Abstract
The Phosphatidyl inositol-3 kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) and c-Met signaling pathways are often deregulated in cancer. The two pathways are interconnected and at least c-Met has been implicated in drug resistance. The aim of the study was to assess in ovarian cancer preclinical models, the efficacy and tolerability of a dual PI3K mTOR inhibitor (PF-05212384 or gedatolisib) and a c-Met inhibitor (crizotinib) either as single agents or in combination. In vitro, both PF-05212384 and crizotinib showed a concentration dependent activity in the two ovarian cancer cell lines. The combination of the two did not result in synergistic activity. A subline resistant to gedatolisib was obtained and showed an increased expression of MDR-1 gene. In vivo results show that crizotinib alone did not display any activity in all the tumors investigated, while PF-05212384 alone had some marginal activity. The combination of the two resulted in all the experiments superior to single agents with a good tolerability. Considering that crizotinib did not show activity in the models used, the results indicate that crizotinib is able to potentiate the activity of PF-05212384. Although the activity of the combination was not striking in these three models of ovarian cancer, due to the good tolerability of the combination, the results would suggest the possibility to combine the two drugs in settings in which gedatolisib or crizotinib alone have already some significant activity.
Introduction
The PI3K/Akt/mTOR pathway is among the most frequently altered signaling network in human cancers [1], [2], [3]. It is involved in several physiological and pathological processes, including cancer, thus making it an attractive pathway to target.
The PI3Ks are members of a conserved family of lipid kinases which are grouped into three classes, I (the most studied in cancer), II and III, according to their substrate preference and sequence homology [4], [5]. Activation of PI3K leads to the activation of several proteins, among which Akt plays a central role. Activated Akt is in turn able to phosphorylate many target proteins and regulate many cellular functions. The main consequences of Akt activation, relevant to cancer, are cell survival, proliferation and growth. Among the downstream effectors, an important role is played by the mTOR. mTOR is present in two distinct complexes inside the cells, a rapamycin and nutrient-sensitive multiprotein complex (mTORC1) and a growth factor sensitive but nutrient insensitive and rapamycin insensitive complex (mTORC2). Interestingly mTOR, when assembled in the mTORC2 complex, is able to phosphorylate Akt, thus suggesting that mTOR can function either upstream or downstream to Akt [6], [7], [8], [9].
Several molecules are being tested for their ability to inhibit the PI3K/Akt/mTOR pathway. There are available molecules with high specificity for specific isoforms of PI3K, molecules with pan inhibitory activity, as well as dual inhibitors able to simultaneously block the activity of two members of the same pathway. Several inhibitors of PI3K/Akt/mTOR pathway are now under preclinical and clinical development and some of them have been clinically approved in selected malignancies, as is the case of the mTOR inhibitors everolimus and temsirolimus for pancreatic neuroendocrine tumors and renal cell carcinoma [10], [11], of the delta isoform specific PI3K inhibitor idelalisib for relapsed chronic lymphocytic leukemia and indolent lymphomas [12], [13].
Despite the strong efficacy of the inhibitors in the pharmacological modulation of the PI3K/Akt/mTOR pathway at preclinical level, their therapeutic clinical efficacy has been well below the expectancies. This has been ascribed to several factors: their lack of specificity, which does not cause an effective inhibition because of off-targets effects limiting the dosages, the existence of feedback loops, as is for example the case of Akt activation following mTORC1 inhibition by rapalogues, and finally the development of resistance [14], [15], [16].
Finally the PI3K/Akt/mTOR pathway is interconnected to different other receptor-mediated signaling which can work together to sustain the growth of cancer cells. Among these, c-Met receptor has been shown to activate PI3K/Akt/mTOR pathway [17], [18] and to play an important role in drug resistance [19]. In this context we assessed in vivo, in ovarian cancer xenografts and patient-derived xenograft (PDX) with altered PI3K/Akt/mTOR pathway, the activity of the dual PI3K-mTOR inhibitor PF-05212384 (gedatolisib) and crizotinib, a widely used c-Met inhibitor, as single agents or in combination, with the idea that these compounds could synergize, acting at different levels on the same pathway highly deregulated in cancer.
Material and Methods
Cell Cultures and Drugs
The human ovarian cancer cell lines A2780 (with deletion in PTEN gene) and SKOV3 (harboring H1047R mutation in PIK3CA gene) were grown in vitro in RPMI1640 supplemented with 10% FBS.
Crizotinib and PF-05212384 were obtained from Pfizer and solutions prepared as recommended.
