Abstract
Age related decline in thymic function is a well-described process that results in reduced T cell development and thymic output of new naïve T cells. Thymic involution leads to reduced response to vaccines and new pathogens in otherwise healthy individuals; however, reduced thymic function is particularly detrimental in clinical scenarios where the immune system is profoundly depleted such as after chemotherapy, radiotherapy, infection and shock. Poor thymic function and restoration of immune competence has been correlated with increased risk of opportunistic infections, tumor relapse and autoimmunity. Apart from their primary role in sex dimorphism, sex steroid levels profoundly affect the immune system in general and, in fact, age-related thymic involution has been at least partially attributed to the increase of sex steroids at puberty. Subsequently it has been demonstrated that removal of sex steroids, or sex steroid ablation (SSA), triggers physiologic changes that ultimately led to thymic re-growth and improved T cell reconstitution in settings of hematopoietic stem cell transplant (HSCT). Although the cellular and molecular process underlying these regenerative effects are still poorly understood, SSA clearly represents an attractive therapeutic approach to enhance thymic function and restore immune competence in immunodeficient individuals.
Keywords: sex steroid ablation, immune reconstitution, thymus
Introduction
One of the best described consequences of aging is the progressive decline in immunocompetence (1, 2). This deleterious phenomenon involves both quantitative and qualitative changes, including loss of bone marrow and thymic output, reduced proliferation of lymphoid progenitors, and diminished function of mature lymphocytes in the periphery. Consequently, older individuals are more susceptible to microbial infections, have decreased immune surveillance against malignant cells and, almost paradoxically, are more susceptible to certain autoimmune diseases (3-7).
The thymus is the principal organ responsible for the generation and development of naïve T cells that circulate in the periphery (8). Thymopoiesis, that is the process of T cell development, is tightly regulated by the bidirectional crosstalk between developing thymocytes and the thymic stromal compartment; which is composed of non–hematopoietic thymic epithelial cells (TECs), endothelium and fibroblasts, as well as the hematopoietic components such as macrophages and dendritic cells (9). T cell development initiates when circulating bone marrow-derived T-lineage progenitors (CTPs) migrate to the thymus and undergo a series of well-defined developmental steps that ultimately lead to the formation of naïve CD4+ and CD8+singlepositive T cells ready to enter into the circulation and encounter antigens (9-11).
Paradoxical to its critical function in maintaining a functional and effective T cell pool to mediate immunity to new pathogens, the thymus undergoes profound age-related degeneration (12-15). This process starts early in life, but becomes more prominent from the onset of puberty. Although in humans the physical size of the thymus remains unchanged, thymic spaces are progressively replaced by adipose tissue during aging that occurs extensively after the age of 15 (16, 17). This process leads to a dramatic decrease in thymic output that is estimated to have plummeted to approximately 90% of its original function by the age of 30 (18).
Age-related thymic involution is problematic for the aged response to new pathogens and in vaccinations. For example, only approximately 30-40% of people over the age of 65 are capable of responding to the influenza vaccine (19-22). Thymic involution also limits thymic regeneration resulting in prolonged time of recovery following immune suppression such as common cancer cytoreductive therapies like chemotherapy or radiation therapy (23-25). Reduced thymic function is particularly critical for older recipients of allogeneic hematopoietic stem cell transplantation (allo-HSCT), who experience a prolonged period of post-transplant T cell deficiency after thymic damage due to cytoreductive conditioning (26-30). Insufficient recovery of thymopoiesis has been intrinsically linked to an increased risk of opportunistic infections and adverse clinical outcome (31, 32). Although young recipients can recover thymic function within months, old patients, whose thymic function is already impaired by the immune senescence, exhibit a long period of T cell deficiency; with an inverse correlation between T cell recovery and age in cancer patients after chemotherapy (28, 33, 34).
Restoration of immune competence, and in particular T cell recovery, is critically dependent on residual thymic function. Therefore, understanding the processes that trigger the decline in thymic function during aging, and developing strategies that can reverse these effects, represent a clinical challenge with the potential to generate therapeutic strategies to rejuvenate the immune system and improve overall outcome in immune compromised patients.
Although several promising strategies to rebuild the thymus and immune recovery have been proposed, including Keratinocyte Growth Factor (KGF), IL-7, IL-12, IL-22, FMS-Related Tyrosine Kinase 3 Ligand (Flt3L), Leptin, Ghrelin, Insulin-Like Growth Factor-1 (IGF-1), Op9-DL1 cultured Pre T cells and Growth Hormone (GH) (35-43); one of the most widely studied has been sex steroid ablation (SSA). Here we provide a brief overview on the effects of sex steroids on immune system and on SSA as a therapeutic tool to enhance thymic function and immune recovery in immunodeficient recipients.
