Abstract
Recent findings regarding the influence of the microbiota in many inflammatory processes have provided a new way to treat diseases. Now, one may hypothesize that the origin of a plethora of diseases is related to the health of the gut microbiota and its delicate, although complex, interface with the epithelial and immune systems. The ‘westernization' of diets, for example, is associated with alterations in the gut microbiota. Such alterations have been found to correlate directly with the increased incidence of diabetes and hypertension, the main causes of chronic kidney diseases (CKDs), which, in turn, have a high estimated prevalence. Indeed, data have arisen showing that the progression of kidney diseases is strictly related to the composition of the microbiota. Alterations in the gut microbiota diversity during CKDs do not only have the potential to exacerbate renal injury but may also contribute to the development of associated comorbidities, such as cardiovascular diseases and insulin resistance. In this review, we discuss how dysbiosis through alterations in the gut barrier and the consequent activation of immune system could intensify the progression of CKD and vice versa, how CKDs can modify the gut microbiota diversity and abundance.
Chronic kidney diseases (CDKs) are characterized by progressive glomerular, tubular and interstitial damage that results in scar tissue and impaired renal function. Patients with CKDs experience increased blood pressure, decreased erythropoietin synthesis, the development of metabolic acidosis and accumulation of high levels of metabolic end products, namely, the uraemic toxins. CKDs are a global health issue with an increasing estimated prevalence of 8–16%.1 Diabetes, hypertension and glomerulonephritis are the leading causes of CKDs worldwide and considering the high incidence of such conditions, the number of people suffering from CKDs has tended towards a sustained increase.1 In the last decade, developed and developing countries have been adopting major changes in dietary habits, in which fibres, fruits and vegetables have been replaced with fat, sugar and high amounts of salt found in fast foods and processed foods.2 These changes in nutritional habits due to fast-food consumption are significantly associated with increases in body weight, body mass index and insulin resistance,3 which are consequently associated with the development of diabetes and hypertension and, therefore, have a direct impact on the annual estimated rates of CKDs.
It is becoming clear that the local and systemic consequences of kidney damage might largely result from changes in the gut microbiota. Microbiota is a term coined to describe the population of bacteria, viruses and fungi that live in a commensal, symbiotic or pathogenic way within a live host. The gut microbiota is harboured within the whole intestine and comprises bacteria from different phyla. The human microbiota is composed of almost 100 trillion bacterial cells colonizing the outer and inner surfaces of the human body, and the microbiota present in the gut is considered the most dense and biodiverse ecosystem in the world, represented by seven great bacterial phyla—Actinobacteria, Bacteroidetes, Firmicutes, Fusobacteria, Proteobacteria, Tenericutes and Verrucomicrobia—of which Bacteroidetes and Firmicutes together comprise 90%.4
This review is focused on the recent findings regarding the importance of maintenance of a healthy microbiota to the progression and development of CKDs. We summarized the negative effects of renal function loss on microbiota composition as well as the consequences of disordered gut microbial diversity in renal function. In addition, we show how some therapies using probiotics, prebiotics and symbiotics could be a promising intervention to address dysbiosis-linked CKDs.
Gut microbiota and diseases
The findings about the gut microbiota over the past decade have surprised the scientific community showing its crucial role for the development and homeostasis of the host by building and modulating the immune system and improving intestinal defence to face opportunistic pathogens, by synthesizing vitamins, by biotransforming conjugated bile acids and by extracting energy from fermenting non-digestible carbohydrates.5, 6 Due to all these beneficial actions, there has been growing emphasis on considering the microbiota as a functional ‘organ' and counting it as part of our eukaryotic cell pool is plausible. Thus, as an ‘organ', diseases of the microbiota that affect their health—diversity and variability—also called dysbiosis, represent a great challenge because they would not only disturb local intestinal homeostasis but might influence a wide range of extra-intestinal complications.5
Disturbance in the composition of the microbiota has been shown to be strongly related to the incidence of inflammatory diseases, supporting a key role of a commensal microbiota in host homeostasis.7 Currently, it is possible to assert that the gut microbiota is involved in several aspects of host homeostasis, for exanple, tissue and cell metabolism, and physiological and immune system functions. These connections dictate disease susceptibility in different organs, such as the intestines, brain, liver, kidneys and so on.8 Many factors can influence the composition of the gut microbiota such as diet, which is able to modulate its composition quickly7 and at the species level.9 Considering the gut as a hypoxic compartment where carbohydrate and proteins constitute the major nutritive resources that reach intestine without being metabolized prior, fermentation is the primary or only way through which these bacteria obtain energy. The intestinal microbial glycobiome is very relevant by encoding specific enzymes that potentially metabolize non-digested carbohydrates (or resistant starch, RS) allowing the host to extract more energy and to generate beneficial products from indigestible polysaccharides.10 A diet rich in RS allows plant-derived polysaccharides to reach the colon in a non-metabolized form to be degraded by resident saccharolytic bacteria microbiota, increasing the amount of short-chain fatty acids (SCFAs).11 In particular, acetate, propionate and butyrate are carbohydrate fermentation secondary products that show a pivotal role in energy homeostasis, host nutrition and a multifactorial role in human health and inflammation.12 Thorburn et al. elegantly showed that mice fed a diet enriched in RS for 3 weeks were resistant to developing allergic airway disease compared with mice fed normal or no-fibre diets. The authors related this protection to altered gut microbial ecology, characterized by the increasing abundance of the Bacteroidetes phylum and high serum acetate levels.13 Moreover, the benefits of a high RS diet in shaping the gut microenvironment was further demonstrated as the offspring from pregnant mice provided with high-RS diets or acetate in drinking water also failed to develop allergic airway disease in adulthood, as evidenced by a reduction of total cells and eosinophils in bronchoalveolar lavage fluid, Th2 cytokine levels and lung inflammation.13 However, the end products of carbohydrates and protein fermentation may be widely diverse, with different and opposing effects on the gut. Although undigested carbohydrates are the main resource of energy for bacteria generating methane and SCFAs as end products, undigested protein metabolism produces potentially toxic end products such as ammonia, thiols, phenols and indols.14
