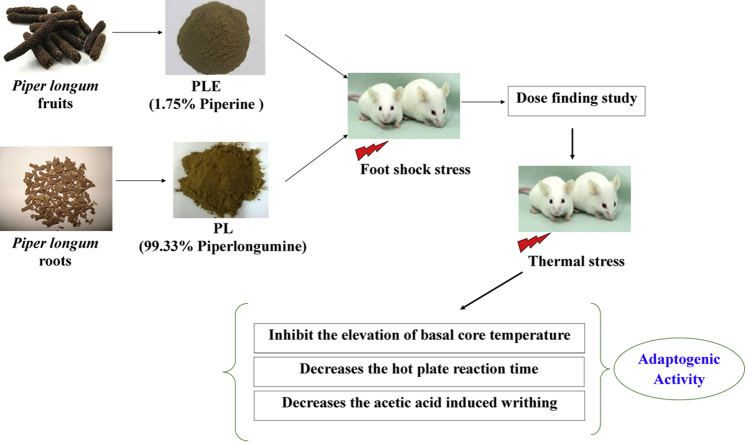
Abstract
Aim
To compare stress resistance increasing and analgesic activities of piperlongumine and a methanolic Piper longum fruit extract (PLE).
Methods
Efficacies of a single and repeated daily oral doses (1–256 mg/kg/day) of PLE, piperlongumine, and 50 mg/kg/day doxycycline against foot shock stress triggered alteration in body weights and core temperatures, and of their 11 daily doses on antidepressants like activity in tail suspension test and on pentobarbital induced sedation in male mice were compared. In another experiment, analgesic activities of single and repeated daily 5 mg/kg oral doses of piperlongumine and PLE in mice hot plate test and in acetic acid induced writing tests were compared with those of aspirin and doxycycline.
Results
After their single oral doses no effects of piperlongumine or PLE or doxycycline were observed in the footshock stress induced hyperthermia test or in hot plate test. However, significant effects of piperlongumine and PLE in both the tests were observed after their 5 or more daily doses. Both of them also dose dependently suppressed daily handling and repetitive testing triggered alterations in body weights and core temperatures. Their doxycycline like antidepressant activity in tail suspension test and aspirin like analgesic effects in acetic acid writhing test were observed after their 11 daily 5 mg/kg oral dose.
Conclusion
Piperlongumine is another bioactive secondary metabolite of P. longum and other plants of piper species with stress response suppressing, analgesic, and anti-inflammatory activities. Its bactericidal activities can also contribute to its therapeutically interesting bio-activity profile.
Keywords: Piper longum, Piperine, Piperlongumine, Physiological stress responses, Hot plate test
Graphical abstract
1. Introduction
Piper longum Linn, commonly known as long piper or Indian long piper, is a plant of Piperaceae family cultivated for harvesting its fruits used in several Asiatic and other countries as a spices and seasoning. Its diverse medicinal uses have also been known in Ayurvedic and other traditionally known systems of medicine and health care since antiquity.1 Like several other plants widely used in modern Indian system of medicine, P. longum is now often pharmacologically classified as an adaptogenic or stress response regulating medicinal herbs with a broad spectrum of therapeutically interesting bioactivities.2, 3, 4, 5 Piperlongumine, piperine and other amide alkaloids with pungent tastes have been identified as the major and structurally unique bioactive secondary metabolites of the plant.6, 7 However, these and numerous other alkaloidal amides of P. longum are also encountered in varying quantities in other plants of the Piperaceae family which are also often used in Chinese and other traditionally known systems of medicine.8, 9 Piperlongumine is now attracting considerable attention of modern drug discoverers as a lead structure for obtaining functionally novel drug leads against inflammatory disorders and cancer.10, 11 However, most extracts of P. longum and other plants of the Piperaceae family often used in modernized versions of Ayurvedic formulation are in general analytically standardized, or analytically characterized, by their piperine contents only.12, 13
Trikatu is one such herbal mixture containing equal parts of P. longum and P. nigrum fruits and Zingiber officinalis root powder.14 This mixture is often used in many Ayurvedic formulations commonly prescribed for treatments of gastric and abdominal disorders, asthma, bronchitis, coughs, dysentery, pyrexia, insomnia, colic and intestinal infection. Diverse stress response regulating potentials of all the three plant used in this mixture have been reported,15 and such properties of P. longum extracts and their analgesic, ulcer protecting and diverse other therapeutically interesting bioactivities of piperine in animal models are also well known.16, 17, 18, 19 However, as yet no reports on the role of piperlongumine in stress response modulating, pain relieving, and diverse other traditionally known medicinal uses of extracts P. longum and other plants of Piperaceae family have appeared.
Piperlongumine and piperine are structurally analogous bioactive molecules and both of them have been reported to possess antimicrobial activities.20 It is now becoming increasingly apparent that gut microbiota play a crucial role in regulating physiological stress responses,21 and that depending on their doses and treatment regimen used, bactericidal and other agents with modulating effects on gut microbial ecology can have diverse health benefits.22, 23 For example, appropriate doses and treatment regimen of doxycycline like antibiotics can suppress physiological stress response and can reset the neuro-hormonal status regulated by gut microbiota.24 Gastric ulcer protective, anticonvulsant, antidepressant, neuro-protective and other brain function modulating activities of the antibiotic have been reported also.25, 26, 27, 28 Therefore, it was of interest to experimentally very the possibility that like medicinally used P. longum extracts and doxycycline, pure piperlongumine also possess stress response suppressing and analgesic activities. Results of the very first experiments conducted to experimentally verify such possibilities are described and discussed in this communication.
The reported experiments constitute a part of our ongoing efforts directed towards translating Ayurvedic therapeutic principles in terms of molecular concepts of modern medicine and to obtain therapeutic leads from traditionally known medicinal plants potentially useful for prevention and cure of psychosomatic disorders commonly associated with systemic inflammation.29 In this study, a pharmacologically well characterized mouse bioassay procedure evolving from our efforts to estimate therapeutically interesting doses and dosing regimen of stress response regulating medicinal plants and their bioactive constituents30, 31, 32 was used for comparing the efficacies of doxycycline like adaptogenic efficacies of piperlongumine and a commercially available P. longum extract in mice. In a further experiment, aspirin like analgesic efficacies of their stress response suppressing doses and treatment regimen were compared in a slightly modified version of the bioassay procedures used in the pilot dose finding experiments.
2. Materials and methods
2.1. Animals
Adult male Swiss albino mice (25 ± 5 g) were acquired from Central Animal House of Institute of Medical Sciences, Banaras Hindu University (Registration Number: 542/AB/CPCSEA), and they were acclimatized to laboratory conditions for one week before starting the experiments. For experimental purposes, six animals per group were housed in polypropylene cages (28 × 19 × 12.5 cm) with saw dust beddings and free access to standard rodent diet and water. They were maintained at 25 ± 1 °C ambient temperature and relative humidity of 50 ± 10% and 12:12 h light and dark cycle (light on at 06:00 and off at 18:00). Principles of laboratory animal care (NIH publication 85-23, revised in 1985) guidelines were always followed and before start of the experiments, approval from Central Animal Ethical Committee of the University was obtained (Dean/2014/CAEC/729, dated 07 August 2014).
2.2. Plant extract, drugs and chemicals
Together with their analytical characteristics, the P. longum fruit extract analytically characterized to contain 1.75% piperine (PLE) and almost pure piperlongumine (99.33%) isolated from P. longum roots used in this study were generously supplied by Sami Labs Limited, Bengaluru, India. The extract PLE is a methanolic extract of dried and powdered fruits of P. longum fruits and purity of piperlongumine and piperine contents of the PLE sample were established by HPLC using acetonitrile and water as mobile phase.
Doxycycline was acquired from Sigma Aldrich, Bengaluru, India; Pentobarbitone sodium from Loba Chemicals Pvt. Ltd., Mumbai, India; Aspirin from HiMedia Laboratories Pvt. Ltd., Mumbai, India; and Carboxymethyl cellulose (CMC) from Central Drug House, Delhi, India. All other chemicals and reagents used in this study were of highest purity commercially available in India.
