Abstract
Purpose
To report the presence of normal limbal epithelium detected by in vivo confocal laser scanning microscopy (IVCM) in three cases of clinically diagnosed total limbal stem cell deficiency (LSCD).
Methods
This is a retrospective case report consists of three patients who were diagnosed with total LSCD based on clinical exam and/or impression cytology. Clinical data including ocular history, presentation, slit-lamp examination, IVCM and impression cytology were reviewed.
Results
The etiology was chemical burn in three cases. One patient has two failed penetrating keratoplasty. Another had allogeneic keratolimbal transplantation but the graft failed one year after surgery. The third patient had failed amniotic membrane transplantation. These three patients presented with signs of total LSCD including the absence of normal Vogt palisades, complete superficial vascularization of the peripheral cornea, non-healing epithelial defects, and corneal scarring. Impression cytology was performed in two cases to confirm the presence of goblet cells in two cases. Each patient however still had distinct areas of corneal and/or limbal epithelial cells detected by IVCM.
Conclusions
Residual normal limbal epithelial cells could be present in eyes with clinical features of total LSCD. IVCM appears to be a more accurate method to evaluate the degree of LSCD.
Keywords: limbal stem cell deficiency, in vivo confocal microscopy, limbal stem cell, diagnosis
Introduction
The epithelium of the ocular surface is maintained by the limbal stem cells (LSCs), which reside in the limbus.1-3 LSCs and their progenitor cells are responsible for normal epithelium repair. The limbus serves an additional purpose as a barrier to prevent migration of the conjunctival epithelium. Dysfunction and/or loss of the LSC population will lead to limbal stem cell deficiency (LSCD).4-6 Etiologies of LSCD include Stevens-Johnson's syndrome, chemical burns, thermal burns, chronic contact lens wear, chronic infectious keratitis, and multiple ocular surgeries.5
The hallmark of LSCD is the presence of conjunctival cells on the corneal surface. Overlapping exam findings with other ocular surface conditions complicates diagnosis of LSCD. The standard diagnostic test of LSCD is impression cytology that detects goblet cells on the cornea.7 However, false negative impression cytology results are often in conditions with concomitant goblet cell deficiency such as Stevens-Johnsons syndrome and chronic topical glaucoma medications uses.8 Improvements in live imaging of the ocular surface using in vivo confocal laser scanning microscopy (IVCM) have allowed new methods in assessing the health of the ocular surface at the cellular level. The morphology of corneal and limbal epithelium detected by IVCM has been well described previously in the literature as cells with distinct hyperreflective borders, dark cytoplasm, and without easily visible nuclei.9-12 Changes in tissue and cellular architecture of the corneal and limbal epithelium have been well characterized in LSCD patients.13-15 Representative images of corneal and limbal epithelium of the normal eye and of eye with total LSCD are shown in Figure 1.
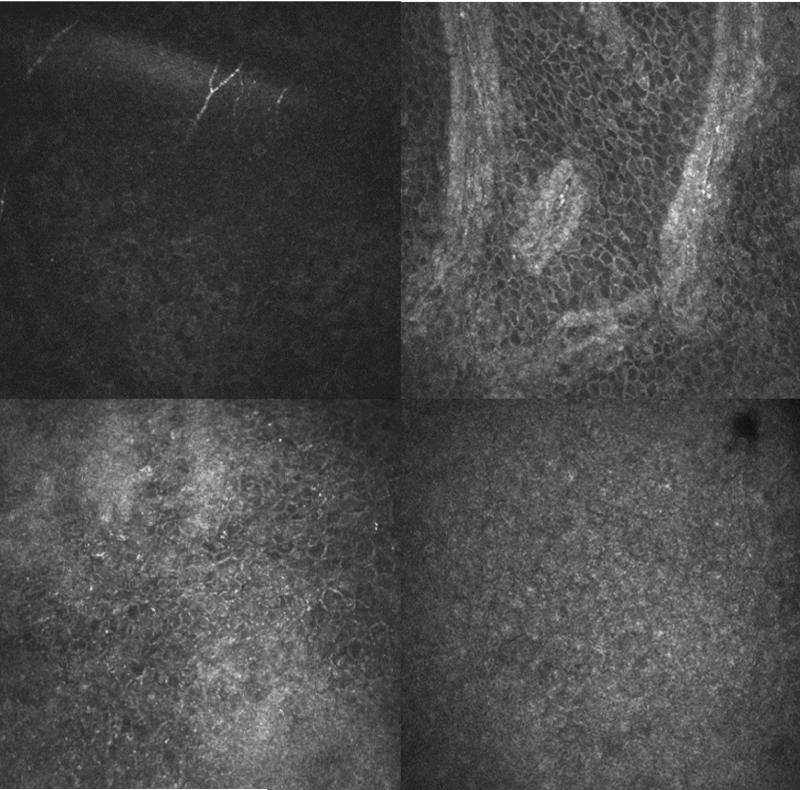
FIGURE 1.
