Abstract
Purpose
MicroRNAs are small non-coding RNAs which regulate gene expression at the post-transcriptional level. We reported that levels of microRNA (miR)-29 family are decreased in Fuchs endothelial corneal dystrophy (FECD) patient corneas. The miR-29 family regulates production of extracellular matrix (ECM) proteins. Accumulation of ECM proteins in Descemet’s membrane is an important pathologic change in FECD. In this study, we transfected miR-29b into human corneal endothelial cells and tissues and evaluated ECM protein expression levels.
Methods
Immortalized Fuchs human corneal endothelial cell line (iFECD) was established by infection of FECD patient’s corneal endothelial cells with hTERT lentivirus. MiR-29b was transfected into iFECD and expression levels of ECMs (COL1A1, COL4A1, LAMC1) were evaluated with quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) and Western blot. Expression level of LAMC1 protein in miR-29b transfected donor corneal endothelium was also evaluated by Western blot.
Results
Compared with control, miR-29b expression level after transfection of iFECD was increased to 335.6 (±91.0)% and ECM expression levels were significantly decreased. Compared with control, qRT-PCR demonstrated reduction of ECM to the following levels: COL1A1: 1.9 (±0.4)%; COL4A1: 7.1 (±1.7)%; LAMC1: 21.5 (±2.7)%. Western blot showed reduced protein expression: COL1A1: 4.8 (±3.2)%; COL4A1: 42.5 (±25.0)%; and LAMC1: 44.8 (±3.1)%. In miR-29b transfected corneal tissue, LAMC1 protein expression level was decreased to 14.4 (±20.5)%.
Conclusions
Over-expression of miR-29b decreased ECM protein production in human corneal endothelial cells. Thus, miR-29 replacement therapy might be a new treatment strategy for FECD aimed at reducing pathologic production of ECM proteins in Descemet’s membrane.
Keywords: Fuchs endothelial corneal dystrophy, corneal endothelium, miR-29b, extracellular matrix, microRNA
Introduction
Fuchs endothelial corneal dystrophy (FECD) is a leading cause for corneal transplantation in the United States and world-wide1–4. Corneal transplantation has been the only definitive treatment for FECD although newer, non-surgical treatments appear promising5. Recent studies have indicated important roles for a variety of pathophysiologic processes in FECD, including oxidative stress, unfolded protein response activation, endoplasmic reticulum stress, autophagy, and apoptosis6–12.
MicroRNAs are small (~22 nucleotides), single-stranded RNAs regulating eukaryotic gene expression at the post-transcriptional level13,14. They cause mRNA destabilization and cleavage or direct translational repression13. These short RNAs are predicted to regulate as many as 60% of human protein-coding genes with a given microRNA potentially binding up to 4500 human gene targets within 3’ untranslated regions13.
In non-ocular tissue, a role for miR-29b as a regulator of fibrosis including ECM expression control15–20 has been proposed. It also has been shown that miR-29 plays a critical role in the pathology of eye diseases, including pterygium21, cataract22, glaucoma23–25 and FECD26. In trabecular meshwork cells, miR-29 is a critical regulator of ECM expression 23–25. We have previously described a comparison of miRNA profiles between FECD and normal corneal endothelial cells and demonstrated widespread miRNA downregulation in FECD26. In particular, endothelial expression of miR-29 family members was decreased, which might contribute to increased sub-endothelial ECM accumulation in FECD26. In FECD patient corneas, it is known that components of abnormal ECM excrescences (guttae) include: collagen type 1 alpha 1 (COL1A1), collagen type 4 alpha 1 (COL4A1) and laminin gamma 1 (LAMC1)27,28.
Thus, we hypothesized that miR-29 replacement could be a potential target of future conservative therapeutic approaches in FECD. To this end, we investigated the effects of over-expressing microRNA-29b on extracellular matrix protein expression levels in immortalized cells and normal human corneal endothelial tissue.