Effect of sex steroids on thymic function
The age-related decline in thymic function is a well-known process conserved in all vertebrates. However, although it has been the topic of intensive research for many years, the mechanisms driving this phenomenon are still poorly understood. Several possible mechanisms have been proposed, including aging of the BM and the depletion in the supply of T-cell progenitors, reduced expression from the supporting micro-environment of thymopoietic cytokines (i.e.IL-7, KGF), increase in TGF-beta, down regulation of the critical TEC factor FoxN1, block in TCR rearrangement and decreased proliferation and increased apoptosis of early thymic progenitors (ETPs) (44-50). It is increasingly evident that thymic involution is a complex process involving the interplay of many mechanisms, however, it is also becoming increasingly apparent that the thymic stromal cell compartment, and in particular the TECs, are believed to represent one of the most sensitive compartments in the process of thymic aging (51).
Although there is evidence to suggest that thymic involution begins immediately after birth, the rate of decline in size and function is more pronounced from the onset of puberty; fitting the increase in circulating sex steroids at that time. Due to this temporal connection, the increase in sex steroids during puberty has been proposed to contribute to the process of thymic involution. This model of thymic involution has been further validated by several studies demonstrating that administration of sex steroids (androgens or estrogens) in young pre-pubertal mice promotes thymic involution (52-58).
All steroid hormones mediate their biological effects through the interaction with specific receptors. Androgen receptors (AR) and estrogen receptor (ER) are expressed in male and female thymii in both the hematopoietic and stromal compartments (56, 59-61). In most instances, after binding their respective sex steroid, the receptor translocates to the nucleus where it directly mediates changes in the expression of target genes. A direct effect of sex steroids on thymocytes has been proposed where testosterone can induce apoptosis of CD4+CD8+ double positive thymocytes though the up regulation of TNF-alpha; and estrogens can induce thymic atrophy by eliminating ETPs and inhibiting proliferation of beta selected thymocytes (62, 63). However, despite this direct negative regulation of the thymocyte compartment by sex steroids, studies using transgenic mice defective in AR function on either the hematopoietic compartment or the stromal compartment, suggested that the presence of a functional of AR within the thymic stromal components but not on the thymocytes is required for sex-steroid mediated thymic atrophy (58, 64). Although the possibility that some of the effects of androgens resides in the hematopoietic cells cannot be excluded, these studies highlight thymic stromal cells, and in particular TECs, as principal targets of the negative actions of sex steroids.
While there is still some debate over the role of sex steroids in the initiation of thymic involution as well as their effects on thymic function (Table 1), SSA represents a rational strategy to boost thymic function and promote its rejuvenation in young and adult mice in steady state condition as well as following immune insults (Figure 1).
Table 1.
| Major questions still open for the biological effects of sex steroids and SSA on thymic function: |
|---|
| What is the role of sex steroids in the initiation and progression of age-related thymic involution? |
| What are the physiological targets of sex steroids (and SSA) within the thymus? |
| How durable is sex steroid ablation-mediated thymic regeneration? What causes the normalization of the thymus after SSA? |
Figure 1.
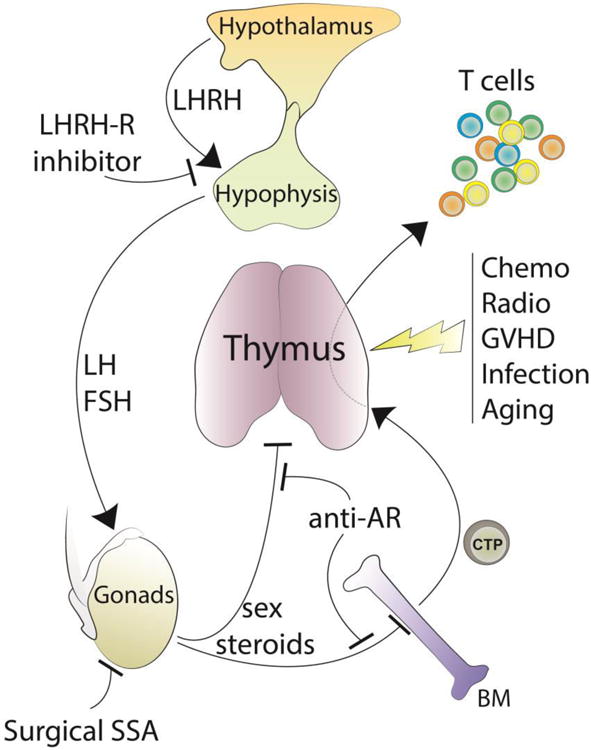
Overview of the hypothalamic–pituitary–gonadal axis and its implication in regulating thymic function. SSA using LHRH-R inhibitors or anti-AR, blocks the negative effects of sex steroids on BM and thymus and promotes their rejuvenation in steady-state conditions as well as following immune insults.
