Abstract
Pleurotus cornucopiae (Oyster mushroom, Tamogitake) has long been eaten as a functional food for enhancement of the immune system, but its effectiveness has not been well confirmed in humans. To this end, we set up a double-blind placebo-controlled human clinical trial to investigate the potential of Oyster mushrooms with respect to the up-regulation of the immune system. The subjects ingested Oyster mushroom extract for 8 weeks. We measured the serum cytokine levels involved in regulation of the immune system, including interferon (IFN)-γ, interleukin (IL)-4, IL-5, IL-10, IL-12, IL-13, and tumor-necrosis factor (TNF)-α. We found that intake of Oyster mushroom extract elevated IFN-γ (P = 0.013) and IL-12, whereas serum levels of IL-10 and IL-13 and other cytokines were minimally changed. We also measured natural killer (NK) cell activity, the levels of which tended to increase, but not significantly. Taken together, these facts suggest that Oyster mushrooms have the potential to enhance the immune system, through Th1 phenotype potentiation as the macrophage-IL-12 – IFN-γ pathway. This results in activation of the cell-mediated immune system as exemplified by up-regulation of NK cell activity. Oyster mushroom extract may be beneficial for the prevention of various diseases, including infectious diseases and cancer, due to its stimulation of the immune system.
Keywords: IFN-γ, NK cell, Oyster mushroom, Th1/Th2, Clinical trial
Graphical abstract
1. Introduction
Various edible mushrooms have been used as functional foods and as important sources of complementary medicine beneficial for the prevention of diseases.1 They have been used as folk medicine all over the world since ancient times. For example, Agaricus blazei Murill mushroom is consumed as a food and for its possible medicinal value.2 Its dried fruiting bodies have been used as stimulant and as an auxiliary treatment of various diseases, including cancer.
Pleurotus species are commonly called Oyster mushrooms. There are about 40 species of these mushrooms distributed worldwide in both temperate and tropical zones of the world.3 The production amount of Oyster mushrooms now ranks second among the cultivated mushrooms in the world. In Japan, Oyster mushrooms of Pleurotus cornucopiae (Tamogitake) with a yellow pileus are commonly sold in markets. Oyster mushrooms are grown and harvested in relatively cool-climate areas in Japan, around Hokkaido and Tohoku. These mushrooms have been traditionally consumed by local people to prevent diseases such as hypertension and cancer.4
The immune system is the body's self-defense system against infectious diseases, immunodeficiencies and cancer growth. The immune system is a complex involving many white blood cells, e.g., macrophages and lymphocytes, proteins and chemicals. A healthy immune system contains elements that are usually well balanced with one another. In a compromised immune system, components are unbalanced and unable to protect the body against harmful agents or processes.
It has been well recognized that Interferon (IFN)-γ coordinates a diverse array of cellular programs through transcriptional regulation of immunomodulatory genes.5 IFN-γ is involved in up-regulation of pathogen recognition, antigen processing and presentation, cellular proliferation and apoptosis relevant to immunomodulation, and leukocyte migration. IFN-γ plays an important role in the integration of signaling and response by other cytokines such as TNF-α and IL-4 and in pathogen-associated molecular patterns.
IFN-γ plays an important role against infection and tumor growth. This molecule orchestrates leukocyte attraction and directs growth, maturation, and differentiation of many cell types, in addition to enhancing natural killer cell activity by regulating B cell function in terms of immunoglobulin production and class switching.6 As negative regulators of IFN-γ production, IL-4, IL-10, transforming growth factor-β, and glucocorticoids are well recognized. In this study, we investigated whether consumption of Oyster mushroom extract by humans would up-regulate IFN-γ and in turn up-regulate the immune system. We designed a double-blind, placebo-controlled study to investigate the effectiveness of Oyster mushroom extract with respect to immune potentiation.
2. Materials and methods
2.1. Subjects
Through screening, we recruited Japanese 100 volunteers and selected 47 subjects without any medication. At the start of this trial, 47 subjects (5 males and 42 females, age range 34–64 years) were enrolled. During screening, subjects with a recent history of gastrointestinal disorders, pregnancy, severe acute and chronic diseases, surgery, severe allergic reaction to food, or current use of any medication were excluded. The clinical intervention was conducted as a double-blind, placebo-controlled trial. At randomization, the 47 eligible subjects were randomly assigned to one of two groups (Oyster mushroom group and placebo group) with adjustment by age, sex, and natural killer (NK) cell activity.
Prior to the start of the trial, 6 persons were excluded because of health problems, such as low ingestion rate (less than 80%) of test foods, and intake of prohibited drugs or foods. As a result, 41 persons were subjected to analysis, 21 and 20 for the placebo and the test meal, respectively. The mean subject age, height, body weight (BW), body mass index (BMI), body fat percentage (BFP) and NK cell activity for each group are reported in Table 1. These data of the placebo and test groups were not statistically significant, indicating the appropriate allotment of test and placebo groups.
Table 1.