Materials and Methods
Cell Culture
An immortalized FECD human corneal endothelial cell line (iFECD) was established by infection of an FECD patient’s corneal endothelial cells with hTERT lentivirus. Culture and use of human FECD cells was conducted after patient informed consent via a Johns Hopkins Medicine Institutional Review Board approved protocol (NA_00023119). iFECD was seeded in culture dishes with OptiMEM-1 (ThermoFisher Scientific, Waltham, MA) supplemented with 8% fetal calf serum, antibiotic/antimycotic solution (10,000 units penicillin (base), 10,000 µg streptomycin (base), and 25 µg of amphotericin B/ml) (Invitrogen, Grand island, NY), 50 µg/ml gentamicin (Invitrogen), 5ng/ml EGF, 20ng/ml NGF, 100µg bovine pituitary extract, 20µg/ml ascorbic acid, 200mg/l calcium chloride and 0.08% chondroitin sulfate. The medium was changed every two days until the cells reached confluence.
Transfection of miR-29b Psh to iFECD
MiR-29b mimic containing a short hairpin structure with a stem loop (miR-29b Psh, Bonac, Fukuoka, Japan) was used in this study. The stem loop contained proline derivatives similar to PnkRNA, as previously described29. MiR-29b was transfected into iFECD using miR-29b Psh with lipofectamine RNAiMAX (Invitrogen, San Diego, CA) using standard protocols. The iFECD cells were seeded in 6-well plates (5×105 cells/well) at 50% confluency and were used at 70% for transfection the next day. The miR-29b Psh (150µl) and lipofectamine (150µl) were mixed and added to 3ml culture media in each well, followed by incubation at 37°C. Final concentration of miR-29b Psh was 100nM. After 48 hours incubation, total RNA and protein were extracted as described below.
Transfection of miR-29b Psh to human corneal tissue
Six human corneas from three donors were obtained from Lions VisionGift (Portland, OR). Corneas were maintained in preservation media (Optisol-GS; Bausch & Lomb, Rochester, NY) at 4°C. Standard eye bank protocol for informed consent and for protection of donor confidentiality was used. The donor corneas were unsuitable for transplantation. Each cornea was divided into halves and incubated at 37°C for transfection. As with the case of iFECD transfection, cell culture medium containing Psh-lipofectamine complex (Psh final concentration was 100nM) was used. After 48 hours incubation, Descemet’s membrane with endothelial cells were stripped and proteins were extracted for Western blot (see below).
RT-PCR
Total RNA samples were extracted from cultured cells using the RNeasy Mini Kit (QIAGEN, Venlo, Netherlands). Complementary DNA samples were synthesized using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA), and Taqman MicroRNA Reverse Transcription Kit (Applied Biosystems). Quantitative reverse transcriptase polymerase chain reaction (RT-PCR) was performed using TaqMan Universal Master Mix II, no UNG (Applied Biosystems), preamplified cDNA products (diluted 1:20; 5 ul), nuclease free water, and human specific primers (Applied Biosystems, Table 1) in a 20 µL reaction volume. A no-template control was included in each experiment to confirm the absence of DNA contamination. All assays used similar amplification efficiency, and a ΔCT experimental design was used for relative quantification. Data analysis was performed using StepOne™ software (Version 2.2, Applied Biosystems). COL1A1, COL4A1, and LAMC1 were normalized to GAPDH whereas miR-29b was normalized to RNU48.
Table 1.
Primer sets used for quantitative reverse transcriptase polymerase chain reaction.