Representative confocal images of a normal eye (top row) and total limbal stem cell deficiency eye (bottom row). The central cornea (top left) and limbus (top right) at the basal cell layer are shown in a normal eye. Distinct individual epithelial cells are observed in both the normal cornea and limbus. The central cornea (bottom left) and temporal limbus (bottom right) at the basal cell layer are shown in the total limbal stem cell deficiency eye. Few epithelial cells with borders and dark cytoplasm can be seen in the cornea. Only few visible cells with clear borders can be detected in the limbus.
Total LSCD is defined by 360 degree involvement of the ocular surface implying complete dysfunction or absence of LSCs. The corneal surface is completely conjunctivalized, which is presented clinically as recurrent and/or persistent corneal epithelial defect, irregular and opaque epithelium, loss of normal limbal structures, absence of the palisade of Vogt with or without vascularization of the cornea. Diagnosis of total LSCD is largely based on clinical presentation and confirmed by impression cytology. In this study, we report the presence of normal limbal epithelial cells detected by IVCM in three cases of total LSCD defined by clinical presentation. This study was approved by the Institution Review Board at the University of California, Los Angeles (IRB# 10-001601).
Case Presentation
Case 1
A 68 year old female with a history of severe bilateral chemical burn at birth by 10% silver nitrate was referred for the management of persistent non-healing ulcer of the left eye. She had two failed penetrating keratoplasties (PKP) in the left eye, and one PKP in the right eye. She was pseudophakic in both eyes and had secondary glaucoma on multiple topical medications at the time of consult. She complained of sharp shooting pains in the left eye.
On examination, her best corrected visual acuity (BCVA) was 20/300 on the right and hand motion on the left eye. Intraocular pressure (IOP) was 16 on the right and 22 on the left by tonopen. The PKP had mild stromal edema and stromal haze on the right. There was superficial corneal neovascularization from 2 to 8 o'clock. No epithelial defect or infiltrates was present. The left eye presented with an opaque and edematous graft with 360 degrees of superficial and deep corneal neovascularization (Figure 2). A central epithelial defect measured 1×3.3 mm was present. Diagnosis of total LSCD was made in the left eye and partial LSCD in the right eye based on clinical exam. She was treated with topical 0.3% gatifloxacin four times daily and aggressive lubrication. The epithelial defect healed eventually. Subsequent corneal impression cytology showed goblet cells in the superior nasal quadrant of the left eye and confirmed the diagnosis of LSCD. She underwent Boston Type I keratoprosthesis and simultaneous Ahmed shunt implantation for the left eye. Her VA improved to 20/400, which was her BCVA after her first PKP.
