Abstract
Acorus calamus is a plant commonly used as a traditional herbal medicine and possesses the wide range of pharmacological applications. The present study investigated the diuretic and antiurolithiatic activities of an ethanolic extract of Acorus calamus L. (Family: Araceae) rhizome (EEAC). For diuretic activity, three doses of EEAC (250, 500 and 750 mg/kg) were studied, and measurement of the urinary volume and electrolytes (Na+ and K+) concentration were taken as evaluation parameters. On the other hand, ethylene glycol induced urolithiasis (0.75% v/v in drinking water for 28 days) was used to study the antiurolithiatic effect of EEAC at the oral dose of 750 mg/kg in male Wistar albino rats. CYSTONE (750 mg/kg, p.o.) was used as a standard reference drug in the present study. After completion of the 28-days respective treatments, the level of various urolithiatic promoters in the biological samples (urine, serum and kidney homogenate) and renal function were used as criteria for assessing the antiurolithiatic effect of EEAC. Results indicate that, the EEAC (750 mg/kg, p.o.) produced significant increase in urine volume (p < 0.001) and urinary excretion of Na+ and K+ electrolytes (p < 0.05) in a pattern comparable to that of furosemide. In ethylene glycol induced urolithiatic model, EEAC significantly (p < 0.05) decreased excretion and deposition of various urolithiatic promoters as compared to urolithiatic control in a pattern comparable to that of CYSTONE. The EEAC supplementation also prevents the impairment of renal functions. The antiurolithiatic mechanism is mediated possibly through diuretic and nephroprotective actions of the active compounds of rhizomes.
Keywords: Acorus calamus, Calcium oxalate, Diuresis, Ethylene glycol, Urolithiasis
Graphical abstract
1. Introduction
Urolithiasis is one such disease that after extensive research in the field of urology has remained incurable in allopathy. It is a process of stone formation which occurs either in the kidney (commonly known as nephrolithiasis) and or in any part of the urinary tract, including the ureters (known as ureteral stone) and bladder (bladder stone). Urolithiasis has an important effect on the health care system with a prevalence of >10% and an expected recurrence rate of ∼50%.1 The worldwide incidence of urolithiasis is quite high, and more than 80% of urinary calculi are calcium oxalate (CaOx) stones alone or CaOx mixed with calcium phosphate.2 Epidemiological studies revealed that the nephrolithiasis is more prevalent in men (12%) than in women (6%) and is more prevalent between the ages of 20–40 in both sexes.3
Various sophisticated investigations, including radiological and other laboratory techniques are not sufficiently helpful to elucidate the exact causes and mechanisms of stone formation.4 However, various factors that might be responsible for the formation of stone have been extensively studied recently. It is believed that when the urine becomes saturated with insoluble materials because of excretion rates are excessive which leads to crystals formation and aggregation to form a stone.5 Urolithiasis needs both preventive and curative therapy. Currently, there are no satisfactory drugs in modern medicine, which can dissolve the stone and therefore physicians remain to be depending on alternative systems of medicine for better relief.5 The other medical management of urolithiasis mainly involves the surgical removal of stones. Techniques such as extracorporeal shock wave lithotripsy (ESWL) and percutaneous nephrolithotomy (PCNL) do not assure the prevention of recurrence of the stone. Moreover, they cause side effects such as hemorrhage, hypertension, tubular necrosis and subsequent fibrosis of the kidney.6 In the traditional systems of medicine, most of the remedies were taken from plants and that were proved to be useful in various disease conditions. Noteworthy, traditional herbal medicines are efficacious and have lesser side effects compared to modern drugs and also reduce the recurrence rate of the renal stone. The vast Ayurvedic literature claims a number of plants to be useful in the treatment of urinary stones; still many plants need to be exploited for their pharmacological actions.7
Acorus calamus L. (Family: Araceae) commonly known as ‘sweet flag', a well-known medicinal plant of the Indian medicinal system, has been used traditionally in a wide variety of ailments.8, 9, 10 The major chemical constituents of A. calamus rhizome are alkaloids, flavonoids, gums, lectins, mucilage, phenols, quinone, saponins, sugars, tannins, and triterpenes.11 The essential oil components of rhizomes comprise mainly α-asarone, β-asarone, γ-asarone, isoeugenol acorenone, iso-acorone, Z-sesquilavandulol and dehydroxy isocalamendiol.12 In laboratory experiments, the rhizomes of A. calamus have been reported various pharmacological activities which include antidiabetes,13 antiproliferative and immunosuppressive,14 antidiarrheal,15 hypolipidemic,16 antioxidant,17 diuretic,18 and nephroprotective19 activities. As per the indigenous system of medicine, A. calamus has been used for the treatment of insomnia, epilepsy, hysteria, loss of memory, remittent fevers and neurosis.20 The rhizomes of the plant possess diuretic and antioxidant properties. Furthermore, the rhizome of the plant has also a significant nephroprotective effect. Based on this literature background, we hypothesized that A. calamus rhizomes might be effective in urolithiatic condition. However, there is a lack of scientific study reporting its antiurolithiatic activity in experimental induced renal stone formation in a laboratory animal. Hence, in the present study, the ethanolic extract of A. calamus evaluated against ethylene glycol induced renal calculi in Wistar albino rats.