The striking effects of increased consumption of these ‘western foods', characterized by low amounts of RS, have been associated with profound changes on the gut microbiota and its by-products and are unquestionable regulators of host homeostasis.12 This evidence was strengthened when the faecal microbiota of urban European children, who were on a diet based on consumption of high fat and lower fibres, was compared from those of African children on a diet essentially based on foods rich in fibres and low fats.15 African children showed a significant enrichment in bacteria belonging to the Bacteroidetes genus, including an abundance of bacteria from the genera Prevotella and Xylanibacter, both efficient at digesting fibre and producing SCFAs, and the depletion of the Firmicutes genus. In addition, European children showed significant enrichment of Enterobacteriaceae (Shigella and Escherichia); the population of fibre-digesting bacteria was almost abrogated.15 This difference in diet quality is considered a strong factor that could explain why the incidence of asthma and inflammatory disease in African children is lower than in European children.16, 17 Excluding the supposed alleged interference of ethnicities, rural Africans showed microbial predominance of the genus Prevotella and more abundant butyrate-producing groups of bacteria, and African Americans showed an enrichment in Bacteroides and lower abundance of saccharolytic bacteria.18
Due to the diversity and varied composition of the gut microbiota, a plethora of diseases have been associated with the predominance of different species of bacteria, which suggests that different diseases might have different impacts in gut microbiota and vice versa.7, 9 For instance, analyses of stools from infants and children up to 5 years of age provided evidence that dysbiosis in the beginning of life is related to a predominance of lung inflammation. According to Arrieta et al., the relative abundance of the bacterial genera Lachnospira, Veillonella, Faecalibacterium and Rothia combined with reduced production of SCFAs was markedly decreased during the first 100 days of life of infants who developed asthma. The therapeutic inoculation of these bacteria taxa into germ-free (GF) mice previously inoculated with the stool from asthmatic patients significantly attenuated airway inflammation in their adult offspring, highlighting the strong potential of microbial composition in inflammatory conditions such as asthma.19 Indeed, approaches by which microbiota was transferred into GF mice noted its influence for the development of diseases. GF mice colonized with the stool from obese people showed significant increases in body mass and adiposity compared with those mice colonized with uncultured faecal microbiota from lean subjects. Such differences in body composition were correlated with the increasing fermentation of SCFAs in mice that received the ‘lean faeces' and increased metabolism of branched-chain amino acids in mice that received the ‘obese faeces'.20
Gut microbiota and CKDs
Recently, a promising connection among the unbalanced gut microbiota, poorly ingested enriched-fibre foods and the progression of CKDs has been established.21, 22 Microbial DNA analyses comparing stools from patients with end-stage renal disease (ESRD) and healthy persons showed a significant increase in the relative abundance for 190 bacterial operational taxonomic units (OTUs), mostly belonging to the Pseudomonadaceae family in the ESRD group, whereas healthy individuals showing an abundance of OTUs belonged to the Sutterellaceae, Bacteroidaceae and Lactobacillaceae families.21 Similar results were obtained with an experimental model of CKD; 5/6 nephrectomy resulted in significant differences in OTU abundance, of which the Bacteroidetes and Firmicutes families, especially Lactobacillaceae and Prevotellaceae, were markedly less prevalent in nephrectomized rats.21
Advanced renal failure impairs glomerular filtration and tubular secretion in CKD patients culminating in increased serum levels of nitrogen compounds (urea and uric acid), which in turn, may reach the intestines and promote the overgrowth of bacterial species that are prone to generate uraemic toxins by fermentation, outnumbering the protective species such as Lactobacilli.21, 23 High urea concentrations in the plasma lead to increased concentrations in the gastrointestinal tract, where it is metabolized by urease-producing bacteria. The main final product resulting from the urea metabolism is ammonia, which increases intraluminal pH, so therefore triggering intestinal disorder and unbalance of gut homeostasis.24
It is now clear that the intestine acts as an important coadjutant when the kidney fails to excrete metabolic products from diet. Hatch et al. have shown that rats submitted to 5/6 nephrectomy had lower renal clearance of oxalate and a higher plasma oxalate concentration than control animals, which was compensated by colon excretion.25 This change in the oxalate flux was observed only in the large intestine, with no changes in flux in the small intestine.25 In addition, the deficient colonization of Oxalobacter formigenes—a gram-negative anaerobic bacterium that degrades oxalate—in the intestinal tract of idiopathic calcium oxalate stone patients was associated with increased urinary oxalate excretion, which attributed to decreased intestinal oxalate degradation and consequently more oxalate available for absorption.26 In addition, the colon has a major role in the excretion of urea and uric acids when renal function declines, replacing the kidney as the primary site for the excretion of these metabolites.27
Indirect effects of the gut microbiota on CKD
Notwithstanding the paucity of studies evaluating RS or SCFA-producing bacteria in CKDs, a RS diet can be beneficial in other contexts. For instance, high amounts of RS can promote insulin sensitivity and reduce body fat.28, 29 The consumption of high-amylose maize RS is related to improved insulin sensitivity in women with insulin resistance28 and diminishes postprandial hyperglycaemia in patients with type 2 diabetes.29 It appears these effects can be partly attributed to the stimulated secretion of gut-secreted hormones glucagon-like peptide-1 and peptide YY,30 two hormones that are both recognized for their anti-obesity effects. These findings become important in the context of CKDs because obesity and, by consequence, type 2 diabetes, are relevant risk factors for kidney failure. Thus, a RS diet can even contribute indirectly to improved kidney function by regulating underlying disturbances that lead to CKDs.
The beneficial effects of ingesting high-fibre diets and SCFAs are also related to the impaired progression of colitis.31 interleukin (IL)-10-deficient mice, which develop colitis spontaneously, have experienced significant amelioration of those symptoms when fed high-fibre diets.32 This protection was associated with an increase of butyrate-producing bacteria as well as the reduction in pro-inflammatory cytokines IL-1β, tumor necrosis factor and IL-23,32 bolstering the idea that the modulation of gut microbiota so as to bias the microbiota to SCFA-producing bacteria may favour an anti-inflammatory profile.
