Abstract
Background
Curcumin (diferuloylmethane) has been associated with the inhibition of angiogenesis, as well as the prevention of cancers and inflammatory processes. The aim of this study was to assess the efficacy of curcumin in suppressing angiogenesis in the cultured endothelial cells of rat aortic rings.
Methods
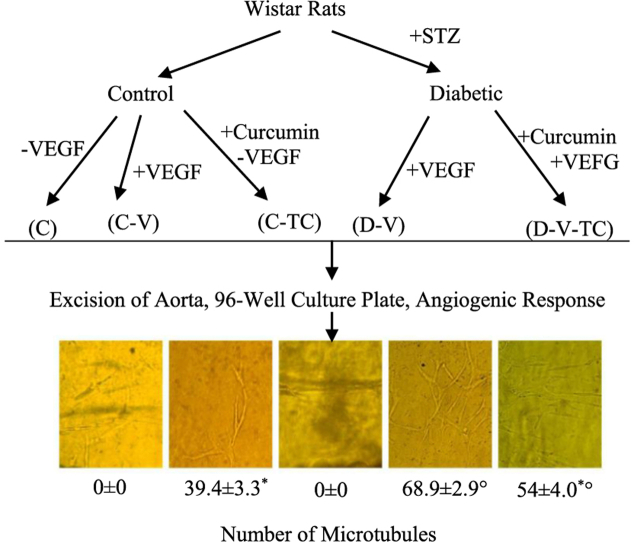
Eight-week-old male Wistar rats were randomized into five groups each with a different treatment and cell culturing paradigm: controls cultured in the absence of VEGF (vascular endothelial growth factor) (C), controls cultured in the presence of VEGF (C-V), controls treated with curcumin and then cultured in media lacking VEGF (C-TC), diabetics cultured in media supplemented with VEGF (D-V) and diabetics treated with curcumin and then cultured in media supplemented with VEGF (D-V-TC). Each group consisted of 8 animals. Diabetes was induced in by streptozotocin (STZ; 60 mg/kg body weight, IV). After 8 weeks, animals were sacrificed and their aortas were excised. Ring-shaped explants were embedded in a 96-well culture plate. Angiogenesis response was measured by counting the number of primary microtubules in each well.
Results
Optic microscopy revealed that the D-V group had the highest number of microvessels, while angiogenesis was not observed in the C or C-TC groups. The number of primary microtubules was significantly lower in the D-V-TC group compared to the D-V group (P < 0.05). The D-V-TC group had a significantly higher number of microvessels compared to the C-TC group (P < 0.05).
Conclusion
Curcumin attenuates angiogenesis response in stertozotocin-induced diabetic rats.
Keywords: Angiogenesis, Aortic ring assay, Curcumin, VEGF, Diabetes mellitus
Abbreviations: C, controls in the absence of VEGF; C-V, controls in the presence of VEGF; C-TC, controls treated with curcumin cultured in the absence of VEGF; D-V, diabetics in a culture containing VEGF; D-V-TC, diabetics treated with curcumin in a culture containing VEGF; DM, diabetes mellitus; VEGF, vascular endothelial growth factor; DMSO, dimethyl sulfoxide; CPCSEA, Committee for the Purpose of Control and Supervision of Experiments on Animals; PBS, phosphate buffer saline; MMP, matrix metalloproteinases; UPA, urokinase plasminogen activator; NF-kB, nuclear factor kappa; AP-1, activator protein 1
Graphical abstract
1. Introduction
Curcumin (Diferuloylmethane) is the principal component and active ingredient of turmeric (Curcuma longa), an organic extract of Curcuma, a rhizomatous herbaceous perennial plant.1 This phytochemical has long been used as a food additive and coloring agent.2 Accumulating evidence suggests that curcumin exhibits various biofunctions.3 Emerging data have revealed that curcumin possesses remarkable anti-oxidant, anti-inflammatory, anti-angiogenesis, and anti-carcinogenic properties.4, 5, 6, 7, 8, 9
Diabetes mellitus (DM) is a major worldwide concern and its long-term complications involve various organ systems.10 Hyperglycemia plays a definite role in the clinical manifestation of DM.11 However, excessive angiogenesis, the formation of new blood vessels from pre-existing vasculatures,12 may also be an important factor in some long-term pathological conditions, including diabetic nephropathy and retinopathy. In vitro angiogenesis assays contribute to the investigation of both angiogenic inducer and inhibitor agents.13 The rat aortic ring model is a well-established in vitro assay for assessing angiogenesis,13 and many consider it the best system for simulating physiological conditions found in vivo.14 Furthermore, it has the advantage of promoting all of the key steps of the angiogenesis process.15 Vascular endothelial growth factor (VEGF) is a prominent proangiogenic mediator16 and therefore can be used as a local inducer of angiogenesis with the intent of monitoring the potency of inhibitory factors.14
In the study presented here, we explored the angiogenic effects of curcumin on cultured endothelial cells derived from the streptozotocin (STZ)-induced diabetic rat aortic rings.