Sex steroid ablation as a therapeutic tool to rejuvenate the thymus
Studies published as early as the beginning of the twentieth century reported that the removal of gonads in cattle and guinea pigs sustained thymic size and architecture in adult animals, providing the first evidence that SSA have positive effect on thymic growth (65, 66). Subsequently, several studies have shown that surgical SSA increases thymic cellularity, restores thymic architecture and organization and enhances thymopoiesis in young and adult animals (67-70). Castration of 9 month old mice rapidly reverses thymic atrophy, restoring the level of CD4+ CD8+ SP, DP and all developing thymocytes starting form the more immature CD25+CD44+CD117+ ETPs, to the same level of thymocytes from 2-month old mice (71). Moreover, Heng and colleagues demonstrated that surgical SSA provided functional benefits in old mice, increasing T cell responsiveness to tumor antigens and enhancing viral clearance (72). Importantly, the T cell repertoire of young mice after surgically castration was similar to that of sham-castrated controls, suggesting that thymic regeneration after SSA does not lead to the peripheral expansion of particular T cell clones (73, 74). In addition to its potential for improving thymopoiesis in aged animals, several studies have also found that immune recovery was accelerating after SSA in recipients of autologous and allogeneic HSCT, and after chemotherapy (75-77). In particular, removal of sex steroids led to an increase in LSKs, common lymphoid progenitors (CLPs), pro-B, pre-B and immature B cells in castrated mice (78).Enhanced immune recovery was also observed in all thymocytes subsets and in peripheral T and B cells following HSCT. In models of allo-HSCT,T cell function and GVL effects were intact while GVHD was not exacerbated (76, 79).
Therapeutically, castrate levels of sex steroids can be achieved in a transient and reversible manner using compounds originally developed for prostate cancer and breast cancer patients. These include targeting upstream signaling events such as luteinizing hormone releasing hormone (LHRH), or directly blocking sex steroid receptors. Previous studies have reported that mice treated with the LHRH-agonist Lupron, or ASC-J9® (an anti-AR), showed increased number of lymphoid and myeloid progenitors in the bone marrow and increased thymic and splenic recovery after allo-HSCT (64, 79).
Although most studies examining SSA have focused on male mice, primarily due to the ease of surgical castration versus ovariectomy, previous reports have shown that both surgical and chemical SSA can also positively impact thymic function in female mice (70, 80-82). However, the specific mechanisms of thymic regeneration after SSA in female mice in comparison to male mice has not been extensively studied; in particular, the relative contributions of estrogen and androgens in male versus female mice are currently unclear.
Clinical trials of SSA have shown that an LHRH-agonist in a small cohort of prostate cancer patients between 60 and 77 years enhances thymic function (75).A significant increase was observed in naïve CD4+ and CD8+ T cells and NK counts 4 months after treatment. Analysis of thymic function by measuring recent thymic emigrants by T-cell receptor excision circles (TRECs) revealed that 8 of the 10 patients showed increase in total TREC+cells/milliliter blood compared with pretreatment conditions. A nonrandomized pilot study involving patients with allogeneic or autologous HSCT showed significant enhancement of naïve (TREC+) T cell regeneration after transplant when the LHRH-Agonist Goserelin, which was administered 21 days prior transplant. Moreover, analysis of V-beta CD3 regions by spectra typing on FACS purified CD4+ and CD8+ T cells showed a significant increase in diversity in LHRH-agonist treated patients in the allogeneic transplant setting (83).
These clinical studies demonstrated that agonists for the LHRH-receptor represent a valid and rational strategy to enhance thymic function, not just in immune compromised patients, but also during normal aging. The continued interest in developing more potent sex steroid blockers for cancer patients, such as the most recent AR inhibitors and LHRH-Antagonists (that have the advantage of bypassing the surge in sex steroids observed with LHRH-Agonists (84)), may provide additional therapeutic tools to reverse sex steroid action and rapidly trigger the recovery of the thymic function (figure 1). Moreover, as our understanding of the molecular regulators of SSA-mediated regeneration, new targets open up for sustained thymic regeneration without the systemic effects of SSA.
Conclusions
Sustained thymopoiesis is a critical process that allows for the recovery of immune competence after thymic injury. This is particular critical for older allo-HSCT recipients whose thymus and immune function are already profoundly impaired due immune senescence. The clinical and preclinical studies highlighted in this review promote SSA as a valid approach to boost thymic regeneration and enhance immune recovery, providing the bases for novel immune regenerative therapies.