In Vitro Cytotoxicity Assays
The growth inhibitory activity of crizotinib and PF-05212384 was determined in vitro by using the MTS test. Cells were seeded in 96 wells plates and after 24 hours treated with increasing concentrations of the drugs for further 72 hours. Survival curves were plotted as percentages of untreated controls, consisted of at least six replicates for each time point and represented the average mean and SD of at least three independent experiments.
Real-Time PCR
RNA was extracted by using Maxwell 16LEV simplyRNA Cells kit (Promega). RNA was retro-transcribed to cDNA using High Capacity cDNA Reverse Transcription Kit (Life Technologies). Differences in MDR-1 gene expression were determined by real-time RT-PCR performed with Sybr Green PCR master mix (Promega) and the dissociation curve was evaluated for each gene. Samples were then normalized using the expression of the housekeeping gene (actin) and their levels were compared to control samples. Real-time PCR was done using the 7900HT Sequence Detection System (Applied Biosystems).
The c-Met gene copy number was assessed using the TaqMan Copy Number Assay (Applied Biosystems) with the ABI 7900, Applied Biosystems. hTERT copy number was used as reference gene.
Animals and In Vivo Activity
Female NCr-nu/nu mice (6 weeks old) were obtained from ENVIGO RMS srl (Correzzana, Italy). They were maintained under specific pathogen free conditions, housed in isolated vented cages and handled using aseptic procedures. The IRCCS-Istituto di Ricerche Farmacologiche Mario Negri adheres to the principles set out in the following laws, regulations, and policies governing the care and use of laboratory animals: Italian Governing Law (D. lg 26/2014; Authorization n.19/2008-A issued March 6, 2008 by Ministry of Health); Mario Negri Institutional Regulations and Policies providing internal authorization for persons conducting animal experiments (Quality Management System Certificate- UNI EN ISO 9001:2008 – Reg, No.6121); the NIH Guide for the Care and Use of Laboratory Animals (2011 edition) and EU directives and guidelines (EEC Council Directive 2010/63/UE). The Statement of Compliance (Assurance) with the Public Health Service (PHS) Policy on Human Care and Use of Laboratory Animals was recently reviewed (9/9/2014) and will expire on September 30, 2019 (Animal Welfare Assurance #A5023–01).
Human A2780 and SKOV-3 cells (5x106 cells/mouse) were injected subcutaneously (sc) in immunodeficient mice. The human ovarian PDX MNHOC218 [20], [21], containing three copies of the c-Met gene, was implanted sc as viable tumor fragments (2x2 mm) through trocar needles.
When the tumor reached approximately 100–150 mm3, they were treated with PF-05212384 10 mg/kg intravenous (iv) Q4dx4, crizotinib 50 mg/kg per os (po) every day or with the combination of the two drugs.
Tumor growth was measured with a Vernier caliper every 2 to 3 days and tumor weights (mg = mm3) were calculated using the formula: (length [mm]*width [mm]2)/2. The efficacy of the treatment was expressed as best tumor growth inhibition [%T/C = (mean tumor weight of treated tumors/mean tumor weight of control tumors)*100]. For all the experiments, a T/C <42% is considered the minimum level for activity [22].
Western Blotting Analysis
Total proteins were purified from snap frozen tumors after homogenation in 50 mM Tris HCl pH 7.4, 250 mM NaCl, 0.1% Nonidet NP-40, 0.5 mM EDTA and NaF 50 mM, in the presence of aprotinin, leupeptine and phenyl-methyl-sulfonyl-fluoride (PMSF) as proteases inhibitors (ratio 1: 1 w/v). Lysates were cleared by centrifuging at 13,000×g for 20 min and protein concentration was determined using a Biorad assay kit (BioRad, Italy). Around 30 μg of total protein extracts were loaded on 12% SDS-PAGE and then transferred to a PVDF membrane. Primary antibodies anti phopho-Akt (Ser473), p-Akt (Thr308), Akt, p-4EBP1 (Thr37/46), 4EBP1, p-S6 (Ser235/236) and S6 were purchased from Cell Signaling Technology; anti p-ERK (Tyr204) and ERK were purchased from Santa Cruz Biotechnology. Antibody binding was revealed by peroxidase labeled secondary antibodies and visualized using enhanced chemioluminescence (Amersham, Italy).
Statistical Analyses
The statistical analyses were performed using Graphpad Prism version 6. Specific tests used to analyze specific experiments are indicated in the legends coupled to the figures. Differences between groups were considered statistically significant when the P ≤ .05.