2.3. Animal grouping and drug administration
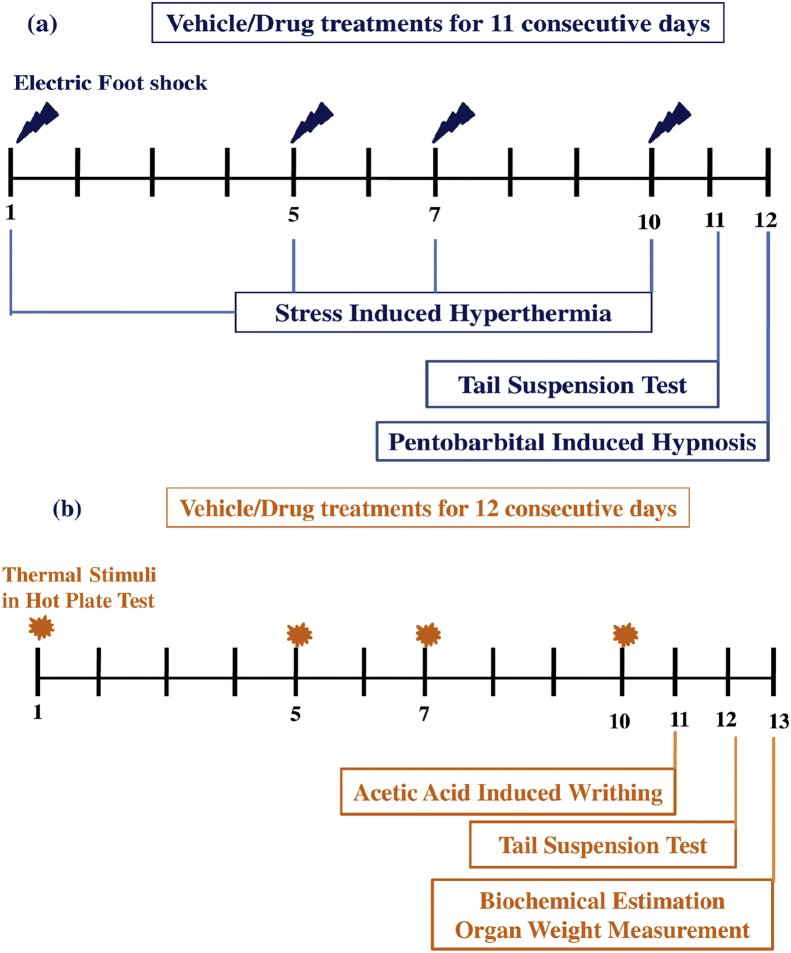
In each of the two pilot experiments conducted to estimate stress response modulating doses and dosing regimen of PLE and piperlongumine, seven groups of six animals each were used. One of them (vehicle treated control group) was treated daily with 0. 3% CMC (10 ml/kg/day), and another one with 50 mg/kg/day doxycycline suspended in CMC for 11 consecutive days. Other five groups were similarly treated either with graded oral daily doses (1, 4, 16, 64 and 256 mg/kg) of piperlongumine or PLE suspended in CMC. In these experiments, 1 h after oral treatments on days 1, 5, 7 and 10 of the experiments all animals were subjected to foot shock stress triggered hyperthermia tests, and 1 h after the treatments on the 11th day of the experiments they were subjected to tail suspension test for potential antidepressant. One day thereafter all groups (without further treatments) were subjected to pentobarbitone sleep tests for assessing longer lasting effects of treatments on brain functions or on drug metabolizing enzymes. Further details of these experiments are summarized in Fig. 1a.
Fig. 1.
(a) Summary of the experimental procedures used in the dose finding experiments and (b) for comparing analgesic and activities of single and repeated doses of test agents.
In a further experiment conducted to compare aspirin or doxycycline like analgesic effects of 11 daily oral doses of 5 mg/kg PLE, or of piperlongumine, six groups of six animals each were used. Hereupon, one control group (Control-HPT) was treated with CMC but not subjected to hot plate test on days 1, 5, 7 and 10 of the experiment and another one (Control + HPT) was treated with CMC and also subjected to hot plate test on those days. The remaining four groups in this experiment were daily treated either with 20 mg/kg/day aspirin, or with 50 mg/kg/day doxycycline or with 5 mg/kg/day piperlongumine or PLE and subjected to hot plate test on those days. The animals used in this experiment were pre selected ones for their sensitivity to hot plate test. Pre-selection of the animals were done one day before the start of the experiment. For such purposes, animals were placed on a hot plate maintained at 55 ± 1 °C and their reaction time was recorded. Only mice which reacted within 15 s and which did not show large variations in their response times when tested on four separate occasions (each 15 min apart) were used in the experiment. On the 11th day of this experiment, and 1 h after the days treatments, all animals of all groups were subjected to acetic acid induced writhing test for analgesics, and on the 12th day of the experiment to tail suspension test for antidepressants. Further details of this experiment are summarized in Fig. 1b.
Body weight and basal core temperatures of all groups in all the three reported experiments were recorded on all treatment and observational days. Except when mentioned, all tests on all days of a given experiment were conducted 1 h after treatments.
2.4. Foot shock stress induced hyperthermia (FSIH) test
This test was conducted by placing individual mouse from each group in a black box (24 × 29 × 40 cm) with a grid floor for 1 min. Electric foot shock through the grid floor (2 mA, 50 Hz of 2 ms duration) was delivered for stress induction. Five consecutive foot shocks of 2 mA at 10 s interval were given starting at 10 s after the animal was placed in the cage. At the end of a minute the animal was placed back in its home cages and 10 min thereafter its rectal temperature was re-recorded by using a digital thermometer and a digital probe.33 Calculated differences between this and basal core temperature of a mouse recorded before subjecting it foot shock stress (basal core temperature) was used as an index for stress induced hyperthermic response of the animal.
2.5. Tail suspension test
Immobility time during tail suspension was recorded according to the method described in.34 On the 11th day and 60 min after that day's oral treatments, each mouse was individually suspended in an upside down posture by the tail from the hook (50 cm above the floor) on which it was fixed with an adhesive tape placed approximately 1 cm from the tip of the tail. After initial vigorous movements, the mouse assumes an immobile posture and the total duration of immobility during the 5 min test session were calculated as immobility time.
2.6. Potentiation of pentobarbitone induced hypnosis
In the dose finding experiments, effects of 11 daily treatments on pentobarbitone (40 mg/kg, i.p) induced sedation and sleep were assessed 24 h after the last doses of test agents. Onset time of sleep (loss of righting reflex) and duration of sleep after pentobarbital challenge were recorded.35 Basal rectal temperatures of all groups were recorded before pentobarbitone injection on this day of the experiment. No apparent physical, physiological and behavioral alterations were observed during the entire duration of both the experiments.
2.7. Hot plate test
On the 1st, 5th, 7th and 10th day of the experiment, individual mice from each test group was tested by gently placing it on a hot plate maintained at 55 ± 1 °C and by recording its reaction time in seconds for forepaw licking or jumping.36 The time for forepaw licking or jumping on the heated plate of the analgesiometer was expressed as its reaction time. For preventing any thermal injury to a mouse, the time allowed to the animal to stay on the hot plate was 30 s.37
2.8. Acetic acid induced writhing test
This test was conducted on the 11th day of the experiments. In this test, acetic acid (0.1 ml of 1% v/v solution) was intraperitoneally injected and the numbers of writhes for the subsequent 20 min observational period were counted.38
2.9. Plasma glucose, insulin, and cortisol level and organs weights
On 13th day of the experiment conducted to compare aspirin like effects of doxycycline, piperlongumine and PLE, animals were sacrificed by decapitation (without any treatments). Immediately before that, their blood samples were collected by orbital puncture in EDTA coated tubes kept in ice, and centrifuged thereafter at 1000 × g for 20 min at 4 °C to separate plasma (Compufuge CPR-30 Plus, with Rotor No. 8; REMI, India). Plasma glucose level was estimated by biochemical enzyme test kit (ERBA diagnostics Mannheim GmbH, Germany) and plasma insulin level was estimated using Enzyme-Linked Immunosorbent Assay (ELISA) test kit (Chemux BioSciences, Inc., USA). Plasma cortisol was estimated using ELISA kit (DSI S.r.l., Italy). All biochemical estimations were done by using an absorbance micro-plate reader (iMarkTM- Bio-Rad Laboratories, California, USA) according to instructions manual of respective enzyme test kits. Immediately after blood collections, adrenal glands and spleen of the animals were dissected out, washed under slowly running tap water and weighed after removing adhered water by gently drying them on sheets of filter papers.39
2.10. Statistical analysis
Mean ± standard errors of mean (SEM) were calculated for the observed values in each experimental group. Statistical analysis was done by one-way analysis of variances (ANOVA) followed by Student Newman Keuls multiple comparison tests. When stated, two way ANOVA followed by Bonferroni post hoc test and t-test were performed. GraphPad Prism-5 (GraphPad Software, Inc. La Jolla, California, USA) and Origin-Pro 8 (OriginLab Corporation, Massachusetts, USA) software were used for statistical analysis and drawing graph. P-values less than 0.05 were considered as statistically significant.