Questions about how the microbiota and intestinal immune system have co-evolved in harmony, shaping a non-inflammatory environment, have become increasingly clear when we consider SCFAs as cell function modulators. In this sense, SCFAs are related to the increased number and suppressive activity of colonic and peripheral regulatory T cells by limiting proliferation of effector CD4+ T cells.33, 34 In addition to T cells, macrophages are regulated and conditioned to reduce the production of proinflammatory cytokines such as nitric oxide, IL-6 and IL-12 in the presence of SCFA.35 Furthermore, SCFAs improve intestinal transit, stool consistency and luminal pH.11 These findings gain importance as long as one considers the immunological branch of CKDs, which is important for macrophage activation and accumulation that appears to contribute to the onset and development of CKDs.36, 37
Still, SCFAs may also interfere in subtypes of kidney cells, such as epithelial cells,38 and control blood pressure regulation by inducing renin secretion,39 strengthening the idea that strategies that can increase SCFA levels in intestine systemically may contribute to the prevention or amelioration of CKDs. Taking into account the kidney—gut axis, these approaches could be used to modulate the gut microbiota either by increasing the proportion of SCFA-producing bacteria or increasing the supply of carbohydrates that reach the gut in a non-metabolized form. In light of this idea, Van Beneden et al.40 reported that treatment with valproic acid (a compound with similar structure of a SCFA) was able to protect mice from proteinuria and kidney injury in a model of focal segmental glomerulosclerosis induced by doxorrubicin. Our group reported that treatment with acetate-producing bacteria can increase acetate levels in the caecum and in the serum, which in turn, ameliorated acute kidney injury,38 demonstrating the therapeutic potential of these molecules. However, more strategies and studies are needed to address the potential of other SCFA, probiotics and prebiotics in the progression of CKD.
CKD triggers dysbiosis
Kidneys remove substances and toxic compounds that come from cell metabolism from the bloodstream to maintain body homeostasis. It is reasonable to hypothesize that impaired renal function due to diminished glomerular filtration rate and tubular absorption leads to increased levels of compounds that should be eliminated, thus accumulating toxicity. In cases when end products of purine metabolism such as urea, uric acid and oxalates, which are predominantly excreted by kidneys via a complex interplay of glomerular filtration, secretion processes and tubular reabsorption, reach high levels in the bloodstream, the colon become the major excretory organ to maintain body homeostasis.25, 27 This adaptive response leads to severe consequences for the gut environment. Serum urea accumulation during CKD increases urea influx into the intestinal lumen, where urease-producing bacteria hydrolyze it into ammonia and ammonium hydroxide, consequently increasing intestinal pH and promoting mucosal irritation and structural alterations to the gut barrier. Such physiological alterations are associated with dysbiosis, bacterial translocation and endotoxaemia, potentially enhancing kidney inflammation.11, 21
CKD exacerbates renal inflammation
A healthy gut environment ensures that defence mechanisms impair the translocation of toxic substances and microbes from the intestinal lumen into the bloodstream. The dynamic equilibrium between the symbiotic microbiota and the host is established by many factors such as the maintenance intestinal barrier integrity, IgA secretion and a balanced immunological response on the intestinal wall. Several lines of evidence support that advanced CKD impairs intestinal epithelial barrier structure and function allowing endotoxin and other bacterial components to cross the intestinal wall and reach systemic circulation. Rats that underwent 5/6 nephrectomy and the uraemic milieu found in CKD are related to the marked depletion of key protein constituents of colonic epithelial tight junctions such as of occludin, claudin-1, zonula occludens-1 and reduced transepithelial resistance, which denote increased permeability and epithelial barrier dysfunction.41 Corroborating these results, changes in the intestinal barrier were also found in in vitro experiments using plasma taken from uraemic patients.42 The disturbance promoted by CKD on the intestinal wall through the loss of tight junctions facilitates the translocation of microbes, lipopolysaccharide and toxic bacterial products to the bloodstream and could enhance the inflammatory conditions of the CKD patient. The presence of endotoxin and gut bacterial DNA fragments in the blood of haemodialysated43 and non-haemodialysated patients44 suggests the gastrointestinal tract as the origin of endotoxins and directly associates severe systemic inflammation in CKD patients with the magnitude of endotoxaemia in the absence of infections.
Once microbes have crossed the intestinal barrier, they may reach the kidneys to be recognized by pattern recognition receptors, such as toll-like receptors (TLRs), NOD-like receptors (NLRs) and the NLRP3 inflammasome that activate local immune cell responses. Considering that the development of chronic injuries is characterized by non-resolving inflammation, the presence of endotoxins in the kidney could exacerbate the progression of a local damage through the increased expression and activation of recognition systems on inflammatory cells and also on renal parenchymal cells, which culminates in exacerbated renal injury and more severe loss of kidney function. Taking into account that renal parenchymal cells and intrarenal immune cells express several families of pattern recognition receptors, such as TLRs, NLRs and the NLRP3 inflammasome, they are able to recognize endotoxins that come from a leaky gut into secretion of pro proinflammatory cytokines and chemokines.45
The sustained activation of TLRs in the kidney was shown to have a key role during progression of kidney disease and local inflammation.36, 46, 47 Renal cells constitutively express TLR2 and TLR4 predominantly in the renal epithelial cells of distal and proximal tubules, and this expression increases during the inflammatory process.48 The presence of bacteria and endotoxins in the bloodstream, mimicked by an experimental model of sepsis in mice, has shown the contribution of TLR2, TLR4 and adaptive protein Myd88 on the development of acute kidney injury secondary to sepsis.46 However, deficiency of TLR2, TLR4 and adaptive protein Myd88 was also associated with decreased interstitial fibrosis, lower M2 macrophage infiltration, and the reduced production of pro-inflammatory cytokines and chemokines in the kidney.36, 47 Mice induced to develop diabetic nephropathy showed the overexpression of NOD2 in renal biopsies of diabetic mice and patients,49 and as TLRs, the expression of NLRs by renal parenchymal cells and intrarenal immune cells could also exacerbate renal inflammation in a scenario where gut endotoxins reach the blood because of colon leakage. The activation of NOD2 in podocytes leads to the stimulation of ERK2 and nuclear factor-kB, and, concomitantly, the secretion of proinflammatory and profibrotic mediators. NOD2-deficient mice have shown lower scarring and renal inflammation as well as protection from the hyperglycaemia-induced reduction in nephrin expression, an essential protein involved in the maintenance of podocyte integrity.49 Furthermore, uraemic patients undergoing dialysis treatment exhibited elevated production of reactive oxygen species and activated NLRP3 inflammasome possibly induced by mitochondrial dysfunction.50 Although no correlation has been established between the prevalence of CKD with dysbiosis caused by pathobionts of the Enterobacteriaceae family, particularly with Proteus mirabilis colonization, intestinal injury caused by this species induces the recruitment and robust NLRP3-dependent IL-1β release by monocytes. Enhanced tissue pathology associated with P. mirabilis colonization was not observed in NLPR3-deficient mice,51 reinforcing the idea that dysbiosis and leakage of the gut barrier can somehow improve kidney inflammation.