Results
In Vitro Activity of Crizotinib and PF-05212384
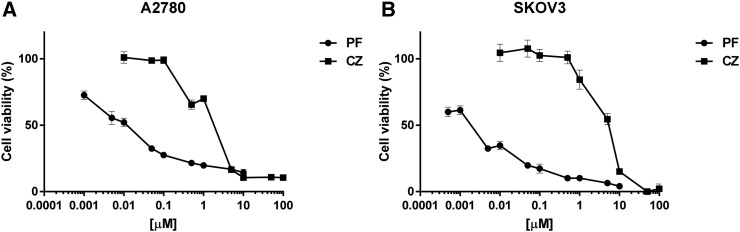
The in vitro inhibitory activity of crizotinib and PF-05212384 in A2780 and SKOV3 cells is reported in Figure 1 panels A and B, respectively. For both drugs, a concentration dependent activity was found, with SKOV3 cells slightly more sensitive to PF-05212384 treatment and A2780 cells slightly more sensitive to crizotinib.
Figure 1.
In vitro activity of PF-05212384 and crizotinib as single agents. Response of A2780 (A) and SKOV3 (B) cell lines treated with increasing concentrations of PF-05212384 (PF) and crizotinib (CZ) for 72 h, detected by MTS assay. The average of 3 independent experiments and SD are shown.
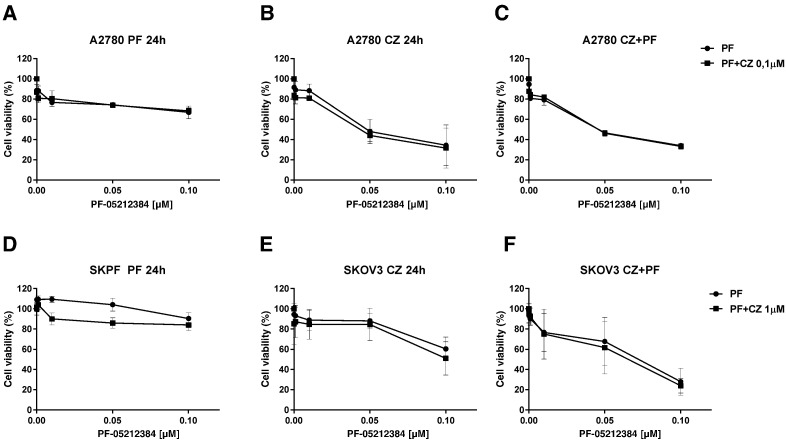
The combination of PF-05212384 and crizotinib was tested in vitro using drugs concentrations non-cytotoxic as single treatments. This did not result in a greater response in both A2780 and SKOV3 cell lines (Figure 2). The effect observed was, in fact, ascribable to PF-05212384 treatment, as similar results were obtained in response to either the single-drug or to the combined treatments. This finding was strengthened by the use of a different treatment schedule. Indeed, to verify whether the effect observed was dependent on the experiment schedule, cells were treated for 24 hours with one of the two drugs and for the remaining 48 hours with the other one. After 24 hours of treatment with PF-05212384 (Figure 2, panels A and D), cells did not respond significantly to either the drug singly or to the combination. When crizotinib was followed after 24 hours by PF-05212384 treatment (Figure 2, panels B and E), a similar concentration-dependent growth inhibition was observed in PF-05212384-treated cells and in cells treated with the combination, thus indicating that crizotinib was almost inactive. These results were in agreement with those obtained after the simultaneous treatment with the two drugs for 72 hours, which, however, was slightly more effective (Figure 2, panels C and F).
Figure 2.
In vitro activity of the combination of PF-05212384 and crizotinib. Response of A2780 and SKOV3 cells treated with PF-05212384 as single agent or in combination with crizotinib, detected by MTS assay. Cells were treated for 72 h with non cytotoxic concentrations of the two drugs using different schedules: PF-05212384 was administered for 24 h and followed by crizotinib for 48 h (A and D), crizotinib was followed after 24 h by PF-05212384 (B and E) or the two drugs were given simultaneously (C and F). The average of 3 independent experiments and SD are shown.