Characteristics of the subjects in the placebo and Oyster mushroom intake groups.
| Characteristic | Oyster mushroom | Placebo | P-value |
|---|---|---|---|
| Number of subjects | n = 20 | n = 21 | – |
| Number of males (male %) | 2 (10.00%) | 2 (9.52%) | 0.678 |
| Age (years) | 51.25 ± 8.80 | 50.24 ± 8.70 | 0.713 |
| Height (cm) | 159.50 ± 4.55 | 158.40 ± 5.32 | 0.482 |
| Body weight (kg) | 53.87 ± 7.07 | 53.65 ± 7.18 | 0.924 |
| BMI (kg/m2) | 21.21 ± 2.85 | 21.39 ± 2.88 | 0.837 |
| Body fat percentage (%) | 27.26 ± 6.20 | 27.82 ± 6.42 | 0.777 |
| NK cell activity (%) | 35.00 ± 10.90 | 32.62 ± 12.32 | 0.517 |
Values shown are mean ± standard deviation (SD). Statistical analysis was performed by Student's t-test for age, height, body weight, BMI, body fat percentage, and natural killer cell activity, and by chi-square test for gender.
2.2. Study design
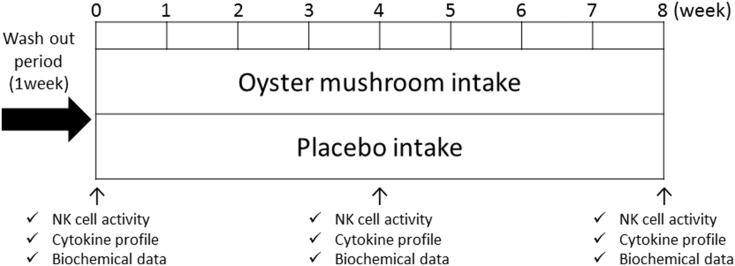
The clinical study was conducted as double-blind, placebo-controlled trail. The time schedule for this clinical study is shown in Fig. 1. Few clinical studies have been performed to evaluate efficacy of Oyster mushroom on immune reaction. Based on an experimental results on clinical studies7, 8 we hypothesized that 8 week intake of Oyster mushroom extract would be sufficient to assess the potential efficacy. The primary endpoint was NK cell activity, and secondary endpoints were parameters relevant to Th1/Th2 balance, including IFN-γ, IL-10, IL-12, IL-13, IL-4, IL-5 and TNF-α. In addition, a set of biomarkers for liver and renal functions were measured to assess safety of Oyster mushroom extract and placebo.
Fig. 1.
Time schedule (in weeks) for this clinical study. Hematological measurement was conducted at baseline (week 0), and weeks 4 and 8.
We performed body composition (BW, BMI, and BFP) measurements at baseline (week 0) and post-intervention (weeks 4 and 8) for the two groups. At all three time points, a medical interview, check of vital signs, blood sample collection, and hematological examinations were performed as well. During the course of this study, subjects were advised to keep diaries of their daily activities, including food consumption, drug intake and exercise.
2.3. Test meal preparation
The test meals (Oyster mushroom extract and placebo) were kindly provided by Three B Co., Inc. (Nanporo-Cho, Hokkaido, Japan), and contained 100% water extract of Oyster mushroom (Tamogitake) extract (80 mL per pouch; Table 2). Oyster mushrooms contained β-glucan 24 mg/meal, whereas the placebo contained no β-glucan. Other than β-glucan contents, the test meal and placebo were not significantly different except that sodium content was higher in the placebo (64 mg/meal) than in Oyster mushroom extract (5.7 mg/meal). The placebo contained various materials: water, tea (0.5%), oyster sauce (0.5%) and caffeine-free coffee (0.1%) for coloring. For preparation, in brief, the fruit bodies of Oyster mushroom were directly extracted with water at 94 °C for 10 min. After concentration, the insoluble parts were removed through filtration of 0.2 mm. For the last 15 years, no serious complaints about Oyster mushroom extract have been reported. The meal was produced and prepared under ISO9001.
Table 2.
Composition of Oyster mushroom compared with placebo per 80 ml.
| Component | Oyster mushroom | Placebo |
|---|---|---|
| Calories (kcal) | 2.4 | 2.1 |
| Water (g) | 97.9 | 80.5 |
| Proteins (g) | 0.9 | 0.0 |
| Lipids (g) | 0.1 | 0.0 |
| Carbohydrates (g) | 0.5 | 0.4 |
| Ash (g) | 0.3 | 0.3 |
| Sodium (mg) | 5.7 | 64 |
| β-glucan (mg) | 24 | – |
2.4. Physical and hematological examination
Blood samples were taken for testing on the starting day and after 4 and 8 weeks of ingestion of Oyster mushroom extract. In addition to the medical interview by a doctor, body composition (BW, BMI, and BFP) were measured. Vital signs (blood pressure upon arrival, pulse rate, body temperature) were also taken. General blood tests included complete blood count (CBC) [white blood cells (WBC), red blood cells (RBC), hemoglobin (Hb), hematocrit (Ht), and platelet count (Plt)], liver function [aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transpeptidase (γ-GTP), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH)], renal function [blood urea nitrogen (BUN), creatinine (CRE), and ureic acid (UA)], blood lipid profile [total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), and Triglyceride (TG)], and blood glucose profile [fasting plasma glucose (FPG) and hemoglobin A1c (HbA1c)].
The hematological examinations were performed by Sapporo Clinical Laboratory, Inc. (Sapporo, Japan). Each subject's body composition and blood pressure were measured with a body composition analyzer DC-320 (Tanita Corp, Tokyo, Japan) and an Omron HEM-7080IC automatic blood pressure monitor (Omron Colin Co., Ltd., Tokyo, Japan).