Dysbiosis and IgA nephropathy
Although it is still inconclusive whether CKD development is a consequence of an unhealthy microbiota—or vice versa—many lines of evidence support how dysbiosis could be linked with the progression of kidney diseases. The immunological impact of dysbiosis—also seen in gastrointestinal diseases such as coeliac disease, Crohn's disease and ulcerative colitis52—could trigger the activation of the gut mucosa occasioned by disruptions of intestinal cell tight junctions, allowing bacterial translocation and colonic establishment of the Th1 response and the overproduction of polymeric IgA by B cells. It is well known that the secretion of IgA in the gut has a key role in maintaining the balance between commensal and pathogenic bacteria, whereas IgA is the most abundant immunoglobulin in mucosal secretions and it is strictly involved in pathogenesis of IgA nephropathy (IgAN).53, 54, 55, 56
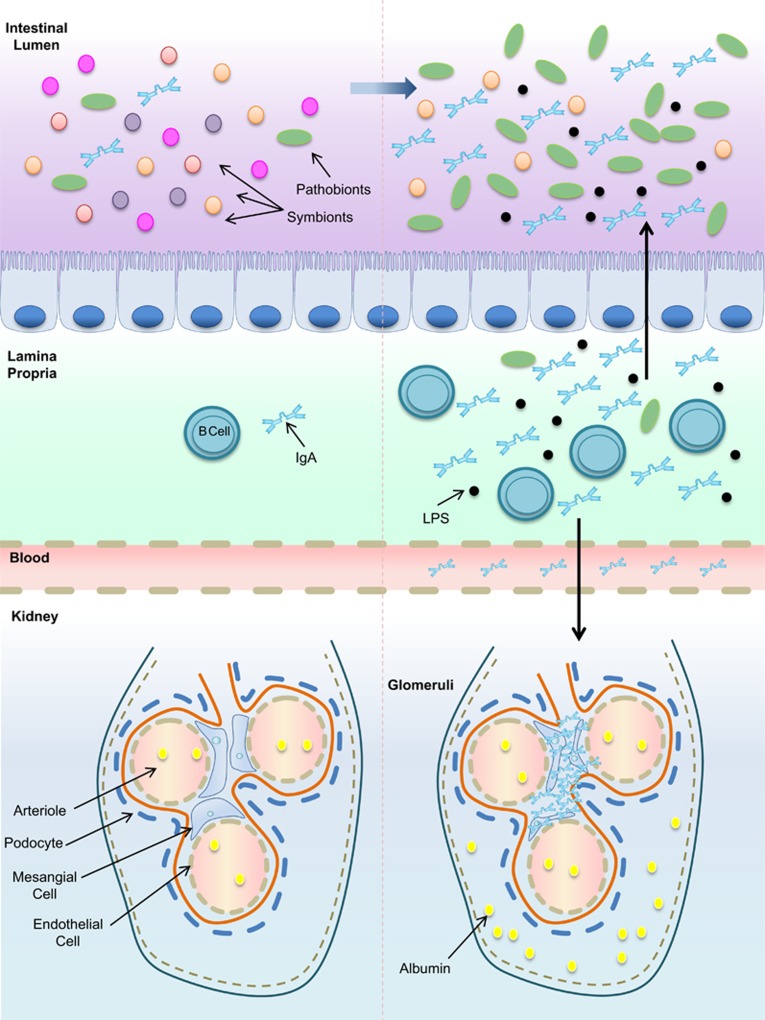
Considered an hyperactive mucosal system response to germs present at the mucosal surface, IgAN is a disorder described as the deposition of IgA immune complexes in the mesangial area, leading to blood and protein excretion in urine (Figure 1).57 Patients with IgAN show dysbiosis characterized by altered composition of the main bacterial phyla (Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria) compared with healthy subjects, with a decrease in total anaerobic bacteria densities (Clostridium, Enterococcus, Lactobacillus, Leuconostoc and Bifidobacterium).53 In contrast, patients with primary and secondary IgAN showed altered duodenal histopathological findings and increased intestinal permeability.58 Constant exposure to endotoxins induced epithelial cells to secrete B-cell-activating factor of the tumor necrosis factor family (BAFF) and the proliferation-inducing ligand (APRIL), both related to the maintenance of tolerogenic immune responses to the microbiota. Mice overexpressing BAFF (BAFF-Tg) have shown high levels of aberrantly glycosylated serum polymeric IgA and, consequently, IgA mesangial deposition.59 However, in these mice, BAFF did not drive IgA deposition by itself in the kidney or elevated levels of it in the serum, but rather, it depended on the presence of bacterial signals. Comparative analyses using GF BAFF-Tg and GF wild-type mice showed that both have similar levels of serum IgA, but GF BAFF-Tg mice showed levels ~100-fold lower than specific pathogen-free BAFF-Tg mice. GF BAFF-Tg mice also showed a decreased frequency of IgA+ plasma cells in the gut and lower IgA deposition in the glomeruli. The re-establishment of commensal flora in GF BAFF-Tg mice was accompanied by a substantially progressive increase in levels of serum IgA, increased IgA+ plasma cells in the lamina propria and the re-emergence of renal glomerular IgA deposition.59 Patients with IgAN also presented elevated serum levels of BAFF and APRIL.59
Figure 1.
Dysbiosis and IgA nephropathy. The balance of gut homeostasis is ruled by microbial composition and secretion of specific immunoglobulins, among them IgA. Gut microbial composition imbalance and the high secretion of under glycosylated oligomeric IgA against gut bacteria may trigger the development of IgA nephropathy. LPS, lipopolysaccharide.