Characterization of SKOV3 Cell Line Resistant to PF-05212384
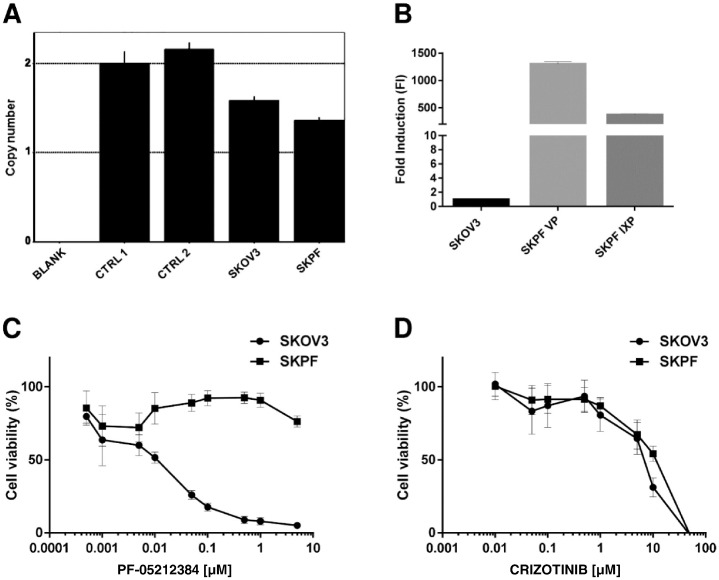
In order to get more insights into the response mechanisms to the combination, attempts were made to have a cell line resistant to PF-05212384 with amplification of c-Met gene. However, SKOV3 cells treated with PF-05212384 developed resistance with normal c-Met gene copies (Figure 3A), but with a very high expression of MDR-1 gene (Figure 3B). Figure 3 reports the activity of PF-05212384 in parental SKOV3 cells and in the resistant subline (SKPF). As it can be seen, the resistant cell line did not respond to the drug anymore (Figure 3, panel C and Table 1). In parallel we have evaluated the activity of crizotinib in SKOV3 and SKPF cells and the drug showed the same activity in both cell lines (Figure 3, panel D).
Figure 3.
Characterization of SKOV3 cell line resistant to PF-05212384. A. c-Met gene copy number in SKOV3 parental cell line and SKOV3 cell line resistant to PF-05212384 (SKPF), assessed using the TaqMan Copy Number Assay and using hTERT copy number as reference gene. B. Relative expression levels of MDR-1 in SKPF at two different culture passages. mRNA expression at basal conditions was assessed by Real-Time PCR and relative expression levels were calculated with ΔΔCt method, setting SKOV3 parental cell line as reference sample (=1). A-B. The histograms represent the average mean and SD of 3 technical replicates. C-D. In vitro activity of PF-05212384 (C) and crizotinib (D) in SKOV3 parental cell line and in SKPF. The average of 3 independent experiments and SD are shown.
Table 1.
Statistical Analysis of PF-05212384 Response in SKOV3 and SKPF, Reported in Figure 3C
| PF-05212384 [μM] |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.0005 | 0.001 | 0.005 | 0.01 | 0.05 | 0.1 | 0.5 | 1 | 5 | |
| SKOV3 vs. SKPF | ns | ns | ns | ** | **** | **** | **** | **** | **** |
The analysis was performed using two-way ANOVA test and Bonferroni post-test for multiple comparisons. ** P < .01, **** P < .0001.
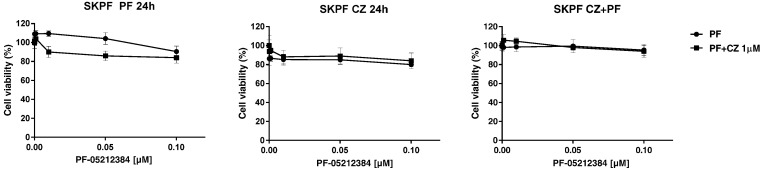
We next analyzed the cytotoxic response of SKPF to the combination of the two drugs (Figure 4). As already seen for the parental cell line, no differences were detected in response to either the single-drug or to the combined treatment. In fact, nor the single treatment with PF-05212384 or the combination were active on the resistant cell line, even when a different schedule was used. The combination was not able to restore SKPF sensitivity, confirming that the addition of crizotinib to PF-05212384 does not enhance the effect of the treatment.
Figure 4.
Dose–response curves of SKPF treated with the combination of PF-05212384 and crizotinib. Response of SKPF treated with increasing concentrations of PF-05212384 as single agent or in combination with a non toxic concentration of crizotinib, detected by MTS assay. Cells were treated for 24 h with one of the two drugs and for 48 h with the other one or the two drugs were given simultaneously for 72 h. The average of 3 independent experiments and SD are shown.