2.5. Cytokine assay by multiplex assays
Secretion of large amounts of IFN-γ (IL-4) is the defining feature of Th1 (Th2) cells, respectively. These cytokines function to directly promote cell-mediated immunity (e.g., IFN-γ) or humoral immunity (e.g., IL-4) and to reciprocally antagonize each other's action. To study this, multiplex beads kits were purchased from LINC Research. Cytokines analyzed in duplicate by each kit included IFN-γ, IL-10, IL-12, IL-13, IL-4, IL-5 and TNF-α. Each multiplex assay was performed in duplicate on two different occasions according to the manufacturer's specifications. Standard curves for each cytokine were generated using the reference cytokine concentrations.
2.6. NK cell activity
Chromium-51 (51Cr) release assay was used for quantification of cytotoxicity to measure NK cell activity.9 The assay was conducted by SRL (Hachioji City, Tokyo, Japan). In brief, target cells are labeled with 51Cr, the label is then released from the target cells by cytolysis. The label was isolated by centrifuging the samples and collecting the supernatants. Supernatants from centrifugation were counted directly in a gamma counter.
2.7. Ethics committee
All subjects provided written informed consent before undergoing any study-related tests, and the study protocol was approved by the Ethics Committee of Hokkaido Information University (a certificate number; 2015-09). The study protocol conformed to the Helsinki Declaration and was registered at the UMIN Clinical Trial Registration System (certificate number UMIN000015208).
2.8. Statistical analysis
The means and standard deviations of subject characteristics were calculated for each group. Changes in subject values were analyzed using Student's t-test comparing means between the test group and the placebo group. To test for normality the Shapiro–Wilk Test was used. The appropriate sample size was statistically determined to obtain a power of 80% with an alpha of 0.05. This study was planned as a feasibility study, but the minimum size needed to detect. We referred to our previous in-house studies relevant to NK cell activities using a mushroom extract, active hexose correlated compounds (AHCC)10 as well as immune enhancement of immune system by Lactobacillus plantarum.11 In order to demonstrate increase of NK cell activity, a sample size of 20 was required under the assumption that increase of NK cell activity is 5% with a standard deviation of 5.5%. Assuming a 10% loss to follow-up, we set the total sample size at 47. Statistical analyses were performed using SPSS Statistic 19 (IBM, Armonk, NY, USA). P-values <0.05 were considered significant.
3. Results
3.1. Physical parameters and blood pressure
We calculated mean values and standard deviations for physical measurement at weeks 0, 4, and 8 after ingestion of test foods for 47 subjects. As for BW, BMI, BFP, and blood pressure, their mean values and standard deviations at the three points 0, 4, and 8 weeks after ingestion were described (Table 3).
Table 3.
Physical parameters and BP.
| Week 0 | Week 4 | Week 8 | ||
|---|---|---|---|---|
| BW (kg) | Oyster mushroom | 53.33 ± 6.79 | 53.54 ± 6.69 | 53.72 ± 6.60 |
| Placebo | 56.20 ± 13.02 | 56.28 ± 13.36 | 56.44 ± 13.27 | |
| BMI | Oyster mushroom | 21.10 ± 2.76 | 21.17 ± 2.70 | 21.24 ± 2.66 |
| Placebo | 21.78 ± 3.83 | 21.81 ± 3.93 | 21.97 ± 3.85 | |
| BFP (%) | Oyster mushroom | 27.62 ± 6.35 | 26.97 ± 6.56 | 28.31 ± 6.13 |
| Placebo | 28.23 ± 6.42 | 28.43 ± 6.31 | 28.64 ± 6.39 | |
| SBP (mmHg) | Oyster mushroom | 114.59 ± 18.68 | 115.45 ± 15.45 | 115.68 ± 20.61 |
| Placebo | 110.84 ± 17.23 | 116.04 ± 18.99 | 113.67 ± 17.81 | |
| DBP (mmHg) | Oyster mushroom | 71.68 ± 13.74 | 71.55 ± 10.82 | 72.14 ± 9.70 |
| Placebo | 70.64 ± 9.78 | 74.36 ± 11.49 | 73.08 ± 10.67 |
Values are mean ± standard deviation (SD). BW; body weight, BMI; body mass index, BFP; body fat percentage, SBP; systolic blood pressure, DBP; diastolic blood pressure.
We found no adverse effects originated from either Oyster mushroom extract or placebo. During the current study, we performed visual analog scale (VAS) as for awakening, mood, eagerness, comfort, fatigability, concentration, and appetite. Among them, we did not find any statistically significant improvement, but the levels of awakening (P = 0.131) and concentration (P = 0.172) were relatively improved by the intake of Oyster mushroom extract in comparison with placebo (data not shown).
3.2. Levels of biomarkers for CBC, liver and renal function, lipid and glucose metabolism
We examined the levels of several biomarkers of CBC, liver and renal functions, and lipid and glucose metabolism. No abnormal changes were found for CBC (WBC, RBC, Hb, Ht, and Plt), liver function (AST, ALT, γ-GTP, ALP, and LDH), and renal function (BUN, CRE, and UA) after the ingestion of Oyster mushroom at either 4 or 8 weeks (Table 4). Likewise, blood lipid profile (TC, LDL-C, HDL-C, and TG) showed no abnormality. Minimal differences were observed between the Oyster mushroom and placebo groups in the parameters of glucose metabolism (FPG and HbA1c). These results show that ingestion of Oyster mushroom is safe in terms of lipid and glucose metabolism, and liver and renal functions.