Acknowledgments
This research was supported by National Institutes of Health award numbers R01-HL069929 (M.R.M. van den Brink), R01-AI080455 (M.R.M. van den Brink), R01-AI101406 (M.R.M. van den Brink), P01-CA023766 (R. J. O'Reilly), and Project 4 of P01-CA023766 (M.R.M. van den Brink). Support was also received from the U.S National Institute of Allergy and Infectious Diseases (NIAID Contract HHSN272200900059C), The Experimental Therapeutics Center of MSKCC funded by Mr. William H. Goodwin and Mrs. Alice Goodwin, The Lymphoma Foundation, Alex's Lemonade Stand, The Geoffrey Beene Cancer Research Center at MSKCC, and The Susan and Peter Solomon Divisional Genomics Program. This project has received funding from the European Union's Seventh Programme for research, technological development and demonstration under grant agreement No [602587]. E.V. was supported by fellowships from the Italian Foundation for Cancer Research and the Italian Society of Pharmacology and a New Investigator Award from the American Society for Blood and Marrow Transplantation. J.A.D. was supported by a CJ Martin fellowship from the Australian National Health and Medical Research Council; a Scholar Award from the American Society of Hematology; and a K99 career transition award from the National Institutes of Health (1K99CA176376).
This article was published as part of a supplement, supported by WIS-CSP Foundation, in collaboration with Gilead, Milteny Biotec, Gamida cell, Adienne Pharma and Biotech, Medac hematology, Kiadis Pharma, Almog Diagnostic.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol. 2010;22:507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yung RL, Julius A. Epigenetics, aging, and autoimmunity. Autoimmunity. 2008;41:329–335. doi: 10.1080/08916930802024889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 5.Burns EA, Leventhal EA. Aging, immunity, and cancer. Cancer control : journal of the Moffitt Cancer Center. 2000;7:513–522. doi: 10.1177/107327480000700603. [DOI] [PubMed] [Google Scholar]
- 6.Castle SC. Clinical relevance of age-related immune dysfunction. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2000;31:578–585. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- 7.Ershler WB, Longo DL. Aging and cancer: issues of basic and clinical science. Journal of the National Cancer Institute. 1997;89:1489–1497. doi: 10.1093/jnci/89.20.1489. [DOI] [PubMed] [Google Scholar]
- 8.Ciofani M, Zuniga-Pflucker JC. The thymus as an inductive site for T lymphopoiesis. Annual review of cell and developmental biology. 2007;23:463–493. doi: 10.1146/annurev.cellbio.23.090506.123547. [DOI] [PubMed] [Google Scholar]
- 9.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 10.Rothenberg EV, Moore JE, Yui MA. Launching the T-cell-lineage developmental programme. Nat Rev Immunol. 2008;8:9–21. doi: 10.1038/nri2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alves NL, Huntington ND, Rodewald HR, Di Santo JP. Thymic epithelial cells: the multi-tasking framework of the T cell “cradle”. Trends Immunol. 2009;30:468–474. doi: 10.1016/j.it.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Flores KG, Li J, Sempowski GD, Haynes BF, Hale LP. Analysis of the human thymic perivascular space during aging. J Clin Invest. 1999;104:1031–1039. doi: 10.1172/JCI7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 14.Kurashima C, Utsuyama M, Kasai M, Ishijima SA, Konno A, Hirokawa K. The role of thymus in the aging of Th cell subpopulations and age-associated alteration of cytokine production by these cells. Int Immunol. 1995;7:97–104. doi: 10.1093/intimm/7.1.97. [DOI] [PubMed] [Google Scholar]
- 15.Sempowski GD, Gooding ME, Liao HX, Le PT, Haynes BF. T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol Immunol. 2002;38:841–848. doi: 10.1016/s0161-5890(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 16.Steinmann GG, Klaus B, Muller-Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scandinavian journal of immunology. 1985;22:563–575. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 17.George AJ, Ritter MA. Thymic involution with ageing: obsolescence or good housekeeping? Immunology today. 1996;17:267–272. doi: 10.1016/0167-5699(96)80543-3. [DOI] [PubMed] [Google Scholar]