2. Materials and methods
2.1. Experimental design
Eight-week-old adult male Wistar rats with an average body weight of 240 ± 20 g (obtained from Pasteur Institute of Iran) were randomly allotted into five groups: controls cultured in the absence of VEGF (C) (Fig. 1), controls cultured in the presence of VEGF (C-V) (Fig. 2), controls treated with curcumin and then cultured in a medium lacking VEGF (C-TC) (Fig. 3), diabetics cultured in media supplemented with VEGF (D-V) (Fig. 4), and diabetics treated with curcumin and then cultured in media supplemented with VEGF (D-V-TC) (Fig. 5). Each group contained eight animals. All rats were provided standard laboratory rodent chow and water ad libitum. After 2 weeks, the experimental rats (groups D-V and D-V-TC) received a single intravenous injection of 60 mg/kg of body weight of STZ,17 whereas the control group received 60 mg/kg of vehicle. STZ was prepared fresh by dissolving in Na-citrate buffer (Sigma-Aldrich), pH = 4.5. In order to verify the diabetic condition, 96 h after the STZ injection, blood glucose levels of the experimental groups were measured using a portable glucose analyzer, Accu-check Blood Glucose Meter (Roche Diagnostics, Basel, Switzerland). Rats with blood glucose levels <15 mmol/L (270 mg/dl) received another injection of STZ. Following the second injection, animals with blood glucose levels higher than 15 mmol/L for 2 weeks were considered to be diabetic.
Fig. 1.

Optic microscopic view of endothelial cells of aortic ring in Group C.
Fig. 2.
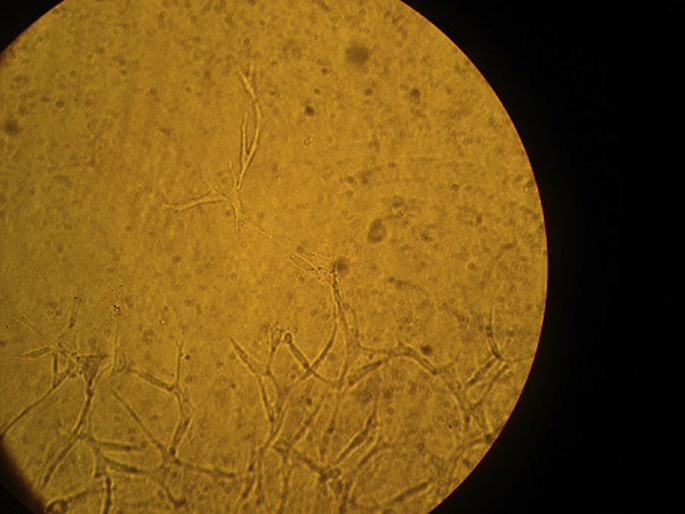
Optic microscopic view of endothelial cells of aortic ring in Group C-V.
Fig. 3.
