Abstract
Objective
To investigate interactions of psychological resources and socioeconomic status in predicting markers of systemic inflammation, as well as potential gender differences and the explanatory role of childhood and adult stress exposures, health behaviors, and negative and positive affect.
Method
We utilized a sample of adults from the Midlife in the United States Survey (MIDUS) who provided biomarker data (N=1,152). SES was operationalized as a composite of education, income, and occupational prestige, and psychological resources as a latent factor measured with optimism, perceived control, and self-esteem. Linear regression models examined these two factors and their interaction in predicting interleukin-6 (IL-6) and C-reactive protein (CRP) measured on average 2 years later, as well as three-way interactions involving gender and the impact of covariate adjustment.
Results
Psychological resources interacted with SES in men (for IL-6: p<.001; for CRP: p=.04) but not in women. In men, greater psychological resources were associated with lower concentrations of IL-6 at lower levels of SES, but higher concentrations of both markers at higher levels of SES. The inverse association between resources and IL-6 at low SES was moderately attenuated upon adjustment for negative affect.
Conclusion
Socioeconomic status might modulate the linkage between psychological resources and systemic inflammation in men. At lower levels of SES, resources may be related to lower inflammation in part through lower negative affect. Associations with higher inflammation at higher SES add to growing evidence suggesting that adaptive psychological characteristics may be associated with markers of poorer physiological function under certain conditions.
Keywords: Socioeconomic status, psychological resources, gender, IL-6, CRP, inflammation
Adaptive psychological functioning appears to be an important health-related resource, potentially encouraging financial and behavioral investments in health and/or buffering the adverse physiological effects of stress (Morozink, Friedman, Coe, & Ryff, 2010; Schöllgen, Huxhold, Schüz, & Tesch-Römer, 2011). Empirically, favorable perceptions and beliefs regarding one’s self, one’s life, and the future, including optimism, perceived mastery and control, and purpose in life, have been repeatedly associated with better self-reported and objective health outcomes (Boehm, Peterson, Kivimaki, & Kubzansky, 2011; Boylan & Ryff, 2015; Infurna & Gerstorf, 2014; Rasmussen, Scheier, & Greenhouse, 2009). Although the preceding constructs are conceptually and empirically distinct, factor analytic results suggest that they tap a general axis of psychological adjustment (Judge, Erez, Bono, & Thoresen, 2002; Matthews, Räikkönen, Gallo, & Kuller, 2008; Schöllgen et al., 2011), which we refer to as “psychological resources” following Gallo and Mathews’ Reserve Capacity Model (Gallo & Matthews, 2003; Matthews & Gallo, 2011).
Reported associations of psychological resources with health outcomes have been independent of sociodemographic characteristics including indicators of socioeconomic status (SES). However, there are some indications that SES and psychological resources may also interact to shape health outcomes (Morozink et al., 2010; Schöllgen et al., 2011; Turiano, Chapman, Agrigoroaei, Infurna, & Lachman, 2014). Conceptually, the health impact of individual psychological characteristics may be contingent upon the broader availability of key economic and social resources for health (e.g., access to health care, a high-quality diet, exercise facilities, etc., as well as health-promoting social norms) as well as the extent of risk exposures (e.g., ambient stressors, deleterious social norms), both of which are unequally distributed by SES (Baum, Garofalo, & Yali, 1999; Turiano et al., 2014). Associations of psychological resources with health status may be stronger at lower levels of SES due to a relative dearth of other health-related resources, as well as greater exposure to stressors that place more onus on individual coping and adjustment. Consistent with this premise, several studies have reported that psychological resources are more strongly related to health outcomes at lower levels of SES (Lachman & Weaver, 1998; Ryff, Radler, & Friedman, 2015; Schöllgen et al., 2011; Turiano et al., 2014). This pattern has extended to biomarkers of physiological function, with stronger associations of life satisfaction with diurnal cortisol slope (Zilioli, Imami, & Slatcher, 2015) as well as psychological well-being with the inflammatory marker interleukin-6 (Morozink et al., 2010) at lower levels of SES. Nevertheless, better psychological functioning may not be uniformly related to better physical health status in all populations, even at low levels of SES. For example, a recent study reported that greater self-control was associated with a marker of faster epigenetic aging in rural African-American youth (Miller, Yu, Chen, & Brody, 2015).
Associations of psychological factors with health outcomes may also vary by gender. Evidence suggests that men tend to assign greater priority to individually-oriented values such as power and autonomy, and women, to interpersonally-oriented values and relationships (Schwartz & Rubel, 2005; Weisgram, Bigler, & Liben, 2010). Notably, Eisenlohr-Moul and Segerstrom (2013) found a corresponding pattern of gender differences in associations of autonomy and sense of relatedness with IL-6. Furthermore, Hagger-Johnson et al. (2012) found a three-way interaction in which SES interacted with neuroticism (which is substantially correlated with perceived control, self-esteem, and optimism; Judge et al., 2002), to predict cardiovascular mortality in women but not men. The limited data at present indicates a need for further study of gender differences in this area, which are potentially important insofar as specific psychological factors may not be equally applicable or beneficial, on average, to health promotion efforts in men and women. For example, there is increasing attention to gender differences in psychosocial interventions for the secondary prevention of coronary heart disease (Bjarnason-Wehrens, Grande, Loewel, Voller, & Mittag, 2007). Additionally, the US National Institutes of Health recently noted the importance of gender differences for intervention development (National Institutes of Health, 2015).
Another gap in the extant research is that most studies have not adjusted for plausible confounders including childhood adversities (e.g., low family SES, child maltreatment) and life stressors, which are associated with poorer psychological functioning and health status as well as lower adult SES (Friedman, Karlamangla, Gruenewald, Koretz, & Seeman, 2015; Taylor, Lehman, Kiefe, & Seeman, 2006). Thus, the apparent importance of psychological resources for health may simply indicate the “long arm” of childhood misfortune or other stress exposures over the life course. The extent of such potential confounding bears upon the expected health impact of policies and interventions designed to promote psychological resources and well-being, particularly in persons of lower SES. Finally, there remains a need for further investigation of the mechanisms underlying any remaining effects of psychological resources on various health indicators. For example, in the reserve capacity model (Gallo & Matthews, 2003; Matthews & Gallo, 2011) psychosocial resources are presumed to influence emotional reactivity to stressors and levels of episodic negative and positive affect, as well as health-related behaviors, which may in turn impact immune and other biological pathways to disease.