Table 4.
Biochemical data.
| Week 0 | Week 4 | Week 8 | ||
|---|---|---|---|---|
| WBC (×103/μl) | Oyster mushroom | 4.75 ± 1.24 | 4.65 ± 1.20 | 4.80 ± 1.33 |
| Placebo | 5.50 ± 1.25 | 5.40 ± 1.38 | 5.51 ± 1.47 | |
| RBC (×104/μl) | Oyster mushroom | 444.05 ± 45.13 | 440.95 ± 47.55 | 447.45 ± 48.15 |
| Placebo | 455.92 ± 48.96 | 452.48 ± 47.42 | 448.46 ± 47.05 | |
| Hb (g/dl) | Oyster mushroom | 13.05 ± 1.69 | 13.05 ± 1.68 | 13.29 ± 1.68 |
| Placebo | 13.63 ± 1.55 | 13.62 ± 1.50 | 13.57 ± 1.46 | |
| Ht (%) | Oyster mushroom | 40.61 ± 3.98 | 40.13 ± 4.04 | 40.85 ± 3.99 |
| Placebo | 42.13 ± 4.08 | 41.73 ± 3.74 | 41.48 ± 3.64 | |
| Plt (×104/μl) | Oyster mushroom | 23.72 ± 5.58 | 24.10 ± 5.72 | 24.69 ± 7.30 |
| Placebo | 22.49 ± 5.47 | 23.37 ± 5.52 | 23.33 ± 4.73 | |
| AST (U/l) | Oyster mushroom | 21.32 ± 3.78 | 22.77 ± 7.70 | 22.77 ± 6.90 |
| Placebo | 23.16 ± 5.91 | 21.84 ± 4.84 | 21.42 ± 5.38 | |
| ALT (U/l) | Oyster mushroom | 18.23 ± 5.50 | 18.14 ± 6.61 | 19.18 ± 7.65 |
| Placebo | 20.08 ± 11.65 | 18.00 ± 9.20 | 18.54 ± 10.23 | |
| γ-GTP (U/l) | Oyster mushroom | 26.68 ± 14.47 | 28.00 ± 19.46 | 30.45 ± 22.25 |
| Placebo | 21.72 ± 10.63 | 20.40 ± 10.97 | 20.00 ± 9.56 | |
| ALP (U/l) | Oyster mushroom | 198.50 ± 41.40 | 204.36 ± 61.27 | 208.73 ± 48.36 |
| Placebo | 188.60 ± 51.06 | 180.36 ± 43.08 | 188.13 ± 52.36 | |
| LDH (U/l) | Oyster mushroom | 195.91 ± 24.09 | 201.77 ± 34.44 | 209.95 ± 35.13 |
| Placebo | 207.44 ± 42.87 | 204.76 ± 34.75 | 204.17 ± 34.87 | |
| BUN (mg/dl) | Oyster mushroom | 13.36 ± 4.23 | 13.66 ± 3.56 | 12.45 ± 3.55 |
| Placebo | 13.51 ± 3.09 | 13.29 ± 3.29 | 12.95 ± 2.90 | |
| CRE (mg/dl) | Oyster mushroom | 0.69 ± 0.11 | 0.71 ± 0.12 | 0.69 ± 0.11 |
| Placebo | 0.69 ± 0.13 | 0.71 ± 0.12 | 0.69 ± 0.12 | |
| UA (mg/dl) | Oyster mushroom | 4.33 ± 1.17 | 4.31 ± 1.12 | 4.25 ± 1.31 |
| Placebo | 4.52 ± 1.32 | 4.54 ± 1.31 | 4.53 ± 1.46 | |
| TC (mg/dl) | Oyster mushroom | 211.77 ± 35.41 | 206.32 ± 30.32 | 211.91 ± 33.39 |
| Placebo | 216.64 ± 35.03 | 213.68 ± 34.27 | 212.54 ± 40.31 | |
| LDL-C (mg/dl) | Oyster mushroom | 122.91 ± 29.38 | 114.32 ± 27.91 | 120.27 ± 30.54 |
| Placebo | 131.00 ± 29.65 | 122.08 ± 28.82 | 122.96 ± 32.16 | |
| HDL-C (mg/dl) | Oyster mushroom | 86.59 ± 19.33 | 81.82 ± 16.82 | 85.23 ± 16.94 |
| Placebo | 80.40 ± 21.40 | 78.56 ± 21.10 | 79.08 ± 20.69 | |
| TG (mg/dl) | Oyster mushroom | 62.91 ± 22.06 | 68.64 ± 27.58 | 64.68 ± 17.51 |
| Placebo | 86.64 ± 70.18 | 85.68 ± 69.49 | 86.13 ± 72.10 | |
| FPG (mg/dl) | Oyster mushroom | 87.05 ± 8.89 | 86.00 ± 8.28 | 87.09 ± 9.55 |
| Placebo | 85.84 ± 5.20 | 86.44 ± 8.94 | 86.63 ± 5.26 | |
| HbA1c (%) | Oyster mushroom | 5.28 ± 0.37 | 5.25 ± 0.36 | 5.28 ± 0.38 |
| Placebo | 5.22 ± 0.31 | 5.19 ± 0.32 | 5.20 ± 0.33 |
Values are mean ± standard deviation (SD). WBC; white blood cells, RBC; red blood cells, Hb; hemoglobin, Ht; hematocrit, Plt; platelet count, AST; aspartate aminotransferase, ALT; alanine aminotransferase, γ-GTP; gamma glutamyl transpeptidase, ALP; alkaline phosphatase, LDH; lactate dehydrogenase, BUN; blood urea nitrogen, CRE; creatinine, UA; ureic acid, TC; total cholesterol, LDL-C; low density lipoprotein cholesterol, HDL-C; high density lipoprotein cholesterol, TG; triglyceride, FPG; fasting plasma glucose, HbA1c; hemoglobin A1c.