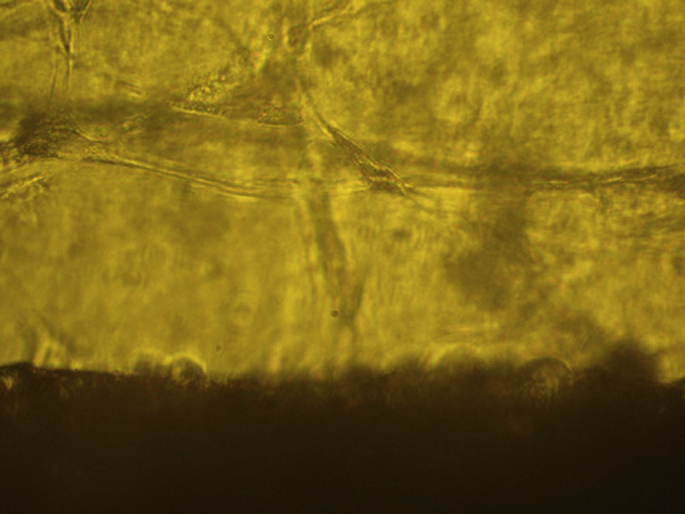
Optic microscopic view of endothelial cells of aortic ring in Group C-TC.
Fig. 4.
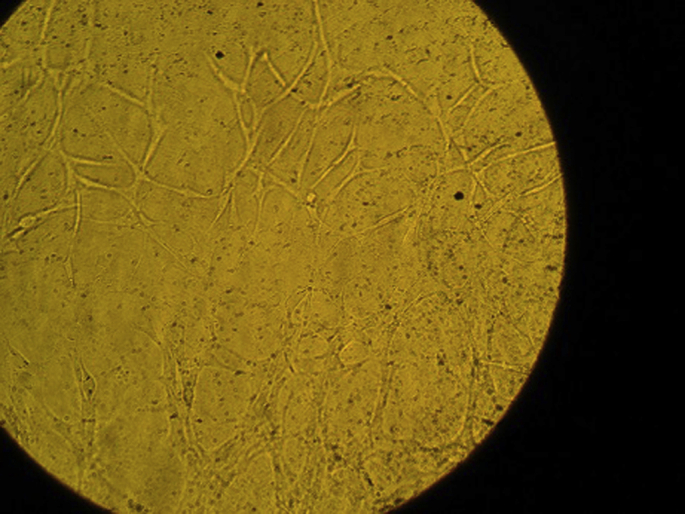
Optic microscopic view of endothelial cells of aortic ring in Group D-V.
Fig. 5.
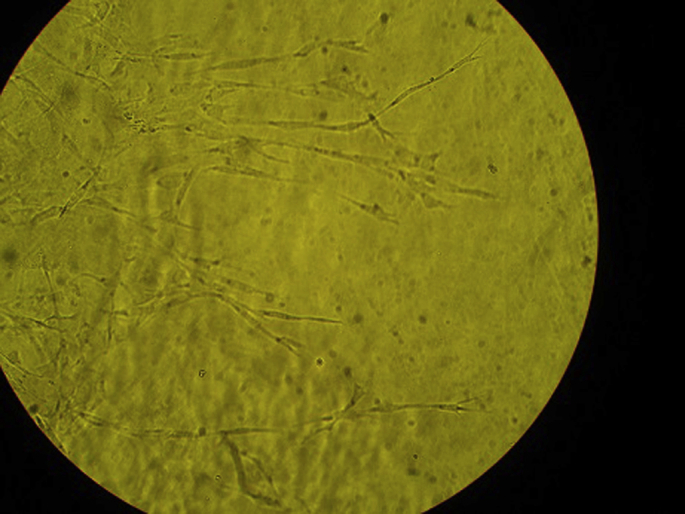
Optic microscopic view of endothelial cells of aortic ring in Group D-V-TC.
Rats in the curcumin-treated groups (groups C-TC and D-V-TC) were given a solution of 100 mg/kg of curcumin per day (dissolved in 2 ml DMSO) by intragastric administration. Treatment with curcumin began 3 days prior to STZ administration and was continued for 8 weeks.