3. Results
3.1. Dose finding experiments
Choices of the dose ranges and treatment regimens of piperlongumine and PLE for these experiments were based on numerous reports by others on therapeutically interesting bioactivities of diverse types of P. longum extracts and of its known bioactive constituents in rodent models. Some such reports have revealed that single oral doses of 5 mg/kg of piperine,40 or 400 mg/kg and higher doses of an aqueous suspension of P. longum roots powder posses analgesics like activities in rodent models,41 and that intraperitoneally administered daily doses between 100 or 1.5 mg/kg of piperlongumine to in mice are effective in suppressing tumor growths.10 Choice of the dose of doxycycline and aspirin for this study was based on our earlier observations revealing their stress response suppressing effects in the bioassay used.42
3.1.1. Body weights
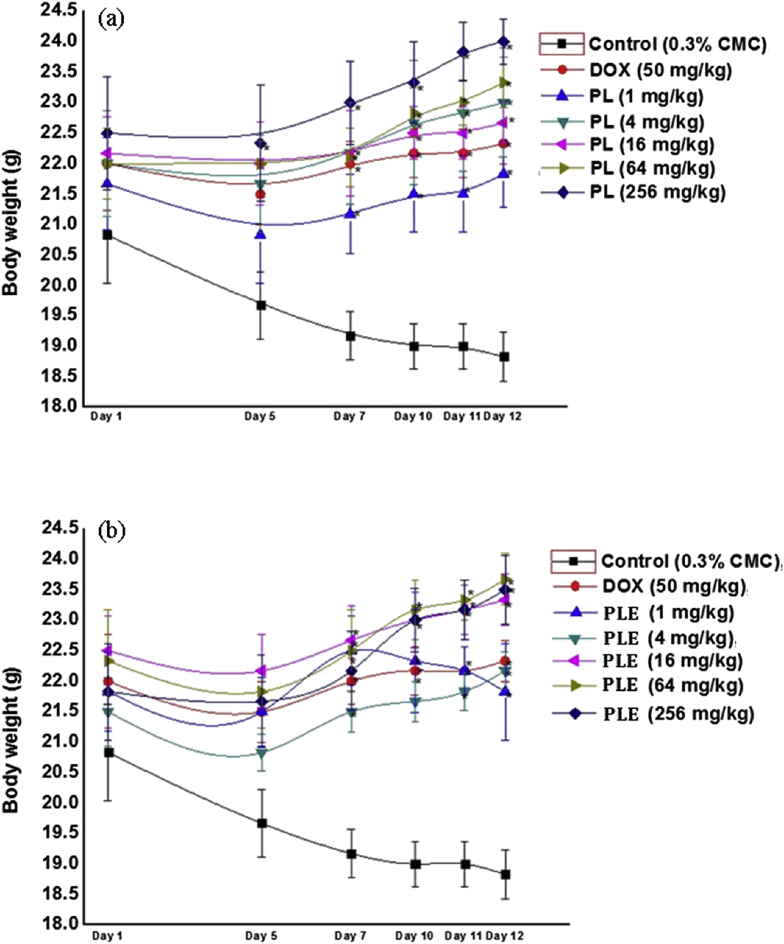
Mean body weights of different groups of animals recorded during the course of the two experiments are summarized in the Fig. 2a and b. As expected from the results of similar experiments in our laboratories using the same bioassay procedure used in this study, body weights of the animals in the CMC treated control groups steadily decreased and those of the doxycycline treated ones remained almost constant during the course of the experiments. Analogous to the observations made for the doxycycline treated groups, body weight losses of all piperlongumine or PLE treated groups were less pronounces on the 5th observational days. On subsequent observational days, mean body weights of most drugs treated ones steadily increased and their body weights on the 11th and 12th days of the experiment were higher than those recorded on the 5th or 1st observational day. The only exception was the 1 mg/kg/day PLE treated group, the mean body weights of which on the 10th and subsequent days were somewhat lower (but not statistically significant) than that recorded for the group on the 7th observation day. These results reveal that minimal effective daily oral doses of piperlongumine or PLE in suppressing the experimental procedures triggered weight losses is 1 mg/kg/day or lower, and that even their 11 highest tested daily oral doses (256 mg/kg/day) have no adverse effects on body weight gain rates of male mice.
Fig. 2.
Effects of occasional foot shock stress on body weights of male mice once daily treated either with piperlongumine (a) or with Piper longum fruits extract (b) for 11 consecutive days. Abbreviations: PL = Piperlongumine, PLE = Piper longum fruits extract, DOX = Doxycycline, CMC = Carboxymethyl cellulose suspension. Values are mean ± SEM (n = 6). * denotes statistically significant difference (Two way ANOVA followed by Bonferroni post hoc test) relative to the corresponding vehicle treated (0.3% CMC) control group (* = p < 0.05).
3.1.2. Basal core temperatures
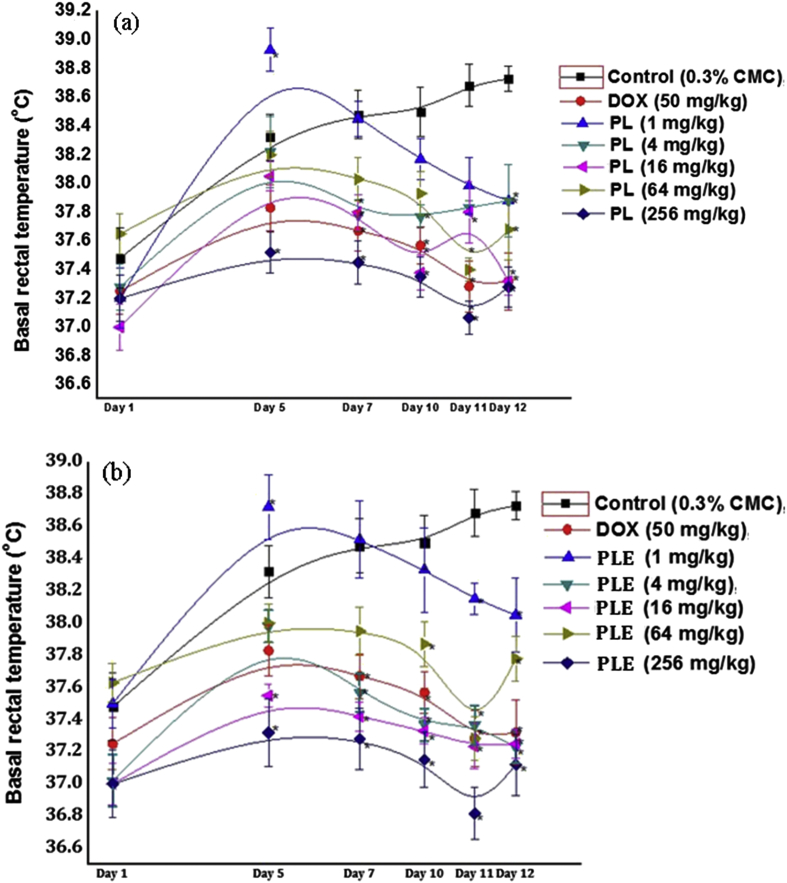
Mean basal rectal temperatures of experimental groups on the 1st days of the experiments were not statistically significantly different from each other, and were within the normal range of male mice colony used in our laboratories. On the 5th day of both the experiments, mean core temperatures of CMC treated control groups recorded before subjecting them to foot shock stress triggered hyperthermia tests were higher, and this experimental procedure triggered increase in basal core temperature increased only slightly on subsequent observational days. Results summarized in Fig. 3a and b revealed that from day 5 onwards, both piperlongumine and PLE treatments dose dependently suppressed such experimental procedures triggered elevations of core body temperatures and that their effects were qualitatively analogous to that of 50 mg/kg/day doxycycline. Estimated minimally effective daily oral doses of both piperlongumine and PLE for this effect were also 1 mg/kg or lower, and observed efficacies of 16 mg/kg/day oral doses of both of them were almost equal to that of 50 mg/kg/day doxycycline.
Fig. 3.
Effects of occasional foot shock stress on basal rectal temperature of male mice once daily treated either with piperlongumine (a) or with Piper longum fruits extract (b) for 11 consecutive days. Values are mean ± SEM (n = 6). * denotes statistically significant difference (Two way ANOVA followed by Bonferroni post hoc test) relative to the corresponding vehicle treated (0.3% CMC) control group (* = p < 0.05).