| Name | Symbol | Reference Sequence |
Assay number |
|---|---|---|---|
| Collagen, type I, alpha 1 | COL1A1 | NM_000088.3 | Hs00164004_m1 |
| Collagen, type IV, alpha 1 | COL4A1 | NM_001845.4 | Hs00266237_m1 |
| Laminin, gamma 1 | LAMC1 | NM_002293.3 | Hs00267056_m1 |
| Glyceraldehyde 3-phosphate dehydrogenase | GAPDH | NM_173979.3 | Hs02758991_g1 |
| microRNA-29b | miR-29b | MIMAT0004515 | hsa-miR-29b: 000413 |
| small nucleolar RNA, C/D box 48 | RNU48 | NR_002745 | RNU48: 001006 |
Western blot
After miR-29b transfection, cultured cells and endothelial cells from corneal tissue were lysed with ice-cold Tissue Protein Extraction Reagent (Thermo Fisher Scientific) supplemented with protease inhibitor (1%) and EDTA (1%). Total protein concentration was measured using a protein assay kit (Thermo Fisher Scientific), and each sample was adjusted to 20 mg/ml. Proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Mini-PROTEAN TGX Gels: Bio-Rad, Hercules, CA) and transferred to PVDF membranes (The PerfectMembrane: GenHunter Corporation, Nashville, TN) that had been soaked in methanol for 1 minute. After blocking with 5% milk for 1 hour, the membranes were then incubated overnight with rabbit anti-COL1A1 antibody (1:1000 dilution; Cell Signaling Technology, Danvers, MA), rabbit anti-COL4A1 antibody (1:1000; Cell Signaling Technology), or rabbit anti-LAMC1 antibody (1:1000; Cell Signaling Technology). The membranes were washed and then incubated with horseradish peroxidase-conjugated donkey anti-rabbit IgG antibody (1:10000 dilution; Cell Signaling Technology) for 30 minutes. The membrane was washed with TBST and antigen was detected using ECL solution (Pierce Biotechnology, Rockford, IL). The membrane then was stripped and restained for GAPDH using rabbit anti-GAPDH antibody (1:10000; Cell Signaling Technology) with 1 hour incubation at room temperature.
Statistical analysis
Statistical analysis was performed using JMP (version 12.1.0, SAS, Cary, NC). RT-PCR and Western blot results were compared using the two-tailed Student’s t-test. P values less than 0.05 were considered significant.
Results
Psh miR-29b induced miR-29b over-expression in iFECD
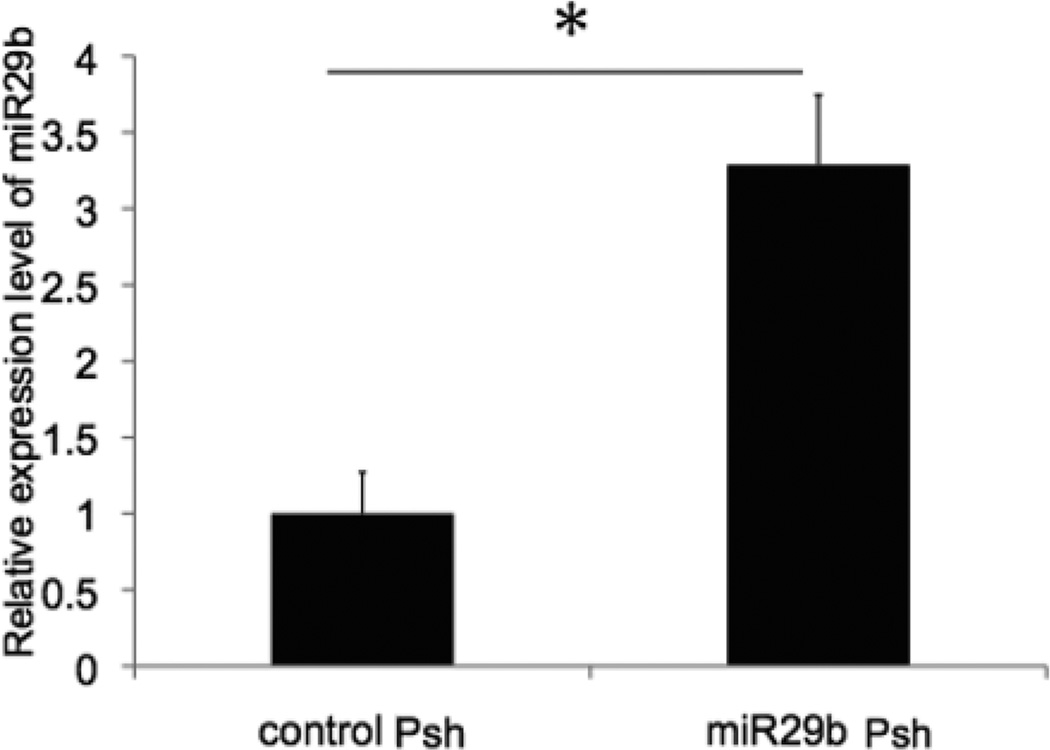
Control Psh or miR-29b Psh was transfected into iFECD with lipofectamine. Forty-eight hours later, transfected cells were collected and miR-29b expression was measured using RT-PCR. Compared with the control group, miR-29b expression level in miR-29b Psh transfected cells was increased 328.7 (±45.7)% (p=0.004) (Figure 1).