3.3. Cytokine profile
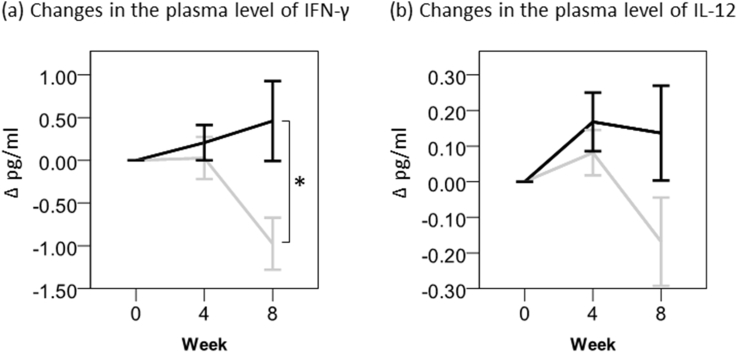
As for Th1-type cytokines, the plasma level of IFN-γ and IL-12 were found to increase with Oyster mushroom consumption (Fig. 2). In particular, IFN-γ, a strong stimulator of NK cells, increased significantly by Oyster mushroom intake compared to the placebo at week 8 (P = 0.013). Even though statistically insignificant, the IL-12 level tended to be increased by Oyster mushroom extract compared to the placebo at week 8 (P = 0.101).
Fig. 2.
Changes in the plasma levels of IFN-γ and IL-12. Values are means ± standard errors (SEs). Black bar, Oyster mushroom; Gray bar, placebo. Blood samples were collected at weeks 0, 4 and 8. The plasma levels of IFN-γ and IL-12 were measured as described in “Materials and Methods.” The statistical analysis was carried out by SPSS. *Statistically significant, P value less than 0.05.
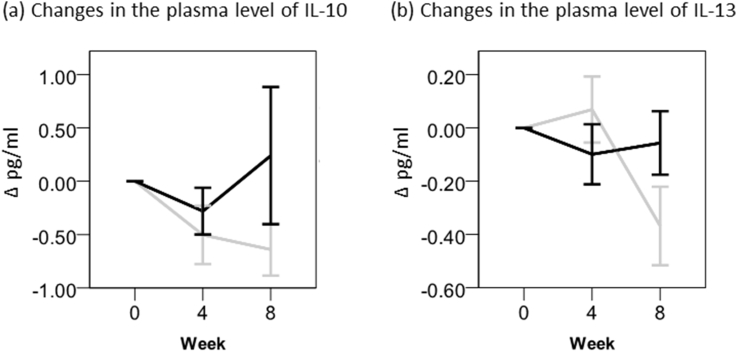
As for Th2-type cytokines, we measured plasma levels of IL-10 and IL-13. The former, IL-10, is known to inhibit secretion of various cytokines by Th1-type cells, macrophages and dendritic cells. Plasma level of this cytokine was minimally changed by the intake of Oyster mushroom extract at week 8 (Fig. 3).
Fig. 3.
Changes in the plasma levels of IL-10 and IL-13. Values are means ± standard errors (SEs). Black bar, Oyster mushroom; Gray bar, placebo. Blood samples were collected at weeks 0, 4 and 8. The plasma levels of IL-10 and IL-13 were measured as described in “Materials and Methods.” The statistical analysis was carried out by SPSS.
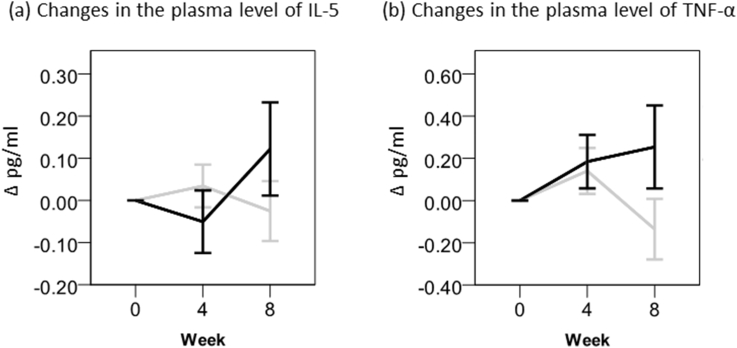
In addition, we measured plasma levels of IL-4, IL-5 and TNF-α to better understand the cytokine profile, and found some increase in the level of Th1-type cytokine TNF-α (P = 0.115) and minimal change in that of Th2-type IL-5 (P = 0.265) at week 8 (Fig. 4). IL-4 plasma levels were too low to be measured in almost subjects (data not shown). Increase of TNF-α is compatible with Th1-type increase and would be functional for suppression of tumor growth. In fact, TNF-α participates in the induction of IFN-γ in an additive fashion in the presence of cofactors such as IFN-α, resulting in NK cell activation. Overall, it was strongly suggested that Oyster mushroom extract stimulates the release of Th1-type cytokines, and minimally affects that of Th2-type cytokines.