To address these gaps in the literature, we investigated the interaction of SES and a latent measure of psychological resources, represented by optimism, perceived control, and self-esteem, in the prospective prediction of two markers of systemic low-grade inflammation: Interleukin-6 (IL-6) and C-reactive protein (CRP). Concentrations of these markers are associated with SES (Friedman & Herd, 2010) as well as stress exposures and affective processes (Hostinar, Lachman, Mroczek, Seeman, & Miller, 2015; Sin, Graham-Engeland, Ong, & Almeida, 2015). In addition, they predict key health outcomes including cardiovascular disease (Danesh et al., 2004), disability (Ferrucci et al., 1999), and all-cause mortality (Harris et al., 1999), making them important outcomes in their own right. Building on the work of Morozink and colleagues (2010), we evaluated interactions between SES and psychological resources in predicting IL-6 and CRP, hypothesizing that resources would be associated with lower levels of these markers at lower levels of SES. Additionally, we hypothesized that these associations would be attenuated upon adjustment for childhood and adult stress exposures, negative and positive affect, and health behaviors. As discussed the literature suggests that interactions of psychological factors and SES may vary by gender, but insufficient information to specify a directional hypothesis (i.e., stronger associations in men or women), so we tested three-way interactions with gender to determine if there were differences in either direction.
Method
Study data were drawn from the Midlife Development in the United States (MIDUS) study. MIDUS was initiated in 1995 with a survey of approximately 7,000 English-speaking adults ages 25 to 74 (MIDUS I). A follow-up survey (MIDUS II) was administered beginning in 2004, with an average follow-up interval of 9 years and a mortality-adjusted response rate of 75%. MIDUS II also added a new oversample consisting of African-Americans between 35 and 85 years of age residing in Milwaukee, Wisconsin. A subsample of MIDUS II participants (N=1,255) subsequently participated in a biomarker project conducted between 2004 and 2009, which involved an overnight visit at one of three clinical research centers and included a collection of fasting blood, urine, and saliva samples. This data collection occurred on average two years after the MIDUS II survey was completed. A detailed description of the biomarker study protocol is available elsewhere (Love, Seeman, Weinstein, & Ryff, 2010). Overall response rates were 43% for the longitudinal sample and 50.5% for the Milwaukee sample. As reported by Love et al. (2010) bivariate comparisons indicated that the biomarker sample was comparable to the broader MIDUS II sample on most sociodemographic characteristics, including age, sex, race/ethnicity, marital status, and income, although participants were more likely to have a college degree (p < .01). Biomarker participants were also similar on self-rated health, chronic conditions, instrumental activities of daily living, exercise, and alcohol use, although they were less likely to smoke (p < .01).
Approximately 92% of biomarker project respondents (N=1,152) had complete data on primary variables including inflammatory markers, sociodemographic covariates, chronic disease and medications, socioeconomic status and psychological resource variables. Respondents with missing data were more likely to be a participant of the Milwaukee sample (32.8% versus 15.3%) had lower scores on the SES composite, t(54.5)=2.35, p=.011, d=.34, and the psychological resources factor, t(74.7)=1.89, p=.032, d=.29, and had higher levels of IL-6, t(98.2)=−2.38, p=.010, d=.27. An additional 124 participants (78 women, 46 men) were missing data on one or more potential explanatory covariates (e.g., stress exposures, health behaviors, positive and negative affect), and were excluded from related analyses.
Measures
Sociodemographic covariates
Sociodemographic covariates included age, gender, racial/ethnic minority status (compared with non-Hispanic whites), and sample (Milwaukee sample versus longitudinal sample).
Inflammatory markers
Inflammatory outcomes included circulating concentrations of interleukin-6 (IL-6), a cytokine closely involved in the regulation of systemic inflammatory processes, and C-reactive protein (CRP), a protein synthesized in the liver and other tissues in response to stimulation by IL-6 and other pro-inflammatory cytokines (Gruenewald & Kemeny, 2007). IL-6 was measured from serum using an enzyme-linked immunosorben assay (ELISA; R & D Systems, Minneapolis, MN), and CRP from plasma with a particle enhanced immunonepholometric assay (BNII nephelometer from Dade Behring, Deerfield, IL). The laboratory intra- and inter-assay coefficients of variance were in acceptable ranges. A base-10 logarithm transformation was applied to IL-6 and CRP variables to reduce skew in the distributions. Although CRP values exceeding 10.0 mg/L may reflect acute inflammation resulting from active infection or injury and are often discarded, results of prior studies suggest that doing so may result in a loss of meaningful outcome variance (O’Connor et al., 2009). Therefore, CRP values above 10.0 mg/L were retained (52 cases, with a mean of 20.4 and range of 10.4 to 61.7 mg/L) in primary analyses. We also performed sensitivity analyses, both truncating extreme values at 10.0 mg/L as well as excluding them altogether.
Socioeconomic status (SES)
A composite measure of socioeconomic status was constructed by averaging standardized scores (z-scores) on education, household income, and occupational prestige, measured at MIDUS II for consistency across the entire sample and weighted equally. Composite measures of SES capture the aggregate effects of multiple socioeconomic indicators and are often used when there is an interest in a general gradient of socioeconomic standing, as opposed to the role of a particular facet of SES such as education (Hagger-Johnson et al., 2012). A prior study (Friedman & Herd, 2010) reported that education did not have main effects on IL-6 or CRP after adjustment for income in this cohort. Nevertheless, education might still play a role in moderating associations of psychological factors with inflammatory markers and was included in the composite. Some authors (Kline, 2011) have argued that since education, income, and occupation are typically understood as causes rather than outcomes of SES, they are more appropriately represented as formative rather than reflective indicators (as in a composite or principal components analysis). In a principal components analysis, the three indicators exhibited similar loadings (0.52 – 0.61) on a dominant first component, which was correlated at .999 with the SES composite. Educational attainment was measured on a 12-point scale (e.g., 1=no school/some grade school; 5=high school degree; 9=four-year college degree/B.A., 12=advanced graduate/professional degree). Past (if retired or unemployed) or current occupational status was measured using the Duncan socioeconomic index (SEI) score, a rating of occupational prestige (Hauser & Warren, 1996). Total household income was calculated in MIDUS by summing reported annual income from various sources, and was adjusted for household size.
