Abstract
BACKGROUND: Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death globally. Mechanistic target of rapamycin (mTOR) is frequently up-regulated in HCC and plays an important role in HCC tumorigenesis. Tumors with loss of tuberous sclerosis complex 2 (TSC2), a negative regulator of mTOR signaling, tend to respond well to mTOR inhibitors. We analyzed TSC2 expression status in Korean patients with HCC and evaluated the correlation between TSC2 loss and response to the mTOR inhibitor, everolimus. METHODS: We retrospectively assessed 36 patients with advanced HCC who had received sorafenib at a single center in Korea between 2008 and 2014, and for whom tumor specimens were available for TSC2 immunohistochemical analysis (IHC). Three patient-derived tumor cell lines (PDCs) were analyzed by western blotting to determine TSC2 expression and drug sensitivity to mTOR. RESULTS: Twelve of 36 patients (33.3%) showed low to undetectable levels of TSC2 expression. No significant differences were observed in progression-free survival (PFS) or overall survival with sorafenib treatment based on TSC2 expression status. Two patients were treated with everolimus after sorafenib failure; one patient, with moderate TSC2 expression, experienced stable disease with a PFS of 5.8 months; the other, with high TSC2 expression, experienced rapid progression. PDC models demonstrated that the TSC2-low HCC PDC line was significantly more sensitive to everolimus than the TSC2-high HCC PDC lines. CONCLUSION: Loss of TSC2 may predict improved response to everolimus in HCC patients, but further studies are needed to confirm the predictive role of TSC2 expression for everolimus treatment.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the second leading cause of cancer-related deaths in the world [1]. The majority of patients diagnosed with HCC have advanced disease, and many are not eligible for potentially curative therapies, such as surgical therapies and loco-regional procedures [2]. Previous studies evaluating cytotoxic chemotherapy for the treatment of patients with advanced HCC failed to demonstrate a significant improvement in overall survival [3]. Recently, sorafenib, an oral multi-targeted tyrosine kinase inhibitor, has been shown to produce a significant improvement in overall survival in two phase III trials for the treatment of patients with HCC, establishing sorafenib as the only standard systemic treatment in advanced HCC [4], [5]. However, the benefits of sorafenib are mostly transient and modest, and there remains an unmet need for more effective novel therapies to improve the poor survival outcomes of treatment for advanced HCC.
The mammalian target of rapamycin (mTOR), which is regulated by the PI3K/Akt signaling pathway, is a key regulator of growth and proliferation of tumor cells [6]. Up-regulation of mTOR signaling has been reported in approximately 40% to 60% of patients with HCC, and is associated with early recurrence and poor prognosis [7], [8]. Everolimus, a rapamycin analog, inhibits the mTOR pathway and blocks tumor growth in xenograft models of human HCC [8]. However, treatment with everolimus in advanced HCC patients for whom sorafenib failed has shown no significant improvement in overall survival in a large, randomized, placebo-controlled phase III clinical trial (EVOLVE-1) [9].
An important inhibitor of mTOR activity is the tuberous sclerosis complex (TSC), which is composed of TSC1 and TSC2 [10]. Growth factor regulation of mTOR occurs largely through regulation of the GTPase activating protein (GAP) activity of the TSC1/TSC2 protein complex for the Ras family member Rheb [11]. Phosphorylation of TSC2 by Akt, or other kinases that inactivate TSC2, activates its downstream target Rheb, which stimulates phosphorylation and activation of the mTOR complex [12]. Everolimus is an effective treatment for TSC manifestations, a rare disease associated with mutations in TSC1 and TSC2 that result in high mTOR activity [13]. A recent retrospective study reported that patients with loss of TSC2 tended to respond better to everolimus, which suggests that TSC2 status could predict a selective response to everolimus [14].
Based on these findings, we aimed to analyze TSC2 expression status in Korean patients with advanced HCC and to evaluate the correlation between TSC2 expression status and the response of the mTOR inhibitor, everolimus. Furthermore, we examined the antitumor activity of everolimus based on TSC2 expression status through patient-derived tumor cell (PDC) models.