- 18.Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. The Journal of pathology. 2007;211:144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA : the journal of the American Medical Association. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 20.Fiore AE, Bridges CB, Cox NJ. Seasonal influenza vaccines. Current topics in microbiology and immunology. 2009;333:43–82. doi: 10.1007/978-3-540-92165-3_3. [DOI] [PubMed] [Google Scholar]
- 21.McElhaney JE, Dutz JP. Better influenza vaccines for older people: what will it take? J Infect Dis. 2008;198:632–634. doi: 10.1086/590435. [DOI] [PubMed] [Google Scholar]
- 22.Nikolich-Zugich J. T cell aging: naive but not young. J Exp Med. 2005;201:837–840. doi: 10.1084/jem.20050341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maraninchi D, Gluckman E, Blaise D, Guyotat D, Rio B, Pico JL, Leblond V, Michallet M, Dreyfus F, Ifrah N, et al. Impact of T-cell depletion on outcome of allogeneic bone-marrow transplantation for standard-risk leukaemias. Lancet. 1987;2:175–178. doi: 10.1016/s0140-6736(87)90763-x. [DOI] [PubMed] [Google Scholar]
- 24.Storek J, Gooley T, Witherspoon RP, Sullivan KM, Storb R. Infectious morbidity in long-term survivors of allogeneic marrow transplantation is associated with low CD4 T cell counts. Am J Hematol. 1997;54:131–138. doi: 10.1002/(sici)1096-8652(199702)54:2<131::aid-ajh6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 25.Maury S, Mary JY, Rabian C, Schwarzinger M, Toubert A, Scieux C, Carmagnat M, Esperou H, Ribaud P, Devergie A, Guardiola P, Vexiau P, Charron D, Gluckman E, Socie G. Prolonged immune deficiency following allogeneic stem cell transplantation: risk factors and complications in adult patients. Br J Haematol. 2001;115:630–641. doi: 10.1046/j.1365-2141.2001.03135.x. [DOI] [PubMed] [Google Scholar]
- 26.Small TN, Papadopoulos EB, Boulad F, Black P, Castro-Malaspina H, Childs BH, Collins N, Gillio A, George D, Jakubowski A, Heller G, Fazzari M, Kernan N, MacKinnon S, Szabolcs P, Young JW, O'Reilly RJ. Comparison of immune reconstitution after unrelated and related T-cell- depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93:467–480. [PubMed] [Google Scholar]
- 27.Storek J, Joseph A, Espino G, Dawson MA, Douek DC, Sullivan KM, Flowers ME, Martin P, Mathioudakis G, Nash RA, Storb R, Appelbaum FR, Maloney DG. Immunity of patients surviving 20 to 30 years after allogeneic or syngeneic bone marrow transplantation. Blood. 2001;98:3505–3512. doi: 10.1182/blood.v98.13.3505. [DOI] [PubMed] [Google Scholar]
- 28.Storek J, Witherspoon RP, Storb R. T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life. Bone marrow transplantation. 1995;16:413–425. [PubMed] [Google Scholar]
- 29.Small TN, Papadopoulos EB, Boulad F, Black P, Castro-Malaspina H, Childs BH, Collins N, Gillio A, George D, Jakubowski A, Heller G, Fazzari M, Kernan N, MacKinnon S, Szabolcs P, Young JW, O'Reilly RJ. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93:467–480. [PubMed] [Google Scholar]
- 30.Lehrnbecher T, Foster C, Vazquez N, Mackall CL, Chanock SJ. Therapy-induced alterations in host defense in children receiving therapy for cancer. Journal of pediatric hematology/oncology. 1997;19:399–417. doi: 10.1097/00043426-199709000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Wils EJ, van der Holt B, Broers AE, Posthumus-van Sluijs SJ, Gratama JW, Braakman E, Cornelissen JJ. Insufficient recovery of thymopoiesis predicts for opportunistic infections in allogeneic hematopoietic stem cell transplant recipients. Haematologica. 2011;96:1846–1854. doi: 10.3324/haematol.2011.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim DH, Sohn SK, Won DI, Lee NY, Suh JS, Lee KB. Rapid helper T-cell recovery above 200 × 10 6/l at 3 months correlates to successful transplant outcomes after allogeneic stem cell transplantation. Bone marrow transplantation. 2006;37:1119–1128. doi: 10.1038/sj.bmt.1705381. [DOI] [PubMed] [Google Scholar]