Psychological resources
Similar to the approach in other studies (Matthews et al., 2008; Schöllgen et al., 2011) we operationalized psychological resources using a factor score whose indicators included optimism, perceived control, and self-esteem, measured at MIDUS II. The use of a factor score removes measurement error in the indicators and is a recommended approach for assessing the joint effects of psychosocial resource variables (Clark et al., 2012; Gallo & Matthews, 2003). In a principal axis factor analysis, a dominant single factor emerged and explained 63.5% of the variance. Factor loadings were .73 for optimism, .77 for perceived control, and .80 for self-esteem. Psychometric information for each indicator is described below.
Optimism
Optimism/pessimism was measured using the Life Orientation Test-Revised (LOT-R; Scheier, Carver, & Bridges, 1994), a six-item measure of the tendency to expect positive outcomes. Items (e.g., “I expect more good things to happen to me than bad”) are assessed on a four-point scale. The LOT-R had good internal consistency (Cronbach’s alpha=.80) in the analytic sample. Positively and negatively worded items appear to load on different factors, and there is debate in the literature as to whether these factors reflect distinct constructs of optimism and pessimism or method effects. As is common in the literature (Rasmussen et al., 2009) we utilized the overall LOT-R score.
Perceived control
The measure of perceived control was based on items developed by Lachman and Weaver (1998), assessing both personal mastery and perceived constraints. Personal mastery, which refers to a sense of efficacy in obtaining goals, was measured using four items (e.g., “I can do just about anything I really set my mind to”) rated on a seven-point scale. Perceived constraints, which refers to perceived lack of control over life outcomes, was measured using eight items (e.g., “What happens in my life is often beyond my control”). These items were combined to create a 12-item composite scale representing overall perceived control (Infurna & Gerstorf, 2014), which had high internal consistency (α=.91) in the analytic sample.
Self-esteem
Self-esteem was measured using seven items (e.g., “On the whole, I am satisfied with myself”), rated on a seven-point scale, from the Rosenberg self-esteem scale(Rosenberg, 1965). The self-esteem scale showed adequate internal consistency (α=.77).
Childhood and adult stress exposures
Childhood maltreatment was measured using the childhood trauma questionnaire (CTQ; Scher, Stein, Asmundson, McCreary, & Forde, 2001), a 28-item self-report measure of various types of maltreatment, administered at the biomarker clinic visit. Items (i.e., “People in my family hit me so hard that it left me with bruises or marks”) are rated on a scale of 1 (“never true”) to 5 (“very often true”). Scores on five subscales (physical abuse, sexual abuse, emotional abuse, physical neglect, and emotional neglect) were summed to obtain a total maltreatment score. Following Gruenewald et al. (2012) and parallel to the adult SES composite, we created a composite measure of childhood socioeconomic disadvantage based on retrospective reports at MIDUS I (MIDUS II for Milwaukee participants) of the highest level of parental education (0=some college or higher, 1=high school/GED, 2=less than high school), whether the family was ever on welfare or ADC (Aid to Dependent Children) for a period of 6 months or more (0= no; 1=yes), and whether the family was better or worse off financially than the average family at the time (0=better off, 1=about the same, 2=worse off). Scores were summed to generate a total score (range 0–5).
Adult stress exposures included stressful life events and financial strain, both of which were associated with markers of inflammation in prior studies (Hostinar et al., 2015; Sturgeon et al., 2015). The former was assessed using a checklist measure of up to 20 events (e.g., death of a family member, divorce, physical or sexual assault, extended unemployment) administered at MIDUS II. A total life events score was calculated by summing the number of reported events. Financial strain at MIDUS II was measured by summing two items. The first asked participants, “How difficult is it for you (and your family) to pay your monthly bills?” with responses rated on a four-point scale from “not at all difficult” to “very difficult” (range 0–3). The second asked participants whether they (and their families living with them) have “more money than you need,” “just enough for your needs,” or “not enough to meet your needs?” (range 0–2).
Negative and positive affect
Negative affect (NA; α=.91) and positive affect (PA; α=.93) were measured at MIDUS II with items asking about the past-month frequency of various negative (e.g., “so sad nothing could cheer you up”; “jittery”) and positive (e.g., “cheerful” “calm and peaceful”) affective states, with items rated on a five-point Likert scale. Several items were created for use in the original MIDUS survey, with others drawn from the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988). To assess negative affect at the time of the biomarker collection, we derived a factor score from several scales asking about the past-week frequency of various symptoms of affective distress. These included the General Distress- Depressive (α=.90) and Anxious Symptoms (α=.80) subscales and the Anxious Arousal subscale (α=.77) from the Mood and Anxiety Symptom Questionnaire (MASQ), as well the Center for Epidemiologic Studies Depression Scale (CESD; (α=.90)). A single factor explained 66% of the variance, and was correlated at approximately .6 with NA measured at MIDUS II. We also examined the Positive Affect Scale from the MASQ. A detailed description of the preceding scales is available elsewhere (Ryff, Weinstein, & Seeman, 2012).
Health covariates
All health-related covariates were measured at the biomarker study clinic visit. We included dummy variables for antihypertensive, cholesterol, and antidepressant medications (O’Connor et al., 2009). A measure of chronic conditions was operationalized as the self-reported number of up to fourteen doctor-diagnosed medical conditions (e.g., heart disease, stroke, high blood pressure, diabetes, cancer) associated with either IL-6 or CRP in bivariate analyses. Smoking status was measured as a three-category variable, with dummy variables comparing former and current smokers to lifetime non-smokers. Hours per week of exercise was measured as a weighted average of light (e.g., “light housekeeping”; 1 × number of hours), moderate (e.g., “brisk walking”; 2 × number of hours), and vigorous activities (e.g., “high intensity aerobics”; 3 × number of hours), accounting for seasonal differences(Friedman et al., 2015). Finally, body mass index (kg/m2) was calculated from measured height and weight.