Figure 1. Over-expression of miR-29b in immortalized Fuchs dystrophy corneal endothelial cells.
Control Psh and miR-29b Psh were transfected into iFECD. Compared to control, miR-29b expression level in the miR-29b Psh group was increased 3.3 fold (*p < 0.05, n = 3 per each condition, bar = standard deviation).
ECM mRNA and protein expression was decreased in iFECD over-expressing miR-29b
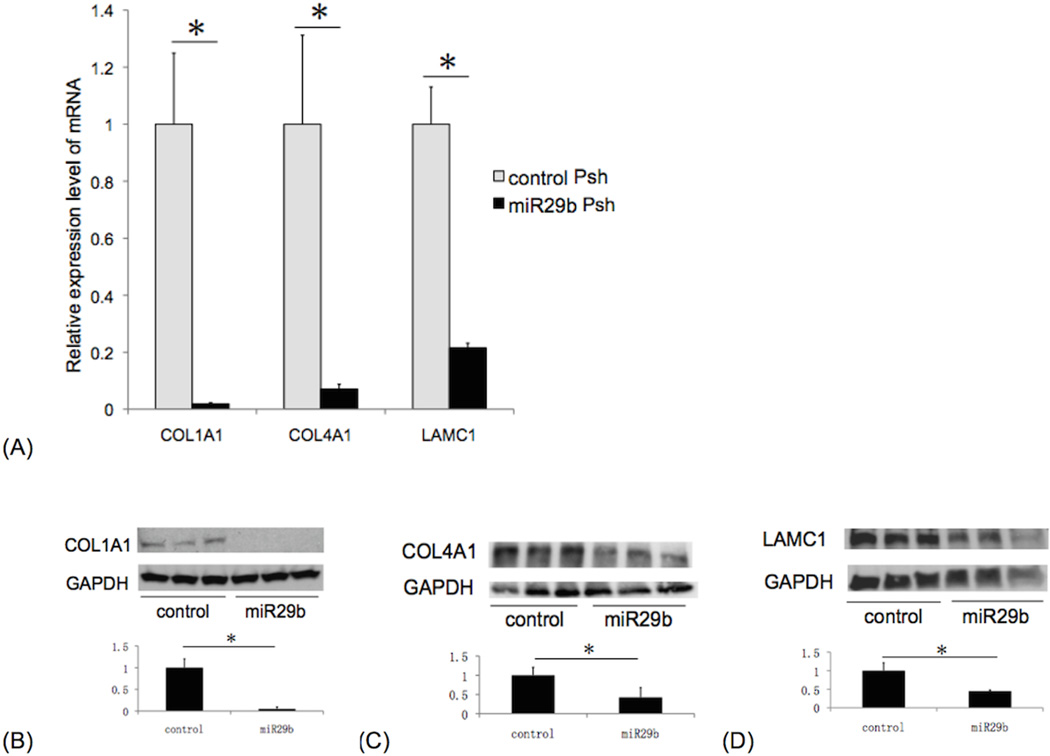
ECM gene expression levels by RT-PCR in immortalized Fuchs corneal endothelial cells transfected with miR-29b Psh were significantly decreased to the following percent of expression levels relative to control Psh transfected cells (mean ± standard deviation): COL1A1: 1.9 (±0.4)% (p=0.021); COL4A1: 7.1 (±1.7)% (p=0.035); LAMC1: 21.5 (±2.7)% (p=0.007) (Figure 2A). ECM protein expression levels in iFECD transfected with miR-29b Psh were significantly decreased to the following percent of expression levels in control Psh transfected cells (mean ± standard deviation): COL1A1: 4.8 (±3.2)% (p=0.001); COL4A1: 42.5 (±25.0)% (p=0.038); LAMC1: 44.8 (±3.1)% (p=0.011) (Figure 2B, 2C, 2D).
Figure 2. ECM mRNA and protein expression in miR-29b Psh transfected immortalized Fuchs dystrophy corneal endothelial cells.