Fig. 4.
Changes in the plasma levels of TNF-α and IL-5. Values are means ± standard errors (SEs). Black bar, Oyster mushroom; Gray bar, placebo. Blood samples were collected at weeks 0, 4 and 8. The plasma levels of TNF-α and IL-5 were measured as described in “Materials and Methods.” The statistical analysis was carried out by SPSS.
3.4. Effects of Oyster mushroom on NK cell activity
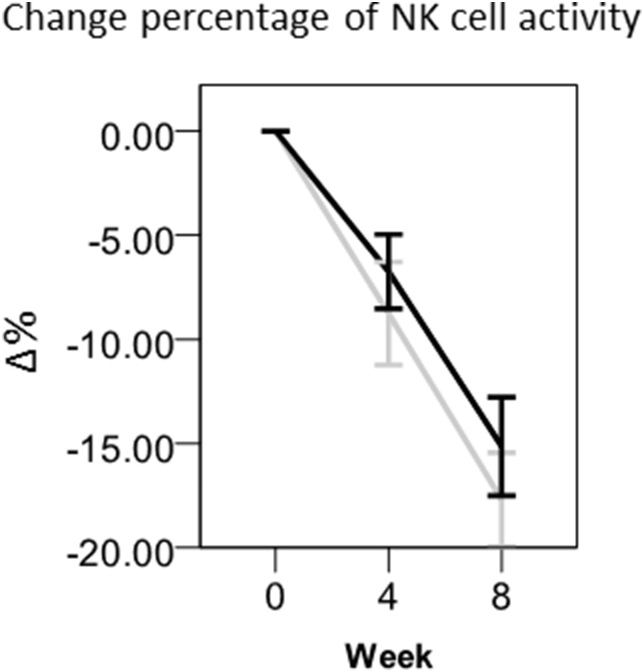
To further confirm the effect of Oyster mushroom extract on activation of the immune system, particularly cell-mediated immunity, we evaluated NK cell activity. Because of up-regulation of INF-γ, the increase of NK cell activity was expected. Accordingly, we found some increase of NK cell activity, even though it was not statistically significant at week 8 (Fig. 5).
Fig. 5.
Changes in the percentage of NK cell activity. Values are means ± standard errors (SEs). Black bar, Oyster mushroom; Gray bar, placebo. Chromium-51 (51Cr) release assays were used for the precise and accurate quantification of cytotoxicity. The method of measuring NK cell activity was described in “Materials and Methods.” The statistical analysis was carried out by SPSS.
4. Discussion
The fruit body of P. Cornucopiae (Oyster mushroom, Tamogitake) has traditionally been used as a health food for the prevention of hypertension, diabetes and cancer.12 In the present study, we examined the effect of Oyster mushroom extract in the human diet, focusing on the activation of immune reactions by evaluating cytokine profiles, particularly the Th1/Th2 balance. For example, IL-4 inhibits IFN-γ-dependent activation of macrophage effector function. Conversely, IFN-γ antagonizes the IL-4 dependent induction of IgE receptors.13, 14, 15 In this study, we demonstrated that 8-week ingestion of Oyster mushroom extract enhanced IFN-γ production in a randomized, double-blind, placebo-controlled, parallel-group clinical study. A strong IFN-γ producer, IL-12 was also increased, but to a lesser extent. On the other hand, Th2-type cytokines such as IL-10 and IL-13 were minimally changed, and IL-4 was undetected because of low production.
It is unclear on the reason why Th2-type cytokines were minimally changed by the intake of Oyster mushroom extract in spite of increase of IFN-γ. Oyster mushroom extract may have unknown functional molecules to modulate the event of Th1/Th2 balance. As another possible cause of lower level of Th2-type cytokines, the assay system, multiplex beads kits used in the study, was relatively less sensitive to Th2-type cytokines than Th1-type cytokines, e.g., IFN-γ. Currently, we plan another clinical trial to answer these questions, in which higher amount of Oyster mushroom extract is used to produce sufficient amount of Th1- and Th2-type cytokines to obtain more definite results.
IFN-γ is major product of Th1-type cells and drives the immune response toward a Th1 phenotype. IFN-γ achieves this by promoting characteristic Th1 effector mechanisms, innate cell-medicated immunity via activation of NK cells, and macrophage activation.16 As for NK cell activity, the increase we measured was relatively lower than expected from the increased level of IFN-γ. The reason for the minimal increase of NK cell activity in this study remains to be elucidated. According to information from the SRL engaged in the assay of NK cell activity, subjects with less than 20% (normal range; 18–40% in SRL) of NK cell activity are appropriate to be included for assessment of immune response. Thus, it is considered that subjects recruited in this study had a relatively high NK cell activities, 35.0% and 32.6% for Oyster mushroom extract and placebo, respectively. Accordingly, the relatively higher NK cell activities of the subjects might cause minimal change of NK cell activity.