2. Material and methods
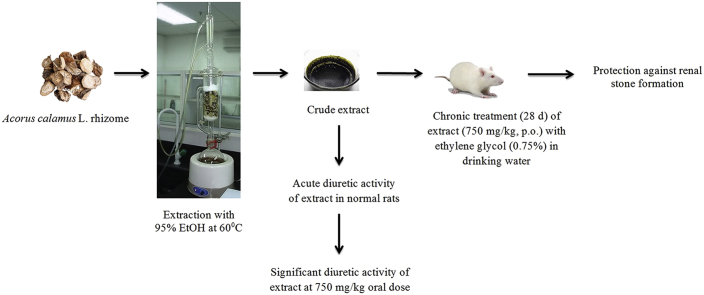
2.1. Identification, collection and extraction of plant material
The rhizomes of A. calamus were purchased from the Sanjivani Ayurvedic Store, Rajkot, India and were identified by Prof. Vishal Muliya, Taxonomist, Department of Botany, Christ College, Rajkot, India. The rhizomes of A. calamus were powdered with a mechanical grinder to obtain a coarse powder. Equal quantity of powder was passed through 40 mesh sieve to get a coarse powder of desired particle size. The powdered material was subjected to successive extraction with ethanol (95% v/v) in a Soxhlet apparatus at 60 °C. Appearance of colorless solvent in the siphon tube was taken as the end point of extraction. The extracts were concentrated to ¾ of its original volume by distillation. The concentrated extracts were taken in a china dish and evaporated on a thermostat controlled water bath until it forms a thick paste. The extract was dried and stored in a refrigerator at 4 °C in a glass bottle throughout the study. The dried, crude concentrated extracts were labeled as EEAC. The yield was found to be 12.8% w/w.
2.2. Chemicals
Ethylene glycol was purchased from Sulab laboratory, Baroda, India. Various kits for biochemical estimation of urine and serum were purchased from Span Diagnostics, Surat, India. CYSTONE was purchased from Shrinath Pharmacy, Rajkot, India. All other chemicals and reagents used were of analytical grade and procured from approved chemical suppliers.
2.3. Preliminary phytochemical analysis
The EEAR was subjected to qualitative analysis of the various phytoconstituents by standard methods.21 It revealed the presence of carbohydrates, glycosides, alkaloids, tannins and phenolic compounds.
2.4. Animals
Healthy adult Wistar male rats weighing 150–200 g of equivalent age groups, were procured from Zydus Research Center, Ahmedabad, India. Upon arrival, the rats were randomly housed under hygienic conditions in polypropylene cages (3 per cage) to minimize isolation stress, if any. The animal facility was well-ventilated and maintained at an ambient temperature of 24 ± 2 °C having 50–60% relative humidity with 12-h light and dark cycle. The animals were acclimatized to the laboratory conditions for one week prior to the experiments and provided with standard diet (JS Exports, Kanpur India) and water ad libitum. The use and care of the animals in this experimental protocol was approved by the Institutional Animal Care and Ethics Committee (Approval Number: SJT/025-2011) following guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animal (CPCSEA), Government of India.
2.5. Preliminary phytochemical screening
Qualitative chemical tests were conducted for preliminary phytochemical screening of the ethanolic extract of A. calamus to know the nature of phytoconstituents present in extract.
2.6. Diuretic activity of EEAC in rats
The method described by Lipschitz was employed for the assessment of the diuretic activity of EEAC.22 The animals were fasted for 18 h prior to experiment allowing only water during the fasting period. Thirty (30) healthy albino Wistar rats were selected and divided into five groups consisting of six rats in each group. Group I: Normal control group; received sodium carboxymethyl cellulose (CMC, 0.5% w/v) orally at the dose of 10 ml/kg. Group II: Standard group: received furosemide orally at the dose of 15 mg/kg. Group III to Group V served as test groups and received EEAC orally at the dose of 250, 500 and 750 mg/kg respectively. After 1 h of respective treatments, the animals were kept in metabolic cages individually for the collection of urine. The urine was collected for 7 h after the dose was administered. The bladder was emptied by pulling the base of the tail of each rat. Diuretic assay parameters such as total urine volume and urinary excretion of Na+ and K+ were measured in collected urine. The total difference in the collected urine volume of the respective test group was compared with the standard and control group. Furthermore, the ratio of urine volume of the test group and the control group was calculated as a diuretic index. Estimation of Na+ and K+ content of the urine samples treated with EEAC at different doses as well as other groups was done by flame photometry.23 The urinary Na+ and K+ content of the respective test groups were also compared with that of control and standard group.
2.7. Ethylene glycol-induced urolithiasis model in rats
Ethylene glycol-induced urolithiasis model was used to assessing the urolithiatic activity in albino Wistar rats.24, 25 Animals were divided into four different groups, each containing six animals. The normal control group maintained on a standard laboratory diet and drinking water ad libitum and treated with vehicle (1% CMC) for 28 days. All remaining groups received calculi inducing treatment for 28 days, comprised 0.75% v/v ethylene glycol in drinking water. The urolithiatic control group received vehicle (1% sodium CMC) by oral gavage once daily for 28 days. The EEAC group treated with 750 mg/kg of EEAC by oral gavage once daily for 28 days, while the positive control group treated with 750 mg/kg of CYSTONE by oral gavage once daily for 28 days.