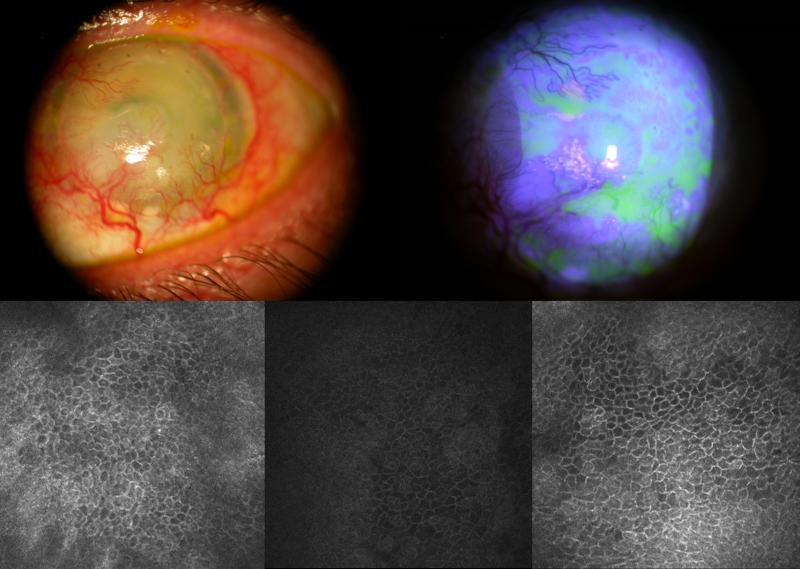
FIGURE 2.
Slit lamp photo of left eye of patient 1 (top left) shows extensive superficial corneal neovascularization, opacity of the corneal epithelium and keratinization in the inferior corneal surface. Fluorescein staining (top right) shows irregularity and stippling staining pattern. Confocal images of the superior limbus (bottom left), nasal limbus (bottom middle), and inferior limbus (bottom right) show the presence of normal limbal epithelial cells.
Four days prior to the combined procedure, IVCM of the left eye was performed on the central cornea, and four locations of the limbus: superior, nasal, temporal and inferior quadrants. IVCM of the superior limbus, nasal limbus, and inferior limbus detected clusters of limbal epithelial layers that consisted of normal superficial, wing and basal layer (Figure 2). The cells were of normal limbal morphology: small cell size, dark cytoplasm and distinct cell-cell borders in a regular pattern. These limbal epithelial cells appear smaller in the superior limbus with minimal heterogeneity. Limbal epithelial cells in the nasal limbus appear to have greater variety in cell size and shape. Areas of typical conjunctival cells and significant subepithelial fibrosis were observed in many locations of the limbus.
Case 2
A 47 year old man with a history of bilateral alkaline chemical burn more than 20 years ago who underwent transplantation of a keratolimbal graft to his left eye 7 years prior was initially referred for management of the failed keratolimbal graft. The graft failed twelve months after the initial transplantation. He had no history of ocular surgery on his right eye.
On exam of the right eye, the visual acuity was counting fingers. There was 360 degrees of corneal neovascularization of the peripheral cornea. The central cornea was hazy (Figure 3). Impression cytology could not be performed on the patient due to patient intolerance.
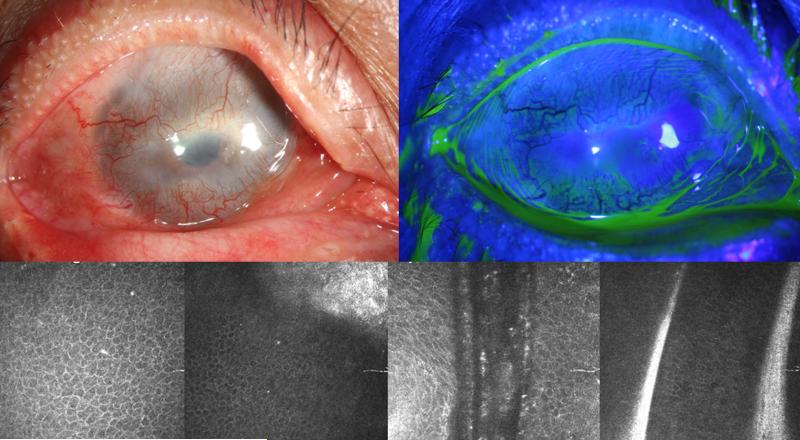
FIGURE 3.