In Vivo Activity of Crizotinib and PF-05212384
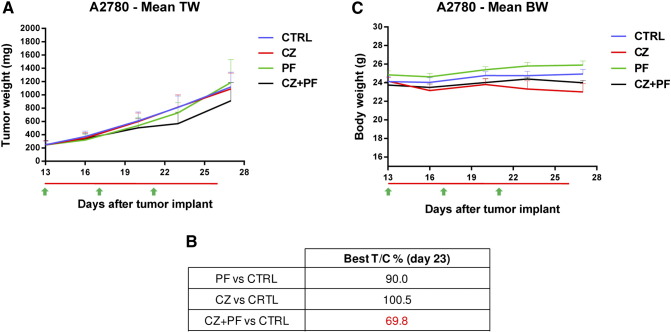
When tested in vivo, A2780-bearing mice showed a very limited activity of both crizotinib and PF-05212384 (Figure 5A). The combination of the two showed some activity. The T/Cx100 values were close to 100 for both drugs as single agent and reached roughly 70 for the combination (Figure 5B). The increased activity was not associated with increased toxicity, as judged by the body weight of the animals that was, for all the groups tested, similar to that of vehicle-treated mice (Figure 5C).
Figure 5.
Antitumor activity of PF-05212384, crizotinib or the combination in A2780 xenografts. A. Tumor growth inhibition activity of A2780 xenografts, treated with PF-05212384 10 mg/kg iv Q4dx4 (green arrows), crizotinib 50 mg/kg po every day (red line) or with the combination of the two drugs for 13 days. B. Percentage of tumor growth inhibition (T/C%) calculated for all the treatment groups. C. Body weight of A2780 bearing mice treated with PF-05212384, crizotinib or the combination. Means and SEM are shown. Statistical analysis was performed using two-way ANOVA test and Bonferroni post-test for multiple comparisons and no differences were detected.
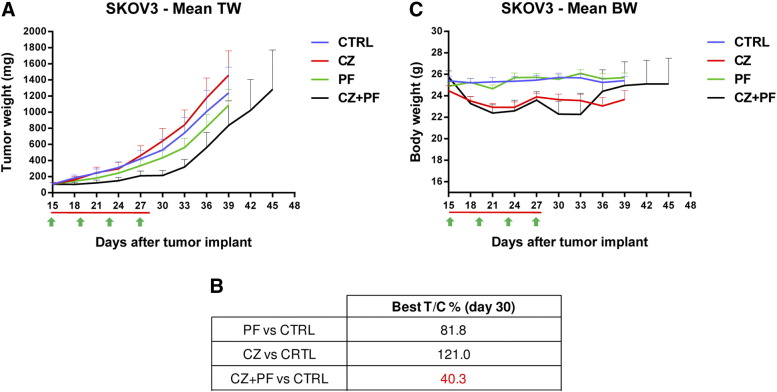
A similar picture was observed in SKOV3 xenografts (Figure 6A). In this case, in agreement with what observed in vitro, this tumor better responded to PF-05212384 with a T/Cx100 value of 82, while the growth of tumors treated with crizotinib was even faster than that of untreated mice. Interestingly the combination resulted active with a T/Cx100 of 40 (Figure 6B). Given that crizotinib was completely uneffective in this model, the combination seemed to have a higher antitumor activity. Again also in this model the combination was well tolerated with no differences in body weight decrease between combination and single agents (Figure 6C).
Figure 6.
Antitumor activity of PF-05212384, crizotinib or the combination in SKOV3 xenografts. A. Tumor growth inhibition activity of SKOV3 xenografts, treated with PF-05212384 10 mg/kg iv Q4dx4 (green arrows), crizotinib 50 mg/kg po every day (red line) or with the combination of the two drugs for 13 days. B. Percentage of tumor growth inhibition (T/C%) calculated for all the treatment groups. C. Body weight of SKOV3 bearing mice treated with PF-05212384, crizotinib or the combination. Means and SEM are shown. Statistical analysis was performed using two-way ANOVA test and Bonferroni post-test for multiple comparisons and no differences were detected.