RT-PCR (A): Compared to control Psh transfected cells, miR-29b Psh transfected cells showed the following levels of ECM gene expression (mean ± standard deviation): COL1A1: 1.9 (±0.4)%; COL4A1: 7.1 (±1.7)%; LAMC1: 21.5 (±2.7)% (*p < 0.05, n=3 per each condition, bar: standard deviation). Western blot: compared to control Psh transfected cells, miR-29b Psh transfected cells showed the following levels of ECM protein expression (mean ± standard deviation): (B) COL1A1: 4.8 (±3.2)% , (C) COL4A1: 42.5 (±25.0)% , and (D) LAMC1: 44.8 (±3.1)% (*p < 0.05, n = 3 per each condition, bar: standard deviation).
LAMC1 protein expression in human corneal endothelium in situ was decreased after miR-29b Psh transfection
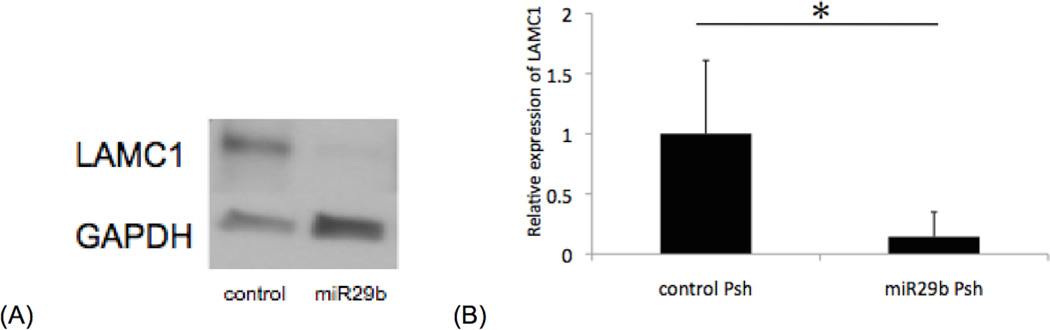
We sought to determine the effects of miR-29b Psh transfection into human corneal endothelium in situ. Human corneoscleral tissue was transfected with miR-29b Psh for 48 hours, and stripped Descemet’s membrane/endothelium was used for Western blot analysis. LAMC1 protein expression level in corneal endothelium transfected with miR-29b Psh was significantly decreased to 14.4 (±20.5)% of control Psh transfected corneal endothelium (p=0.017, Figure 3A, 3B).
Figure 3. LAMC1 protein expression in miR-29b Psh transfected human corneal endothelium in situ.
(A) Representative immunoblot demonstrating decreased LAMC1 protein expression in miR-29b Psh transfected corneal endothelium. (B) LAMC1 protein expression level in miR-29b Psh transfected corneal endothelium is 14.4 (±20.5)% of control Psh transfected corneal endothelium level (*p < 0.05, n = 6 per each condition, bar: standard deviation).
Discussion
MicroRNAs regulate eukaryotic gene expression through mRNA destabilization and cleavage or direct translational repression13,14. These short RNAs are considered to be important regulators of gene expression in normal and some disease states. Thus, they could represent potentially attractive therapeutic targets.
The present study investigated the effects of over-expression of miR-29b on ECM mRNA and protein levels in iFECD and human corneal endothelium. First, miR-29b expression was increased using miR-29b Psh transfection in iFECD. Over-expressed miR-29b in iFECD down-regulated ECM expression. Further, using ex vivo human corneal tissue, over-expression of miR-29b also down-regulated ECM expression in corneal endothelium. The latter finding supports the potential feasibility of modifying miR-29b levels in vivo to alter ECM protein production.
Our previous study profiling miRNAs between FECD and normal endothelial cells demonstrated widespread miRNA downregulation in FECD including significant effects on the miR-29 family26. This finding was associated in FECD corneas with increased sub-endothelial accumulation of ECM proteins which are targets of miR-2926. Furthermore, decreased endothelial expression of DICER1, an important component of miRNA biogenesis, was demonstrated. These results raise the interesting possibility that altered miRNA biogenesis caused by a decrease in DICER1 may contribute to reduction of miRNA abundance. Recent studies demonstrate cytotoxicity of DICER1 depletion in various tissues, and this down-regulation may be triggered by oxidative stress30–32. These findings suggest the interesting possibility that oxidative stress in FECD may contribute to DICER1 depletion and a subsequent decrease in miRNA levels with further consequences in FECD pathogenesis9.