2.7.1. Collection and analysis of urine
After 28 days of an experimental period, all animals were kept in individual metabolic cages with the hydration of 15 ml of water, and urine samples of 24 h were collected. Animals had free access to drinking water during the urine collection period. A drop of concentrated hydrochloric acid was added to the collected urine and stored at 4 °C. After urine collection, total urinary excretion of calcium, oxalate, phosphate and uric acid were measured by various biochemical kits (Span Diagnostics, Surat, India) according to manufacturer's instruction.
2.7.2. Serum analysis
After urine collection on 29th-day blood was collected retro-orbitally under mild anesthetic condition and animals were sacrificed by cervical decapitation. Serum was separated by centrifugation at 12,000 rpm for 5 min and analyzed for calcium, phosphate, urea, uric acid and creatinine by using various biochemical kits (Span Diagnostics, Surat, India) according to the manufacturer's instruction.
2.7.3. Preparation of kidney homogenate and biochemical estimation
The abdomen was cut open to remove both kidneys from each animal. Isolated kidneys were cleaned from extraneous tissue and rinsed with ice-cold physiological saline and dried at 80 °C in a hot air oven. A sample of 100 mg of the dried kidney was boiled in 10 ml of 1N hydrochloric acid for 30 min and homogenized. The homogenate was centrifuged at 2000 rpm for 10 min, and the supernatant was separated. The supernatant was analyzed for calcium, phosphate, oxalate and uric acid by using various biochemical kits (Span Diagnostics, Surat, India) according to the manufacturer's instruction.
2.8. Statistical analysis
All values were expressed as mean ± SEM (standard error of mean) of six rats (n = 6). The statistical analysis was done by analysis of variance (ANOVA) followed by Dunnett's multiple comparison tests. The value of p < 0.05 was considered as significant. The statistical software used was GraphPad Prism (version 5.0).
3. Results
3.1. Preliminary phytochemical screening
The EEAC was subjected to qualitative analysis of the various phytoconstitutents by standard methods. It revealed the presence of carbohydrates, glycosides, alkaloids, tannins and phenolic compounds.
3.2. Effect of EEAC on diuresis
EEAC did not produce a significant increase in urine volume at the dose of 250 and 500 mg/kg, while EEAC at the dose of 750 mg/kg showed significantly (p < 0.001) increase in the urine volume (with good diuretic index; >1.5) as compared to a normal control group. Furthermore, the effect of EEAC (750 mg/kg) was also comparable with that of the standard diuretic agent, furosemide (Table 1). The EEAC at the dose of 750 mg/kg showed significantly (p < 0.05) increase in urinary electrolytes Na+ and K+ excretion as compared to the control group. Furosemide also significantly (p < 0.05) enhanced the urinary Na+ and K+ excretion when compared to the control group (Table 1).
Table 1.
Effect of EEAC on urine volume, diuretic index, urinary Na+ and K+ excretion in normal rats.
| Group | Dose (p.o.) | Volume of urine (ml) | Diuretic index | Na+ (mEq/L) | K+ (mEq/L) | Na+/K+ |
|---|---|---|---|---|---|---|
| Control | 10 ml/kg | 1.88 ± 0.18 | – | 62.50 ± 8.73 | 9.59 ± 0.79 | 6.51 |
| Furosemide | 15 mg/kg | 4.02 ± 0.17∗ | 2.14 | 94.64 ± 10.13∗ | 13.88 ± 0.80∗∗ | 6.81 |
| EEAC 1 | 250 mg/kg | 1.78 ± 0.13 | 0.95 | 66.25 ± 5.58 | 9.45 ± 0.64 | 7.01 |
| EEAC 2 | 500 mg/kg | 2.21 ± 0.14 | 1.18 | 77.41 ± 9.23 | 11.16 ± 0.59 | 6.93 |
| EEAC 3 | 750 mg/kg | 3.78 ± 0.11∗ | 2.01 | 82.47 ± 3.80∗ | 12.06 ± 0.47∗ | 6.83 |
All values are expressed as mean ± SEM of 6 rats (n = 6), *p < 0.001, when compared to control, p.o. = per oral, mEq/L = Milli equivalents per liter.
3.3. Effect of EEAC on ethylene glycol-induced urolithiasis
3.3.1. Effect of EEAC on body weight, urine volume, and kidney weight
Administration of ethylene glycol (0.75% v/v in drinking water) produced significant (p < 0.05) reduction in body weight of urolithiatic control rats. These changes were significantly (p < 0.001) prevented in CYSTONE and EEAC treated groups as compared to urolithiatic control rats. Significant (p < 0.001) decrease in 24-h urine volume (ml) was observed in urolithiatic control rats while EEAC showed significant (p < 0.001) improvement in urinary output as compared to urolithiatic control animals. Furthermore, there was significant (p < 0.05) increase in wet weight of kidney (g) in urolithiatic control rats, which was significantly (p < 0.05) prevented by EEAC and CYSTONE. The same scenario was observed in dry kidney weight. (Table 2).
Table 2.