3.1.3. Foot shock stress induced hyperthermia (FSIH)
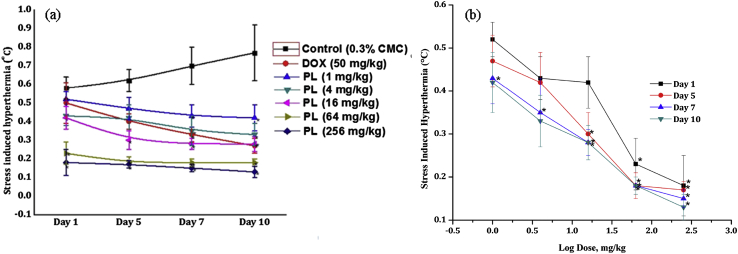
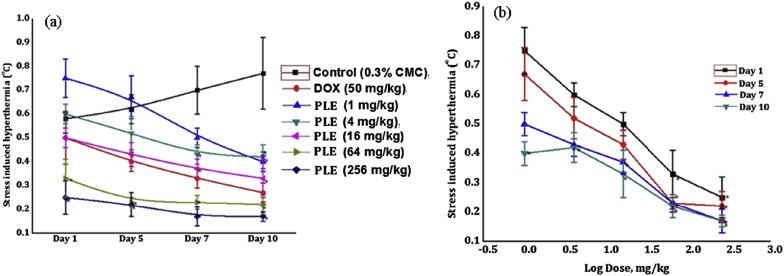
Magnitude of transient hyperthermic responses of the vehicle treated control groups observed 10 min after exposures to foot shocks on the first observational day was somewhat lower than on subsequent days of the experiment. Such stress triggered hyperthermic responses of the groups were always between 0.5 to 0.7 °C (Fig. 4, Fig. 5a). No statistically significant effects of a single oral dose of 50 mg/kg doxycycline, or of 1, 4, and 16 mg/kg piperlongumine or PLE were observed. However, on this observational day, the foot shock stress triggered hyperthermic responses of the 64 and 256 mg/kg piperlongumine or PLE were significantly lower than those of the corresponding control groups. Doxycycline like and daily dose dependant efficacies of both piperlongumine and PLE in this test were observed on all observational days of the experiments and their efficacies increased with the numbers of treatment days. Efficacies of 4 mg/kg/day piperlongumine or of 16 mg/kg/day PLE observed in suppressing FSIH on all test days were almost equal to those of 50 mg/kg/day doxycycline. Dose response curves of piperlongumine and PLE in suppressing FISH are shown in Fig. 4, Fig. 5b respectively.
Fig. 4.
(a) Effects of daily oral doses of piperlongumine in mice foot shock stress induced hyperthermia test on the 1st, 5th, 7th and 10th observational days of the dose finding experiment and (b) its log dose response curves drawn from the observe values on different observational days. Values are mean ± SEM (n = 6). * denotes statistically significant difference (Two way ANOVA followed by Bonferroni post hoc test) relative to the vehicle treated (0.3% CMC) control group (* = p < 0.05).
Fig. 5.
(a) Effects of daily oral doses of piperlongumine in mice foot shock stress induced hyperthermia test on the 1st, 5th, 7th and 10th observational days of the dose finding experiment and (b) its log dose response curves drawn from the observe values on different observational days. Values are mean ± SEM (n = 6). * denotes statistically significant difference (Two way ANOVA followed by Bonferroni post hoc test) relative to the vehicle treated (0.3% CMC) control group (* = p < 0.05).
3.1.4. Tail suspension test
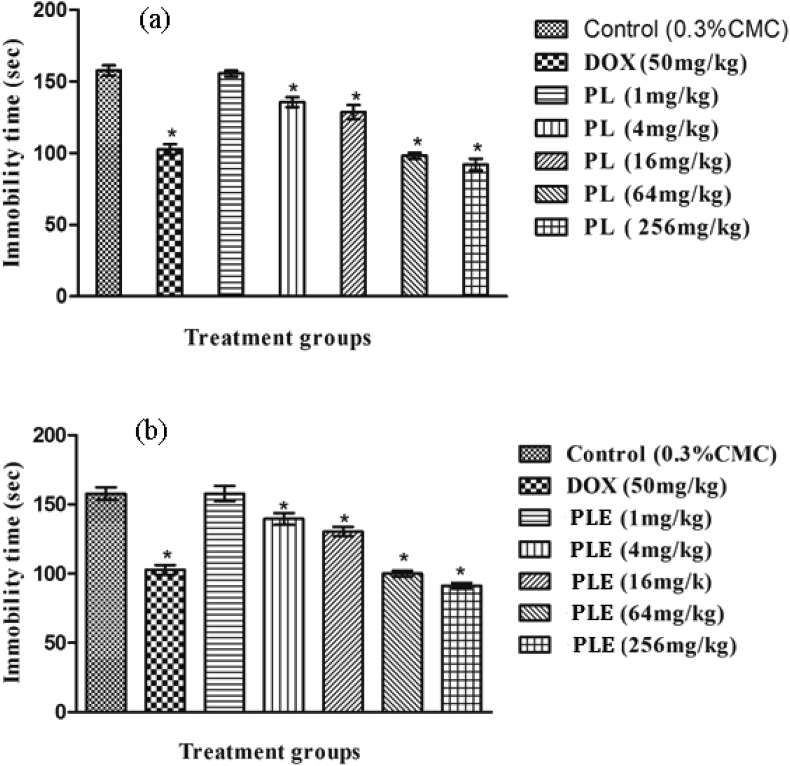
Results summarized in Fig. 6a and b reveal that mean immobility time of doxycycline treated animals in both the experiments was significantly lower than those of the vehicle treated ones. Statistically significant and dose dependant effects of both piperlongumine and PLE were observed after their daily 4–256 mg/kg oral doses. In this test, the observed effects of 50 mg/kg/day doxycycline were almost equal to those of 64 mg/kg/day piperlongumine or PLE.
Fig. 6.
Effects of 11 daily oral doses of (a) of piperlongumine or (b)Piper longum fruits extract on tail suspension test in male mice. Values are mean ± SEM (n = 6). * denotes statically significant difference (one way ANOVA followed by t-test) relative to control group (* = p < 0.05).
3.1.5. Pentobarbitone induced sleep test
No statistically significant effects of doxycycline or piperlongumine or PLE were observed in this test conducted 24 h after their 11 consecutive daily doses (results not shown). This test is often uses for estimating inducing potentials of test agents on drug metabolizing enzymes, or of their sedative effects. Therefore, these observations might indicate that even fairy high daily doses (264 mg/kg/day) of piperlongumine or PLE do not have any longer lasting effects on such enzymes involved in pentobarbitone metabolism, or that they do not induce sedation in male mice.
3.2. Analgesic and other stress response modulating activities
Earlier observations in our laboratories have revealed that fairly low daily oral doses (20 mg/kg/day) of salicylic acid also suppresses physiological stress responses in the same mouse bioassay used for the dose finding experiments in this study.30 Since aspirin is a pro-drug of salicylic acid, and aspirin like bioactivities of P. longum and other plants of the Piperaceae family enriched in amide alkaloids have been reported,43, 44 further experiment was conducted to compare the efficacy of stress response suppressing daily oral doses (5 mg/kg/day) of piperlongumine and PLE with those of aspirin or doxycycline. In this experiment, an additional CMC treated control group was tested in parallel, but was not subjected to hot plate test for analgesics (CON–HPT group). This was necessary for estimating the effects of repeated testing in hot plate test on diverse other responses quantified in this experiment.
