscience at the University of California, Riverside, both under the mentorship of Dr. Manuela Martins-Green. His research focused on the development of a preclinical animal model for chronic and impaired wound healing, and deciphering the underlying mechanism in problematic wounds. Zubair A. Karim, PhD, is a Research Assistant Professor at Western University of Health Sciences. His expertise is in the area of the platelet secretory machinery. Fadi T. Khasawneh, PhD, is an Associate Professor of Pharmaceutical Sciences at the Western University of Health Sciences. He is an expert in platelet biology and signaling. Manuela Martins-Green, PhD, Professor of Cell Biology at the University of California at Riverside is a wound healing biologist who studies normal and abnormal processes of healing, including roles of insulin in burn treatment and the development of a chronic wound mouse model.
References
- 1.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen 1994;2:165–170 [DOI] [PubMed] [Google Scholar]
- 3.Fivenson DP, Faria DT, Nickoloff BJ, et al. Chemokine and inflammatory cytokine changes during chronic wound healing. Wound Repair Regen 1997;5:310–322 [DOI] [PubMed] [Google Scholar]
- 4.Loots Ma, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol 1998;111:850–857 [DOI] [PubMed] [Google Scholar]
- 5.Dhall S, Do D, Garcia M, et al. A novel model of chronic wounds: importance of redox imbalance and biofilm-forming bacteria for establishment of chronicity. PLoS One 2014;9:e109848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhall S, Wijesinghe DS, Karim ZA, et al. Arachidonic acid-derived signaling lipids and functions in impaired healing. Wound Repair Regen 2015;23:644–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mustoe Ta, O'Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg 2006;117(7 Suppl):35S–41S [DOI] [PubMed] [Google Scholar]
- 8.Roberts AEL, Kragh KN. The limitations of in vitro experimentation in understanding biofilms and chronic infection. J Mol Biol 2015;427:3646–3661 [DOI] [PubMed] [Google Scholar]
- 9.Hurlow J, Couch K, Laforet K, Bolton L, Metcalf D, Bowler P. Clinical biofilms: a challenging frontier in wound care. Adv Wound Care 2015;4:295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark RAF. Basics of cutaneous wound repair. J Dermatol Surg Oncol 1993;19:693–706 [DOI] [PubMed] [Google Scholar]
- 11.Projahn D, Koenen RR. Platelets: key players in vascular inflammation. J Leukoc Biol 2012;92:1167–1175 [DOI] [PubMed] [Google Scholar]
- 12.Hawiger J. Mechanisms involved in platelet vessel wall interaction. Thromb Haemost 1995;74:369–372 [PubMed] [Google Scholar]
- 13.Lentz BR. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog Lipid Res 2003;42:423–438 [DOI] [PubMed] [Google Scholar]
- 14.Schoenwaelder SM, Yuan Y, Josefsson EC, et al. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood 2009;114:663–667 [DOI] [PubMed] [Google Scholar]
- 15.Heemskerk JWM, Bevers EM, Lindhout T. Platelet activation and blood coagulation. Thromb Haemost 2002;88:186–193 [PubMed] [Google Scholar]
- 16.Petreaca ML, Do D, Dhall S, et al. Deletion of a tumor necrosis superfamily gene in mice leads to impaired healing that mimics chronic wounds in humans. Wound Repair Regen 2012;20:353–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi W, Karim ZA, Whiteheart SW. Protein expression in platelets from six species that differ in their open canalicular system. Platelets 2010;21:167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhall S, Do DC, Garcia M, et al. Generating and reversing chronic wounds in diabetic mice by manipulating wound redox parameters. J Diabetes Res 2014;2014:e562626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karim ZA, Alshbool FZ, Vemana HP, et al. Third hand smoke: impact on hemostasis and thrombogenesis. J Cardiovasc Pharmacol April 2015;66:177–182 [DOI] [PubMed] [Google Scholar]