Effect of EEAC on various physical parameters of ethylene glycol induced urolithiasis in Wistar rats.
| Group | Dose (p.o.) | Physiological parameter |
|||
|---|---|---|---|---|---|
| % Change in body weight | Diuresis (ml) | Wet kidney weight (g) | Dry kidney weight (g) | ||
| Normal control | 10 ml/kg | 5.80 ± 0.74 | 9.39 ± 0.75 | 1.425 ± 0.12 | 0.854 ± 0.02 |
| Urolithiatic control | 10 ml/kg | −8.20 ± 1.01# | 6.91 ± 0.57# | 2.29 ± 0.05# | 1.02 ± 0.03# |
| EEAC | 750 mg/kg | 3.79 ± 0.30∗∗ | 16.90 ± 0.65∗∗ | 1.772 ± 0.05∗∗ | 0.991 ± 0.04 |
| CYSTONE | 750 mg/kg | 3.86 ± 0.35∗∗ | 18.17 ± 0.80∗∗ | 1.677 ± 0.06∗∗ | 0.882 ± 0.03∗ |
All values are expressed as mean ± SEM of 6 rats (n = 6); #p < 0.05 when compared to normal control, *p < 0.05 and **p < 0.001 when compared to urolithiatic control, p.o. = per oral.
3.3.2. Effect of EEAC on urine analysis
The urinary excretion of various urolithiatic promoters such as calcium, oxalate, phosphate and uric acid was measured. There were significant (p < 0.05) increase in the urinary excretion of calcium, oxalate, phosphate and uric acid in the urine of urolithiatic control rats as compared to normal control rats. However, supplementation with EEAC displayed a significant (p < 0.05) reduction in urinary excretion of calcium, oxalate, phosphate and uric acid as compared to urolithiatic control rats. Furthermore, these results are also comparable with the standard drug, CYSTONE that also significantly (p < 0.05) reduced urolithiatic promoters in the urinary excretion of urolithiatic control rats (Table 3).
Table 3.
Effect of EEAC on urinary excretion of various urolithiatic factors of ethylene glycol induced urolithiasis in Wistar rats.
| Group | Dose (p.o.) | Urine parameter (mg/24 h) |
|||
|---|---|---|---|---|---|
| Calcium | Oxalate | Phosphate | Uric acid | ||
| Normal control | 10 ml/kg | 5.10 ± 0.41 | 0.69 ± 0.12 | 6.07 ± 0.28 | 3.65 ± 0.19 |
| Urolithiatic control | 10 ml/kg | 9.73 ± 0.35# | 2.21 ± 0.25# | 10.28 ± 0.32# | 6.16 ± 0.11# |
| EEAC | 750 mg/kg | 6.34 ± 0.20∗ | 1.04 ± 0.18∗ | 7.18 ± 0.23∗ | 4.24 ± 0.11∗ |
| CYSTONE | 750 mg/kg | 6.23 ± 0.29∗ | 0.95 ± 0.15∗ | 7.21 ± 0.21∗ | 4.29 ± 0.13∗ |
All values are expressed as mean ± SEM of 6 rats (n = 6), #p < 0.001 when compared to normal control and * p < 0.001 when compared to urolithiatic control, p.o. = per oral.
3.3.3. Effect of EEAC on serum analysis
Renal stone induction causes impairment of renal functions and resulting in the serum elevation of glomerular and tubular damage markers. In the present study, the rats treated with ethylene glycol alone showed significantly (p < 0.01) elevation of various serum markers including calcium, phosphate, uric acid, blood urea nitrogen and creatinine compared with the normal control rats. However, EEAC along with ethylene glycol produced significantly (p < 0.05 and p < 0.01 and p < 0.001) reduction in serum calcium, phosphate, uric acid, blood urea nitrogen and creatinine compared with the ethylene glycol alone-treated controls and the results are comparable with the value observed with the standard drug, CYSTONE (Table 4).
Table 4.
Effect of EEAC on serum concentration of various urolithiatic factors of ethylene glycol induced urolithiasis in Wistar rats.
| Group | Dose (p.o.) | Serum parameter (mg/dl) |
||||
|---|---|---|---|---|---|---|
| Calcium | Phosphate | Uric acid | Urea | Creatinine | ||
| Normal control | 10 ml/kg | 7.22 ± 0.35 | 3.35 ± 0.16 | 7.18 ± 0.28 | 7.18 ± 0.28 | 0.50 ± 0.05 |
| Urolithiatic control | 10 ml/kg | 9.42 ± 0.29# | 4.40 ± 0.17# | 8.38 ± 0.19# | 8.38 ± 0.19# | 1.65 ± 0.06# |
| EEAC | 750 mg/kg | 7.53 ± 0.40∗∗∗ | 3.47 ± 0.22∗ | 7.34 ± 0.15∗∗ | 7.34 ± 0.15∗ | 0.72 ± 0.04∗∗ |
| CYSTONE | 750 mg/kg | 7.27 ± 0.31∗∗∗ | 3.32 ± 0.21∗∗ | 7.28 ± 0.17∗∗∗ | 7.28 ± 0.17∗∗ | 0.61 ± 0.04∗∗ |
All values are expressed as mean ± SEM of 5 rats (n = 6), #p < 0.01 when compared to normal control and * p < 0.05, **p < 0.01 and ***p < 0.001 when compared to urolithiatic control, p.o. = per oral.
3.3.4. Effect of EEAC on kidney homogenate analysis
The deposition of urolithiatic promoters in the renal tissues, namely calcium, oxalate, phosphate and uric acid were recorded. However, those promoters were found to be significantly (p < 0.05) higher in the renal tissue of ethylene glycol alone treated urolithiatic control rats compared to the normal control rats. EEAC treatment with ethylene glycol produced significantly (p < 0.01 and p < 0.001) reduction in the deposition of urolithiatic promoters compared with urolithiatic control rats (Table 5).