2.2. Animal care
Rats were housed in individual and separate cages in a temperature (23 ± 3 °C) and humidity (50 ± 10%) controlled vivarium with a 12 h light/dark cycle. Body weight was recorded weekly and food intake was monitored daily. All animals had free access to water and chow throughout the study. All animal protocols used were in accordance with the guidelines for care and use of laboratory animals provided by the CPCSEA and was approved by the animal ethics review committee of our institution.
2.3. Rat aortic ring bioassay
At the end of 8 weeks, animals were sacrificed by inhalation of anesthetics under a fume hood. The thoracic aorta was surgically excised. After deliberate removal of periaortic fibro-adipose tissue, the aorta was sectioned into 2 mm ring-like segments. The ring-shaped explants were rinsed in PBS (Sigma-Aldrich, USA; pH = 7.4) and were embedded in a solution containing 1.6 g/L of gentamicin dissolved in 50 ml PBS (pH = 7.4). Containers were maintained at 4 °C. The culture medium was prepared in a laminar-flow cabinet by mixing a culture media of Hams F12 (pH = 7.4, Sigma-Aldrich) and Dulbecco's modified Eagle's medium (DMEM, pH = 7.4, Sigma-Aldrich) in a ratio of 50:50. Both media were filtered through a 0.22 μm hydrophilic cartilage membrane (Durapore, EMD Millipore). Immediately, after aortic ring embedding, 2.5 mg/L VEGF (Sigma-Aldrich) and 20% fetal calf serum (FCS, Sigma-Aldrich) were added to the culture medium of groups C-V, D-V and D-V-TC.
To prepare the culture media, a 96-well culture plate (Greiner Bio-One, Germany) was covered with fibrin gel and was allowed to set-up for 1 h at 37 °C, 5% CO2. Fibrin gel was obtained by combining 2.5 μl thrombin (Sigma-Aldrich; concentration: 500 ku/L) and 250 μl fibrinogen (Sigma-Aldrich; concentration: 8 g/L), under the laminar-flow cabinet. Then, the aortic explant was embedded in each fibrin-coated well and 1 mL of culture media was added to the medium and was covered with an additional layer of fibrin gel. The rings were incubated at 37 °C, 5% CO2 for 7 days. All assays were performed in duplicates.
An inverted optic microscope (Motic AE31) was used to monitor the angiogenesis progress in individual wells. One week after the incubation, the aortic outgrowth was evaluated by counting new microvessels every 3 days for 2 weeks, according to the criteria proposed by Nicosia et al.15 Rings were photographed on day 7. A hemocytometer device was used to determine the number of new microtubules. A Mann–Whitney U test was employed to assess the angiogenic response between groups. A two-tailed P-value <0.05 was considered statistically significant.
3. Results
Optic microscopy revealed proliferation of endothelial cells into the surrounding culture media. New endothelial cells had distinct nuclei, clear borders, and distinguished cellular membranes. Capillary sprouts, consisting of endothelial cells, were formed and migrated toward the fibrin gel. Generation of primary microtubules (tubulogenesis) and extension of new branches into the fibrin gel was also noted. The gradual extension of microtubules and convergence of capillary sprouts lead to formation of new vasculatures that covered the periaortic space.
To determine whether curcumin would affect neovessel outgrowth in diabetes mellitus, animals were randomized in four cohorts of diabetic and non-diabetic, curcumin-treated and non-curcumin treated. The results showed angiogenesis response in all groups where VEGF was added to medium, whereas groups C and C-TC, which did not receive VEGF, failed to generate new blood vessels.
The angiogenesis response we observed in the two diabetic cohorts (groups D and D-TC) demonstrated a reduced number of microvessels in the D-TC group (P < 0.05). The comparison of two curcumin treated cohorts (groups C-TC and D-V-TC) showed a higher number of new microvessels in diabetic animals (D-V-TC group; P < 0.05). Overall, group D-V had the most angiogenesis response and group C-TC showed the least number of primary tubules (Table 1).
Table 1.