Interestingly, in several gastrointestinal diseases in which dysbiosis is associated, such as Crohn's disease and ulcerative colitis, a high frequency of IgAN has been reported.60 Taking into account that IgA molecules are the most abundant in mucosal secretions, the development of polymeric IgA, which is more effective in binding to pathogens, deposits were interestingly related to the presence of commensal flora and the circulation of corresponding specific IgA antibodies.59 However, the contact with alimentary components and intestinal flora in a spontaneous murine strain overexpressing IgA led to a threefold increase in the number of intestinal IgA-producing plasma cells, with the concomitant downregulation of secretory IgA excretion into the intestinal lumen after the 10th week of age, culminating in high IgA levels in circulation and renal IgA deposits.54
CKD, the gut microbiota and its metabolites
Considering that CKD patients are often under dietary restrictions to avoid hyperkalemia, pharmacological approaches, such as phosphate-bindings agents and, eventually, antibiotics to treat current infections, certainly reinforce an unbalanced biochemical milieu that shapes the composition and function of microbiota. CKD patients with high dietary consumption of fibres have lower inflammation status, with low levels of reactive serum C-reactive protein and lower mortality compared with CKD patients fed regular- or low-fibre diets.61 Surprisingly, the high intake of fibres and fibre-rich plant foods was also associated with a significantly lower risk of renal cell carcinoma.62 Taking into account the wide availability of nitrogen products in the gut, Wong et al.63 reported that the overgrowth of nitrogen compound-metabolizing bacteria such as urease-, uricase-, p-cresol- and indol-producing microbes in ESRD supplant the number of bacteria containing enzymes converting dietary fibre to SCFAs. According to a study by Wong et al.,63 among 19 microbial families predominant in ESRD patients, 12 possessed urease, 5 possessed uricase, and 4 possessed indole and p-cresol-forming enzymes, whereas 2 families possessing butyrate-forming enzymes were shown to be decreased when compared with control patients. High levels of serum urea in CKD patients lead to its influx into the gastrointestinal tract where it is hydrolyzed by urease-producing bacteria generating as a final product ammonium hydroxide, which elevates the gut's luminal pH and causes mucosal damage and irritation. Massive quantities of urea and uric acid in the gut of ESRD patients alter the gut milieu and favour nitrogen compounds-metabolizing bacteria overgrowth.
High serum levels of indoxyl sulfate and p-cresyl sulfate- negatively correlate with kidney function and have been considered essential factors in the development of systemic inflammation, and predictors of mortality and cardiovascular disease in CKD patients.64 Further, both indoxyl sulfate and p-cresyl sulfate were found to be associated with vascular stiffness, aortic calcification and, consequently, higher cardiovascular mortality64 being reported even as a vascular toxin inducer of oxidative stress in endothelial cells.65 The increased accumulation of these metabolites, which are not completely removed during haemodialysis, can interfere with many biological functions66 by stimulating the generation of reactive oxygen species that induce the activation of the NF-kB pathway, resulting in the secretion of proinflammatory cytokines, chemokines and adhesion molecules.65, 67
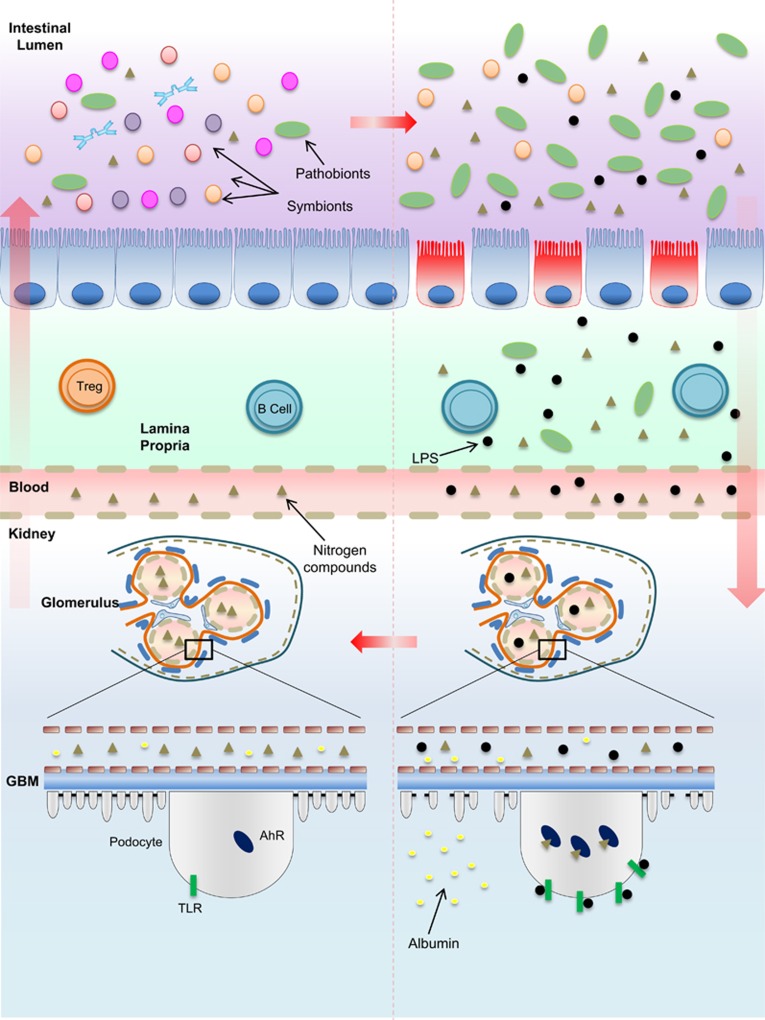
As shown recently by Wong et al.,63 a reduced dietary fibre diet in ESRD patients was associated with higher colonic pH due to increased levels of indoxyl sulfate and p-cresol sulfate. These patients also had a decreased number of bacteria that were able to produce butyrate. The administration of indole, a precursor of indoxyl sulfate, in 5/6 nephrectomized rats triggered glomerulopathy and tubule-interstitial damage in healthy mice with increased levels of creatinine and blood urea nitrogen, and the reduced clearance of creatinine, inulin and p-aminohippuric acid.68 Curiously, urine analysis could not detect indole levels but only its metabolite indoxyl sulfate. One of the detrimental effects of high levels of indoxyl sulfate in the blood can be associated with its potential to damage tubular cells69 and podocytes (Figure 2).41
Figure 2.
Dybiosis and the evolution of kidney damage. In a healthy intestinal microflora, symbiotic bacteria coexist with pathogenic bacteria. The outnumbered presence of symbionts is supposed to maintain gut homeostasis by degrading resistant carbohydrates and proteins that escape from gastric metabolism. An imbalance of the gut biochemical milieu either due to the changes in dietary habits, in which the supply of non-metabolized proteins that reach the intestine is higher than the supply of carbohydrates, or due to the biochemical alterations resulting from reduced kidney function, which impairs the excretion of metabolism products such as urea or acid, may reinforce or contribute to dysbiosis. The reduction of mucus production, loss of tight junctions, leakage of the gut barrier and bacterial translocation initiated due to the overproduction of nitrogen products by urease and uricase-producing bacteria generate high levels of indole sulfate and p-cresil, which, along with endotoxins and LPS, cross the lamina propria to reach the kidneys. The activation of TLRs and other pattern recognition receptors by endotoxins as well as the binding of AhRs by indoxyl sulfate in podocytes are involved in podocyte death and, consequently, the loss of renal selectivity, increased permeability to high-molecular-weight proteins, such as albumin, and renal failure. The damage is propagated to other cells on subjacent glomeruli and progressively contributes to decreased renal clearance. Higher levels of nitrogen products in the blood achieve the intestinal lumen and reinforce, in a vicious cycle, dysbiosis. AhR, aryl hydrocarbon receptor; GBM, glomerular basement membrane. LPS, lipopolysaccharide; TLR, Toll-like receptor; Treg, regulatory T cell.