Material and Methods
Patient Selection
We collected and reviewed the medical records of 36 patients with advanced or metastatic HCC who were treated with sorafenib between 2008 and 2014 at a single center in Korea. Patients with histologically diagnosed HCC, whose tumor specimens were available for immunohistochemical (IHC) staining of TSC2 expression were eligible for the study. Clinical information including age, sex, etiology, Eastern Cooperative Oncology Group (ECOG) performance status, Barcelona Clinic Liver Cancer (BCLC) staging system, Child-Pugh class, macroscopic vascular invasion, extrahepatic spread, alpha-fetoprotein (AFP), and previous treatment were extracted from hospital records. This study was approved by the institutional review board of the Samsung Medical Center.
TSC2 Immunohistochemical Analysis
Formalin-fixed paraffin-embedded tissue including both HCC and adjacent non-tumorous liver were sectioned with 4 μm thickness. IHC study was performed using Bond-Max auto-immunostainer (Leica Biosyatem, Melbourne, Australia). Antigen retrieval was performed with ERI buffer at pH 6.0 for 20 minutes. The sections were incubated with rabbit monoclonal antibody to TSC2 (#4308, 1:200; Cell Signaling technology) for 15 minutes at 37 °C. Bond Polymer Refine Detection kit (Leica) was used for chromogenic reaction. No immunoreactivity was observed in tissue sections used as negative control in which primary antibody was replaced by isotype-matched irrelevant antibody. Positive control (human normal kidney) showed cytoplasmic TSC2 expression in epithelial cells of convoluted tubules.
IHC staining was assessed by a pathologist (C.K. Park) without knowledge of the patients' characteristics. The sections were scored by combining the proportion and intensity of the stained cells as reported previously [14]. The proportion of stained tumor cells was determined semiquantitatively and the staining intensity was classified as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The histo-score (H-score) of each tumor was calculated by multiplication of the proportion of stained cells and the staining intensity [14].
Cell Culture
HCC cells were obtained from pericardial effusions and ascites drained for therapeutic purposes after obtaining written informed consent. The protocol was approved by the Institutional Review Board (IRB) at the Samsung Medical Center. The cells were cultured in RPMI 1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS).
Cell Viability Assay
After pathologic confirmation, cells were seeded at 5 × 103 cells on 96-well plates and incubated for 24 hours at 37°C. Cells were then treated with different doses of everolimus (3-fold serial dilutions starting at 10 μM/ml) and incubated for 5 days at 37°C. Cell growth inhibition was determined using a CellTiter-Glo Luminescent Cell Viability assay (Promega, Madison, WI, USA) according to the manufacturer's protocol. The absorbance value of each well was measured with a microplate reader set at 490 nm. All experiments were performed in triplicate.
Western Blot Analyses
HCC cells were cultured in RPMI 1640 medium with 10% FBS at 37°C. Cells were seeded at a concentration of 1 × 106 cells per 100-mm dish and incubated at 37°C for 24 hours. Cells were then incubated with the drug (1 μM everolimus) in RPMI 1640 medium with 10% FBS at 37°C for an additional 3 days. Total cells were lysed in lysis buffer containing 20 mM HEPES (pH 7.4), 150 mM NaCl, 1 mM MgCl2, 1 mM EDTA, 2 mM EGTA, 10% glycerol, 1% Triton X-100, 1 μg/ml of leupeptin, and 1 μg/ml of aprotinin. Equal amounts (30 μg) of cell lysates were dissolved in 8% Bis-Tris gels with MOPS running buffer (Invitrogen, Carlsbad, CA, USA), transferred onto a nitrocellulose membrane and incubated overnight at 4 °C with the following primary specific antibodies: rabbit anti-human Tuberin/TSC2 (D93F12) Ab (1:1000 dilution), rabbit anti-human phosphorylated mTOR (Ser2448) Ab (1:700 dilution), rabbit anti-human mTOR Ab (1:700 dilution) from Cell Signaling Technologies (Beverly, MA, USA), and b-tubulin (1:5000 dilution) from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Membranes were incubated with HRP-conjugated anti-rabbit or anti-mouse secondary antibodies (1:3000 dilution) for 3 hours and bands were visualized using the ECL Western Blotting Detection System (GE Healthcare, Buckinghamshire, UK).
Statistical Analysis
Progression-free survival (PFS) and overall survival was calculated using the Kaplan-Meier method and compared using the log-rank test. P < .05 was considered statistically significant.