In the early-onset form of FECD, it has been shown that missense mutations Q455K and L450W in the gene encoding the α2 subunit of collagen VIII (COL8A2) are associated with disease pathogenesis27,33, and collagen type VIII is a component of guttae34. Although the α1 subunit of collagen VIII has been reported to be regulated by miR-29b35, to our knowledge, COL8A2 has not described as a miR-29b target.
In this study, we successfully transfected miR-29b into human corneal endothelial cells by incubation with miR-29b Psh. This result suggests that miR-29b could form the basis of an efficient treatment of FECD in the future. Presently miRNA-based therapies for a variety of diseases are in preclinical or clinical trial stages36. Potential approaches for FECD treatment could include delivery of oligonucleotides, plasmids, or adeno-associated virus encoding miR-29b via topical or intracameral route36.
Increased ECM protein expression is a hallmark feature of FECD. In the present study, miR-29b Psh was used to transfect iFECD and human corneal tissue, resulting in reduced ECM protein expression. Since the Psh platform is DICER independent, we hypothesize that any potential decrease in DICER levels in these cells would not affect the ability to over-express miR-29b. Thus, miR-29b Psh therapy could be a feasible approach for future FECD treatment and warrants further study.
Acknowledgments
The authors thank Lions VisionGift (Portland, OR) for providing corneas used in this study.
Financial Support: Grants from the Richard Lindstrom/Eye Bank Association of America Research Fund (T.T.), Japan Eye Bank Association (T.T), the J. Willard and Alice S. Marriott Foundation, Margaret Andrews, Edward Colburn, Lorraine Collins, Richard Dianich, Mary Finegan, Barbara and Peter Freeman, Stanley Friedler, MD, Ida Jeffries, Herbert Kasoff, Diane Kemker, Jean Mattison, Florenz Ourisman, Lee Silverman, Norman Tunkel, PhD, and the National Eye Institute EY 019874 (all to A.S.J.).
Footnotes
No authors have conflicts of interest or proprietary interests in the contents of this manuscript.
References
- 1.Al-Yousuf N. Penetrating keratoplasty: indications over a 10 year period. Brit J Ophthalmol. 2004;88:998–1001. doi: 10.1136/bjo.2003.031948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorrepaal SJ, Cao KY, Slomovic AR. Indications for penetrating keratoplasty in a tertiary referral centre in Canada, 1996–2004. Can J Ophthalmology. 2007;42:244–250. [PubMed] [Google Scholar]
- 3.Ghosheh FR, Cremona FA, Rapuano CJ, et al. Trends in penetrating keratoplasty in the United States 1980–2005. Int Ophthalmol. 2007;28:147–153. doi: 10.1007/s10792-007-9177-z. [DOI] [PubMed] [Google Scholar]
- 4.Gain P, Jullienne R, He Z, et al. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016;134:167–173. doi: 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- 5.Koizumi N, Okumura N, Ueno M, et al. New therapeutic modality for corneal endothelial disease using Rho-associated kinase inhibitor eye drops. Cornea. 2014;33:S25–S31. doi: 10.1097/ICO.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 6.Borderie VM, Baudrimont M, Vallée A, et al. Corneal endothelial cell apoptosis in patients with Fuchs' dystrophy. Invest Ophthalmol Vis Sci. 2000;41:2501–2505. [PubMed] [Google Scholar]
- 7.Buddi R, Lin B, Atilano SR, et al. Evidence of oxidative stress in human corneal diseases. J Histochem Cytochem. 2002;50:341–351. doi: 10.1177/002215540205000306. [DOI] [PubMed] [Google Scholar]