However, unlike tubular cells, podocytes are specialized terminally differentiated cells that are unable to replicate and with limited regeneration skills that surround the outer side of the glomeruli. Podocytes emit cytoplasmic projections called foot process, which connect with adjacent foot processes from other podocytes to form a structure called slit diaphragm. The slit diaphragm is composed by specific negatively charged proteins, which repel other high-molecular-weight proteins from the blood, thus keeping the glomerular filtration.70
The extensive activation of aryl hydrocarbon receptor, an indoxyl sulfate ligand, decreased podocyte viability over time and impaired podocyte function. Podocytes chronically exposed to indoxyl sulfate manifested prominent foot process effacement, a wrinkled pattern of podocin and synaptopodin, and increased glomerular vimentin levels. Indoxyl sulfate exposure can also cause the reorganization of the actin cytoskeleton from stress fibres to predominantly cortical actin and induce a pro-inflammatory phenotype in mouse and human podocytes (Figure 2).41 Preliminary experiments in which aryl hydrocarbon receptor was knocked down in mouse podocytes induced the recovered expression of some functional slit diaphragm-related genes.41
In contrast, SCFAs have been associated with attenuated kidney damage by protecting against oxidative stress in human tubular cells and improving mitochondrial biogenesis.38 Indeed, substantial changes observed in colonic microbial metabolism in CKD patients can be more attributed to dietary restrictions than to loss of renal function per se.71 In light of recent findings, SCFAs have been considered to be a great regulator of gut homeostasis by serving as a major source of nutrients for colonocytes and promoting the differentiation of effector T cells, regulatory T cells and colonic immunoregulation.72 Emerging data have explored the effects of SCFAs and their potential to regulate systemic inflammation at other sites in the body beyond the gut. In this scenario, SCFAs are able to modulate the inflammatory process on renal tissue by decreasing the capacity of dendritic cells to maturate and to induce CD4+ and CD8+ T-cell proliferation.38 Moreover, due to low levels of fibre, SCFAs are supplanted by an excess of by-products derived from protein metabolism and such products, as indoxyl sulfate, a tryptophan metabolite, have been shown to contribute to progression of kidney injury.
Recently, a cross-sectional study assessed free serum indoxyl sulfate and p-cresyl sulfate in 40 CKD patients under a therapy utilizing prebiotics. According to this study, the ratio of protein to fibre was directly associated with increased levels of serum indoxyl sulfate and p-cresyl sulfate. Particularly, the increased ingestion of fibres was correlated with lower levels of p-cresyl sulfate but not indoxyl sulfate.73 Levels of p-cresyl sulfate were significantly reduced in haemodialysis patients who received daily doses of oligofructose-enriched inulin for 4 weeks.74 Symbiotic therapy was also shown to significantly decrease the levels of p-cresyl sulfate in predialysis adults with CKD after altering the stool microbiome, particularly with depletion of Ruminococcaceae and enrichment of Bifidobacteria.75
Considering the use of probiotics, treatment with Bifidobacteria in mice subjected to an ischaemia/reperfusion experimental model reduced the disruption of tight junctions in the gut and the associated consequences such as bacterial translocation, endotoxin levels and pro-inflammatory cytokine release, whereas the increased concentration of SCFA resulted in recovery of gut homeostasis.76 Part of the recovery could be attributed to increased levels of acetate produced by Bifidobacterium adolescentis and Bifidobacterium longum.38 Furthermore, the daily ingestion of a probiotic formulation containing a mix of Lactobacillus acidophilus, Bifidobacterium longum and Streptococcus thermophiles showed a significant reduction in blood urea nitrogen levels without adverse effects in CKD patients.77
Together, these data show that alterations to the gut microbiota during CKDs are the major predictors of decreased kidney function and CKD progression because the presence of these uraemic toxins were found to be associated with decreased podocyte function. However, more studies are needed to clarify how these toxins are related to those events and to determine whether the analysis of the gut microbiota may be used as a predictor of decreased kidney function during CKD.
Future directions
The understanding of the physiological functions of the gut microbiome and the consequences of its dysbiosis has propelled the scientific community to seek various ways of re-establishing symbiosis. The potential beneficial effect of probiotics, prebiotics and symbiotics (co-administration of pro- and prebiotics) appears to be a promising alternative to control progression of CKD. To restore a symbiotic environment in the gut during the progression of CKD, new approaches have emerged. The use of probiotics, prebiotics and symbiotics have become an inexpensive, healthful, non-invasive promising alternative. However, there is a lack of information about the adverse effects of probiotic intervention studies, although controlled trials do not indicate an increased risk. More basic and clinical research must be done to thoroughly understand the symbiotic environment and the role of dysbiosis in the progression of CKD as well as its associated complications.
It is becoming clear that the interplay of kidney diseases with the gut microbiota will lead to improvements in both dysbiosis and renal injury. Strong evidence sustains that kidney injury is a relevant factor that triggers dysbiosis. However, it is still unclear in what case could dysbiosis initiate kidney injury before any other primary or secondary causes. Studies to determine whether a specific species of bacteria could be strictly involved in the development of kidney diseases, which is identified as a supposed promoter in the development of asthma,19 should be encouraged. Furthermore, the benefits and consequences of microbiota fermentation by-products on renal cells such as mesangial cells and podocytes require extensive investigation. The molecular basis of the pathogenesis involved in the kidney—gut axis necessitates finding new questions and answers.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (grant numbers 2012/02207-2, 2012/15205-4 and 2015/18121-4) and by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, INCT Complex Fluids; grant number 140739/2008-4) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES–PNPD). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
The authors declare no conflict of interest.