3.2.1. Body weights and basal rectal temperatures
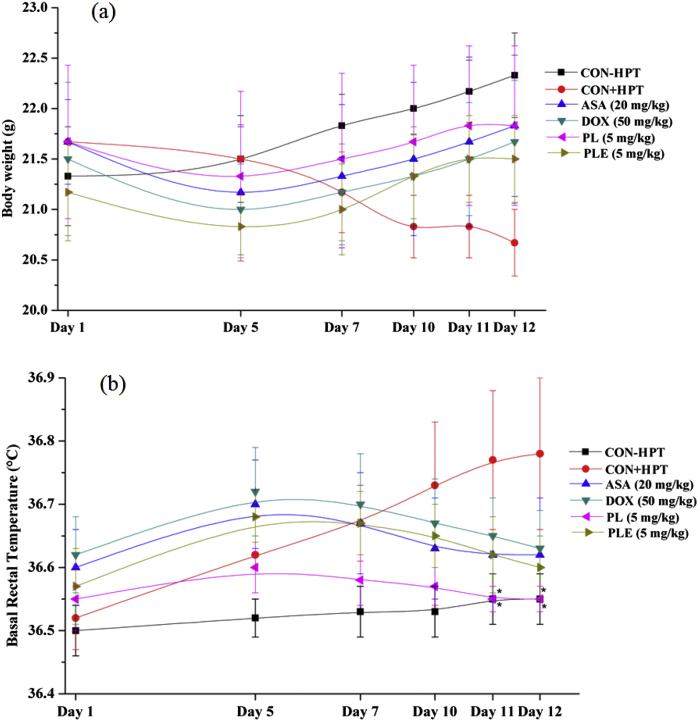
These results are summarized in Fig. 7a and b. Mean body weight of the CMC treated control group not subjected to hot plate test (CON-HPT) increased steadily during the course of the experiment, and mean basal core temperatures of this group remained almost constant during the entire experiment. In contrast, the mean body weights of the control group subjected to hot plate tests (CON + HPT) steadily decreased from day 5 onwards of the test, and its mean basal core temperatures from the 5th experimental day onwards were higher than that recoded on the 1st day of the experiment. These changes caused by repeated testing on the hot plate test were less pronounced in all drug treated groups. These observations are qualitatively similar to those made in the two dose finding experiments, and reveal that 5 mg/kg daily oral doses of piperlongumine and PLE are high enough for antagonizing weight losses and elevation of body temperature caused by occasional exposures to hot plate test. Qualitatively, these observed effects of piperlongumine and PLE were quite analogous to those of doxycycline or of aspirin.
Fig. 7.
Effects of occasional thermal stress on mean body weights (a) and basal rectal temperatures (b) of male mice treated with piperlongumine (PL), Piper longum fruits extract (PLE), Doxycycline (DOX), Aspirin (ASA), or carboxymethyl cellulose (CON + HPT) for 12 consecutive days and subjected to hot plate tests on days 1st, 5th, 7th, and 10th day of experiment. The carboxymethyl cellulose treated control group (CON-HPT) was not subjected to hot plate test. Values are mean ± SEM (n = 6). * denotes statistically significant difference (Two way ANOVA followed by Bonferroni post hoc test) relative to (CON + HPT) group (* = p < 0.05).
3.2.2. Hot plate test
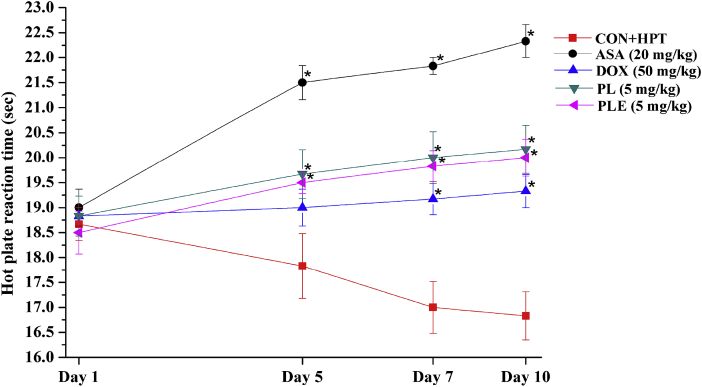
Mean reaction times of all test groups were almost identical on the first observational day of the experiment, and that of the doxycycline treated one remained almost constant on all subsequent observational days (Fig. 8). As judged by their reaction times, sensitivity of the CMC treated control group (CMC + HPT group) in the test gradually decreased, and that of the aspirin treated one increased on the 5th, 7th and 10th days of the experiment. The reaction times of the 5 mg/kg/day piperlongumine of PLE treated groups increased also slightly (but not significantly) on those observational days. In comparison to the observed effects of 20 mg/kg/day aspirin, the efficacies of 5 mg/kg/day piperlongumine and PLE in this test were much lower, but were almost equal to those of 50 mg/kg/day doxycycline.
Fig. 8.
Mean reaction times of male mice treated with piperlongumine (PL), Piper longum fruits extract (PLE), Doxycycline (DOX), Aspirin (ASA), or carboxymethyl cellulose to control group (CON + HPT) subjected to hot plate test on days 1, 5, 7, and 10. Values are mean ± SEM (n = 6). *denotes statistically significant difference (Two way ANOVA followed by Bonferroni post hoc test) relative to CON + HPT group (*p < 0.05).
3.2.3. Acetic acid writhing test
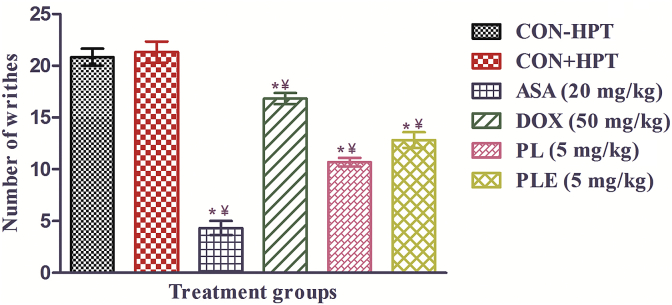
Mean numbers of writhes in both the CMC treated control groups were almost identical whereas that of the aspirin (20 mg/kg/day) treated one was much lower. Doxycycline (50 mg/kg/day) like statistically significant effects of both piperlongumine and PLE were also observed in this test (Fig. 9). Efficacies of 5 mg/kg/day piperlongumine or PLE in suppressing the number of writhers were somewhat higher than that of 50 mg/kg/day doxycycline, but much lower than that of the low dose aspirin tested (20 mg/kg/day).
Fig. 9.
Mean number of writhes induced by intraperitoneal injections of acetic acid in male mice treated with piperlongumine (PL), Piper longum fruits extract (PLE), Doxycycline (DOX), Aspirin (ASA), or carboxymethyl cellulose to two control groups for 10 consecutive days. (CON + HPT) – subjected to hot plate test on days 1, 5, 7, and 10. (CON-HPT) – not subjected to hot plate tests. Values are mean ± SEM (n = 6). *denotes statistically significant difference (One way ANOVA followed by student t-test) relative to CON + HPT group (*p < 0.05). ¥ denotes statistically significant difference (One way ANOVA followed by student t-test) relative to CON-HPT group (¥p < 0.05).
3.2.4. Tail suspension test and other observations
No statistically significant differences between the mean immobility times of all test groups in the tail suspension test were observed. Mean plasma glucose, insulin and cortisol levels of all test groups were also not significantly different from one another. Therefore, these results are not shown here.
However, such were not the observations made to judge the effects of treatments on spleen and adrenal glands weights. These results are summarized in Table 1. Mean absolute and relative adrenal gland weight of CMC treated control group subjected to hot plate tests were lower than those of the control group not subjected to the test. Observed adrenal gland hypertrophy in control group subjected to hot plate test was significantly less pronounced only in the doxycycline treated group. Although, the mean spleen weights of the control group subjected to hot plate test were significantly lower than that of the other control group, such was not the case for their relative spleen weights. These differences could be due to differences in amounts of blood or other extracellular fluids in the spleens of the two groups. Absolute spleen weights of all the drug treated groups were also statistically significantly lower than that of the control group not subjected to hot plate test. Spleen hypertrophy observed in the CMC treated control group subjected to hot plate tests was significantly less pronounced in the doxycycline and PLE treated groups only. These observations could indicate that the modes and sites of actions of PLE and the antibiotic doxycycline are not like those of piperlongumine and aspirin. However, further dose response and other studies are necessary to experimentally verify this possibility.
Table 1.
Effect of 11 daily oral doses of piperlongumine (PL), Piper longum extract (PLE), aspirin (ASA), and doxycycline (DOXY) on absolute and relative weights of adrenal glands and spleen of male mice subjected to hot plate test on days 1, 5, 7 and 10 of treatments.
| Treatment groups | Absolute organ weight (mg) |
Relative organ weight (mg/g of body weight) |
||
|---|---|---|---|---|
| Adrenal glands | Spleen | Adrenal glands | Spleen | |
| CON-HPT | 15.33 ± 0.61* | 143.33 ± 1.12* | 0.69 ± 0.03* | 6.43 ± 0.15 |
| CON + HPT | 20.17 ± 0.54 | 130.83 ± 0.95 | 0.90 ± 0.03¥ | 5.87 ± 0.12 |
| ASA (20 mg/kg) | 17.83 ± 0.79 | 140.83 ± 0.95* | 0.80 ± 0.04 | 6.32 ± 0.11 |
| DOX (50 mg/kg) | 16.67 ± 0.80* | 136.67 ± 0.61*¥ | 0.75 ± 0.04* | 6.13 ± 0.13 |
| PL (5 mg/kg) | 18.50 ± 0.96 | 138.67 ± 1.36¥ | 0.83 ± 0.05 | 6.11 ± 0.13 |
| PLE (5 mg/kg) | 17.67 ± 0.49 | 135.67 ± 1.50*¥ | 0.79 ± 0.03 | 6.08 ± 0.12 |
Values are mean ± SEM (n = 6). *denotes statistically significant difference (One way ANOVA followed by Student t-test) relative to CON + HPT group (* = p < 0.05). ¥ denotes statistically significant difference (One way ANOVA followed by Student t-test) relative to CON–HPT group (¥ = p < 0.05).