Statistical Analysis
We utilized linear regression analysis to examine main and interaction effects of SES and psychological resources in models predicting IL-6 and CRP, controlling for sociodemographic characteristics, chronic disease burden, and medications. By treating these covariates as confounders we took a conservative approach, as they could also be mediators of any associations with inflammatory markers (Gallo & Matthews, 2003). Next, we tested three-way interactions of SES, psychological resources, and gender, and given evidence of differences, proceeded to test interactions in gender-stratified models. To facilitate the interpretation of simple slopes, psychological resources and SES were restandardized in the male and female subsamples separately. We then examined the extent to which the interaction term and simple slopes were altered upon further adjustment for different sets of potential confounders or mediators. Only cases with complete data on all covariates were included in these analyses (i.e., the analytic sample remained constant). Regression models were estimated with cluster-robust standard errors due to the inclusion of 134 sets of twins or siblings in the analytic sample. Supplementary analyses examined interactions with separate indicators of SES and psychological resources. As the data are unlikely to be missing completely at random (MCAR), we performed sensitivity analyses using full information maximum likelihood (FIML) to handle missing data. FIML provides unbiased estimates when data are missing at random (MAR) or dependent on observed factors, a more realistic scenario than MCAR (Graham, 2009).
Results
Descriptive statistics for the sample are shown in Table 1. Study participants ranged in age from 35 to 86, with an average age of approximately 57.4 (SD=11.5). The sample was predominantly white (78%) and relatively well-educated, with roughly 40% having a college degree. In bivariate analyses, psychological resources and SES were positively correlated and related in expected directions to stress exposures, health behaviors, and inflammatory markers.
Table 1.
Descriptive Statistics
| Variables | % / Mean (SD) | Range |
|---|---|---|
| Demographic covariates | ||
| Age | 57.4 (11.5) | 35 – 86 |
| Female gender | 56.7% | -- |
| White race | 79.8% | -- |
| Medical conditions | 2.25 (1.86) | 0 – 10 |
| Medications | ||
| Blood pressure medication | 36.7% | -- |
| Cholesterol medication | 27.7% | -- |
| Antidepressant medication | 13.9% | -- |
| Socioeconomic indicators | ||
| Education | 7.53 (2.53) | 1 – 12 |
| Income | 51,596 (42,027) | 0 – 212,132 |
| Occupational prestige (SEI) | 40.90 (14.25) | 13.85 – 80.53 |
| Psychological variables | ||
| Optimism (Life Orientation Test-R) | 23.70 (4.78) | 6 – 30 |
| Perceived control | 5.62 (0.98) | 1.92 – 7 |
| Self-esteem (Rosenberg Scale) | 38.05 (7.41) | 11 – 49 |
| Negative affect (MIDUS II) | 1.55 (0.57) | 1 – 4.72 |
| Positive affect (MIDUS II) | 3.54 (0.69) | 1 – 5 |
| Childhood adversity variables | ||
| Socioeconomic disadvantage | 1.95 (1.46) | 0 – 6 |
| Childhood trauma (CTQ) | 38.3 (14.5) | 25 – 114 |
| Adult stress exposures | ||
| Life events | 3.11 (2.13) | 0 – 11 |
| Financial strain | 2.15 (1.67) | 0 – 6 |
| Bio-behavioral risk variables | ||
| Current smoker / former smoker | 14.3% / 32.3% | -- |
| Hours of exercise per week | 4.53 (6.87) | -- |
| Body mass index (BMI) | 29.62 (6.52) | -- |
| Inflammatory markers | ||
| IL-6 (pg/mL) | 2.98 (2.95) | 0.16 – 23.0 |
| CRP (ug/mL) | 3.04 (4.87) | 0.14 – 61.7 |
Notes. N = 1,152. SEI = Socioeconomic index; CTQ = Childhood trauma questionnaire; IL-6 = Interleukin-6; CRP = C-reactive protein
Full-sample Analyses
Table 2 shows regression coefficients for models predicting IL-6 and CRP in the full sample. As shown, there were no statistically significant main effects of psychological resources, whereas higher SES was marginally related to lower IL-6 (B=−.02, p=.07) and significantly related to lower CRP (B=−.04, p=.01). Psychological resources interacted with SES to predict IL-6 (B=.02, p=.029), but not CRP (p=.24). In addition, there was a statistically significant three-way interaction of resources, SES, and gender in predicting IL-6 (B=−.05, p=.047). A three-way interaction for CRP was similar in magnitude, but did not reach statistical significance (p=.12). Given overall evidence of gender differences, we proceeded with gender-stratified models.
Table 2.
Unstandardized Regression Coefficients From Linear Models Predicting IL-6 and CRP in Full Sample (N=1,152)
| Variable | IL-6 | CRP | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Step 1 | Step 2 | Step 3 | Step 1 | Step 2 | Step 3 | |
|
| ||||||
| B | B | B | B | B | B | |
| Age | .003** | .003** | .003** | −.004** | −.004* | −.004* |
| Female gender | .016 | .015 | .025 | .122** | .121** | .132** |
| Minority race/ethnic. | .006 | .010 | .013 | .012 | .015 | .015 |
| Milwaukee sample | .173** | .161** | .156** | .141* | .131* | .134* |
| Chronic disease | .029** | .029** | .030** | .049** | .050** | .051** |
| Blood pressure med. | .052* | .051* | .051* | .117** | .117** | .115** |
| Cholesterol med. | −.001 | −.004 | −.005 | −.087* | −.090** | −.092** |
| Antidepressant med. | .053 | .054 | .049 | .064 | .065 | .062 |
| SES composite | −.018 | −.021* | −.014 | −.041* | −.043** | −.058** |
| Psychological resources | −.011 | −.007 | −.006 | .020 | .023 | .041 |
| SES * Psych. resources | -- | .023* | .046** | -- | .019 | .044* |
| SES* Psych.* Female | -- | -- | −.040* | -- | -- | −.047 |
Notes. IL-6 = Interleukin 6; CRP = C-reactive Protein.
p<.05,
p<.01
Gender-stratified Analyses
In gender-stratified models, interactions of psychological resources and SES were significant in models predicting both inflammatory markers in men (IL-6: B=.05, p<.001; CRP: B=.05, p=.04), but neither in women (p=.67; p=.84). We proceeded to examine simple slopes for associations of psychological resources with IL-6 and CRP in men, at different levels of the moderator SES.