Results
TSC2 IHC Expression Status and Survival Outcomes
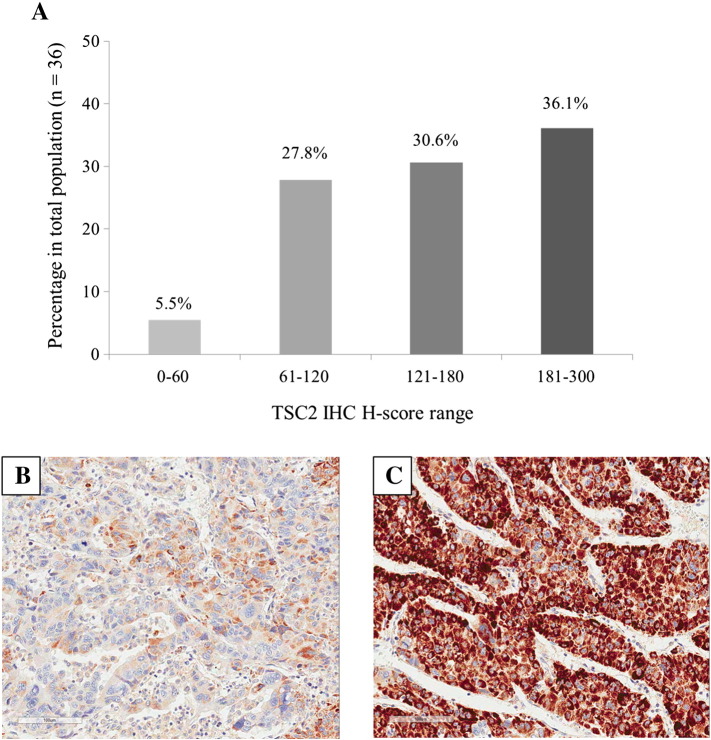
We retrospectively assessed 36 patients with advanced HCC who had tumor specimens available for TSC2 immunohistochemical analysis. Most patients did not have macroscopic vascular invasion (75%), and had Barcelona Clinic Liver Cancer stage C disease (94.4%). The most common etiology of HCC was hepatitis B virus (HBV) and the disease was rated as Child-Pugh class A in 34 (94.4%) patients. All patients had received sorafenib and the median PFS of sorafenib treatment was 2.5 months. Tumor specimens for TSC2 IHC analysis were obtained from liver (66.7%), lung (19.4%) and other metastatic sites (13.9%). The median time interval between acquisition of histologic specimen for TSC2 IHC analysis and starting sorafenib treatment was 11.7 months. Immunoreactivity for TSC2 was observed in the cytoplasm of tumor cells. Of the 36 patients, two had minimal TSC2 IHC expression (TSC2 H-score = 0-60), 10 had low TSC2 IHC expression (TSC2 H-score = 61-120), 11 had moderate TSC2 IHC expression (TSC2 H-score = 121-180), and 13 had high TSC2 IHC expression (TSC2 H-score = 181-300) (Figure 1, A and B). Based on previous research, in which TSC2 immunostaining scores from 0 to 120 were defined as TSC2 null/low [14], 12 (33.3%) patients were considered TSC2 null/low. There were no significant differences in median PFS or overall survival (OS) with sorafenib treatment between TSC2 null/low patients and the others (2.5 months vs. 2.0 months, P = .054; 15.5 months vs. 14.1 months, P = .348, respectively). Patients' baseline characteristics and survival outcomes following sorafenib treatment are described in Table 1.
Figure 1.
TSC2 IHC histo-score (H-score) range distribution in 36 hepatocellular carcinomas and two examples of IHC staining for TSC2 in hepatocellular carcinoma. (A) H-scores were divided into four groups: 0 to 60 (minimal), 61 to 120 (low), 121 to 180 (moderate), and 181 to 300 (high). The percentage of each range is marked at the top of the bars. (B and C) Immunostaining of TSC2 show low cytoplasmic expression (B) and high cytoplasmic expression (C) in hepatocellular carcinoma (×200).
Table 1.