4. Discussion
Observations reported in this article are the very first ones revealing analgesic and stress response suppressing potentials of piperlongumine and indicating that doxycycline like antimicrobial activities of piperine or of other bioactive constituents of P. longum could also be involved in its broad spectrum of therapeutically interesting bioactivities in animal models. Although their single lower oral doses tested had no effects on foot shock stress induced hyperthermia or in hot plate test for analgesics, both piperlongumine and PLE were quite effective in both the tests after daily treatments. In the dose finding experiments, even their 1 mg/kg daily oral dose were effective in protecting the animals from intermittent foot shock stress triggered body weight losses and slight elevation in basal core temperatures, and their 11 daily 4 mg/kg doses significantly shortened their immobility period in the tail suspension test for anti-depressants also. All observed effects of PLE and piperlongumine quantified in this study continued to increase with increasing numbers of treatment days, and such were also the observations made with the antibiotic doxycycline treated animals. Therefore, it is apparent that like for doxycycline and other adaptogenic or stress response regulating phytochemicals and herbal extracts,45, 46 pharmacological observations made after single oral doses of piperlongumine and PLE must not necessarily be very predictive of their efficacies that can be expected after their regular oral intake.
Analogous were also the observations made with aspirin in the hot plate test used in this study as occasional noxious stimuli (instead of foot shock). It was interesting to note though, that the mean reaction time of aspirin treated group in the test observed after its five or more daily oral doses remained constantly higher than those observed after its single low oral dose (20 mg/kg), whereas those of the PLE, piperlongumine, or doxycycline treated ones remained almost constant on all observational days. These observations made with low dose aspirin in this study are analogous to those reported elsewhere47 revealing its anti-stress, anxiolytic and antidepressant like activities in stressed mice and rats. They indicate that biological mechanisms and processes involved in the observed protective effects of low dose PLE and piperlongumine against foot shock stress triggered central hypersensitivity to pain are not identical to those involved in such effects of low dose aspirin. However, the possibility that after their higher daily oral doses PLE or piperlongumine also possesses aspirin like analgesic activities in hot plate test remains open.
Since aspirin like analgesic activities of piperlongumine, PLE, and doxycycline were also observed in the acetic acid writhing test after their 11 daily oral doses, it is apparent that they are also effective suppressors of inflammatory pain. Such efficacies of piperlongumine and PLE observed after their 5 mg/kg daily doses were almost equal and more pronounced than that observed after 10 fold higher daily doses of doxycycline. These observations indicate that apart from their bactericidal activities other peripheral mechanisms and processes could also be involved in observed anti-nociceptive effects of PLE and piperlongumine, or that the bactericidal activity profiles of PLE and piperlongumine are not identical to that of doxycycline. In any case, it remains certain that both piperlongumine and PLE are desensitizers of stress triggered central hypersensitivity to pain perception and they possess non-steroidal anti-inflammatory agents like anti-nociceptive activity.
Nociception triggered by noxious stimuli activates nociceptors, and repeated exposures to thermal stimuli outside the brain also alter central sensitivity to pain in hot plate test.48, 49, 50 Tissue injury and other peripheral noxious stimuli induces exaggerated releases of prostaglandins and other mediators and neurotransmitters regulating central sensitivity to pain perception, and inhibition of prostaglandin biosynthesis by aspirin is involved in its anti-inflammatory and analgesic activities.51 Similarities observed between the activity profiles of aspirin and PLE and piperlongumine in this study could indicate that modulation of prostaglandin homeostasis are involved in their modes of actions. However, since prostaglandins are also involved in the functions of the hypothalamic-pituitary-adrenal (HPA) axis through adrenergic and other neuro-hormonal processes regulating stress responses triggered by repeated testing,52 it could as well be that modulatory effects of PLE and piperlongumine on such processes are also involved in their observed analgesic activities. Therefore, efforts to verify such possibilities and to estimate the durations of actions of low dose piperlongumine, PLE and its bioactive constituents involved in its suppressing central hypersensitivity to pain are now being made in our laboratories.
The very first report pointing out adaptogenic potentials of a P. longum extract appeared in 1999,2 and since then the numbers of reports reconfirming stress resistance increasing activities of diverse types of P. longum extracts or for identifying their bioactive constituents have continued to increase.3 Although piperine and piperlongumine are now often considered to be the quantitatively major bioactive constituent of the plant, our observations revealed that only 1 or 5 mg daily oral doses of PLE containing only 1.75% piperine is as effective as the same daily oral doses of pure piperlongumine in increasing stress triggered alterations in body weight and temperatures or central hypersensitivity to nociceptive responses and adrenal gland weights. Therefore, it is apparent that other extractable bioactive constituent of the plant must also be involved in its observed high efficacy for stress resistance increasing and antinociceptive activities. These observations indicate that either other constituents of PLE are several fold more efficacious stress response modifier and analgesics than piperine and piperlongumine, or that the observed high efficacy of the extract is due to the synergistic effects between diverse such molecules constituting the major bulk of PLE.
To our judgments, the latter mentioned possibility is more plausible one than the other. All crude plant extracts contain numerous other non-nitrogenous molecules with stress resistance increasing phytochemicals after their fairly low daily oral doses. Observations made during our efforts to identify them from several other edible and other plants often used in Ayurvedic and other traditionally known systems of medicine have revealed that not only aromatic phenolic acids, but also fumaric acid and vitamins like ascorbic or nicotinic acids are effective in stress triggered alterations in body weights and temperature in laboratory rodents after their daily low oral doses.30, 31, 32, 47, 53, 54, 55, 56 Like in the present study, a consistent observation during those studies has also been that their anti-stress efficacies do not depend only on their daily doses but also on the number of treatment days. Most phytochemicals identified to date with such properties possess bactericidal activities and bioavailability of numerous of them (as judged by their circulating blood levels observed after their low oral doses) are fairly low or almost negligible. Since numerous such bactericidal phytochemicals ubiquitously present in all herbal extracts, they could also contribute to the high efficacy of the P. longum extract observed in this study.
In any case, observations made in the present study with doxycycline add further experimental evidences in favor of the working hypothesis that alterations in gut microbial ecology caused by repeated daily oral doses of antibiotics and other bactericidal phytochemicals are involved in their stress response modulating and other bioactivities. They also suggest that the bioassay procedure used in this study for comparing analgesic activities of aspirin and doxycycline with PLE and piperlongumine is well suited for identifying other bioactive constituents of PLE, or for better understanding of synergism between piperine and diverse other PLE constituents potentially involved in its observed nociceptive activities in stressed mice. Numerous reports have not only reconfirmed bactericidal activity of piperlongumine and it has recently been reported also that it also possess protective effects against bacterial toxins triggered abnormalities on barrier function of epithelial cells.57 Piperine also possess bactericidal activities, and it is also well known that pungency of piperine, capsaicin, and structurally diverse amide alkaloids are caused by activation of the heat- and acid-sensing ion channel TRPV 1.58 Such pain sensing transient receptor potential (TRP) channels function as molecular sensors of enviorenmental stimuli and initiate activity in pathways when they sense signs of tissue damage and inflammation.59 Although it has been reported that single oral 5 or 10 mg/kg doses of piperine posses anti-inflammatory activity and inhibits prostaglandin release in rodents, such low single oral doses of piperine have no significant analgesics like effects in 55 °C hot plate or in acetic acid induced writhing test.60
Therefore, it seems reasonable to assume that desensitization of TRPV1 and/or other receptors and biological processes involved in nociceptive responses, evolves only slowly after their repeated daily oral doses of the piperine containing extract PLE tested. Since more recent observations in our laboratories42 have revealed that 11 daily oral doses of PLE, piperlongumine, aspirin, or doxycycline to non-stressed mice do not have any significant effects in hot plate or foot shock stress induced hyperthermia tests, or on their body weights and basal rectal temperatures, it seems certain that their low dose anti-nociceptive effects observed in this study is mainly due to their anti-stress activities. However, since excellent bioavailability of oral 5 mg/kg piperlongumine has been observed in mice,10, 61 it could as well be that its primary sites of actions do not reside inside the gastro-intestinal tract only. However, the question whether such are also the cases for other alkyl amides, or for other PLE constituents, cannot yet be answered with any certainty.