Mean ± standard deviation of number of new microtubules assessed by Hemocytometer device in Wistar rats of specified groups. Mann–Whitney U test was employed to assess the angiogenic response between groups.
| Wistar rat group | Group C | Group C-V | Group C-TC | Group D-V | Group D-V-TC |
|---|---|---|---|---|---|
| Mean ± Standard deviation | 0 ± 0 | 39.375 ± 3.335* | 0 ± 0 | 68.875 ± 2.850 φ | 54.625 ± 4.033* φ |
*, φ show a two-tailed P-value of <0.05 between specified groups.
4. Discussion
In this study, we evaluated the angiogenic response of aortic rings obtained from a rat model of diabetes. The aortic outgrowth was evaluated by counting the new microvessels every 3 days for 2 weeks, 1 week after the incubation. We showed that the D-V-TC group had a lower angiogenic response compared with the D-V group, a result that illustrates the inhibitory effect of curcumin on angiogenesis in Wistar rats with diabetes. Curcumin, a natural derivate of turmeric has been implicated to show potent chemopreventive effects in some cancer models.6, 7 Previous investigations indicate that part of the anti-carcinogenic activity of curcumin could be due to inhibition of angiogenesis. Numerous studies have inspected the efficacy of curcumin in angiogenesis; however, most have investigated the inhibitory effect of curcumin on angiogenesis in cancers and have not explored its role in diabetes.
Angiogenesis is a complex process involving several steps. Secretion of proangiogenic growth factors initiates the process by stimulating the endothelial cells. Next, proteinases such as MMP and UPA will degrade the basal membrane and endothelial cells proliferate into the surrounding matrix. Endothelial cells migrate, forming a sprout, and proliferation follows the tip cells, which move along the growth factor gradient. Primary tubules begin to form and smooth muscle cells are recruited, and eventually the new vessel matures.18, 19 To evaluate the angiogenesis response, we measured the generation of primary microtubules (tubulogenesis) and extension of new branches into the fibrin gel. The gradual extension of microtubules and convergence of capillary sprouts lead to the formation of new vasculatures that covered the periaortic space, indicating angiogenesis.
Angiogenesis is a dynamic process that occurs throughout life, as many factors affect the demand for oxygen resulting in capillaries growing and regressing accordingly. Sprouting angiogenesis plays a pivotal role in the maintenance of healthy tissues and the pathogenesis of many chronic diseases. Maintaining control over abnormal angiogenesis is the goal of many different treatment paradigms. Decreased angiogenesis could be an effective therapeutic intervention in diabetic retinopathy and nephropathy.20, 21 Excessive secretion of VEGF is implicated to play an important role in diabetic kidney diseases and VEGF suppression has been shown to have protective effects in diabetic nephropathy.20
Several molecular pathways have been proposed in an attempt to unveil the mechanism of the curcumin effect on the angiogenic response, and curcumin has been shown to mediate multiple cell signaling pathways. Many studies have implicated that curcumin suppresses the transcription and expression of NF-kB, including cyclin D1, MMP, VEGF, and AP-1.22, 23, 24 Curcumin was found to downregulate MMP-9 and UPA.25 A study exploring the efficacy of curcumin on tumor angiogenesis reported inhibition of several gene expressions including VEGF, angiopoietin 1 and 2 in particular neoplastic cells.26 Furthermore, curcumin was demonstrated to exert an inhibitory effect on protein kinase C and some cell adhesion molecules.27
In the current work, we sought to evaluate the impact curcumin may have on the angiogenesis process in aortic rings of sterptozotocin-induced diabetic rats. We found that curcumin effectively prevented the angiogenic response in aortic ring models in both the diabetic and non-diabetic environment. Several investigators have examined curcumin's influence in diabetic retinopathy and nephropathy20, 28 and found that curcumin demonstrated protective effects on the kidney and retina of animals with diabetes mellitus.29, 30 However, no previous study has evaluated the effect of curcumin in an aortic ring assay of diabetic rats. Our results are consistent with emerging data suggesting that curcumin may function as an antiangiogenic agent.9, 26, 31 Nevertheless, curcumin also appears to be a stimulator of angiogenesis in wound healing.32
It should be noted that aortic ring model might face some disadvantages, while it is a practical in vitro assay of the typical angiogenesis known to occur in microvessels. Moreover, there is the possibility of variable angiogenic responses, which we attempted to overcome by using duplicate assays and measurements. Furthermore, poor bioavailability and fast metabolism could limit clinical utilization of curcumin.