Patients Baseline Characteristics and Survival Outcomes of Sorafenib Treatment Based on TSC2 IHC Status
| Variables | TSC2-Null/Low⁎ (N = 12) | TSC2-Moderate/High (N = 24) | Total (N = 36) |
|---|---|---|---|
| Median age, years (range) | 61 (43-75) | 57 (35-85) | 57 (35-85) |
| Gender, n (%) | |||
| Male | 9 (75.0) | 34 (100.0) | 33 (91.7) |
| Female | 3 (25.0) | 0 (0) | 3 (8.3) |
| Cause of disease, n (%) | |||
| Hepatitis B | 10 (83.3) | 18 (75.0) | 28 (77.8) |
| Hepatitis C | 0 | 1 (4.2) | 1 (2.8) |
| Unknown | 2 (16.7) | 5 (20.8) | 7 (19.4) |
| ECOG performance status, n (%) | |||
| 0 | 7 (58.3) | 15 (62.5) | 22 (61.1) |
| 1 | 5 (41.7) | 9 (37.5) | 14 (38.9) |
| BCLC stage | |||
| B (intermediate) | 0 | 2 (8.3) | 2 (5.6) |
| C (advanced) | 12 (100.0) | 22 (91.7) | 34 (94.4) |
| Macroscopic vascular invasion, n (%) | 3 (25.0) | 6 (25.0) | 9 (25.0) |
| Extrahepatic spread, n (%) | 11 (91.7) | 21 (87.5) | 32 (88.9) |
| Lung | 9 (75.0) | 13 (79.2) | 22 (61.1) |
| Lymph node | 1 (8.3) | 5 (20.8) | 6 (16.7) |
| Child-Pugh class | |||
| A | 11 (91.7) | 23 (95.8) | 34 (94.4) |
| B | 1 (8.3) | 1 (4.2) | 2 (5.6) |
| AFP ≥ 200 ng/mL, n (%) | 5 (41.7) | 6 (25.0) | 11 (30.6) |
| Previous therapy | |||
| Liver resection | 9 (75.0) | 22 (91.7) | 31 (86.1) |
| Locoregional therapy | |||
| Transarterial chemoembolization | 7 (58.3) | 21 (87.5) | 28 (77.8) |
| Radiofrequency ablation | 4 (33.3) | 12 (50.0) | 16 (44.4) |
| Radiotherapy | 10 (83.3) | 8 (33.3) | 18 (50) |
| Metasectomy | 5 (41.7) | 8 (33.3) | 13 (36.1) |
| Systemic chemotherapy | 3 (25.0) | 2 (8.3) | 5 (13.9) |
| Site of tumor specimen acquisition | |||
| Liver | 8 (66.7) | 16 (66.7) | 24 (66.7) |
| Lung | 2 (16.7) | 5 (20.8) | 7 (19.4) |
| Other metastatic lesion | 2 (16.7) | 3 (12.5) | 5 (13.9) |
| Interval from specimen acquisition to starting sorafenib treatment, months | 8.6 | 11.7 | 11.7 |
| Progression free survival, months | 2.5 | 2.0 | 2.5 |
| Overall survival, months | 15.5 | 14.1 | 14.1 |
ECOG, Eastern Cooperative Oncology Group; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein.
TSC2 IHC scores ranging from 0 to 120 were defined as TSC2 null/low.
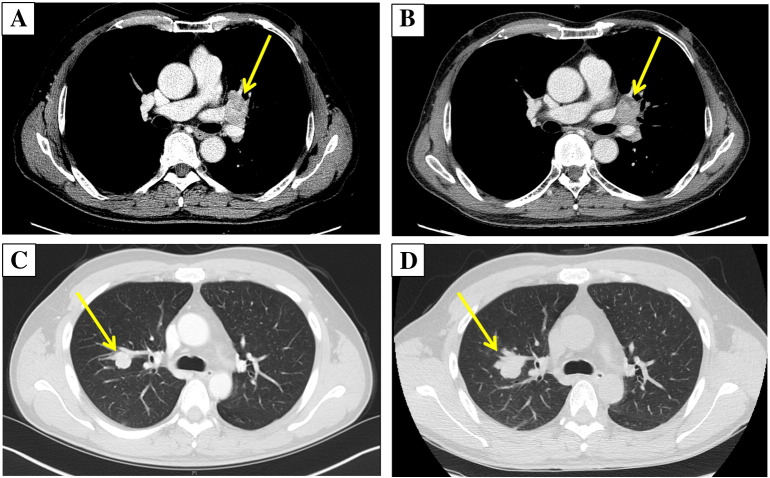
Two (5.6%) patients received everolimus after sorafenib failure. The TSC2-high patient (TSC2 H-score = 230) experienced a shorter PFS of 2.1 months compared with the TSC2-moderate patient (TSC2 H-score = 150), whose PFS was 5.8 months (Table 2). Figure 2 illustrates the computed tomography (CT) scan images at baseline and following two cycles of everolimus treatment for these two patients. The TSC2-moderate HCC patient with lung and brain metastasis experience stable disease after two cycles of everolimus treatment, whereas the TSC2-high HCC patient with lung metastasis experienced rapid progression of the lung metastasis.