Slit lamp photo of right eye of patient 2 (top left) shows 360 degree of corneal neovascularization and pannus. Fluorescein staining (top right) shows irregular corneal epithelium. Confocal images of the central cornea wing cell layer (bottom left), and central cornea (bottom middle-left), superior limbus (bottom middle-right), and inferior limbus (bottom right) show the presence of normal limbal epithelium.
On IVCM, cells typically representing the morphology of normal cornea and limbal epithelial are observed (Figure 3). Central wing cell layer shows cells with clear bright borders, dark cytoplasm, and small bright nuclei in the center. The central basal layer shows similar cells with smaller diameters in addition to presence of a subbasal nerve. The superior and inferior limbal basal cell layer also show presence of patches of normal basal epithelial cells. Few dendritic cells are observed in these frames of the epithelium. No typical structure of Palisade of Vogt was present. A couple of atrophic stromal cords were observed.
Case 3
A 48 year old man with history of binocular alkaline chemical burn 1.5 years presented in clinic with decreased vision. He had amniotic membrane transplant in both eyes after binocular injury. On exam of the left eye, BCVA was counting fingers and the IOP was normal. Corneal neovascularization could be appreciated 360 degrees with greater extension in the inferior and superior quadrants (Figure 4). Extensive fibrosis and central haze is appreciated with minimal superior sparing of the cornea. Impression cytology was negative for goblet cells on both the cornea and conjunctiva.
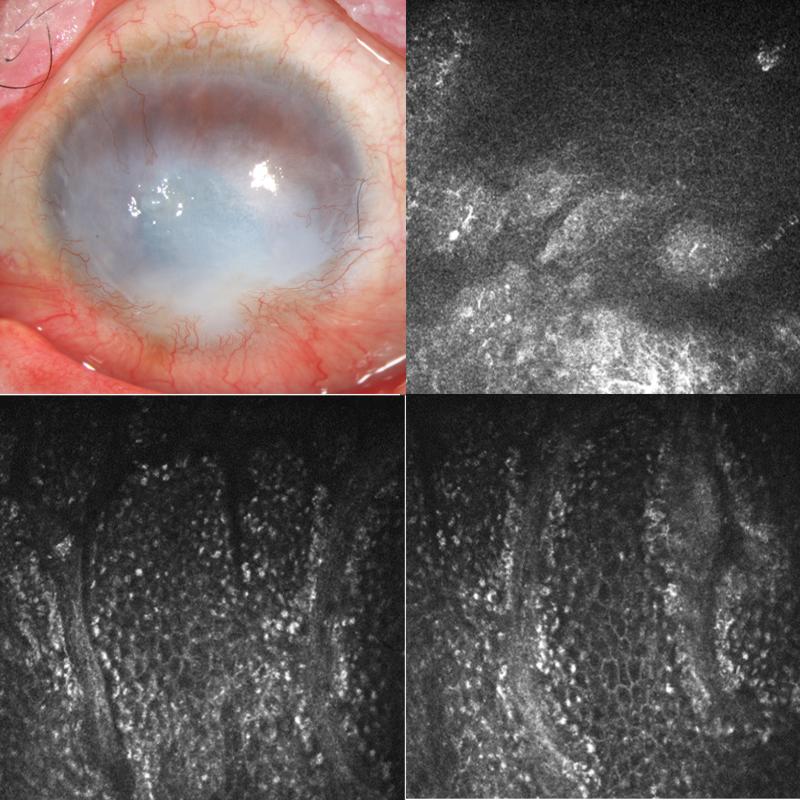
FIGURE 4.
Slit lamp photo of left eye of patient 3 (top left) shows 360 degree of superficial corneal neovascularization, inferior pannus and corneal scarring. Confocal images of the central cornea (top right), superior limbus (bottom left and bottom right) show the presence of normal basal limbal epithelial cells.