References
- Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013; 382: 260–272. [DOI] [PubMed] [Google Scholar]
- Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 2005; 81: 341–354. [DOI] [PubMed] [Google Scholar]
- Pereira MA, Kartashov AI, Ebbeling CB, Van Horn L, Slattery ML, Jacobs DR Jr et al. Fast-food habits, weight gain, and insulin resistance (the CARDIA study): 15-year prospective analysis. Lancet 2005; 365: 36–42. [DOI] [PubMed] [Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M et al. Diversity of the human intestinal microbial flora. Science 2005; 308: 1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G et al. The gut microbiota and host health: a new clinical frontier. Gut 2016; 65: 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 2013; 54: 2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505: 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldszmid RS, Trinchieri G. The price of immunity. Nat Immunol 2012; 13: 932–938. [DOI] [PubMed] [Google Scholar]
- Chung WS, Walker AW, Louis P, Parkhill J, Vermeiren J, Bosscher D et al. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol 2016; 14: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS et al. Metagenomic analysis of the human distal gut microbiome. Science 2006; 312: 1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane GT, Macfarlane S. Fermentation in the human large intestine: its physiologic consequences and the potential contribution of prebiotics. J Clin Gastroenterol 2011; 45: S120–S127. [DOI] [PubMed] [Google Scholar]
- Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and "western-lifestyle" inflammatory diseases. Immunity 2014; 40: 833–842. [DOI] [PubMed] [Google Scholar]
- Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun 2015; 6: 7320. [DOI] [PubMed] [Google Scholar]
- Evenepoel P, Meijers BK, Bammens BR, Verbeke K. Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl 2009; 76: S12–S19. [DOI] [PubMed] [Google Scholar]
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 2010; 107: 14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014; 20: 159–166. [DOI] [PubMed] [Google Scholar]
- Berthon BS, Macdonald-Wicks LK, Gibson PG, Wood LG. Investigation of the association between dietary intake, disease severity and airway inflammation in asthma. Respirology 2013; 18: 447–454. [DOI] [PubMed] [Google Scholar]
- Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr 2013; 98: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 2015; 7: 307ra152. [DOI] [PubMed] [Google Scholar]
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013; 341: 1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int 2013; 83: 308–315. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Liu SM, Lau WL, Khazaeli M, Nazertehrani S, Farzaneh SH et al. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS ONE 2014; 9: e114881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AJ, McKain N, Wallace RJ. Ammonia production by human faecal bacteria, and the enumeration, isolation and characterization of bacteria capable of growth on peptides and amino acids. BMC Microbiol 2013; 13: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JY. The gastrointestinal tract in uremia. Dig Dis Sci 1993; 38: 257–268. [DOI] [PubMed] [Google Scholar]
- Hatch M, Freel RW, Vaziri ND. Intestinal excretion of oxalate in chronic renal failure. J Am Soc Nephrol 1994; 5: 1339–1343. [DOI] [PubMed] [Google Scholar]
- Siener R, Bangen U, Sidhu H, Hönow R, von Unruh G, Hesse A. The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney Int 2013; 83: 1144–1149. [DOI] [PubMed] [Google Scholar]
- Hatch M, Vaziri ND. Enhanced enteric excretion of urate in rats with chronic renal failure. Clin Sci (Lond) 1994; 86: 511–516. [DOI] [PubMed] [Google Scholar]
- Gower BA, Bergman R, Stefanovski D, Darnell B, Ovalle F, Fisher G et al. Baseline insulin sensitivity affects response to high-amylose maize resistant starch in women: a randomized, controlled trial. Nutr Metab (Lond) 2016; 13: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Chang DM, Wu DJ, Peng HY, Chuang LM. Assessment of blood glucose regulation and safety of resistant starch formula-based diet in healthy normal and subjects with type 2 diabetes. Medicine (Baltimore) 2015; 94: e1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan MJ, Martin RJ, Raggio AM, McCutcheon KL, Brown IL, Birkett A et al. High-amylose resistant starch increases hormones and improves structure and function of the gastrointestinal tract: a microarray study. J Nutrigenet Nutrigenomics 2012; 5: 26–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun 2015; 6: 6734. [DOI] [PubMed] [Google Scholar]
- Valcheva R, Hotte N, Gillevet P, Sikaroodi M, Thiessen A, Madsen KL. Soluble dextrin fibers alter the intestinal microbiota and reduce proinflammatory cytokine secretion in male il-10-deficient mice. J Nutr 2015; 145: 2060–2066. [DOI] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013; 341: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013; 504: 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA 2014; 111: 2247–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga TT, Correa-Costa M, Guise YF, Castoldi A, de Oliveira CD, Hyane MI et al. MyD88 signaling pathway is involved in renal fibrosis by favoring a TH2 immune response and activating alternative M2 macrophages. Mol Med 2012; 18: 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Costa M, Braga TT, Felizardo RJ, Andrade-Oliveira V, Perez KR, Cuccovia IM et al. Macrophage trafficking as key mediator of adenine-induced kidney injury. Mediators Inflamm 2014; 2014: 291024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Oliveira V, Amano MT, Correa-Costa M, Castoldi A, Felizardo RJ, de Almeida DC et al. Gut Bacteria Products Prevent AKI Induced by Ischemia-Reperfusion. J Am Soc Nephrol 2015; 26: 1877–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 2013; 110: 4410–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beneden K, Geers C, Pauwels M, Mannaerts I, Verbeelen D, van Grunsven LA et al. Valproic acid attenuates proteinuria and kidney injury. J Am Soc Nephrol 2011; 22: 1863–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichii O, Otsuka-Kanazawa S, Nakamura T, Ueno M, Kon Y, Chen W et al. Podocyte injury caused by indoxyl sulfate, a uremic toxin and aryl-hydrocarbon receptor ligand. PLoS ONE 2014; 9: e108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri ND, Goshtasbi N, Yuan J, Jellbauer S, Moradi H, Raffatellu M et al. Uremic plasma impairs barrier function and depletes the tight junction protein constituents of intestinal epithelium. Am J Nephrol 2012; 36: 438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossola M, Sanguinetti M, Scribano D, Zuppi C, Giungi S, Luciani G et al. Circulating bacterial-derived DNA fragments and markers of inflammation in chronic hemodialysis patients. Clin J Am Soc Nephrol 2009; 4: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Jiang H, Shi K, Ren Y, Zhang P, Cheng S et al. Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology (Carlton) 2012; 17: 733–738. [DOI] [PubMed] [Google Scholar]