Table 5.
Effect of EEAC on various urolithiatic factors in kidney homogenate of ethylene glycol induced urolithiasis in Wistar rats.
| Group | Dose (p.o.) | Kidney homogenate parameter (mg/100 mg kidney weight) |
|||
|---|---|---|---|---|---|
| Calcium | Oxalate | Phosphate | Uric acid | ||
| Normal control | 10 ml/kg | 2.29 ± 0.14 | 0.72 ± 0.05 | 1.38 ± 015 | 1.80 ± 0.05 |
| Urolithiatic control | 10 ml/kg | 4.62 ± 0.16# | 1.47 ± 0.12# | 2.36 ± 0.16# | 2.67 ± 0.26# |
| EEAC | 750 mg/kg | 2.60 ± 0.20∗∗ | 0.75 ± 0.06∗∗ | 1.48 ± 0.14∗ | 1.72 ± 0.08∗ |
| CYSTONE | 750 mg/kg | 2.56 ± 0.12∗∗ | 0.70 ± 0.06∗∗ | 1.26 ± 0.15∗∗ | 1.61 ± 0.09∗∗ |
All values are expressed as Mean ± SEM (n = 6); #p < 0.01 when compared to normal control, *p < 0.01 and **p < 0.001 when compared to urolithiatic control, p.o. = per oral.
4. Discussion
In the present study, we demonstrated diuretic and antiurolithiatic activities of an ethanolic extract of A. calamus (EEAC) in experimentally induced animal models. Initially, the diuretic activity of three different doses (250, 500 and 750 mg/kg) of EEAC has been carried out to establish an effective dose for chronic antiurolithiatic activity. The administration of a single oral dose of EEAC (250, 500 and 750 mg/kg) markedly increases diuresis as indicated by increase urinary flow. Additionally, acute treatment with EEAC also increases dose dependent excretion of both Na+ and K+ electrolytes. However, a significant diuretic activity has been observed with the highest dose (750 mg/kg) which also showed marked kaliuresis and natriuresis. Moreover, the increase in the ratio of concentration of excreted Na+ and K+ for the EEAC indicates that the EEAC increases Na+ excretion to a greater extent than K+ which is essential quality of a good diuretic agent with lesser hyperkalaemic side effect. Furosemide, a standard diuretic used in this study also increased urine volume and excretion of Na+ and K+ suggested that stimulation of diuresis by EEAC could be similar to that of furosemide. Such rapid and acute diuretic activity of EEAC may be due to active phytoconstituents such as alkaloids, steroids, tannins, phenolic compound, terpenoids and flavonoids, which are present in EEAC detected by phytochemical analysis in our study.
In the present study, we also examined the protective effects of EEAC in ethylene glycol induced renal calculi in albino Wistar rat. Urinary supersaturation of various stone-forming elements is commonly considered to be one of the causative factors in the stone formation.26 Renal calcium oxalate (CaOx) crystal deposition induced by ethylene glycol is the most appropriate animal model that is frequently used to mimic the stone formation in humans. Previously reported studies showed that after the 28 days period of ethylene glycol (0.75% v/v) administration in the drinking water of laboratory animals significantly cause renal stone formation that mainly consist CaOx by increasing the urinary concentration of oxalate.27, 28, 29, 30, 31 Furthermore, Robinson et al has demonstrated that 0.75% v/v of ethylene glycol supplement in drinking water for 90 days in laboratory rats did not produce any carcinogenicity or gastric ulcer.32 Ethylene glycol is readily absorbed in the intestine and is metabolized in the liver to oxalate leading to hyperoxaluria. The oxalate precipitates in the urine as CaOx due to its poor solubility. CaOx crystals and high oxalate levels in nephrons damage epithelial cells, inducing heterogeneous crystal nucleation and causing aggregation of crystals.33, 34 The urinary system of male rats also resembles that of humans therefore it is rational to use this disease model to examine the protective effects of chronic treatment of EEAC. To our knowledge, this is the first study to use ethylene glycol induced renal calculi rat model in investigating the protective effects of the EEAC on urolithiasis.
Weight loss observed in urolithiatic rats is due to anorexia and disturbances in carbohydrates, proteins or fat metabolism that is affected by the ingestion of ethylene glycol. The present study showed a significant reduction in body weight of urolithiatic rats that was effectively suppressed by EEAC treatment. Furthermore, urine volume was also significantly reduced in urolithiatic rats that might be due to crystal and stone formation in renal tissue. It is reported that the increase in the urinary excretion volume facilitates the removal of small crystals and reduces the chance of these crystals to grow or aggregate.35 However, supplementation of EEAC markedly improves the urinary excretion volume (diuresis) and thereby hastens the process of dissolving the pre-formed stones and prevention of new stone formation in the urinary system. An increase in dry and wet kidney weight is also observed in urolithiatic control rats due to deposition of various urolithiatic promoters in the kidney. Conversely, EEAC decreases kidney weight by suppressing the deposition of various urolithiatic promoters in the kidney.