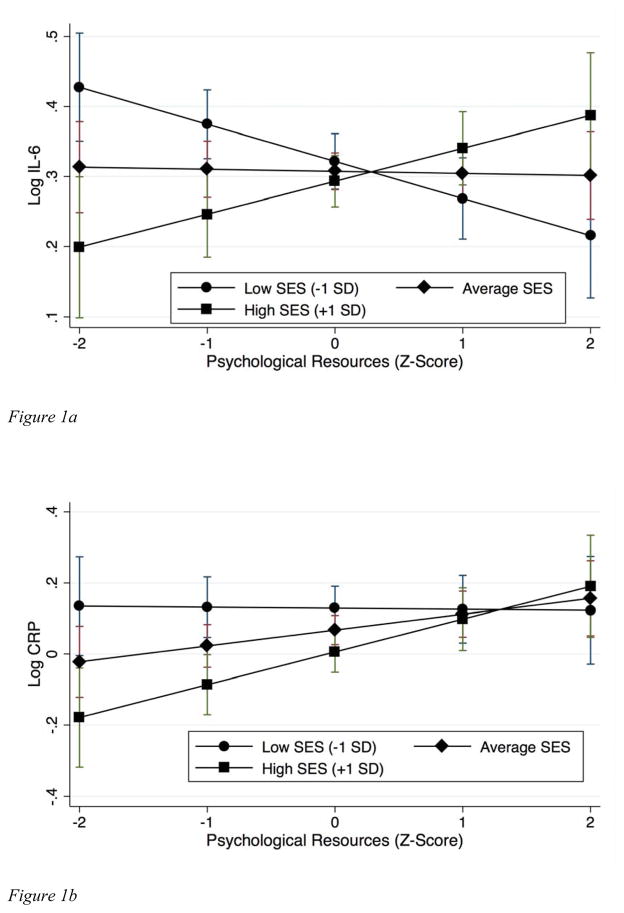
IL-6
Examination of simple slopes indicated that greater psychological resources were associated with lower IL-6 at lower levels of SES, with a statistically significant association, B=−.04, p=.016 at .75 SD below the mean of SES (approximately 25% of the sample SES distribution). Conversely, resources were associated with higher IL-6 at higher levels of SES, with a significant association (B=.05, p=.033) at 1 SD above the mean (20% of the distribution). The coefficient for the interaction of resources and SES was not appreciably attenuated (<10%) upon adjustment for any covariates and remained significant at p=.002 or less. The main effect term for psychological resources (at average SES: B=−.003, p=.86) remained non-significant in all covariate-adjusted models. Adjustment for childhood and adult stress exposures attenuated the association at low SES (1 SD below the mean) but accentuated the association at high SES by 10–15% (determined as (bunadjusted – badjusted) / bunadjusted; Weng, Hsueh, Messam, & Hertz-Picciotto, 2009). Adjustment for NA measured at MIDUS II also attenuated the association at low SES and accentuated the association at high SES, both by 35% (15% for NA measured at the biomarker visit), with further adjustment for PA having no additional effect. Adjustment for smoking, exercise, and BMI attenuated associations at both levels of SES by less than 10%.
CRP
Examination of simple slopes revealed non-significant associations of greater resources with lower CRP at low levels of SES, and significant associations with higher CRP at higher levels of SES. Associations were larger than for IL-6 (B=.09, p=.005 at 1 SD above the mean on SES) and significant across a wider range of SES (+0.25 SD and above, or 40% of the distribution; see Figure 1b). The interaction was not appreciably attenuated (<10%) and remained significant in all covariate-adjusted models. The main effect term for psychological resources (at average SES: B=.04, p=.09) was accentuated by 30–60% in models adjusted for stress exposures as well as NA and PA, and minimally altered (<5%) by health behaviors. The association at high SES (+1 SD) was more modestly accentuated (10–20%) in these models.
Figure 1.
Figure 1a. Simple slopes for associations of psychological resources with IL-6 at different levels of SES in men.
Figure 1b. Simple slopes for associations of psychological resources with CRP at different levels of SES in men.
Sensitivity and Supplementary Analyses
Results of sensitivity analyses truncating and excluding values of CRP exceeding 10.0 mg/L were similar. In analyses using FIML interactions were equivalent or stronger in magnitude. Supplementary analyses performed in both the full sample and the male subsample indicated that three-way and two-way interactions involving the SES composite were driven primarily by income and occupational status, with weaker and non-significant interactions involving education. With respect to psychological resources, interactions were strongest and uniformly significant for perceived control, whereas they were weakest and predominantly non-significant for optimism.
Discussion
In a sample of middle-aged and older adults, we found that socioeconomic status interacted with psychological resources in predicting markers of systemic inflammation in men but not women. Similar to the results of a prior study (Morozink et al., 2010), greater psychological resources were associated with lower IL-6 at lower levels of SES. However, at higher levels of SES, greater psychological resources were associated with higher IL-6 and CRP.
The association of psychological resources with lower IL-6 at lower levels of SES was not substantially explained by childhood and adult stress exposures or health behaviors. Negative affect measured at MIDUS II, rather than by a different set of instruments administered at the biomarker data collection, explained the largest portion of the association. Although substantially correlated, the former measure includes items tapping additional elements of negative affect beyond anxiety and depression, such as irritation and shame. It is also possible that the measure at MIDUS II captures more stable trait-like variance that, due to chronic and cumulative effects, shows stronger associations with these markers even several years later. In laboratory studies, resource variables including optimism and self-esteem were associated with smaller increases in both negative affect and IL-6 in response to acute psychological stressors (Brydon, Walker, Wawrzyniak, Chart, & Steptoe, 2009; O’Donnell, Brydon, Wright, & Steptoe, 2008). Thus, the lower level of IL-6 observed in low SES men with greater psychological resources might result in part from a greater ability to stave off negative emotions in response to stressors. The explanatory role of stress reactivity should be further evaluated in future work.
In lower SES men inverse associations of psychological resources with CRP, a more established predictor of coronary heart disease (Danesh et al., 2004), were not statistically significant. This suggests that in lower SES men resources may have less direct relevance for cardiovascular risk than for other outcomes more strongly linked with IL-6, including physical decline and all-cause mortality (Maggio, Guralnik, Longo, & Ferrucci, 2006).