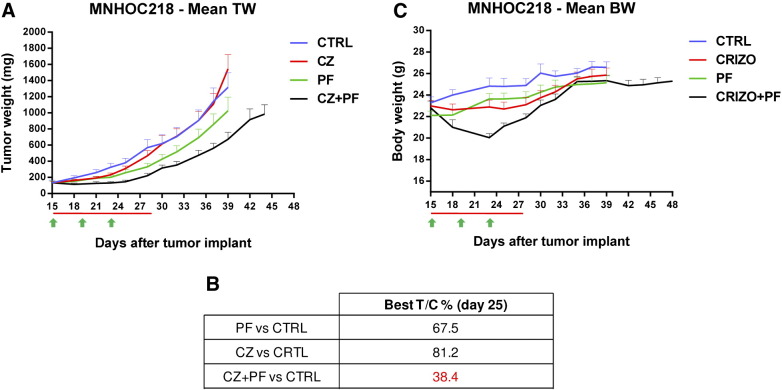
We then moved to a PDX model selecting a tumor with negative staining for PTEN and presenting an additional copy of both c-Met and PIK3CA genes. In this model (Figure 7A and Table 2) crizotinib showed some initial activity, but then the tumors started growing at the same rate of untreated mice. PF-05212384 had some activity with a T/Cx100 of 67 but the combination was much more active reaching a T/Cx100 of 38 (Figure 7B). In this case an initial drop in body weight was observed for the group treated with the combination, which however completely recovered in few days (Figure 7C).
Figure 7.
Antitumor activity of PF-05212384, crizotinib or the combination in the PDX MNHOC218. A. Tumor growth inhibition activity of MNHOC218 PDX, treated with PF-05212384 10 mg/kg iv Q4dx4 (green arrows), crizotinib 50 mg/kg po every day (red line) or with the combination of the two drugs for 13 days. B. Percentage of tumor growth inhibition (T/C%) calculated for all the treatment groups. C. Body weight of MNHOC218 PDX bearing mice treated with PF-05212384, crizotinib or the combination. Means and SEM are shown.
Table 2.
Statistical Analysis of the In Vivo Antitumor Activity Reported in Figure 7A
| Day |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 | 18 | 23 | 25 | 28 | 30 | 32 | 35 | 37 | 39 | 42 | |
| CTRL vs. PF | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| CTRL vs. CZ | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| CTRL vs. CZ + PF | ns | ns | ns | ns | ns | * | ns | * | ** | **** | **** |
| PF vs. CZ | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | **** |
| PF vs. CZ + PF | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | * |
| CZ vs. CZ + PF | ns | ns | ns | ns | ns | ns | ns | * | ** | **** | **** |
The analysis was performed on the mean tumor weight calculated for all the treatment groups, using two-way ANOVA test and Bonferroni post-test for multiple comparisons. *P < .1, ** P < .01, **** P < .0001.
Pharmacodynamic Studies
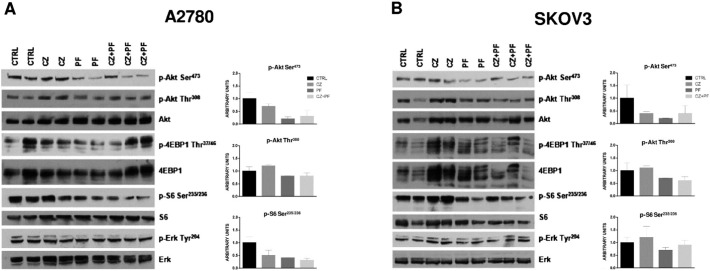
The effect of single agents or of the combination on PI3K and MAPK pathways activation in vivo was determined by Western blot analysis performed on lysates from tumors explanted 24 hours after treatment in A2780 and SKOV3-derived xenografts (Figure 8, A and B, respectively). In A2780 xenograft model crizotinib alone had a partial inhibitory effect and induced a slight increase in the amount of p-Akt (Thr308), whose pattern was almost unaffected in samples treated with PF-05212384 or the combination. PF-05212384 mainly inhibited p-S6 (Ser235/236) and p-Akt (Ser473) and this effect was not boosted by the addition of crizotinib in the combination. A lower extent of p-Akt (Ser473) was also detected in SKOV3-derived xenografts (Figure 8B) upon both single-drug and combined treatments. In this model, Akt (Thr308) and S6 phosphorylation state was marginally affected by PF-05212384 or the combination, whereas crizotinib induced an increase in their phosphorylation levels. Analysis of p-4EBP1 (Thr37/46) and p-ERK (Tyr204) did not reveal clear changes in any of the models used.
Figure 8.
Effects of treatment on PI3K and MAPK pathways molecular effectors. Representative Western blot analysis after 24 hours of treatment with PF-05212384, crizotinib or the combination on A2780 xenografts (A) and SKOV3 xenografts (B). The histograms below the Western blot represent protein band intensities of p-Akt (Ser473), p-Akt (Thr308), p-S6 (Ser235/236) levels normalized with the amount of the relative total protein. Two independent experiments were performed.