It is well recognized that hyperoxaluria is a far more significant risk factor in the pathogenesis of renal stones than hypercalciuria.36 The changes in urinary oxalate levels are relatively much more significant and have about 15-fold greater effect than those of calcium.25, 37, 38 In the present study, ethylene glycol treatment markedly increased the oxalate level in the urine of urolithiatic rats that was reduced by chronic EEAC treatment. The decrease in oxalate excretion by EEAC was might be either due to the inhibition of the formation of oxalate, or the EEAC might interfere with oxalate metabolism. Moreover, literature indicates that the plant extract rich in tannins, flavonoids and isoflavonoids can lead to relaxation of smooth muscle of the urinary and biliary tract which could facilitate the expulsion of stones from the kidney and diminished the size of calculi in rats.31, 39 The phytochemical analysis of EEAC in our study and previously reported studies9, 11, 12, 40 have already confirmed that EEAC is the rich source of tannins, flavonoids, and isoflavonoids.
The ethylene glycol is metabolized to acidic metabolites like hippuric acid, oxalic acid, formic acid and benzoic acids which cause metabolic acidosis and further produced defects in proximal tubule HCO3− reabsorption. Acidosis produces renal calcium leak associated with the gut absorption of calcium and bone calcium release that leads to hypercalciuria and hypercalcemia.41, 42, 43 Moreover, increased urinary calcium is a factor favoring the nucleation and precipitation of CaOx or calcium phosphate from urine and subsequent crystal growth.44 A similar change was observed in the present study, the administration of ethylene glycol cause increased calcium level in urine, serum and kidney homogenate thereby promoting the formation of CaOx stones. The treatment of EEAC reduced calcium in the urine, serum, and kidney homogenate in urolithiatic rats and thereby prevents the formation and aggregation of stone. An increase in urinary phosphate is observed in urolithiatic rats. Increased urinary phosphate excretion along with oxalate stress seems to provide an environment appropriate for stone formation by forming calcium phosphate crystals, which epitaxially induces calcium oxalate deposition.45 Treatment of EEAC restores phosphate level in serum, urine and kidney homogenate of urolithiatic rats, thus reducing the risk of stone formation.
The present study also showed that there were an increased calcium and oxalate levels in kidney homogenate by ethylene glycol treatment in urolithiatic rats. In other words, ethylene glycol increased the deposition of calcium and oxalate contents in renal tissue that was significantly attenuated by EEAC administration. The increase in calcium level in renal tissue might be due to the increased bioavailability of nitric oxide (NO) which in turn activates cGMP (3′, 5′-cyclic guanosine monophosphate) that controls the increase in intracellular calcium levels. Literature indicates that NO donors have the capacity to control the intracellular rise in calcium levels.30, 31, 46 EEAC could decrease the calcium level by increasing the bioavailability of NO to sequester calcium through the cGMP pathway. Furthermore, ethylene glycol also increased oxalate production by increasing substrate availability for various oxalate synthesizing enzymes such as glycolic acid oxidase and lactate dehydrogenase, which catalyze the oxidation and reduction of glyoxalate into glycolate and oxalate.38 These changes facilitate the hyperoxaluria and subsequent CaOx crystal adherence and retention in renal tubules.47 Hence, EEAC might effectively control the level of oxalate by inhibiting the synthesis of oxalate.
In urolithiasis, the glomerular filtration rate (GFR) decreases due to the obstruction to the outflow of urine by stones in the urinary system. Due to this, the waste products, particularly nitrogenous substances such as urea, uric acid and, creatinine get accumulated in blood.48 In the present study, elevation of serum urea, uric acid and, creatinine have been noted which were restored by treatment with EEAC and thereby improved renal functions in urolithiatic condition.
5. Conclusion
In conclusion, EEAC showed remarkable protection from ethylene glycol induced urolithiasis by strongly suppressing various urolithiatic promoters in serum, urine, and kidney tissue. Furthermore, EEAC has a significant diuretic action that can further help to flush those promoters in urine and also increases the dissolution of pre-formed stones and prevention of new stone formation (recurrence). Thus, the present finding emphasizes that the rhizomes of A. calamus possess potential medicinal value and beneficial in the prevention of renal stone. Further studies need to be undertaken to explain detail mechanism(s) of action of A. calamus rhizomes.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors are grateful to S J Thakkar Pharmacy College for providing funding and all necessary facilities to carry out the research work and their constant support.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Knoll T. Stone disease. Eur Urol Suppl. 2007;6:717–722. [Google Scholar]