Associations of psychological resources with higher levels of inflammatory markers at higher levels of SES were unexpected, and were not explained by negative or positive affect, stress exposures, or health behaviors. Prior studies have also reported associations of resource variables with markers of immune function in unexpected directions, including associations of greater optimism (one element of the resources dimension examined here) with higher CRP (Roy et al., 2010). Other research has linked optimism to poorer cellular immunity (which may conceivably co-occur with systemic inflammation; Segerstrom & Miller, 2004), under circumstances of persistent stress (Segerstrom, 2005). One explanatory hypothesis is that optimists exhibit greater engagement with challenging stressors, which is more physiologically demanding than the avoidance characteristic of pessimism, at least in the short term (Segerstrom, 2005). Such a dynamic would appear plausible for perceived control and self-esteem as well. Thus, it may be that higher SES men with greater psychological resources are more likely to take on physiologically demanding occupational or social roles or tasks, which even in the absence of emotional distress may initiate allostatic mechanisms that eventuate in systemic inflammation.
Several studies have found associations of psychological variables related to control and coping with indicators of adverse physiological function in African-Americans (Miller et al., 2015; Subramanyam et al., 2013). These findings are consistent with the theory of John Henryism (James, 1994), which suggests that the sustained effort associated with high levels of active coping in the face of systemic racism and disadvantage exacts a toll on physical health. Researchers have more consistently observed such associations in African-American men than in women, and they appear to be stronger at lower rather than higher SES (Subramanyam et al., 2013). Findings observed here in predominantly white, higher SES men clearly cannot be attributed to the wearing struggle imposed by systemic racism. Rather, they may reflect a similar physiological cost to striving in a context primed to reward such efforts with the trappings of socioeconomic achievement. In a post-hoc analysis a measure of striving derived from scales measuring goal persistence and achievement orientation was associated with both higher IL-6 (B=.04, p=.026) and CRP (B=.07, p=.028) in higher SES men (SES above +.25 SD, the point at which simple slopes for resources were statistically significant for at least one marker; N=188), and when added to the model associations of psychological resources with IL-6 (B=.049, p=.045) and CRP (B=.100, p=.004) were attenuated by 45–50% and no longer significant. This finding provides some support for the proposed mechanism, although given the exploratory nature of the analysis it should be interpreted cautiously and investigated in future work.
There may be various reasons why the interaction of psychological resources and SES was not observed in women. Men appear to place greater importance on the basic value of power, defined as “social status and prestige, control or dominance over people and resources” (Schwartz & Rubel, 2005, p. 1010). As such, when power is lacking due to lower social position (Marmot, 2004), psychological resources involving positive expectancies, a sense of control over outcomes, and self-worth may be particularly palliative in men, whereas this relationship may reverse at higher SES as discussed above. Another possibility is that the pattern of differential slopes seen in Figure 1 is attenuated in women because the distribution of SES is somewhat lower and more compressed, most likely as a consequence of reduced access to high status occupations and lower pay levels within the same occupation (Petersen & Morgan, 1995). Of course, the actuality may involve a combination of these two possibilities, or another entirely. Further research is needed to better understand how race, SES, and gender intersect to moderate associations of psychological characteristics with physical health.
In this study, income and occupation appeared to be more influential than education in modulating the relationship of psychological resources to inflammatory markers in men. Additionally, gender differences and associations with inflammatory markers were largest for perceived control, suggesting that perceptions of control might be particularly influential to adaptive coping in low SES men but to striving or engagement with stressors in high SES men.
There is one pointed theoretical, and one applied, implication of the primary findings observed here. Theoretically, traditional models of dispositional characteristics, coping, stress and physical health presume that “positive” psychological attributes promote effective coping processes, thereby reducing physiological sequelae of stress related to the function of the Hypothalamic-Pituitary-Adrenal (HPA) and Sympathetic-Adrenal-Medullary (SAM) Axes (Lutgendorf & Costanzo, 2003; Taylor & Stanton, 2007). Findings of the present study join others noted above (Miller et al., 2015; Segerstrom, 2005) in suggesting that in some cases, the relationship between putatively adaptive psychological characteristics and physiological markers of stress may be inverted. As theory evolves around psychological attributes and neuroendocrine and immune dysregulation, modulation by life context requires careful consideration. At an applied level, psychosocial interventions aimed at forestalling adverse physiological effects of stress often attempt to bolster psychological resources and well-being (Albus, 2010; Miller & Cohen, 2001). Such interventions might further explore tailoring to socioeconomic context. For instance, enhancing psychological resources might be more effective in reducing inflammation in low SES men, whereas targeting other attributes might be more fruitful among higher SES men.
Findings must be interpreted with a balanced regard for study strengths and limitations. Limitations include the absence of baseline measurement of inflammatory markers, which precluded assessment of change in levels of inflammation over time. Instead, we examined prospective associations with IL-6 and CRP at a single future time point. Negative affect and life events were assessed at baseline, and therefore whether these variables are confounders (i.e., temporally preceding psychological resources) or mediators of the observed associations is empirically ambiguous. Despite considerable sociodemographic diversity and national scope, the MIDUS biomarker sample is not nationally representative. Due circumspection is therefore warranted in generalizing findings to different populations. Finally, it is impossible to randomize individuals to SES or differing levels of psychological attributes, so restraint must be exercised in declaring these associations causal. Strengths of the study include its incorporation of multiple markers of systemic inflammation and indicators of SES, adjustment for a comprehensive set of potential explanatory variables, and sensitivity analyses accounting for missing data. In addition, the sample featured greater representation of African-American participants from the Milwaukee oversample, which was not available in prior studies (Morozink et al., 2010).
In summary, we found that in men psychological resources were associated with lower circulating concentrations of IL-6 at lower levels of SES, but higher concentrations of both IL-6 and CRP at higher levels of SES. Additional research is needed to explain observed gender differences in this pattern and to further investigate underlying mechanisms, potentially including dampened stress reactivity and greater striving or engagement with stressors. On balance however, findings add to a growing body of work suggesting that the socioeconomic context in which individual psychological functioning occurs may shape the nature and direction of its associations with markers of physiological function and disease risk.