As for another possible reason for insignificant enhancement of NK cell activity, the amount of Oyster mushroom extract may not have been sufficient. In terms of the biological actions of Oyster mushrooms, it is well recognized to have the potential to reduce blood pressure, but we found little change of blood pressure in the current study. In this context, we might obtain unequivocal results for the activation of NK cell activity if we use a higher amount of Oyster mushroom extract for subjects with lower level of NK cell activities, but this hypothesis needs further investigation by another clinical trial integrating the current results.
Macrophages respond to a wide range of different cell products (cytokines) during the innate and acquired immune response. Of these, IFN-γ is among the most important. Macrophages activated with IFN-γ induce direct anti-microbial and anti-tumor actions through antigen processing and presentation pathways.17 In brief, the mechanism as for antitumor action involves amplification of Type-1, increases of IFN-γ and Th1 development, enhancement of cytotoxic T-lymphocyte activity, or augmentation of NK cell activation and lytic ability. In this context, it is very likely that Oyster mushroom extract stimulates macrophages by INF-γ which results in enhancement of immune system leading to protection of us from infection and tumorigenesis.
The potential of Oyster mushroom extract to protect us from acute and chronic diseases has already been demonstrated by animal experiments using Sarcom180 mice cells.18 Specifically, it showed that orally administered water extract of Oyster mushroom inhibited the growth of Sarcoma180 in ICR mice, thereby demonstrating an anti-tumor effect. Further, Oyster mushroom extract induced costimulatory molecule expression and IL-12 secretion by dendritic cells. The study also found that CD25/GITR-positive regulatory T cells were decreased in tumor-bearing mice administered Oyster mushroom extract. These previous animal experimental data well support the current results of the human clinical trial demonstrating Oyster mushroom extract showing the potential to increase Th1-type cytokines, namely IFN-γ.
In terms of the mechanism of Oyster mushroom, the constituent(s) responsible for up-regulation of immune response by the current Oyster mushroom extract has not been definitively identified. It has been reported that Oyster mushroom contains beta-d-glucose, ergosterol, mannitol, phenolic compounds, linoleic acid, peptides, and carbohydrates.19 As for β-glucan, many isolated polysaccharides and protein-bound polysaccharides from A. blazei have been shown to have direct anti-tumor activity.20 It was reported that a tea preparation of A. blazei extracts was anti-mutagenic in Chinese hamster lung fibroblasts.21 Similarly to A. blazei, the mushroom Letinus edodes also was observed to be effective in protecting cells from DNA damage.
The constituent or constituents responsible for the anti-mutagenic activity of edible Oyster mushrooms remain to be identified. The Oyster mushroom extract used in this study contains 24 mg/pack of β-glucan, which is considered to be a direct activator of the immune system. In comparison with previous reports from other institutes, the Oyster mushrooms used in this study may contain enough β-glucan to induce immune-protective action against infectious diseases and tumorigenesis.
In recent years, various new foods have attracted attention as health-beneficial foods and as source materials for disease prevention and health promotion supplements. In particular, herbal and fishery medicine derived from plant and fish extracts is being increasingly utilized to prevent a wide variety of diseases despite relatively little knowledge regarding their mechanism of action. In this literature, we demonstrated that Oyster mushroom extract up-regulates the immune system by release of IFN-γ. In this sense, Oyster mushrooms would be one of the promising candidates for prevention of infectious and non-infectious diseases.
Lastly, it would be of interest to try to elucidate the mechanism of action for Oyster mushroom extract in further studies. As for the defense mechanism in terms of cytokine profiles and the immune system, IFN-γ production is controlled by cytokines secreted by antigen-presenting cells (APCs), e.g., macrophages, most notably IL-12 and IL-18.22 These cytokines serve as a bridge to link infection with IFN-γ production in the innate immune response. Macrophage recognition of many pathogens induces secretion of IL-12 and chemokines (e.g., macrophage-inflammatory protein-1α (MIP-1α)).23 These chemokines attract NK cells to the site of inflammation, and IL-12 promotes IFN-γ synthesis in these cells. We are planning to design a human clinical trial focusing on these pathways to elucidate the mechanism of Oyster mushrooms in terms of immune system responses to various diseases, including cancer.
5. Conclusion
In conclusion, we revealed that Oyster mushrooms potentiate the immune system, through Th1 phenotype potentiation as the macrophage-IL-12 – IFN-γ pathway. Thus, Oyster mushroom extract may be beneficial for the prevention of various diseases by stimulation of the immune system.
Source of support
This research was supported in part by the Northern Advancement Center for Science and Technology (NOASTEC) Foundation (26Noastecfood-1).