Table 2.
Patients Profile and Survival Outcomes of Two HCC Patients with Everolimus Treatment Based on TSC2 IHC Expression
| Patient ID | Sex | Age | TSC IHC | Best Response | PFS (months) | Previous Sorafenib Failure | Presence of Hepatitis Virus | Site of Metastasis |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 63 | 150 | Stable disease | 5.8 | Yes | HBV Carrier | Lung, brain |
| 2 | M | 50 | 230 | Progression | 2.1 | Yes | HBV Carrier | Lung |
Figure 2.
Comparison of response with everolimus treatment based on TSC2 IHC expression. (A and B) Two computed tomography (CT) scan images of a HCC patient with lung and brain metastasis whose tumor expressed moderate TSC2 IHC staining (patient ID 1) at baseline (A) and following two cycles of everolimus treatment (B). (C and D) Two computed tomography (CT) scan images of a HCC patient with lung metastasis whose tumor expressed high TSC2 IHC staining (patient ID 2) at baseline (C) and following two cycles of everolimus treatment (D).
TSC2 Loss and Efficacy of Everolimus in PDC Models
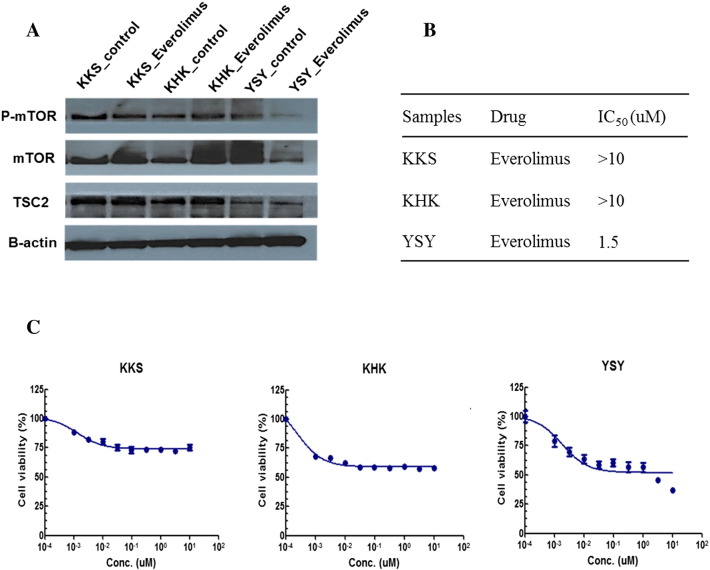
We had previously established three PDCs for testing the antitumor activity of various agents. In order to examine the effect of loss of TSC2 on the antitumor effect of everolimus, we performed western blot analyses of TSC2, mTOR, and phosphorylation of mTOR (p-mTOR). As shown in Figure 3A, one PDC (YSY) showed minimal detectable levels of TSC2 at baseline and significantly decreased levels of mTOR and p-mTOR after treatment with everolimus. In contrast, the other two PDCs (KKS and KHK) with wild-type TSC showed insignificant changes in mTOR and p-mTOR after the same treatment with everolimus.
Figure 3.
TSC2 loss in patient-derived HCC cell could predict everolimus efficacy. (A) Western blot analysis of three HCC cell lines with indicated antibodies. YSY cell line, expressed low TSC2 protein level, showed decreasing the phosphorylation of mTOR by everolimus. (B) Drug sensitivity to everolimus. IC50 values of KKS, KHK, and YSY cell were >10, >10, and 1.5 μM, respectively. (C) Graphs of everolimus IC50 values of three HCC cells.
The half maximal inhibitory concentration (IC50) is a measure of the effectiveness of a substance in inhibiting a specific biological or biochemical function. As shown in Figure 3, B and C, everolimus exhibited promising antitumor activity in the TSC2-low PDC (YSY), with an IC50 value of 1.5 μM/ml compared with the two TSC2-wild PDCs (KKS and KHK), which showed IC50 values above 10 μM/ml.