- 33.Sfikakis PP, Gourgoulis GM, Moulopoulos LA, Kouvatseas G, Theofilopoulos AN, Dimopoulos MA. Age-related thymic activity in adults following chemotherapy-induced lymphopenia. European journal of clinical investigation. 2005;35:380–387. doi: 10.1111/j.1365-2362.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- 34.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Horowitz ME, Magrath IT, Shad AT, Steinberg SM, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. The New England journal of medicine. 1995;332:143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 35.Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, West ML, Smith OM, Holland AM, Tsai JJ, Boyd RL, van den Brink MR. Interleukin-22 drives endogenous thymic regeneration in mice. Science. 2012;336:91–95. doi: 10.1126/science.1218004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dixit VD, Yang H, Sun Y, Weeraratna AT, Youm YH, Smith RG, Taub DD. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007;117:2778–2790. doi: 10.1172/JCI30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alpdogan O, Muriglan SJ, Kappel BJ, Doubrovina E, Schmaltz C, Schiro R, Eng JM, Greenberg AS, Willis LM, Rotolo JA, O'Reilly RJ, van den Brink MR. Insulin-like growth factor-I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation. Transplantation. 2003;75:1977–1983. doi: 10.1097/01.TP.0000070167.81584.A2. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Hsu HC, Stockard CR, Yang P, Zhou J, Wu Q, Grizzle WE, Mountz JD. IL-12 inhibits thymic involution by enhancing IL-7- and IL-2-induced thymocyte proliferation. J Immunol. 2004;172:2909–2916. doi: 10.4049/jimmunol.172.5.2909. [DOI] [PubMed] [Google Scholar]
- 39.Mackall CL, Fry TJ, Bare C, Morgan P, Galbraith A, Gress RE. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood. 2001;97:1491–1497. doi: 10.1182/blood.v97.5.1491. [DOI] [PubMed] [Google Scholar]
- 40.Fry TJ, Sinha M, Milliron M, Chu YW, Kapoor V, Gress RE, Thomas E, Mackall CL. Flt3 ligand enhances thymic-dependent and thymic-independent immune reconstitution. Blood. 2004;104:2794–2800. doi: 10.1182/blood-2003-11-3789. [DOI] [PubMed] [Google Scholar]
- 41.Zakrzewski JL, Kochman AA, Lu SX, Terwey TH, Kim TD, Hubbard VM, Muriglan SJ, Suh D, Smith OM, Grubin J, Patel N, Chow A, Cabrera-Perez J, Radhakrishnan R, Diab A, Perales MA, Rizzuto G, Menet E, Pamer EG, Heller G, Zuniga-Pflucker JC, Alpdogan O, van den Brink MR. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006;12:1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 42.van den Brink MR, Alpdogan O, Boyd RL. Strategies to enhance T-cell reconstitution in immunocompromised patients. Nat Rev Immunol. 2004;4:856–867. doi: 10.1038/nri1484. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg GL, Zakrzewski JL, Perales MA, van den Brink MR. Clinical strategies to enhance T cell reconstitution. Semin Immunol. 2007;19:289–296. doi: 10.1016/j.smim.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen L, Xiao S, Manley NR. Foxn1 is required to maintain the postnatal thymic microenvironment in a dosage-sensitive manner. Blood. 2009;113:567–574. doi: 10.1182/blood-2008-05-156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hauri-Hohl MM, Zuklys S, Keller MP, Jeker LT, Barthlott T, Moon AM, Roes J, Hollander GA. TGF-beta signaling in thymic epithelial cells regulates thymic involution and postirradiation reconstitution. Blood. 2008;112:626–634. doi: 10.1182/blood-2007-10-115618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrew D, Aspinall R. Age-associated thymic atrophy is linked to a decline in IL-7 production. Experimental gerontology. 2002;37:455–463. doi: 10.1016/s0531-5565(01)00213-3. [DOI] [PubMed] [Google Scholar]
- 47.Min H, Montecino-Rodriguez E, Dorshkind K. Reduction in the developmental potential of intrathymic T cell progenitors with age. J Immunol. 2004;173:245–250. doi: 10.4049/jimmunol.173.1.245. [DOI] [PubMed] [Google Scholar]
- 48.Sempowski GD, Hale LP, Sundy JS, Massey JM, Koup RA, Douek DC, Patel DD, Haynes BF. Leukemia inhibitory factor, oncostatin M, IL-6, and stem cell factor mRNA expression in human thymus increases with age and is associated with thymic atrophy. J Immunol. 2000;164:2180–2187. doi: 10.4049/jimmunol.164.4.2180. [DOI] [PubMed] [Google Scholar]
- 49.Sharp A, Kukulansky T, Globerson A. In vitro analysis of age-related changes in the developmental potential of bone marrow thymocyte progenitors. Eur J Immunol. 1990;20:2541–2546. doi: 10.1002/eji.1830201203. [DOI] [PubMed] [Google Scholar]
- 50.Eren R, Zharhary D, Abel L, Globerson A. Age-related changes in the capacity of bone marrow cells to differentiate in thymic organ cultures. Cell Immunol. 1988;112:449–455. doi: 10.1016/0008-8749(88)90315-2. [DOI] [PubMed] [Google Scholar]