Acknowledgments
Source of Funding:
Data used for this research was provided by the longitudinal study titled “Midlife in the United States,” (MIDUS) managed by the Institute on Aging, University of Wisconsin. This research was supported by a grant from the National Institute on Aging (P01-AG020166; R01AG044588).
Footnotes
Conflicts of Interest
None declared.
References
- Albus C. Psychological and social factors in coronary heart disease. Annals of Medicine. 2010;42(7):487–494. doi: 10.3109/07853890.2010.515605. [DOI] [PubMed] [Google Scholar]
- Baum A, Garofalo JP, Yali A. Socioeconomic status and chronic stress: Does stress account for SES effects on health? Annals of the New York Academy of Sciences. 1999;896(1):131–144. doi: 10.1111/j.1749-6632.1999.tb08111.x. [DOI] [PubMed] [Google Scholar]
- Bjarnason-Wehrens B, Grande G, Loewel H, Voller H, Mittag O. Gender-specific issues in cardiac rehabilitation: Do women with ischaemic heart disease need specially tailored programmes? European Journal of Cardiovascular Prevention and Rehabilitation. 2007;14(2):163–171. doi: 10.1097/HJR.0b013e3280128bce. [DOI] [PubMed] [Google Scholar]
- Boehm JK, Peterson C, Kivimaki M, Kubzansky L. A prospective study of positive psychological well-being and coronary heart disease. Health Psychology. 2011;30(3):259–267. doi: 10.1037/a0023124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan JM, Ryff CD. Psychological well-being and metabolic syndrome: Findings from the Midlife in the United States national sample. Psychosomatic Medicine. 2015;77(5):548–558. doi: 10.1097/PSY.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Walker C, Wawrzyniak AJ, Chart H, Steptoe A. Dispositional optimism and stress-induced changes in immunity and negative mood. Brain, Behavior, and Immunity. 2009;23(6):810–816. doi: 10.1016/j.bbi.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE. Socioeconomic status and health: Mediating and moderating factors. Annual Review of Clinical Psychology. 2013;9:723–749. doi: 10.1146/annurev-clinpsy-050212-185634. [DOI] [PubMed] [Google Scholar]
- Clark CJ, Henderson KM, de Leon CF, Guo H, Lunos S, Evans DA, Everson-Rose SA. Latent constructs in psychosocial factors associated with cardiovascular disease: An examination by race and sex. Frontiers in Psychiatry. 2012;3:5. doi: 10.3389/fpsyt.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, … Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. New England Journal of Medicine. 2004;350(14):1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- Eisenlohr-Moul T, Segerstrom S. Autonomy, positive relationships, and IL-6: Evidence for gender-specific effects. British Journal of Health Psychology. 2013;18(2):420–438. doi: 10.1111/j.2044-8287.2012.02085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti M, Cohen HJ, … Havlik RJ. Serum IL-6 level and the development of disability in older persons. Journal of the American Geriatrics Society. 1999;47(6):639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Friedman EM, Herd P. Income, education, and inflammation: Differential associations in a national probability sample (the MIDUS study) Psychosomatic Medicine. 2010;72(3):290–300. doi: 10.1097/PSY.0b013e3181cfe4c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Karlamangla AS, Gruenewald TL, Koretz B, Seeman TE. Early life adversity and adult biological risk profiles. Psychosomatic Medicine. 2015;77(2):176–185. doi: 10.1097/PSY.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: Do negative emotions play a role? Psychological Bulletin. 2003;129(1):10–51. doi: 10.1037/0033-2909.129.1.10. [DOI] [PubMed] [Google Scholar]
- Graham JW. Missing data analysis: Making it work in the real world. Annual Review of Psychology. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Karlamangla AS, Hu P, Stein-Merkin S, Crandall C, Koretz B, Seeman TE. History of socioeconomic disadvantage and allostatic load in later life. Social Science & Medicine. 2012;74(1):75–83. doi: 10.1016/j.socscimed.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald TL, Kemeny ME. Psychoneuroimmunological processes in aging and health. In: Aldwin CM, Park CL, Spiro AI, editors. Handbook of health psychology and aging. New York, NY: Guilford Press; 2007. pp. 97–118. [Google Scholar]
- Hagger-Johnson G, Roberts B, Boniface D, Sabia S, Batty GD, Elbaz A, … Deary IJ. Neuroticism and cardiovascular disease mortality: Socioeconomic status modifies the risk in women. Psychosomatic Medicine. 2012;74(6):596–603. doi: 10.1097/PSY.0b013e31825c85ca. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, … Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. The American Journal of Medicine. 1999;106(5):506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Hauser RM, Warren JR. Socioeconomic indexes for occupations: A review, update, and critique. 1996 (Paper No. 96-01). Retrieved from University of Wisconsin-Madison Center for Demography & Ecology website: http://www.ssc.wisc.edu/cde/cdewp/96-01.pdf.