- 20.Karim ZA, Zhang J, Banerjee M, et al. IkB kinase phosphorylation of SNAP-23 controls platelet secretion. Blood 2013;121:4567–4575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson SP. Arterial thrombosis—insidious, unpredictable and deadly. Nat Med 2011;17:1423–1436 [DOI] [PubMed] [Google Scholar]
- 22.Munnix ICA, Kuijpers MJE, Auger J, et al. Segregation of platelet aggregatory and procoagulant microdomains in thrombus formation regulation by transient integrin activation. Arterioscler Thromb Vasc Biol 2007;27:2484–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merten M, Thiagarajan P. P-selectin expression on platelets determines size and stability of platelet aggregates. Circulation 2000;102:1931–1936 [DOI] [PubMed] [Google Scholar]
- 24.Guo S, Shen S, Wang J, et al. Detection of high-risk atherosclerotic plaques with ultrasound molecular imaging of glycoprotein IIb/IIIa receptor on activated platelets. Theranostics 2015;5:418–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gralnick H, Williams S, Coller B. Fibrinogen competes with von Willebrand factor for binding to the glycoprotein IIb/IIIa complex when platelets are stimulated with thrombin. Blood 1984;64:797–800 [PubMed] [Google Scholar]
- 26.Bombeli T, Schwartz BR, Harlan JM. Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), alphavbeta3 integrin, and GPIbalpha. J Exp Med 1998;187:329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brzoska T, Suzuki Y, Mogami H, Sano H, Urano T. Binding of thrombin-activated platelets to a fibrin scaffold through αIIbβ3 evokes phosphatidylserine exposure on their cell surface. PLoS One 2013;8:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Escolar G, White JG. The platelet open canalicular system: a final common pathway. Blood Cells 1991;17:467–485; discussion 486–495. [PubMed] [Google Scholar]
- 29.Cramer EM, Savidge GF, Vainchenker W, et al. Alpha-granule pool of glycoprotein IIb-IIIa in normal and pathologic platelets and megakaryocytes. Blood 1990;75:1220–1227 [PubMed] [Google Scholar]
- 30.Otterdal K, Smith C, Øie E, et al. Platelet-derived LIGHT induces inflammatory responses in endothelial cells and monocytes. Blood 2006;108:928–935 [DOI] [PubMed] [Google Scholar]
- 31.Liu GZ, Fang LB, Hjelmstrom P, Gao XG. Enhanced plasma levels of LIGHT in patients with acute atherothrombotic stroke. Acta Neurol Scand 2008;118:256–259 [DOI] [PubMed] [Google Scholar]
- 32.Garrido VT, Proença-Ferreira R, Dominical VM, et al. Elevated plasma levels and platelet-associated expression of the pro-thrombotic and pro-inflammatory protein, TNFSF14 (LIGHT), in sickle cell disease. Br J Haematol 2012;158:788–797 [DOI] [PubMed] [Google Scholar]
- 33.Celik S, Langer H, Stellos K, et al. Platelet-associated LIGHT (TNFSF14) mediates adhesion of platelets to human vascular endothelium. Thromb Haemost 2007;98:798–805 [PubMed] [Google Scholar]
- 34.Celik S, Shankar V, Richter A, et al. Proinflammatory and prothrombotic effects on human vascular endothelial cells of immune-cell-derived LIGHT. Eur J Med Res 2009;14:147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krötz F, Sohn HY, Gloe T, et al. NAD (P) H oxidase–dependent platelet superoxide anion release increases platelet recruitment NAD (P) H oxidase–dependent platelet superoxide anion release increases platelet recruitment. Blood 2002;100:917–924 [DOI] [PubMed] [Google Scholar]
- 36.Pignatelli P, Pulcinelli FM, Lenti L, Gazzaniga PP, Violi F. Hydrogen peroxide is involved in collagen-induced platelet activation. Blood 1998;91:484–490 [PubMed] [Google Scholar]
- 37.Karamouzis I, Berardelli R, D'Angelo V, et al. Enhanced oxidative stress and platelet activation in patients with Cushing's syndrome. Clin Endocrinol (Oxf) 2015;82:517–524 [DOI] [PubMed] [Google Scholar]
- 38.Minuz P, Patrignani P, Gaino S, et al. Increased oxidative stress and platelet activation in patients with hypertension and renovascular disease. Circulation 2002;106:2800–2805 [DOI] [PubMed] [Google Scholar]