It remains certain though, that both piperlongumine and PLE are very effective in increasing stress resistance in laboratory rodents, and that both of them can be considered as potential therapeutic leads for prevention of central hypersensitivity to pain accompanying numerous, if not all, chronic inflammatory diseases.62, 63 It has recently been reported that piperlongumine possess gastro protective effects and that fairly low oral doses (4.5 mg/kg) of piperlongumine affords protection against gastric ulcers and acid hyper-secretion.64 Since apart from piperine and piperlongumine, diverse other alkyl amides are encountered in P. longum and other plants of piper species,65 it could as well be that some such alkyl amides are also involved in its observed effects and that PLE, and that PLE and other plant extracts enriched in them are safer therapeutic option for pain prevention than aspirin and other currently available anti-inflammatory drugs. Therefore, efforts to identify the bioactive constituents of PLE involved in its low dose effects observed in this study could not only be useful for more rational pharmacological and analytical standardization of piperine containing P. longum extracts, but also for obtaining structurally and functionally novel therapeutic leads potentially useful for prevention and/or cure of enviorenmental stress and life style associated health problems eventually leading to central sensitivity syndromes.
5. Conclusion
Piperlongumine is an orally active stress resistance increasing alkyl amide with preventive effects against chronic stress triggered central hypersensitivity to pain. In this respect, the tested P. longum extract analytically standardized to contain only 1.75% of another such molecule piperine is as effective as piperlongumine. Further efforts to identify the bioactive constituents of this extract using the bioassay procedures reported in this study could lead to yet other structurally and functionally novel drug leads for prevention and cure of central sensitivity syndromes.
Conflicts of interest
The authors state that they have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Manoj P., Soniya E.V., Banerjee N.S., Ravichandran P. Recent studies on well-known spice, Piper longum Linn. Nat Prod Rad. 2004;3:222–227. [Google Scholar]
- 2.Rege N.N., Thatte U.M., Dahanukar S.A. Adaptogenic properties of six rasayana herbs used in ayurvedic medicine. Phytother Res. 1999;13:275–291. doi: 10.1002/(SICI)1099-1573(199906)13:4<275::AID-PTR510>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Kumar S., Kamboj J., Sharma S. Overview for various aspects of the health benefits of Piper longum linn. fruit. J Acupunct Meridian Stud. 2011;4:134–140. doi: 10.1016/S2005-2901(11)60020-4. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S., Malhotra S., Prasad A.K. Anti-inflammatory and antioxidant properties of piper species: a perspective from screening to molecular mechanisms. Curr Top Med Chem. 2015;15:886–893. doi: 10.2174/1568026615666150220120651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaveri M., Khandhar A., Patel S., Patel A. Chemistry and pharmacology of Piper longum L. Int J Pharm Sci Res. 2010;5:67–76. [Google Scholar]
- 6.Liu H.L., Luo R., Chen X.Q. Identification and simultaneous quantification of five alkaloids in Piper longum L. by HPLC-ESI-MS n and UFLC-ESI-MS/MS and their application to piper nigrum L. Food Chem. 2015;177:191–196. doi: 10.1016/j.foodchem.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 7.Bao N., Ochir S., Sun Z., Borjihan G., Yamagishi T. Occurrence of piperidine alkaloids in Piper species collected in different areas. J Nat Med. 2014;68:211–214. doi: 10.1007/s11418-013-0773-0. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y.H., Morris-Natschke S.L., Yang J., Niu H.M., Long C.L., Lee K.H. Anticancer principles from medicinal Piper (胡椒 Hú Jiāo) plants. J Trad Compl Med. 2014;4:8–16. doi: 10.4103/2225-4110.124811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yadav V., Kumar V. Advances in modern knowledge on dravyaguna of Piper longum during the past five years. Indian J Phamracol. 2014:46. [Google Scholar]
- 10.Bezerra D.P., Pessoa C., de Moraes M.O., Saker-Neto N., Silveira E.R., Costa-Lotufo L.V. Overview of the therapeutic potential of piplartine (piperlongumine) Eur J Pharm Sci. 2013;48:453–463. doi: 10.1016/j.ejps.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Seo Y.H., Kim J.K., Jun J.G. Synthesis and biological evaluation of piperlongumine derivatives as potent anti-inflammatory agents. Bioorg Med Chem Lett. 2014;24:5727–5730. doi: 10.1016/j.bmcl.2014.10.054. [DOI] [PubMed] [Google Scholar]
- 12.Hamrapurkar P.D., Jadhav K., Zine S. Quantitative estimation of piperine in Piper nigrum and Piper longum using high performance thin layer chromatography. J App Pharm Sci. 2011;1:117–120. [Google Scholar]
- 13.Qu H., Lv M., Xu H. Piperine: bioactivities and structural modifications. Mini Rev Med Chem. 2015;15:145–156. doi: 10.2174/1389557515666150101100509. [DOI] [PubMed] [Google Scholar]
- 14.Murunikkara V., Rasool M. Trikatu, an herbal compound as immunomodulatory and anti-inflammatory agent in the treatment of rheumatoid arthritis–an experimental study. Cell Immunol. 2014;287:62–68. doi: 10.1016/j.cellimm.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Neha J., Mishra R.N. Adaptogenic activity of Trikatu megaExt. Int J Res Pharm Biomed Sci. 2011;2:570–573. [Google Scholar]
- 16.Kilari E.K., Rao L.S.N., Sreemanthula S., Kola P.K. Anti-stress and nootropic activity of aqueous extract of Piper longum fruit, estimated by noninvasive biomarkers and Y-maze test in rodents. Environ Exp Biol. 2015;13:25–31. [Google Scholar]
- 17.Bai Y.F., Xu H. Protective action of piperine against experimental gastric ulcer. Acta Pharmacol Sin. 2000;21:357–359. [PubMed] [Google Scholar]
- 18.Li S., Wang C., Wang M., Li W., Matsumoto K., Tang Y. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci. 2007;80:1373–1381. doi: 10.1016/j.lfs.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 19.Wattanathorn J., Chonpathompikunlert P., Muchimapura S., Priprem A., Tankamnerdthai O. Piperine, the potential functional food for mood and cognitive disorders. Food Chem Toxicol. 2008;46:3106–3110. doi: 10.1016/j.fct.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Reddy S.P., Jamil K., Madhusudhan P., Anjani G., Das B. Antibacterial activity of isolates from Piper longum and Taxus baccata. Pharm Biol. 2001;39:236–238. [Google Scholar]
- 21.Dinan T.G., Cryan J.F. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinol. 2012;37:1369–1378. doi: 10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Arnold D.L., Jackson R.W., Waterfield N.R., Mansfield J.W. Evolution of microbial virulence: the benefits of stress. Trends Genet. 2007;23:293–300. doi: 10.1016/j.tig.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Dicks L.M., Botes M. Probiotic lactic acid bacteria in the gastro-intestinal tract: health benefits, safety and mode of action. Benef Microbes. 2010;1:11–29. doi: 10.3920/BM2009.0012. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y., Kasper L.H. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1–12. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh L.P., Mishra A., Saha D., Swarnakar S. Doxycycline blocks gastric ulcer by regulating matrix metalloproteinase-2 activity and oxidative stress. World J Gastroenterol. 2011;17:3310. doi: 10.3748/wjg.v17.i28.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mello B.S.F., Monte A.S., McIntyre R.S. Effects of doxycycline on depressive-like behavior in mice after lipopolysaccharide (LPS) administration. J Psychiatr Res. 2013;47:1521–1529. doi: 10.1016/j.jpsychires.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Cho Y., Jin Son H., Kim E. Doxycycline is neuroprotective against nigral dopaminergic degeneration by a dual mechanism involving mmp-3. Neurotox Res. 2009;16:361–371. doi: 10.1007/s12640-009-9078-1. [DOI] [PubMed] [Google Scholar]