On IVCM, central cornea frames demonstrate some cellular architecture similar to normal appearing epithelium (Figure 4). Groups of polygonal cells with distinct borders and dark cytoplasm can be appreciated in these central cornea images. Image frames of the superior limbus demonstrate characteristic cells of normal epithelium as described previously but larger in diameter compared to corneal epithelial cells. Additionally, Palisade of Vogt structures can be identified with consistently regularly shaped epithelial cells between those structures.
Discussion
The current diagnosis of LSCD mainly relies on clinical examination and could be confirmed by impression cytology. However, even when conjunctival goblet cells cannot be detected on impression cytology, LSCD cannot be excluded. Additionally, impression cytology cannot quantify the degree of LSCD because of sampling bias. The normal corneal epithelium has tight junctions and it is difficult to obtain corneal epithelial cells by impression cytology.8 The conjunctival epithelium is loosely adhered and the yield of the sampling is higher. Therefore, absence of the corneal epithelial cells on the impression cytology does not confirm the absence of these cells on the ocular surface. A staging system to quantify the degree of LSCD is not yet established. In recent years, IVCM has been used to evaluate LSCD and attempt to detect goblet cells on cornea.13 Several reports have further demonstrated that the subbasal nerve plexus, corneal and limbal basal cell density and epithelial thickness correlate with the degree of LSCD.16,17 These quantifiable parameters could be used to stage the degree of LSC function or deficiency.
The current study shows that there are normal limbal epithelial cells and corneal epithelial cells in eyes that clinically present with signs of total LSCD. This finding confirms that clinical exam is not sufficient to accurately determine the degree of LSCD. However, the prevalence of the existence of normal limbal epithelial cells in clinically total LSCD is unknown. This is due to the limitation of the current study. Only four locations of the limbus were imaged and this accounted for less than1/3 of the total limbal areas. Whether there were normal limbal epithelial cells in other locations is unknown. In addition, limbal epithelial cells could be hidden in limbal lacuna in the deep limbal stroma and difficult to detect.18 A larger study to obtain images of the entire limbal region would be necessary to investigate the prevalence of normal limbal epithelial cells in these eyes clinically present with total LSCD.
It is important to differentiate between total LSCD and severe limbal stem cell dysfunction, where the proliferation rate is not enough to compensate for epithelial turnover. Clinically it is extremely difficult to differentiate between deficiency and dysfunction since both can lead to irregular epithelium, staining, and persistent epithelial defects. However, IVCM could play a role identifying histologic and structural conservation of structures such as remains of normal limbal and corneal epithelium. Correlation with the clinical picture and history may aid in the distinguishing of these two entities.
Total LSCD represents an end stage disease, in which restorative vision interventions are limited to keratoprosthesis or LSC transplantation in the form of keratolimbal graft or cultivated LSCs in human.19-24 Determining the degree of LSCD is crucial for appropriate patient management. In patients with partial LSCD, LSC transplantation might not be necessary and perhaps medical management with or without amniotic membrane transplantation might be sufficient to avoid complications associated with keratoprosthesis and LSC transplantation.
An accurate determination of the amount of LSCs is also necessary to evaluate the treatment outcome correctly. Several reports show that the donor cells were no longer detected despite a successful reconstruction of a normal corneal epithelium in successful allogeneic limbal stem cell transplantation.25,26 Only the recipient corneal epithelium was found in more than 50% of the limbal transplants tested. This observation suggests that the recipient LSCs were regenerated to a sufficient amount to repopulate the corneal surface. In light of the finding of our current study, it is possible that there might be residual LSCs in these eyes that were clinically classified as total LSCD. The transplanted donor LSCs and/or their niche cells in the case of keratolimbal graft might provide the necessary trophic factors to revive the residual recipient LSCs and promote their self-renewal. Therefore, in eyes with sufficient amount of residual LSCs, removal of pannus and scar tissue combined with providing trophic factors might be a sufficient treatment modality. However, in eyes that do not have sufficient amount of LSCs, replenish of LSCs by transplantation would be necessary. Concomitant transplantation of niche cells might be also required to ensure the survival of the transplanted LSCs.