Discussion
The PI3K/Akt/mTOR pathway is one of the most deregulated signaling pathway in cancer. Several inhibitors of the pathway have been tested both at preclinical and clinical level. Although a clear predictive marker for the response to PI3K/Akt/mTOR inhibitor is still lacking, it is generally accepted that tumors with activated PI3K pathway respond to treatment with PI3K inhibitors. It is however expected that these tumors, after an initial response, will develop resistance as it has been reported for other PI3K inhibitors.
Among the possible resistance mechanisms, amplification/overexpression of c-Met represents a common mechanism of resistance to chemotherapy, particularly following tyrosine kinase inhibitors (TKI) [23], [24], [25]. There is preclinical evidence that tumors dependent on PI3K can develop resistance through c-Met amplification [26], [27]. Additional experimental evidence indicates a cross-talk between c-Met-dependent and PI3K-dependent signaling which implies that the combination of PI3K and c-Met inhibitors could have clear benefits [27].
In our study, we aimed at combining, in ovarian cancer xenografts and PDX, a dual PI3K mTOR inhibitor (PF-05212384 or gedatolisib) with crizotinib as c-Met inhibitor. The idea was that the presence of a continuous treatment with crizotinib would have reduced the chances to develop resistance to treatment with the PI3K-mTOR inhibitor. We tested initially the combination in vitro to verify that in the two cell lines used (A2780 and SKOV3) there was no synergism, to be sure that the in vivo experiments would have evidentiated a potentiation of the activity through inhibition of the emerging PI3K mTOR-resistant clone. Indeed in our system crizotinib per se was devoid, at the doses used here, of any significant antitumor activity. On the contrary, in all the three models used it was able to increase the activity of PF-05212384. Based on our in vitro lack of additivity/synergism between the two drugs, the increased activity observed could be possibly associated to the ability of crizotinib to block the development of c-Met mediated resistant clone.
In our in vitro system we could not derive a PF-05212384 resistant clone with amplified or overexpressed c-Met, being the clones selected for resistance in vitro associated to an increased expression of the efflux pump MDR-1. This precluded the possibility to test in vivo the activity of the combination in cells with c-Met associated PI3K inhibitors resistant clones. However, we can exclude that the superior antitumor activity of the combination observed in vivo is due to the ability of crizotinib to prevent MDR-1 positive clones, since in vitro the combination tested in cells resistant to PF-05212384 and overexpressing MDR-1 did not result in any significant positive effect compared to PF-05212384 alone.
The availability of a PDX model with three copies of the c-Met gene allowed us to test the combination in a model with a modest increase of c-Met and again we found a superior activity of the combination.
Pharmacodynamic studies, performed in xenografts, showed that crizotinib did not change the ability of PF-05212384 to inhibit the PI3K/Akt/mTOR pathway, again in line with our initial hypothesis.
Conclusions
In summary, the results obtained in the three models show that crizotinib alone did not show any activity in all the tumors investigated, while PF-05212384 alone had some marginal activity. The combination of the two showed higher activity compared to drugs alone. Considering that crizotinib did not show activity in the models used, the results indicate that crizotinib is able to potentiate the activity of PF-05212384.
The activity of the combination, although superior to the drugs alone, is not striking in these three models of ovarian cancer. However, due to the well tolerability of the combination, the results would suggest the possibility to combine the two drugs in settings in which PF-05212384 or crizotinib alone have already some significant activity.
Acknowledgements
The generous contribution of the Italian Association for Cancer Research (AIRC- IG12915 to MB) is gratefully acknowledged. EC is recipient of a FIRC fellowship.
Footnotes
Funding: This study has been supported by an unrestricted grant by Pfizer.
Contributor Information
Alice Iezzi, Email: alice.iezzi@marionegri.it.
Elisa Caiola, Email: elisa.caiola@marionegri.it.
Massimo Broggini, Email: massimo.broggini@marionegri.it.