Discussion
HCC is highly resistant to chemotherapeutic agents, and few treatment options are available for advanced disease. Although large-scale randomized clinical trials have proven that sorafenib improves the probability of survival in patients with advanced HCC [4], [5], no other targeted agents have demonstrated survival benefits against HCC. The PI3K/Akt/mTOR pathway plays a critical role in carcinogenesis [15] and mTOR is an important mediator of the PI3K-Akt pathway, which acts as a central regulator of cell growth and proliferation and progression from G1 to S phase. Despite the high frequency of mTOR activation in HCC, the EVOLVE-1 clinical trial to assess the efficacy of the mTOR inhibitor everolimus found no associated improvement in overall survival [9]. Thus, predictive markers are needed to maximize the efficacy of everolimus in a clinical setting. Hyunh et al. [14] suggested that loss of TSC2 could predict a selective response to everolimus. In that study, which defined TSC2 H-scores of 60 and 120 as the cutoff values for TSC2-null and TSC2-low tumors, respectively, the frequency of TSC2-null or -low IHC expression was higher in Asian patients than in Caucasian patients, up to approximately 40% of 239 patients. Similarly, the frequency of TSC2-null or -low IHC expression in our study was 33.3% of 36 HCC patients. No significant difference between TSC2 expression status and survival outcome was observed with sorafenib treatment, and patients with high TSC2 IHC expression seemed to show a poor response to everolimus.
Tumor heterogeneity can make treatment responses difficult to predict but understanding the molecular basis of tumorigenesis can help to identify treatment targets. TSC1 and TSC2 were identified in 1997 and 1993, respectively, as the genetic loci mutated in the autosomal dominant tumor syndrome TSC [16], [17]. TSC1 and TSC2 act as tumor suppressors in various cancers [18], [19], and several studies have suggested that inactivating mutations in TSC1/2 might be a potent predictive biomarker of potential response to mTOR inhibitors [14], [20], [21], [22]. Hyunh et al. reported that the loss of TSC2, as determined by immunoblot analysis, was associated with increased mTOR activity and decreased Akt phosphorylation [14]. Furthermore, TSC2-null cell lines showed enhanced sensitivity, with greater inhibition of cell proliferation to everolimus than TSC2-wild type cell lines in a patient-derived HCC xenograft study, consistent with the results of our PDC study. PDCs are in vitro cell models generated from freshly resected patient tumors or malignant body fluids that preserve the histologic and genomic features of primary tumor cells [23]. PDC models have been suggested to be promising models for preclinical experiments and to closely resemble the patient tumor genome and clinical response. In our study, everolimus exhibited potent antitumor activity in TSC2-low PDCs compared with TSC2-wild type PDCs.
Because inactivating mutations in TSC1/TSC2 have been identified at low frequency in various common forms of cancer through The Cancer Genome Atlas (TCGA) program [24] and because sequencing to identify mutations cannot always predict protein loss, IHC analysis is a useful approach for identifying the functional status of TSC2. Non-genetic mechanisms of TSC2 loss may result in a relatively higher frequency of TSC2 null/low IHC expression compared with TSC1/2 inactivating mutations. Promoter methylation, phosphorylation, and acetylation might be responsible for silencing of TSC2[25]. TSC2 null or low expression is common in Asian HCC patients, with a frequency of approximately one third. These findings suggest that TSC2 loss might be a potential predictive biomarker for the response to mTOR inhibitors in Asian patients with HCC. However, lack of a clear cutoff value for TSC2 loss limits the validation of TSC2 expression as a predictor of mTOR inhibitors. Standardization of immunostaining and scoring methods for TSC2 expression should be established to improve the utility of TSC2 expression as a predictive biomarker.
In conclusion, although this study has some limitations, such as the retrospective nature of the analysis and the small number of patients, the mTOR inhibitor everolimus exhibited promising antitumor activity in TSC2 null/low HCC patients. Further large prospective studies are needed to identify whether TSC2 loss can serve as a predictive marker for everolimus treatment.
Acknowledgements
This work was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C2750, HI14C3418). Support was also provided by a grant from the 20 by 20 project of Samsung Medical Center (GF01140111). The funders had no role in the design and conduct of the study.
Footnotes
This work was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C2750, HI14C3418). Support was also provided by a grant from the 20 by 20 project of Samsung Medical Center (GF01140111). The funders had no role in the design and conduct of the study.
Contributor Information
Cheol-Keun Park, Email: ckpark@skku.edu.