- 51.Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM, Goodnow CC, Boyd RL. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood. 2006;108:3777–3785. doi: 10.1182/blood-2006-02-004531. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Qiao S, Tuckermann J, Okret S, Jondal M. Thymus-derived glucocorticoids mediate androgen effects on thymocyte homeostasis. FASEB J. 2010;24:5043–5051. doi: 10.1096/fj.10-168724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Windmill KF, Meade BJ, Lee VW. Effect of prepubertal gonadectomy and sex steroid treatment on the growth and lymphocyte populations of the rat thymus. Reproduction, fertility, and development. 1993;5:73–81. doi: 10.1071/rd9930073. [DOI] [PubMed] [Google Scholar]
- 54.Grossman CJ, Sholiton LJ, Blaha GC, Nathan P. Rat thymic estrogen receptor--II. Physiological properties. Journal of steroid biochemistry. 1979;11:1241–1246. doi: 10.1016/0022-4731(79)90191-2. [DOI] [PubMed] [Google Scholar]
- 55.Kuhl H, Gross M, Schneider M, Weber W, Mehlis W, Stegmuller M, Taubert HD. The effect of sex steroids and hormonal contraceptives upon thymus and spleen on intact female rats. Contraception. 1983;28:587–601. doi: 10.1016/0010-7824(83)90109-9. [DOI] [PubMed] [Google Scholar]
- 56.Luster MI, Hayes HT, Korach K, Tucker AN, Dean JH, Greenlee WF, Boorman GA. Estrogen immunosuppression is regulated through estrogenic responses in the thymus. J Immunol. 1984;133:110–116. [PubMed] [Google Scholar]
- 57.Dulos GJ, Bagchus WM. Androgens indirectly accelerate thymocyte apoptosis. Int Immunopharmacol. 2001;1:321–328. doi: 10.1016/s1567-5769(00)00029-1. [DOI] [PubMed] [Google Scholar]
- 58.Olsen NJ, Olson G, Viselli SM, Gu X, Kovacs WJ. Androgen receptors in thymic epithelium modulate thymus size and thymocyte development. Endocrinology. 2001;142:1278–1283. doi: 10.1210/endo.142.3.8032. [DOI] [PubMed] [Google Scholar]
- 59.McCruden AB, Stimson WH. Androgen binding cytosol receptors in the rat thymus: physicochemical properties, specificity and localisation. Thymus. 1981;3:105–117. [PubMed] [Google Scholar]
- 60.Kovacs WJ, Olsen NJ. Androgen receptors in human thymocytes. J Immunol. 1987;139:490–493. [PubMed] [Google Scholar]
- 61.Pearce P, Khalid BA, Funder JW. Androgens and the thymus. Endocrinology. 1981;109:1073–1077. doi: 10.1210/endo-109-4-1073. [DOI] [PubMed] [Google Scholar]
- 62.Zoller AL, Kersh GJ. Estrogen induces thymic atrophy by eliminating early thymic progenitors and inhibiting proliferation of beta-selected thymocytes. J Immunol. 2006;176:7371–7378. doi: 10.4049/jimmunol.176.12.7371. [DOI] [PubMed] [Google Scholar]
- 63.Guevara Patino JA, Marino MW, Ivanov VN, Nikolich-Zugich J. Sex steroids induce apoptosis of CD8+CD4+ double-positive thymocytes via TNF-alpha. Eur J Immunol. 2000;30:2586–2592. doi: 10.1002/1521-4141(200009)30:9<2586::AID-IMMU2586>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 64.Lai KP, Lai JJ, Chang P, Altuwaijri S, Hsu JW, Chuang KH, Shyr CR, Yeh S, Chang C. Targeting thymic epithelia AR enhances T-cell reconstitution and bone marrow transplant grafting efficacy. Mol Endocrinol. 2013;27:25–37. doi: 10.1210/me.2012-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Henderson J. On the relationship of the thymus to the sexual organs: I. The influence of castration on the thymus. The Journal of physiology. 1904;31:222–229. doi: 10.1113/jphysiol.1904.sp001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goodall A. The post-natal changes in the thymus of guinea-pigs, and the effect of castration on thymus structure. The Journal of physiology. 1905;32:191–198. doi: 10.1113/jphysiol.1905.sp001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fitzpatrick FT, Kendall MD, Wheeler MJ, Adcock IM, Greenstein BD. Reappearance of thymus of ageing rats after orchidectomy. The Journal of endocrinology. 1985;106:R17–19. doi: 10.1677/joe.0.106r017. [DOI] [PubMed] [Google Scholar]
- 68.Utsuyama M, Hirokawa K. Hypertrophy of the thymus and restoration of immune functions in mice and rats by gonadectomy. Mech Ageing Dev. 1989;47:175–185. doi: 10.1016/0047-6374(89)90030-4. [DOI] [PubMed] [Google Scholar]
- 69.Olsen NJ, Watson MB, Henderson GS, Kovacs WJ. Androgen deprivation induces phenotypic and functional changes in the thymus of adult male mice. Endocrinology. 1991;129:2471–2476. doi: 10.1210/endo-129-5-2471. [DOI] [PubMed] [Google Scholar]