- 28.Wang D.D., Englot D.J., Garcia P.A., Lawton M.T., Young W.L. Minocycline- and tetracycline-class antibiotics are protective against partial seizures in vivo. Epilepsy Behav. 2012;24:314–318. doi: 10.1016/j.yebeh.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chatterjee S.S., Kumar V. Holistic psychopharmacology and promiscuous plants and principles of ayurveda. Am J Plant Sci. 2012;3:1015–1021. [Google Scholar]
- 30.Langstieh A.J., Verma P., Thakur A.K., Chatterjee S.S., Kumar V. Desensitisation of mild stress triggered responses in mice by a Brasssica juncea leaf extracts and some ubiquitous secondary plant metabolites. Pharmacologia. 2014;5:326–338. [Google Scholar]
- 31.Thakur A.K., Soni U.K., Rai G., Chatterjee S.S., Kumar V. Protective effects of Andrographis paniculata extract and pure andrographolide against chronic stress-triggered pathologies in rats. Cell Mol Neurobiol. 2014;34:1111–1121. doi: 10.1007/s10571-014-0086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thakur A.K., Chatterjee S.S., Kumar V. Adaptogenic potential of andrographolide: an active principle of king of bitter (Andrographis paniculata) J Trad Compl Med. 2015;5:42–50. doi: 10.1016/j.jtcme.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zethof T.J.J., Heyden J.A.M., Tolboom J.T.B.M., Olivier B. Stress induced hyperthermia as a putative anxiety model. Eur J Pharmacol. 1995;294:125–135. doi: 10.1016/0014-2999(95)00520-x. [DOI] [PubMed] [Google Scholar]
- 34.Steru L., Chermat R., Thierry B., Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacol. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 35.Ojima K., Matsumoto K., Tohga M., Watanabe H. Hyperactivity of central noradrenergic and CRF system is involved in social isolation-induced decrease in pentobarbitone sleep. Brain Res. 1995;684:87–94. doi: 10.1016/0006-8993(95)00388-7. [DOI] [PubMed] [Google Scholar]
- 36.Turner R.A. Analgesics. In: Turner R., Ebborn P., editors. Screening Methods in Pharmacology. Academic Press; New York: 1965. pp. 100–102. [Google Scholar]
- 37.Paudel K.R., Das B.P., Rauniar G.P., Sangraula H., Deo S., Bhattacharya S.K. Antinociceptive effect of amitriptyline in mice of acute pain models. Indian J Exp Biol. 2007;45:529–531. [PubMed] [Google Scholar]
- 38.Shinde N.V., Kanase K.G., Shilimkar V.C., Undale V.R., Bhosale A.V. Antinociceptive and anti-inflammatory effects of solvent extracts of Tagetes erectus Linn (Asteraceae) Trop J Pharm Res. 2009;8:325–329. [Google Scholar]
- 39.Salman T.M., Alagbonsi I.A., Biliaminu S.A. Blood glucose-lowering effect of Telfairia Occidentalis: a preliminary study on the underlying mechanism and responses. Biokemistri. 2013;25:133–139. [Google Scholar]
- 40.Tasleem F., Azhar I., Ali S.N., Perveen S., Mahmood Z.A. Analgesic and anti-inflammatory activities of Piper nigrum L. Asian Pac J Trop Med. 2014:S461–S468. doi: 10.1016/S1995-7645(14)60275-3. [DOI] [PubMed] [Google Scholar]
- 41.Vedhanayaki G., Shastri G.V., Kuruvilla A. Analgesic activity of Piper longum Linn. root. Indian J Exp Biol. 2003;41:649–651. [PubMed] [Google Scholar]
- 42.Yadav V., Chatterjee S.S., Majeed M., Kumar V. Long lasting preventive effects of piperlongumine and a Piper longum extract against stress triggered pathologies in mice. J Intercult Ethnopharmacol. 2015;4:277–283. doi: 10.5455/jice.20150921010411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar V., Thakur A.K., Verma S., Yadav V., Chatterjee S.S. Potential of some traditionally used edible plants for prevention and cure of diabesity associated comorbidities. TANG Humanit Med. 2015;5:e8. [Google Scholar]
- 44.Rios M.Y., Olivo H.F. Natural and synthetic alkamides: applications in pain therapy. Stud Nat Prod Chem. 2014:79. [Google Scholar]
- 45.Kumar V., Chatterjee S.S. Single and repeated dose effects of phytochemicals in rodent behavioural models. EC Pharm Sci. 2014;1:16–18. [Google Scholar]
- 46.Panossian A., Wagner H. Stimulating effect of adaptogens: an overview with particular reference to their efficacy following single dose administration. Phytother Res. 2005;19:819–838. doi: 10.1002/ptr.1751. [DOI] [PubMed] [Google Scholar]
- 47.Khan S.A., Chatterjee S.S., Kumar V. Potential anti-stress, anxiolytic and antidepressant like activities of mono-hydroxybenzoic acids and aspirin in rodents: a comparative study. Austin J Pharmacol Ther. 2015;3:1073. [Google Scholar]
- 48.Cesare P., McNaughton P. Peripheral pain mechanisms. Curr Opin Neurobiol. 1997;7:493–499. doi: 10.1016/s0959-4388(97)80028-1. [DOI] [PubMed] [Google Scholar]
- 49.Besson J.M. The neurobiology of pain. Lancet. 1999;353:1610–1615. doi: 10.1016/s0140-6736(99)01313-6. [DOI] [PubMed] [Google Scholar]
- 50.Wilson S.G., Mogil J.S. Measuring pain in the (knockout) mouse: big challenges in a small mammal. Behav Brain Res. 2001;125:65–73. doi: 10.1016/s0166-4328(01)00281-9. [DOI] [PubMed] [Google Scholar]
- 51.Okuse K. Pain signaling pathway from cytokines to ion channel. Int J Biochem Cell Biol. 2007;39:490–496. doi: 10.1016/j.biocel.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 52.Bugajski J. Role of prostaglandins in the stimulation of the hypothalamic-pituitary-adrenal axis by adrenergic and neurohormone systems. J Physiol Pharmacol. 1996;47:559–575. [PubMed] [Google Scholar]
- 53.Thakur A.K., Dey A., Chatterjee S.S., Kumar V. Reverse ayurvedic pharmacology of ashwagandha as an adaptogenic anti-diabetic plant: a pilot study. Curr Trad Med. 2015;1:51–61. [Google Scholar]
- 54.Verma S., Chatterjee S.S., Kumar V. Metformin like stress response modulating effects of turmeric curcuminoids in mice. SAJ Neurol. 2015;1:102. [Google Scholar]
- 55.Shivavedi N., Chatterjee S.S., Kumar V. Evaluation of pharmacologically interesting dose range of ascorbic acid in mice. SAJ Neurol. 2014;1:1–8. [Google Scholar]
- 56.Shivavedi N., Chatterjee S.S., Kumar V. Stress response modulating effects of lactic acid in mice. Ther Targets Neurol Dis. 2014;1:e418. [Google Scholar]
- 57.Möhler H., Pfirrmann R.W., Frei K. Redox-directed cancer therapeutics: taurolidine and piperlongumine as broadly effective antineoplastic agents (review) Int J Oncol. 2014;45:1329–1336. doi: 10.3892/ijo.2014.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McNamara F.N., Randall A., Gunthorpe M.J. Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1) Br J Pharmacol. 2005;144:781–790. doi: 10.1038/sj.bjp.0706040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo J., Walters E.T., Carlton S.M., Hu H. Targeting pain-evoking transient receptor potential channels for the treatment of pain. Curr Neuropharmacol. 2013;11:652–663. doi: 10.2174/1570159X113119990040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sudjarwo S.A. The potency of piperine as antiinflammatory and analgesic in rats and mice. Folia Medica Indones. 2005;41:190–194. [Google Scholar]
- 61.Raj L., Ide T., Gurkar A.U. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Boomershine C.S. Fibromyalgia: the prototypical central sensitivity syndrome. Curr Rheumatol Rev. 2015;11:131–145. doi: 10.2174/1573397111666150619095007. [DOI] [PubMed] [Google Scholar]
- 63.Yunus M.B. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum. 2008;37:339–352. doi: 10.1016/j.semarthrit.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Burci L.M., Pereira I.T., da Silva L.M. Antiulcer and gastric antisecretory effects of dichloromethane fraction and piplartine obtained from fruits of Piper tuberculatum Jacq. in rats. J Ethnopharmacol. 2013;148:165–174. doi: 10.1016/j.jep.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 65.Gutierrez R.M., Gonzalez A.M., Hoyo-Vadillo C. Alkaloids from piper: a review of its phytochemistry and pharmacology. Mini Rev Med Chem. 2013;13:163–193. doi: 10.2174/138955713804805148. [DOI] [PubMed] [Google Scholar]