- 2.Mitra S.K., Gopumadhavan S., Venkataranganna M.V., Sundaram R. Effect of cystone, a herbal formulation, on glycolic acid-induced urolithiasis in rats. Phytother Res. 1998;12:372–374. [Google Scholar]
- 3.Worcester E.M., Coe F.L. Nephrolithiasis. Prim Care. 2008;35:369–391. doi: 10.1016/j.pop.2008.01.005. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt P., Paul P. Analysis of urinary stone constituents using powder X-ray diffraction and FT-IR. J Chem Sci. 2008;120(2):267–273. [Google Scholar]
- 5.Evan A.P. Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr Nephrol. 2010;25:831–841. doi: 10.1007/s00467-009-1116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pietrow P., Preminger G.M. 9th ed. vol. 2. WB Saunders; Philadelphia: 2007. (Campbell-Walsh Urology). [Google Scholar]
- 7.Butterweck V., Khan S.R. Herbal medicines in the management of urolithiasis: alternative or complementary? Planta Med. 2009;75:1095–1103. doi: 10.1055/s-0029-1185719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee P.K., Kumar V., Mal M., Houghton P.J. In vitro acetylcholinesterase inhibitory activity of the essential oil from Acorus calamus and its main constituents. Planta Med. 2007;73:283–285. doi: 10.1055/s-2007-967114. [DOI] [PubMed] [Google Scholar]
- 9.Lai X.Y., Liang H., Zhao Y.Y. [A survey of the studies on chemical constituents and pharmacological activities of Acorus plants] Zhongguo Zhong Yao Za Zhi. 2002;27:161–165. 198. [PubMed] [Google Scholar]
- 10.Shukla P.K., Khanna V.K., Ali M.M., Maurya R., Khan M.Y., Srimal R.C. Neuroprotective effect of Acorus calamus against middle cerebral artery occlusion-induced ischaemia in rat. Hum Exp Toxicol. 2006;25:187–194. doi: 10.1191/0960327106ht613oa. [DOI] [PubMed] [Google Scholar]
- 11.Qiao D., Gan L.S., Mo J.X., Zhou C.X. [Chemical constituents of Acorus calamus] Zhongguo Zhong Yao Za Zhi. 2012;37:3430–3433. [PubMed] [Google Scholar]
- 12.Marongiu B., Piras A., Porcedda S., Scorciapino A. Chemical composition of the essential oil and supercritical CO2 extract of Commiphora myrrha (Nees) Engl. and of Acorus calamus L. J Agric Food Chem. 2005;53:7939–7943. doi: 10.1021/jf051100x. [DOI] [PubMed] [Google Scholar]
- 13.Si M.M., Lou J.S., Zhou C.X. Insulin releasing and alpha-glucosidase inhibitory activity of ethyl acetate fraction of Acorus calamus in vitro and in vivo. J Ethnopharmacol. 2010;128:154–159. doi: 10.1016/j.jep.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 14.Mehrotra S., Mishra K.P., Maurya R. Anticellular and immunosuppressive properties of ethanolic extract of Acorus calamus rhizome. Int Immunopharmacol. 2003;3:53–61. doi: 10.1016/s1567-5769(02)00212-6. [DOI] [PubMed] [Google Scholar]
- 15.Shoba F.G., Thomas M. Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhoea. J Ethnopharmacol. 2001;76:73–76. doi: 10.1016/s0378-8741(00)00379-2. [DOI] [PubMed] [Google Scholar]
- 16.Parab R.S., Mengi S.A. Hypolipidemic activity of Acorus calamus L. in rats. Fitoterapia. 2002;73:451–455. doi: 10.1016/s0367-326x(02)00174-0. [DOI] [PubMed] [Google Scholar]
- 17.Devi S.A., Ganjewala D. Antioxidant Activities of methanolic extracts of sweet-flag (Acorus calamus) leaves and rhizomes. J Herbs Spices Med Plants. 2011;17:1–11. [Google Scholar]
- 18.Bhavna M., Rani S. Screening of Achyranthus aspera, Acorus calamus, Caesalpinia crista for diuretic activity. Blonano Front. 2006;2:53–54. [Google Scholar]
- 19.Palani S.R.S., Praveen R., Parameswaran P., Senthil Kumar B. Therapeutic efficacy of Acorus calamus on acetaminophen induced nephrotoxicity and oxidative stress in male albino rats. Acta Pharm Sci. 2010;52:89–100. [Google Scholar]
- 20.Agarwal S.L., Arora R.B., Dandiya P.C., Singh K.P. A note on the preliminary studies of certain pharmacological actions of Acorus calamus L. J Am Pharm Assoc Am Pharm Assoc. 1956;45:655–656. doi: 10.1002/jps.3030450921. [DOI] [PubMed] [Google Scholar]
- 21.KR K. 19 ed. Nirali Prakashan; Pune: 2009. Practical Pharmacognosy. [Google Scholar]
- 22.Lipschitz W.L., Hadidian Z., Kerpcsar A. Bioassay of diuretics. J Pharmacol Exp Ther. 1943;79:97–110. [Google Scholar]
- 23.Jeffery G.H.M.J., Denny R.C. 5th ed. Addison Wesley Longman Ltd; England: 1989. Vogel's Textbook of Quantitative Chemical Analysis. [Google Scholar]
- 24.Rushton H.G., Spector M. Effects of magnesium deficiency on intratubular calcium oxalate formation and crystalluria in hyperoxaluric rats. J Urol. 1982;127:598–604. doi: 10.1016/s0022-5347(17)53920-8. [DOI] [PubMed] [Google Scholar]