- Leemans JC, Kors L, Anders HJ, Florquin S. Pattern recognition receptors and the inflammasome in kidney disease. Nat Rev Nephrol 2014; 10: 398–414. [DOI] [PubMed] [Google Scholar]
- Castoldi A, Braga TT, Correa-Costa M, Aguiar CF, Bassi ÊJ, Correa-Silva R et al. TLR2, TLR4 and the MYD88 signaling pathway are crucial for neutrophil migration in acute kidney injury induced by sepsis. PLoS ONE 2012; 7: e37584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Costa M, Braga TT, Semedo P, Hayashida CY, Bechara LR, Elias RM et al. Pivotal role of Toll-like receptors 2 and 4, its adaptor molecule MyD88, and inflammasome complex in experimental tubule-interstitial nephritis. PLoS ONE 2011; 6: e29004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS et al. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol 2002; 168: 1286–1293. [DOI] [PubMed] [Google Scholar]
- Du P, Fan B, Han H, Zhen J, Shang J, Wang X et al. NOD2 promotes renal injury by exacerbating inflammation and podocyte insulin resistance in diabetic nephropathy. Kidney Int 2013; 84: 265–276. [DOI] [PubMed] [Google Scholar]
- Granata S, Masola V, Zoratti E, Scupoli MT, Baruzzi A, Messa M et al. NLRP3 inflammasome activation in dialyzed chronic kidney disease patients. PLoS ONE 2015; 10: e0122272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SU, Kamada N, Muñoz-Planillo R, Kim YG, Kim D, Koizumi Y et al. Distinct commensals induce interleukin-1β via NLRP3 inflammasome in inflammatory monocytes to promote intestinal inflammation in response to injury. Immunity 2015; 42: 744–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal SR, Chang EB. The microbial basis of inflammatory bowel diseases. J Clin Invest 2014; 124: 4190–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis M, Montemurno E, Piccolo M, Vannini L, Lauriero G, Maranzano V et al. Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN). PLoS ONE 2014; 9: e99006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata T, Nogaki F, Fagarasan S, Sakiyama T, Kobayashi I, Miyawaki S et al. Increased frequency of surface IgA-positive plasma cells in the intestinal lamina propria and decreased IgA excretion in hyper IgA (HIGA) mice, a murine model of IgA nephropathy with hyperserum IgA. J Immunol 2000; 165: 1387–1394. [DOI] [PubMed] [Google Scholar]
- Papista C, Lechner S, Ben Mkaddem S, LeStang MB, Abbad L, Bex-Coudrat J et al. Gluten exacerbates IgA nephropathy in humanized mice through gliadin-CD89 interaction. Kidney Int 2015; 88: 276–285. [DOI] [PubMed] [Google Scholar]
- Lechner SM, Papista C, Chemouny JM, Berthelot L, Monteiro RC. Role of IgA receptors in the pathogenesis of IgA nephropathy. J Nephrol 2016; 29: 5–11. [DOI] [PubMed] [Google Scholar]
- Coppo R, Amore A, Hogg R, Emancipator S. Idiopathic nephropathy with IgA deposits. Pediatr Nephrol 2000; 15: 139–150. [DOI] [PubMed] [Google Scholar]
- Almroth G, Axelsson T, Müssener E, Grodzinsky E, Midhagen G, Olcén P. Increased prevalence of anti-gliadin IgA-antibodies with aberrant duodenal histopathological findings in patients with IgA-nephropathy and related disorders. Ups J Med Sci 2006; 111: 339–352. [DOI] [PubMed] [Google Scholar]
- McCarthy DD, Kujawa J, Wilson C, Papandile A, Poreci U, Porfilio EA et al. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest 2011; 121: 3991–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiopoulos V, Trompouki S, Hadjiyannakos D, Paraskevakou H, Kamperoglou D, Vlassopoulos D. IgA nephropathy in association with Crohn's disease: a case report and brief review of the literature. Ren Fail 2010; 32: 523–527. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy VM, Wei G, Baird BC, Murtaugh M, Chonchol MB, Raphael KL et al. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int 2012; 81: 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel CR, Park Y, Chow WH, Graubard BI, Hollenbeck AR, Sinha R. Intake of fiber and fiber-rich plant foods is associated with a lower risk of renal cell carcinoma in a large US cohort. Am J Clin Nutr 2013; 97: 1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Piceno YM, Desantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 2014; 39: 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 2009; 4: 1551–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, Dignat-George F et al. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost 2007; 5: 1302–1308. [DOI] [PubMed] [Google Scholar]
- Neirynck N, Vanholder R, Schepers E, Eloot S, Pletinck A, Glorieux G. An update on uremic toxins. Int Urol Nephrol 2013; 45: 139–150. [DOI] [PubMed] [Google Scholar]
- Niwa T, Shimizu H. Indoxyl sulfate induces nephrovascular senescence. J Ren Nutr 2012; 22: 102–106. [DOI] [PubMed] [Google Scholar]
- Niwa T, Ise M, Miyazaki T. Progression of glomerular sclerosis in experimental uremic rats by administration of indole, a precursor of indoxyl sulfate. Am J Nephrol 1994; 14: 207–212. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Takeda M, Tojo A, Sekine T, Cha SH, Khamdang S et al. Role of organic anion transporters in the tubular transport of indoxyl sulfate and the induction of its nephrotoxicity. J Am Soc Nephrol 2002; 13: 1711–1720. [DOI] [PubMed] [Google Scholar]
- Grahammer F, Schell C, Huber TB. The podocyte slit diaphragm—from a thin grey line to a complex signalling hub. Nat Rev Nephrol 2013; 9: 587–598. [DOI] [PubMed] [Google Scholar]
- Poesen R, Windey K, Neven E, Kuypers D, De Preter V, Augustijns P et al. The Influence of CKD on Colonic Microbial Metabolism. J Am Soc Nephrol 2015; 27: 1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 2015; 8: 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Johnson DW, Xu H, Carrero JJ, Pascoe E, French C et al. Dietary protein-fiber ratio associates with circulating levels of indoxyl sulfate and p-cresyl sulfate in chronic kidney disease patients. Nutr Metab Cardiovasc Dis 2015; 25: 860–865. [DOI] [PubMed] [Google Scholar]
- Meijers BK, De Preter V, Verbeke K, Vanrenterghem Y, Evenepoel P. p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant 2010; 25: 219–224. [DOI] [PubMed] [Google Scholar]
- Rossi M, Johnson DW, Morrison M, Pascoe EM, Coombes JS, Forbes JM et al. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): a randomized trial. Clin J Am Soc Nephrol 2016; 11: 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang W, Zuo L, Zhu W, Wang B, Li Q et al. Bifidobacteria may be beneficial to intestinal microbiota and reduction of bacterial translocation in mice following ischaemia and reperfusion injury. Br J Nutr 2013; 109: 1990–1998. [DOI] [PubMed] [Google Scholar]
- Ranganathan N, Ranganathan P, Friedman EA, Joseph A, Delano B, Goldfarb DS et al. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv Ther 2010; 27: 634–647. [DOI] [PubMed] [Google Scholar]