In conclusion, our data indicate that curcumin suppresses the angiogenic response in a rat aortic model of diabetes. Curcumin is a safe and non-toxic phytochemical25, 33 and application of curcumin for the purpose of treatment and prevention of diabetic complications seems practical. However, further investigation, particularly in vivo models and clinical trials, are warranted to fully inspect the angiogenic efficacy of curcumin.
Conflict of Interest
Authors declare no conflict of interests.
Acknowledgments
There are no acknowledgements.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Mohammad Hossein Dehghan, Email: dehghan9@yahoo.com.
Hossein Mirmiranpour, Email: h_mirmiranpoor@yahoo.com.
Sara Faghihi-Kashani, Email: dehsarvi.sara@gmail.com.
Kourosh Kabir, Email: Kabir.kourosh@yahoo.com.
Mehrdad Larry, Email: mehrdad.larry@gmail.com.
Ehsan Zayerzadeh, Email: zayerzadeh@standard.ac.ir.
Salume Salehi, Email: salume.salehi@gmail.com.
References
- 1.Ammon H.P., Wahl M.A. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [Epub 1991/02/01] [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal B.B., Sundaram C., Malani N., Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [Epub 2007/06/16] [DOI] [PubMed] [Google Scholar]
- 3.Fan X., Zhang C., Liu D.B., Yan J., Liang H.P. The clinical applications of curcumin: current state and the future. Curr Pharm Des. 2013;19:2011–2031. [PubMed] [Google Scholar]
- 4.Tilak J.C., Banerjee M., Mohan H., Devasagayam T.P. Antioxidant availability of turmeric in relation to its medicinal and culinary uses. Phytother Res. 2004;18:798–804. doi: 10.1002/ptr.1553. [DOI] [PubMed] [Google Scholar]
- 5.Singh S., Aggarwal B.B. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 6.Schaffer M., Schaffer P.M., Zidan J., Bar Sela G. Curcuma as a functional food in the control of cancer and inflammation. Curr Opin Clin Nutr Metab Care. 2011;14:588–597. doi: 10.1097/MCO.0b013e32834bfe94. [Epub 2011/10/12] [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal B.B., Harikumar K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [Epub 2008/07/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jurenka J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- 9.Noorafshan A., Ashkani-Esfahani S. A review of therapeutic effects of curcumin. Curr Pharm Des. 2013;19:2032–2046. [PubMed] [Google Scholar]
- 10.Wild S., Roglic G., Green A., Sicree R., King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 11.Nathan D.M. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [Epub 1993/06/10] [DOI] [PubMed] [Google Scholar]
- 12.Martin A., Komada M.R., Sane D.C. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23:117–145. doi: 10.1002/med.10024. [Epub 2002/12/25] [DOI] [PubMed] [Google Scholar]
- 13.Blacher S., Devy L., Burbridge M.F. Improved quantification of angiogenesis in the rat aortic ring assay. Angiogenesis. 2001;4:133–142. doi: 10.1023/a:1012251229631. [DOI] [PubMed] [Google Scholar]
- 14.Auerbach R., Lewis R., Shinners B., Kubai L., Akhtar N. Angiogenesis assays: a critical overview. Clin Chem. 2003;49:32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- 15.Nicosia R.F., Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest. 1990;63:115–122. [PubMed] [Google Scholar]
- 16.Johnson K.E., Wilgus T.A. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care. 2014;3:647–661. doi: 10.1089/wound.2013.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akbarzadeh A., Norouzian D., Mehrabi M.R. Induction of diabetes by streptozotocin in rats. Indian J Clin Biochem. 2007;22:60–64. doi: 10.1007/BF02913315. [Epub 2007/09/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adair T.H., Montani J.P. Morgan & Claypool Life Sciences; 2010. Angiogenesis.http://www.ncbi.nlm.nih.gov/books/NBK53238/ Available from: [PubMed] [Google Scholar]