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
We thank Ms. Hiroyo katsuyama for her technical assistance with data management of the clinical trial.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Jose N., Janardhanan K.K. Antioxidant and antitumor activity of Pleurotus florida. Curr Sci. 2000;79:941–943. [Google Scholar]
- 2.Fujimiya Y., Suzuki Y., Oshiman K. Selective tumoricidal effect of soluble proteoglucan extracted from the basidiomycete, Agaricus blazei Murill, mediated via natural killer cell activation and apoptosis. Cancer Immunol Immunother. 1998;46:147–159. doi: 10.1007/s002620050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volz P.A. Early historic cancer chemotherapy work involving basidiomycetous mushrooms. Int J Med Mushrooms. 1999;1:191–194. [Google Scholar]
- 4.De Lima P.L., Delmanto R.D., Sugui M.M. Letinula edodes (Berk.) Pegler (Shiitake) modulates genotoxic and mutagenic effects induced by alkylating agents in vivo. Mutat Res. 2001;496:23–32. doi: 10.1016/s1383-5718(01)00215-7. [DOI] [PubMed] [Google Scholar]
- 5.Schroder K., Hertzog P.J., Ravasi T., Hume D.A. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 6.Carnaud C., Lee D., Donnars O. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 7.Noguchi M., Kakuma T., Tomiyasu K. Effect of an extract of Ganoderma lucidum in men with lower urinary tract symptoms: a double-blind, placebo-controlled randomized and dose-ranging study. Asian J Androl. 2008;10:651–658. doi: 10.1111/j.1745-7262.2008.00336.x. [DOI] [PubMed] [Google Scholar]
- 8.Deng G., Lin H., Seidman A. A phase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients: immunological effects. J Cancer Res Clin Oncol. 2009;135:1215–1221. doi: 10.1007/s00432-009-0562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mickel R.A., Kessler D.J., Taylor J.M., Licktenstein A. Natural killer cell cytotoxicity in the peripheral blood, cervical lymph nodes, and tumor of head and neck cancer patients. Cancer Res. 1988;48:5017–5022. [PubMed] [Google Scholar]
- 10.Takanari J., Hirayama Y., Homma K., Miura T., Nishioka H., Maeda T. Effects of active hexose correlated compound on the seasonal variations of immune competence in healthy subjects. J Evid Based Complement Altern Med. 2015;20:28–34. doi: 10.1177/2156587214555573. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura M., Ohkawara T., Tetsuka K. Effects of yogurt containing Lactobacillus plantarum HOKKAIDO on immune function and stress markers. J Tradit Complement Med. 2016;6:275–280. doi: 10.1016/j.jtcme.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khatun K., Mahtab H., Khanam P.A., Sayeed M.A., Khan K.A. Oyster mushroom reduced blood glucose and cholesterol in diabetic subjects. Mymensingh Med J. 2007;16:94–99. doi: 10.3329/mmj.v16i1.261. [DOI] [PubMed] [Google Scholar]
- 13.Schindler H., Lutz M.B., Röllinghoff M., Bogdan C. The production of IFN-gamma by IL-12/IL-18-activated macrophages requires STAT4 signaling and is inhibited by IL-4. J Immunol. 2001;166:3075–3082. doi: 10.4049/jimmunol.166.5.3075. [DOI] [PubMed] [Google Scholar]
- 14.Fukao T., Frucht D.M., Yap G., Gadina M., O'Shea J.J., Koyasu S. Inducible expression of Stat4 in dendritic cells and macrophages and its critical role in innate and adaptive immune responses. J Immunol. 2001;166:4446–4455. doi: 10.4049/jimmunol.166.7.4446. [DOI] [PubMed] [Google Scholar]
- 15.Hochrein H., Shortman K., Vremec D., Scott B., Hertzog P., O'Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J Immunol. 2001;166:5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 16.Frucht D.M., Fukao T., Bogdan C., Schindler H., O'Shea J.J., Koyasu S. IFN-gamma production by antigen-presenting cells: mechanisms emerge. Trends Immunol. 2001;22:556–560. doi: 10.1016/s1471-4906(01)02005-1. [DOI] [PubMed] [Google Scholar]
- 17.Boehm U., Klamp T., Groot M., Howard J.C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 18.Kato K., Kaihou S., Hagiwara S., Tomiyama T., Hamada H. vol. 46. 2005. A novel antitumor effect of water extract from Pleurotus cornucopia that promotes dendritic cell activation and down-regulation of regulatory T cells in tumor-bearing mice; p. 172. (Proceedings of the 96th Annual Meeting of the American Association for Cancer Research). [Google Scholar]
- 19.Jayakumar T., Sakthivel M., Thomas P.A., Geraldine P. Pleurotus ostreatus, an oyster mushroom, decreases the oxidative stress induced by carbon tetrachloride in rat kidneys, heart and brain. Chem Biol Interact. 2008;176:108–120. doi: 10.1016/j.cbi.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Ito H., Shimura K., Itoh H., Kawada M. Antitumor effects of a new polysaccharide-protein complex (ATOM) prepared from Agaricus blazei (Iwade Strain101) “Himematsutake” and its mechanisms in tumor-bearing mice. Anticancer Res. 1997;17:277–284. [PubMed] [Google Scholar]
- 21.Menoli R.C., Mantovani M.S., Ribeiro L.R., Speit G., Jordão B.Q. Antimutagenic effects of the mushroom Agaricus blazei Murrill extracts on V79 cells. Mutat Res. 2001;496:5–13. doi: 10.1016/s1383-5718(01)00227-3. [DOI] [PubMed] [Google Scholar]
- 22.Fukao T., Matsuda S., Koyasu S. Synergistic effects of IL-4 and IL-18 on IL-12-dependent IFN-gamma production by dendritic cells. J Immunol. 2000;164:64–71. doi: 10.4049/jimmunol.164.1.64. [DOI] [PubMed] [Google Scholar]
- 23.Salazar-Mather T.P., Hamilton T.A., Biron C.A. A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J Clin Invest. 2000;105:985–993. doi: 10.1172/JCI9232. [DOI] [PMC free article] [PubMed] [Google Scholar]