- Hostinar CE, Lachman ME, Mroczek DK, Seeman TE, Miller GE. Additive contributions of childhood adversity and recent stressors to inflammation at midlife: Findings from the MIDUS study. Developmental Psychology. 2015;51(11):1630–1644. doi: 10.1037/dev0000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infurna FJ, Gerstorf D. Perceived control relates to better functional health and lower cardio-metabolic risk: The mediating role of physical activity. Health Psychology. 2014;33(1):85–94. doi: 10.1037/a0030208. [DOI] [PubMed] [Google Scholar]
- Judge TA, Erez A, Bono JE, Thoresen CJ. Are measures of self-esteem, neuroticism, locus of control, and generalized self-efficacy indicators of a common core construct? Journal of Personality and Social Psychology. 2002;83(3):693–710. doi: 10.1037//0022-3514.83.3.693. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 3. New York: The Guilford Press; 2011. [Google Scholar]
- Lachman ME, Weaver SL. The sense of control as a moderator of social class differences in health and well-being. Journal of Personality and Social Psychology. 1998;74(3):763–773. doi: 10.1037//0022-3514.74.3.763. [DOI] [PubMed] [Google Scholar]
- Love GD, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: Protocol, measures, sample, and comparative context. Journal of Aging and Health. 2010;22(8):1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, Costanzo ES. Psychoneuroimmunology and health psychology: An integrative model. Brain, Behavior, and Immunity. 2003;17(4):225–232. doi: 10.1016/s0889-1591(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2006;61(6):575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot MG. The status syndrome: How social standing affects our health and longevity. New York, NY: Henry Holt; 2004. [Google Scholar]
- Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annual Review of Psychology. 2011;62:501. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Räikkönen K, Gallo L, Kuller LH. Association between socioeconomic status and metabolic syndrome in women: Testing the reserve capacity model. Health Psychology. 2008;27(5):576–583. doi: 10.1037/0278-6133.27.5.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S. Psychological interventions and the immune system: A meta-analytic review and critique. Health Psychology. 2001;20(1):47–63. doi: 10.1037//0278-6133.20.1.47. [DOI] [PubMed] [Google Scholar]
- Miller GE, Yu T, Chen E, Brody GH. Self-control forecasts better psychosocial outcomes but faster epigenetic aging in low-SES youth. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(33):10325–10330. doi: 10.1073/pnas.1505063112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozink JA, Friedman EM, Coe CL, Ryff CD. Socioeconomic and psychosocial predictors of interleukin-6 in the MIDUS national sample. Health Psychology. 2010;29(6):626–635. doi: 10.1037/a0021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. Consideration of sex as a biological variable in NIH-funded research. 2015. Notice #NOT-OD-15-102. [Google Scholar]
- O’Connor M, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, … Sloan EK. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain, Behavior, and Immunity. 2009;23(7):887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K, Brydon L, Wright CE, Steptoe A. Self-esteem levels and cardiovascular and inflammatory responses to acute stress. Brain, Behavior, and Immunity. 2008;22(8):1241–1247. doi: 10.1016/j.bbi.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Petersen T, Morgan LA. Separate and unequal: Occupation-establishment sex segregation and the gender wage gap. American Journal of Sociology. 1995:329–365. [Google Scholar]
- Rasmussen HN, Scheier MF, Greenhouse JB. Optimism and physical health: A meta-analytic review. Annals of Behavioral Medicine. 2009;37(3):239–256. doi: 10.1007/s12160-009-9111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M. Society and the adolescent self-image. Princeton, NJ: Princeton Press; 1965. [Google Scholar]
- Roy B, Diez-Roux AV, Seeman T, Ranjit N, Shea S, Cushman M. Association of optimism and pessimism with inflammation and hemostasis in the multi-ethnic study of atherosclerosis (MESA) Psychosomatic Medicine. 2010;72(2):134–140. doi: 10.1097/PSY.0b013e3181cb981b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryff CD, Radler BT, Friedman EM. Persistent psychological well-being predicts improved self-rated health over 9–10 years: Longitudinal evidence from MIDUS. Health Psychology Open. 2015;2(2):2055102915601582. doi: 10.1177/2055102915601582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryff CD, Seeman T, Weinstein M. Documentation for psychosocial constructs and composite variables in MIDUS II biomarker project (P4) 2012 (ICPSR No. 29282). Retrieved from Interuniversity Consortium for Political and Social Research website: https://www.icpsr.umich.edu.
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the life orientation test. Journal of Personality and Social Psychology. 1994;67(6):1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Scher CD, Stein MB, Asmundson GJ, McCreary DR, Forde DR. The childhood trauma questionnaire in a community sample: Psychometric properties and normative data. Journal of Traumatic Stress. 2001;14(4):843–857. doi: 10.1023/A:1013058625719. [DOI] [PubMed] [Google Scholar]
- Schöllgen I, Huxhold O, Schüz B, Tesch-Römer C. Resources for health: Differential effects of optimistic self-beliefs and social support according to socioeconomic status. Health Psychology. 2011;30(3):326–335. doi: 10.1037/a0022514. [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Rubel T. Sex differences in value priorities: Cross-cultural and multimethod studies. Journal of Personality and Social Psychology. 2005;89(6):1010–1028. doi: 10.1037/0022-3514.89.6.1010. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC. Optimism and immunity: Do positive thoughts always lead to positive effects? Brain, Behavior, and Immunity. 2005;19(3):195–200. doi: 10.1016/j.bbi.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin NL, Graham-Engeland JE, Ong AD, Almeida DM. Affective reactivity to daily stressors is associated with elevated inflammation. Health Psychology. 2015;34(12):1154–1165. doi: 10.1037/hea0000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgeon JA, Arewasikporn A, Okun MA, Davis MC, Ong AD, Zautra AJ. The psychosocial context of financial stress: Implications for inflammation and psychological health. Psychosomatic Medicine. 2015 doi: 10.1097/PSY.0000000000000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanyam MA, James SA, Diez-Roux AV, Hickson DA, Sarpong D, Sims M, … Wyatt SB. Socioeconomic status, John Henryism and blood pressure among African-Americans in the Jackson Heart Study. Social Science & Medicine. 2013;93:139–146. doi: 10.1016/j.socscimed.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the Coronary Artery Risk Development in Young Adults Study. Biological Psychiatry. 2006;60(8):819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Stanton AL. Coping resources, coping processes, and mental health. Annual Review of Clinical Psychology. 2007;3:377–401. doi: 10.1146/annurev.clinpsy.3.022806.091520. [DOI] [PubMed] [Google Scholar]
- Turiano NA, Chapman BP, Agrigoroaei S, Infurna FJ, Lachman M. Perceived control reduces mortality risk at low, not high, education levels. Health Psychology. 2014;33(8):883–890. doi: 10.1037/hea0000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weisgram ES, Bigler RS, Liben LS. Gender, values, and occupational interests among children, adolescents, and adults. Child Development. 2010;81(3):778–796. doi: 10.1111/j.1467-8624.2010.01433.x. [DOI] [PubMed] [Google Scholar]
- Weng HY, Hsueh YH, Messam LL, Hertz-Picciotto I. Methods of covariate selection: Directed acyclic graphs and the change-in-estimate procedure. American Journal of Epidemiology. 2009;169(10):1182–1190. doi: 10.1093/aje/kwp035. [DOI] [PubMed] [Google Scholar]
- Zilioli S, Imami L, Slatcher RB. Life satisfaction moderates the impact of socioeconomic status on diurnal cortisol slope. Psychoneuroendocrinology. 2015;60:91–95. doi: 10.1016/j.psyneuen.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]