- 70.Oner H, Ozan E. Effects of gonadal hormones on thymus gland after bilateral ovariectomy and orchidectomy in rats. Archives of andrology. 2002;48:115–126. doi: 10.1080/014850102317267427. [DOI] [PubMed] [Google Scholar]
- 71.Heng TS, Goldberg GL, Gray DH, Sutherland JS, Chidgey AP, Boyd RL. Effects of castration on thymocyte development in two different models of thymic involution. J Immunol. 2005;175:2982–2993. doi: 10.4049/jimmunol.175.5.2982. [DOI] [PubMed] [Google Scholar]
- 72.Heng TS, Reiseger JJ, Fletcher AL, Leggatt GR, White OJ, Vlahos K, Frazer IH, Turner SJ, Boyd RL. Impact of sex steroid ablation on viral, tumour and vaccine responses in aged mice. PLoS One. 2012;7:e42677. doi: 10.1371/journal.pone.0042677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roden AC, Moser MT, Tri SD, Mercader M, Kuntz SM, Dong H, Hurwitz AA, McKean DJ, Celis E, Leibovich BC, Allison JP, Kwon ED. Augmentation of T Cell Levels and Responses Induced by Androgen Deprivation. The Journal of Immunology. 2004;173:6098–6108. doi: 10.4049/jimmunol.173.10.6098. [DOI] [PubMed] [Google Scholar]
- 74.Goldberg GL, Dudakov JA, Reiseger JJ, Seach N, Ueno T, Vlahos K, Hammett MV, Young LF, Heng TS, Boyd RL, Chidgey AP. Sex steroid ablation enhances immune reconstitution following cytotoxic antineoplastic therapy in young mice. J Immunol. 2010;184:6014–6024. doi: 10.4049/jimmunol.0802445. [DOI] [PubMed] [Google Scholar]
- 75.Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, Blazar BR, Millar JL, Malin MA, Chidgey AP, Boyd RL. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 76.Goldberg GL, Alpdogan O, Muriglan SJ, Hammett MV, Milton MK, Eng JM, Hubbard VM, Kochman A, Willis LM, Greenberg AS, Tjoe KH, Sutherland JS, Chidgey A, van den Brink MR, Boyd RL. Enhanced immune reconstitution by sex steroid ablation following allogeneic hemopoietic stem cell transplantation. J Immunol. 2007;178:7473–7484. doi: 10.4049/jimmunol.178.11.7473. [DOI] [PubMed] [Google Scholar]
- 77.Goldberg GL, Sutherland JS, Hammet MV, Milton MK, Heng TS, Chidgey AP, Boyd RL. Sex steroid ablation enhances lymphoid recovery following autologous hematopoietic stem cell transplantation. Transplantation. 2005;80:1604–1613. doi: 10.1097/01.tp.0000183962.64777.da. [DOI] [PubMed] [Google Scholar]
- 78.Dudakov JA, Goldberg GL, Reiseger JJ, Vlahos K, Chidgey AP, Boyd RL. Sex steroid ablation enhances hematopoietic recovery following cytotoxic antineoplastic therapy in aged mice. J Immunol. 2009;183:7084–7094. doi: 10.4049/jimmunol.0900196. [DOI] [PubMed] [Google Scholar]
- 79.Goldberg GL, King CG, Nejat RA, Suh DY, Smith OM, Bretz JC, Samstein RM, Dudakov JA, Chidgey AP, Chen-Kiang S, Boyd RL, van den Brink MR. Luteinizing hormone-releasing hormone enhances T cell recovery following allogeneic bone marrow transplantation. J Immunol. 2009;182:5846–5854. doi: 10.4049/jimmunol.0801458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perisic M, Stojic-Vukanic Z, Pilipovic I, Kosec D, Nacka-Aleksic M, Dikic J, Arsenovic-Ranin N, Leposavic G. Role of ovarian hormones in T-cell homeostasis: from the thymus to the periphery. Immunobiology. 2013;218:353–367. doi: 10.1016/j.imbio.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 81.Min H, Montecino-Rodriguez E, Dorshkind K. Reassessing the role of growth hormone and sex steroids in thymic involution. Clin Immunol. 2006;118:117–123. doi: 10.1016/j.clim.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 82.Umathe SN, Dixit PV, Wanjari MM, Ullewar MP. Leuprolide--a GnRH agonist--prevents restraint stress-induced immunosuppression via sex steroid-independent peripheral mechanism in mice. Int Immunopharmacol. 2008;8:71–79. doi: 10.1016/j.intimp.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 83.Sutherland JS, Spyroglou L, Muirhead JL, Heng TS, Prieto-Hinojosa A, Prince HM, Chidgey AP, Schwarer AP, Boyd RL. Enhanced immune system regeneration in humans following allogeneic or autologous hemopoietic stem cell transplantation by temporary sex steroid blockade. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:1138–1149. doi: 10.1158/1078-0432.CCR-07-1784. [DOI] [PubMed] [Google Scholar]
- 84.van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology. 2008;71:1001–1006. doi: 10.1016/j.urology.2007.12.070. [DOI] [PubMed] [Google Scholar]