References
- 1.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 2.Fry MJ. Structure, regulation and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1994;1226:237–268. doi: 10.1016/0925-4439(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 3.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 4.Djordjevic S, Driscoll PC. Structural insight into substrate specificity and regulatory mechanisms of phosphoinositide 3-kinases. Trends Biochem Sci. 2002;27:426–432. doi: 10.1016/s0968-0004(02)02136-9. [DOI] [PubMed] [Google Scholar]
- 5.Foster FM, Traer CJ, Abraham SM, Fry MJ. The phosphoinositide (PI) 3-kinase family. J Cell Sci. 2003;116:3037–3040. doi: 10.1242/jcs.00609. [DOI] [PubMed] [Google Scholar]
- 6.Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27:5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldo P, Cecco S, Giacomin E, Lazzarini R, Ros B, Marastoni S. mTOR pathway and mTOR inhibitors as agents for cancer therapy. Curr Cancer Drug Targets. 2008;8:647–665. doi: 10.2174/156800908786733513. [DOI] [PubMed] [Google Scholar]
- 9.Abraham RT, Eng CH. Mammalian target of rapamycin as a therapeutic target in oncology. Expert Opin Ther Targets. 2008;12:209–222. doi: 10.1517/14728222.12.2.209. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grunwald V, Thompson JA, Figlin RA, Hollaender N. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 11.Yao JC, Phan AT, Jehl V, Shah G, Meric-Bernstam F. Everolimus in advanced pancreatic neuroendocrine tumors: the clinical experience. Cancer Res. 2013;73:1449–1453. doi: 10.1158/0008-5472.CAN-12-3923. [DOI] [PubMed] [Google Scholar]
- 12.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, Barrientos JC, Zelenetz AD, Kipps TJ, Flinn I. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, Flinn IW, Flowers CR, Martin P, Viardot A. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 15.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yap TA, Bjerke L, Clarke PA, Workman P. Drugging PI3K in cancer: refining targets and therapeutic strategies. Curr Opin Pharmacol. 2015;23:98–107. doi: 10.1016/j.coph.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai AZ, Abella JV, Park M. Crosstalk in Met receptor oncogenesis. Trends Cell Biol. 2009;19:542–551. doi: 10.1016/j.tcb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 19.Maroun CR, Rowlands T. The Met receptor tyrosine kinase: a key player in oncogenesis and drug resistance. Pharmacol Ther. 2014;142:316–338. doi: 10.1016/j.pharmthera.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Ricci F, Fratelli M, Guffanti F, Porcu L, Spriano F, Dell'Anna T, Fruscio R, Damia G. Patient-derived ovarian cancer xenografts re-growing after a cisplatinum treatment are less responsive to a second drug re-challenge: a new experimental setting to study response to therapy. Oncotarget. 2016 doi: 10.18632/oncotarget.7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ricci F, Bizzaro F, Cesca M, Guffanti F, Ganzinelli M, Decio A, Ghilardi C, Perego P, Fruscio R, Buda A. Patient-derived ovarian tumor xenografts recapitulate human clinicopathology and genetic alterations. Cancer Res. 2014;74:6980–6990. doi: 10.1158/0008-5472.CAN-14-0274. [DOI] [PubMed] [Google Scholar]
- 22.Zamai M, VandeVen M, Farao M, Gratton E, Ghiglieri A, Castelli MG, Fontana E, D'Argy R, Fiorino A, Pesenti E. Camptothecin poly[n-(2-hydroxypropyl) methacrylamide] copolymers in antitopoisomerase-I tumor therapy: intratumor release and antitumor efficacy. Mol Cancer Ther. 2003;2:29–40. [PubMed] [Google Scholar]
- 23.Krumbach R, Schuler J, Hofmann M, Giesemann T, Fiebig HH, Beckers T. Primary resistance to cetuximab in a panel of patient-derived tumour xenograft models: activation of MET as one mechanism for drug resistance. Eur J Cancer. 2011;47:1231–1243. doi: 10.1016/j.ejca.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Turke AB, Zejnullahu K, YL W, Song Y, Dias-Santagata D, Lifshits E, Toschi L, Rogers A, Mok T, Sequist L. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CT, Kim H, Liska D, Gao S, Christensen JG, Weiser MR. MET activation mediates resistance to lapatinib inhibition of HER2-amplified gastric cancer cells. Mol Cancer Ther. 2012;11:660–669. doi: 10.1158/1535-7163.MCT-11-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang MK, Zhou HY, Yam JW, Wong AS. c-Met overexpression contributes to the acquired apoptotic resistance of nonadherent ovarian cancer cells through a cross talk mediated by phosphatidylinositol 3-kinase and extracellular signal-regulated kinase 1/2. Neoplasia. 2010;12:128–138. doi: 10.1593/neo.91438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu P, Cheng H, Santiago S, Raeder M, Zhang F, Isabella A, Yang J, Semaan DJ, Chen C, Fox EA. Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway-dependent and PI3K pathway-independent mechanisms. Nat Med. 2011;17:1116–1120. doi: 10.1038/nm.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]