- 25.Karadi R.V., Gadge N.B., Alagawadi K.R., Savadi R.V. Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J Ethnopharmacol. 2006;105:306–311. doi: 10.1016/j.jep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Worcester E.M., Coe F.L. Clinical practice. Calcium kidney stones. N Engl J Med. 2010;363:954–963. doi: 10.1056/NEJMcp1001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selvam R., Kalaiselvi P., Govindaraj A., Bala Murugan V., Sathish Kumar A.S. Effect of A. lanata leaf extract and vediuppu chunnam on the urinary risk factors of calcium oxalate urolithiasis during experimental hyperoxaluria. Pharmacol Res. 2001;43:89–93. doi: 10.1006/phrs.2000.0745. [DOI] [PubMed] [Google Scholar]
- 28.Huang H.S., Ma M.C., Chen J., Chen C.F. Changes in the oxidant-antioxidant balance in the kidney of rats with nephrolithiasis induced by ethylene glycol. J Urol. 2002;167:2584–2593. [PubMed] [Google Scholar]
- 29.Atmani F., Slimani Y., Mimouni M., Hacht B. Prophylaxis of calcium oxalate stones by Herniaria hirsuta on experimentally induced nephrolithiasis in rats. BJU Int. 2003;92:137–140. doi: 10.1046/j.1464-410x.2003.04289.x. [DOI] [PubMed] [Google Scholar]
- 30.Divakar K., Pawar A.T., Chandrasekhar S.B., Dighe S.B., Divakar G. Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem Toxicol. 2010;48:1013–1018. doi: 10.1016/j.fct.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Saha S., Verma R.J. Antinephrolithiatic and antioxidative efficacy of Dolichos biflorus seeds in a lithiasic rat model. Pharm Biol. 2015;53:16–30. doi: 10.3109/13880209.2014.909501. [DOI] [PubMed] [Google Scholar]
- 32.Robinson M., Pond C.L., Laurie R.D., Bercz J.P., Henningsen G., Condie L.W. Subacute and subchronic toxicity of ethylene glycol administered in drinking water to Sprague-Dawley rats. Drug Chem Toxicol. 1990;13:43–70. doi: 10.3109/01480549009011069. [DOI] [PubMed] [Google Scholar]
- 33.Thamilselvan S., Khan S.R., Menon M. Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res. 2003;31:3–9. doi: 10.1007/s00240-002-0286-x. [DOI] [PubMed] [Google Scholar]
- 34.Scheid C.R., Cao L.C., Honeyman T., Jonassen J.A. How elevated oxalate can promote kidney stone disease: changes at the surface and in the cytosol of renal cells that promote crystal adherence and growth. Front Biosci. 2004;9:797–808. doi: 10.2741/1265. [DOI] [PubMed] [Google Scholar]
- 35.Khan S.R., Finlayson B., Hackett R.L. Experimental calcium oxalate nephrolithiasis in the rat. Role of the renal papilla. Am J Pathol. 1982;107:59–69. [PMC free article] [PubMed] [Google Scholar]
- 36.H.T. Lippincott Reven; Philadelphia: 1996. Kidney Stones: Medical and Surgical Management. [Google Scholar]
- 37.Robertson W.G., Peacock M. The cause of idiopathic calcium stone disease: hypercalciuria or hyperoxaluria? Nephron. 1980;26:105–110. doi: 10.1159/000181963. [DOI] [PubMed] [Google Scholar]
- 38.Soundararajan P., Mahesh R., Ramesh T., Begum V.H. Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Indian J Exp Biol. 2006;44:981–986. [PubMed] [Google Scholar]
- 39.Calixto J.B., Santos A.R., Cechinel Filho V., Yunes R.A. A review of the plants of the genus Phyllanthus: their chemistry, pharmacology, and therapeutic potential. Med Res Rev. 1998;18:225–258. doi: 10.1002/(sici)1098-1128(199807)18:4<225::aid-med2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 40.Tkachev A.V., Gur'ev A.M., Yusubov M.S. Acorafuran, a new sesquiterpenoid from Acorus calamus essential oil. Chem Nat Compd. 2006;42:696–698. [Google Scholar]
- 41.Marshall R.W., Cochran M., Hodgkinson A. Relationships between calcium and oxalic acid intake in the diet and their excretion in the urine of normal and renal-stone-forming subjects. Clin Sci. 1972;43:91–99. doi: 10.1042/cs0430091. [DOI] [PubMed] [Google Scholar]
- 42.Sayer J.A., Simmons N.L. Urinary stone formation: Dent's disease moves understanding forward. Exp Nephrol. 2002;10:176–181. doi: 10.1159/000058344. [DOI] [PubMed] [Google Scholar]
- 43.Moochhala S.H., Sayer J.A., Carr G., Simmons N.L. Renal calcium stones: insights from the control of bone mineralization. Exp Physiol. 2008;93:43–49. doi: 10.1113/expphysiol.2007.040790. [DOI] [PubMed] [Google Scholar]
- 44.Lemann J., Jr., Worcester E.M., Gray R.W. Hypercalciuria and stones. Am J Kidney Dis. 1991;17:386–391. doi: 10.1016/s0272-6386(12)80628-7. [DOI] [PubMed] [Google Scholar]
- 45.Ngo T.C., Assimos D.G. Uric acid nephrolithiasis: recent progress and future directions. Rev Urol. 2007;9:17–27. [PMC free article] [PubMed] [Google Scholar]
- 46.Pragasam V., Kalaiselvi P., Sumitra K., Srinivasan S., Varalakshmi P. Counteraction of oxalate induced nitrosative stress by supplementation of l-arginine, a potent antilithic agent. Clin Chim Acta. 2005;354:159–166. doi: 10.1016/j.cccn.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 47.Khan S.R. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res. 2005;33:349–357. doi: 10.1007/s00240-005-0492-4. [DOI] [PubMed] [Google Scholar]
- 48.PB G. Bhalani Publishing House; Mumbai: 1994. Text Book of Medical Laboratory Technology. [Google Scholar]