Due to the challenge of confirming whether there are residual LSCs in eyes present with clinical signs of total LSCD, caution should be taken in the interpretation of results from the experimental data in animal models as well. Non-LSCs, such as mesenchymal stem cells have been suggested to be capable of reconstructing a corneal epithelial surface in animal model of LSCD.27 It has been shown that mesenchymal cells can serve as feeder cells in vitro for the expansion of LSCs.28,29 These mesenchymal cells provide factors for the successful propagation of LSCs. If there are still residual LSCs, it is possible that the transplanted mesenchymal cells provide trophic factors to revive the residual LSCs but not due to transdifferentiation.
In summary, normal limbal epithelial cells and corneal epithelial cells could exist in eyes with clinical signs of total LSCD. Diagnosis and classification of LSCD by clinical exam and the presence of goblet cells is not sufficient. IVCM appears to be a more accurate tool to assess the degree of LSCD.
Acknowledgments
FUNDING DISCLOSURES: This work was supported in part by an unrestricted grant from Research to Prevent Blindness. S.X.D received grant support from the National Eye Institute grants (5P30EY000331 and 1R01EY021797) and a California Institute for Regenerative Medicine (TR2-01768 and BF1-01768).
Footnotes
CONFLICT OF INTEREST: no conflicts of interest to disclose
References
- 1.Tseng SC. Concept and application of limbal stem cells. Eye. 1989;3(Pt 2):141–157. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- 2.Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983;24:1442–1443. [PubMed] [Google Scholar]
- 3.Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–722. doi: 10.1016/s0161-6420(89)32833-8. discussion 722-703. [DOI] [PubMed] [Google Scholar]
- 4.Dua HS, Saini JS, Azuara-Blanco A, et al. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol. 2000;48:83–92. [PubMed] [Google Scholar]
- 5.Sejpal K, Bakhtiari P, Deng SX. Presentation, diagnosis and management of limbal stem cell deficiency. Middle East Afr J Ophthalmol. 2013;20:5–10. doi: 10.4103/0974-9233.106381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Secker GA, Daniels JT. Corneal epithelial stem cells: deficiency and regulation. Stem Cell Rev. 2008;4:159–168. doi: 10.1007/s12015-008-9029-x. [DOI] [PubMed] [Google Scholar]
- 7.Puangsricharern V, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102:1476–1485. doi: 10.1016/s0161-6420(95)30842-1. [DOI] [PubMed] [Google Scholar]
- 8.Poli M, Burillon C, Auxenfans C, et al. Immunocytochemical Diagnosis of Limbal Stem Cell Deficiency: Comparative Analysis of Current Corneal and Conjunctival Biomarkers. Cornea. 2015 doi: 10.1097/ICO.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 9.Dua HS, Miri A, Alomar T, et al. The role of limbal stem cells in corneal epithelial maintenance: testing the dogma. Ophthalmology. 2009;116:856–863. doi: 10.1016/j.ophtha.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Jalbert I, Stapleton F, Papas E, et al. In vivo confocal microscopy of the human cornea. Br J Ophthalmol. 2003;87:225–236. doi: 10.1136/bjo.87.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi A, Sugiyama K. In vivo corneal confocal microscopic findings of palisades of Vogt and its underlying limbal stroma. Cornea. 2005;24:435–437. doi: 10.1097/01.ico.0000151542.15736.da. [DOI] [PubMed] [Google Scholar]
- 12.Miri A, Al-Aqaba M, Otri AM, et al. In vivo confocal microscopic features of normal limbus. Br J Ophthalmol. 2012;96:530–536. doi: 10.1136/bjophthalmol-2011-300550. [DOI] [PubMed] [Google Scholar]