Ho Yeong Lim, Email: hoylim@skku.edu.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 3.Yeo W, Mok TS, Zee B, Leung TW, Lai PB, Lau WY, Koh J, Mo FK, Yu SC, Chan AT. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532–1538. doi: 10.1093/jnci/dji315. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 6.Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 7.Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, Tovar V, Roayaie S, Minguez B, Sole M. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972–1983. doi: 10.1053/j.gastro.2008.08.008. [1983.e1971–1911] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou L, Huang Y, Li J, Wang Z. The mTOR pathway is associated with the poor prognosis of human hepatocellular carcinoma. Med Oncol. 2010;27:255–261. doi: 10.1007/s12032-009-9201-4. [DOI] [PubMed] [Google Scholar]
- 9.Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, Poon RT, Blanc JF, Vogel A, Chen CL. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312:57–67. doi: 10.1001/jama.2014.7189. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–190. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 12.Demetriades C, Plescher M, Teleman AA. Lysosomal recruitment of TSC2 is a universal response to cellular stress. Nat Commun. 2016;7:10662. doi: 10.1038/ncomms10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curatolo P, Bjornvold M, Dill PE, Ferreira JC, Feucht M, Hertzberg C, Jansen A, Jozwiak S, Kingswood JC, Kotulska K. The Role of mTOR Inhibitors in the Treatment of Patients with Tuberous Sclerosis Complex: Evidence-based and Expert Opinions. Drugs. 2016;76:551–565. doi: 10.1007/s40265-016-0552-9. [DOI] [PubMed] [Google Scholar]
- 14.Huynh H, Hao HX, Chan SL, Chen D, Ong R, Soo KC, Pochanard P, Yang D, Ruddy D, Liu M. Loss of Tuberous Sclerosis Complex 2 (TSC2) Is Frequent in Hepatocellular Carcinoma and Predicts Response to mTORC1 Inhibitor Everolimus. Mol Cancer Ther. 2015;14:1224–1235. doi: 10.1158/1535-7163.MCT-14-0768. [DOI] [PubMed] [Google Scholar]
- 15.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 16.European Chromosome 16 Tuberous Sclerosis Consortium Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–1315. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 17.van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den Ouweland A, Halley D, Young J. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–808. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 18.Chakraborty S, Mohiyuddin SM, Gopinath KS, Kumar A. Involvement of TSC genes and differential expression of other members of the mTOR signaling pathway in oral squamous cell carcinoma. BMC Cancer. 2008;8:163. doi: 10.1186/1471-2407-8-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knowles MA, Hornigold N, Pitt E. Tuberous sclerosis complex (TSC) gene involvement in sporadic tumours. Biochem Soc Trans. 2003;31:597–602. doi: 10.1042/bst0310597. [DOI] [PubMed] [Google Scholar]
- 20.Kwiatkowski DJ, Choueiri TK, Fay AP, Rini BI, Thorner AR, de Velasco G, Tyburczy ME, Hamieh L, Albiges L, Agarwal N. Mutations in TSC1, TSC2, and MTOR Are Associated with Response to Rapalogs in Patients with Metastatic Renal Cell Carcinoma. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagle N, Grabiner BC, Van Allen EM, Amin-Mansour A, Taylor-Weiner A, Rosenberg M, Gray N, Barletta JA, Guo Y, Swanson SJ. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N Engl J Med. 2014;371:1426–1433. doi: 10.1056/NEJMoa1403352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voss MH, Hakimi AA, Pham CG, Brannon AR, Chen YB, Cunha LF, Akin O, Liu H, Takeda S, Scott SN. Tumor genetic analyses of patients with metastatic renal cell carcinoma and extended benefit from mTOR inhibitor therapy. Clin Cancer Res. 2014;20:1955–1964. doi: 10.1158/1078-0432.CCR-13-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JY, Kim SY, Park C, Kim NK, Jang J, Park K, Yi JH, Hong M, Ahn T, Rath O. Patient-derived cell models as preclinical tools for genome-directed targeted therapy. Oncotarget. 2015;6:25619–25630. doi: 10.18632/oncotarget.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, Meyerson M, Gabriel SB, Lander ES, Getz G. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang WG, Sampson J, Martin TA, Lee-Jones L, Watkins G, Douglas-Jones A, Mokbel K, Mansel RE. Tuberin and hamartin are aberrantly expressed and linked to clinical outcome in human breast cancer: the role of promoter methylation of TSC genes. Eur J Cancer. 2005;41:1628–1636. doi: 10.1016/j.ejca.2005.03.023. [DOI] [PubMed] [Google Scholar]