- 19.Demir R., Kayisli U.A., Cayli S., Huppertz B. Sequential steps during vasculogenesis and angiogenesis in the very early human placenta. Placenta. 2006;27:535–539. doi: 10.1016/j.placenta.2005.05.011. [Epub 2005/07/21] [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa T., Sato W., Kosugi T., Johnson R.J. Uncoupling of VEGF with endothelial NO as a potential mechanism for abnormal angiogenesis in the diabetic nephropathy. J Diabetes Res. 2013;2013 doi: 10.1155/2013/184539. 184539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tremolada G., Del Turco C., Lattanzio R. The role of angiogenesis in the development of proliferative diabetic retinopathy: impact of intravitreal anti-VEGF treatment. Exp Diabetes Res. 2012;2012:728325. doi: 10.1155/2012/728325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunnumakkara A.B., Guha S., Krishnan S., Diagaradjane P., Gelovani J., Aggarwal B.B. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 23.Mehta H.J., Patel V., Sadikot R.T. Curcumin and lung cancer-a review. Target Oncol. 2014;9:295–310. doi: 10.1007/s11523-014-0321-1. [DOI] [PubMed] [Google Scholar]
- 24.Bae M.K., Kim S.H., Jeong J.W. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol Rep. 2006;15:1557–1562. [PubMed] [Google Scholar]
- 25.Aggarwal B.B., Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Gururaj A.E., Belakavadi M., Venkatesh D.A., Marme D., Salimath B.P. Molecular mechanisms of anti-angiogenic effect of curcumin. Biochem Biophys Res Commun. 2002;297:934–942. doi: 10.1016/s0006-291x(02)02306-9. [DOI] [PubMed] [Google Scholar]
- 27.Bhandarkar S.S., Arbiser J.L. Curcumin as an inhibitor of angiogenesis. Adv Exp Med Biol. 2007;595:185–195. doi: 10.1007/978-0-387-46401-5_7. [DOI] [PubMed] [Google Scholar]
- 28.Suryanarayana P., Saraswat M., Mrudula T., Krishna T.P., Krishnaswamy K., Reddy G.B. Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Investig Ophthalmol Vis Sci. 2005;46:2092–2099. doi: 10.1167/iovs.04-1304. [DOI] [PubMed] [Google Scholar]
- 29.Wu W., Geng H., Liu Z., Li H., Zhu Z. Effect of curcumin on rats/mice with diabetic nephropathy: a systematic review and meta-analysis of randomized controlled trials. J Tradit Chin. 2014;34:419–429. doi: 10.1016/s0254-6272(15)30041-8. [DOI] [PubMed] [Google Scholar]
- 30.Aldebasi Y.H., Aly S.M., Rahmani A.H. Therapeutic implications of curcumin in the prevention of diabetic retinopathy via modulation of anti-oxidant activity and genetic pathways. Int J Physiol Pathophysiol Pharmacol. 2013;5:194–202. [PMC free article] [PubMed] [Google Scholar]
- 31.Hahm E.R., Gho Y.S., Park S., Park C., Kim K.W., Yang C.H. Synthetic curcumin analogs inhibit activator protein-1 transcription and tumor-induced angiogenesis. Biochem Biophys Res Commun. 2004;321:337–344. doi: 10.1016/j.bbrc.2004.06.119. [Epub 2004/09/11] [DOI] [PubMed] [Google Scholar]
- 32.Kant V., Gopal A., Kumar D. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J Surg Res. 2015;193:978–988. doi: 10.1016/j.jss.2014.10.019. [Epub 2014/12/03] [DOI] [PubMed] [Google Scholar]
- 33.NTP toxicology and carcinogenesis studies of turmeric oleoresin (CAS No. 8024-37-1) (major component 79%-85% curcumin, CAS No. 458-37-7) in F344/N rats and B6C3F1 mice (feed studies) Natl Toxicol Program Tech Rep Ser. 1993;427:1–275. [PubMed] [Google Scholar]