- 13.Nubile M, Lanzini M, Miri A, et al. In vivo confocal microscopy in diagnosis of limbal stem cell deficiency. Am J Ophthalmol. 2013;155:220–232. doi: 10.1016/j.ajo.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Deng SX, Sejpal KD, Tang Q, et al. Characterization of limbal stem cell deficiency by in vivo laser scanning confocal microscopy: a microstructural approach. Arch Ophthalmol. 2012;130:440–445. doi: 10.1001/archophthalmol.2011.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Q, Deng SX, Xu J. In vivo confocal microscopy of congenital aniridia-associated keratopathy. Eye. 2013 doi: 10.1038/eye.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan EH, Chen L, Yu F, et al. Epithelial Thinning in Limbal Stem Cell Deficiency. Am J Ophthalmol. 2015;160:669–677. e664. doi: 10.1016/j.ajo.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 17.Chan EH, Chen L, Rao JY, et al. Limbal Basal Cell Density Decreases in Limbal Stem Cell Deficiency. Am J Ophthalmol. 2015;160:678–684. e674. doi: 10.1016/j.ajo.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarei-Ghanavati S, Ramirez-Miranda A, Deng SX. Limbal Lacuna: A Novel Limbal Structure Detected by In Vivo Laser Scanning Confocal Microscopy. Ophthalmic Surg Lasers Imaging. 2011;42:e129–e131. doi: 10.3928/15428877-20111201-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan CC, Biber JM, Holland EJ. The modified Cincinnati procedure: combined conjunctival limbal autografts and keratolimbal allografts for severe unilateral ocular surface failure. Cornea. 2012;31:1264–1272. doi: 10.1097/ICO.0b013e31823f8e95. [DOI] [PubMed] [Google Scholar]
- 20.Basu S, Taneja M, Narayanan R, et al. Short-term outcome of Boston Type 1 keratoprosthesis for bilateral limbal stem cell deficiency. Indian J Ophthalmol. 2012;60:151–153. doi: 10.4103/0301-4738.94060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sejpal K, Yu F, Aldave AJ. The Boston keratoprosthesis in the management of corneal limbal stem cell deficiency. Cornea. 2011;30:1187–1194. doi: 10.1097/ICO.0b013e3182114467. [DOI] [PubMed] [Google Scholar]
- 22.Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 23.Basu S, Fernandez MM, Das S, et al. Clinical outcomes of xeno-free allogeneic cultivated limbal epithelial transplantation for bilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96:1504–1509. doi: 10.1136/bjophthalmol-2012-301869. [DOI] [PubMed] [Google Scholar]
- 24.Holland EJ. Management of Limbal Stem Cell Deficiency: A Historical Perspective, Past, Present, and Future. Cornea. 2015 doi: 10.1097/ICO.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 25.Williams KA, Brereton HM, Aggarwal R, et al. Use of DNA polymorphisms and the polymerase chain reaction to examine the survival of a human limbal stem cell allograft. Am J Ophthalmol. 1995;120:342–350. doi: 10.1016/s0002-9394(14)72164-6. [DOI] [PubMed] [Google Scholar]
- 26.Henderson TR, Findlay I, Matthews PL, et al. Identifying the origin of single corneal cells by DNA fingerprinting: part II-- application to limbal allografting. Cornea. 2001;20:404–407. doi: 10.1097/00003226-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Rohaina CM, Then KY, Ng AM, et al. Reconstruction of limbal stem cell deficient corneal surface with induced human bone marrow mesenchymal stem cells on amniotic membrane. Trans Res. 2014;163:200–210. doi: 10.1016/j.trsl.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Nakatsu MN, Gonzalez S, Mei H, et al. Human Limbal Mesenchymal Cells Support the Growth of Human Corneal Epithelial Stem/Progenitor Cells. Invest Ophthalmol Vis Sci. 2014 doi: 10.1167/iovs.14-14999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omoto M, Miyashita H, Shimmura S, et al. The use of human mesenchymal stem cell-derived feeder cells for the cultivation of transplantable epithelial sheets. Invest Ophthalmol Vis Sci. 2009;50:2109–2115. doi: 10.1167/iovs.08-2262. [DOI] [PubMed] [Google Scholar]