- 8.Engler C, Kelliher C, Spitze AR, et al. Unfolded protein response in fuchs endothelial corneal dystrophy: a unifying pathogenic pathway? Am J Ophthalmol. 2010;149:194–202. doi: 10.1016/j.ajo.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jurkunas UV, Bitar MS, Funaki T, et al. Evidence of oxidative stress in the pathogenesis of fuchs endothelial corneal dystrophy. Am J Pathol. 2010;177:2278–2289. doi: 10.2353/ajpath.2010.100279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jurkunas UV, Rawe I, Bitar MS, et al. Decreased expression of peroxiredoxins in Fuchs' endothelial dystrophy. Invest Ophthalmol Vis Sci. 2008;49:2956–2963. doi: 10.1167/iovs.07-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jun AS, Meng H, Ramanan N, et al. An alpha 2 collagen VIII transgenic knock-in mouse model of Fuchs endothelial corneal dystrophy shows early endothelial cell unfolded protein response and apoptosis. Hum Mol Genet. 2011;21:384–393. doi: 10.1093/hmg/ddr473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng H, Matthaei M, Ramanan N, et al. L450W and Q455K Col8a2 knock-in mouse models of Fuchs endothelial corneal dystrophy show distinct phenotypes and evidence for altered autophagy. Invest Ophthalmol Vis Sci. 2013;54:1887–1897. doi: 10.1167/iovs.12-11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winter J, Jung S, Keller S, et al. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 15.Sengupta S, Boon den JA, Chen I-H, et al. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc Natl Acad Sci USA. 2008;105:5874–5878. doi: 10.1073/pnas.0801130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapinas K, Kessler CB, Delany AM. miR-29 suppression of osteonectin in osteoblasts: Regulation during differentiation and by canonical Wnt signaling. J Cell Biochem. 2009;108:216–224. doi: 10.1002/jcb.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du B, Ma LM, Huang MB, et al. High glucose down-regulates miR-29a to increase collagen IV production in HK-2 cells. FEBS letters. 2010 doi: 10.1016/j.febslet.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa T, Iizuka M, Sekiya Y, et al. Suppression of type I collagen production by microRNA-29b in cultured human stellate cells. Biochem Bioph Res Co. 2010;391:316–321. doi: 10.1016/j.bbrc.2009.11.056. [DOI] [PubMed] [Google Scholar]
- 19.van Rooij E, Sutherland LB, Thatcher JE, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Hassan MQ, Jafferji M, et al. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem. 2009;284:15676–15684. doi: 10.1074/jbc.M809787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelsvold DH, Utheim TP, Olstad OK, et al. miRNA and mRNA expression profiling identifies members of the miR-200 family as potential regulators of epithelial-mesenchymal transition in pterygium. Exp Eye Res. 2013;115:189–198. doi: 10.1016/j.exer.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubo E, Hasanova N, Sasaki H, et al. Dynamic and differential regulation in the microRNA expression in the developing and mature cataractous rat lens. J Cell Mol Med. 2013;17:1146–1159. doi: 10.1111/jcmm.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luna C, Li G, Qiu J, et al. Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Mol Vis. 2009;15:2488–2497. [PMC free article] [PubMed] [Google Scholar]
- 24.Villarreal G, Oh D-J, Kang MH, et al. Coordinated regulation of extracellular matrix synthesis by the microRNA-29 family in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52:3391–3397. doi: 10.1167/iovs.10-6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luna C, Li G, Qiu J, et al. Cross-talk between miR-29 and Transforming Growth Factor-Betas in Trabecular Meshwork Cells. Invest Ophthalmol Vis Sci. 2011;52:3567. doi: 10.1167/iovs.10-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthaei M, Hu J, Kallay L, et al. Endothelial cell microRNA expression in human late-onset Fuchs' dystrophy. Invest Ophthalmol Vis Sci. 2014;55:216–225. doi: 10.1167/iovs.13-12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottsch JD, Zhang C, Sundin OH, et al. Fuchs corneal dystrophy: aberrant collagen distribution in an L450W mutant of the COL8A2 gene. Invest Ophthalmol Vis Sci. 2005;46:4504–4511. doi: 10.1167/iovs.05-0497. [DOI] [PubMed] [Google Scholar]
- 28.Okumura N, Minamiyama R, Ho LT, et al. Involvement of ZEB1 and Snail1 in excessive production of extracellular matrix in Fuchs endothelial corneal dystrophy. Lab Invest. 2015;95:1291–1304. doi: 10.1038/labinvest.2015.111. [DOI] [PubMed] [Google Scholar]
- 29.Hamasaki T, Suzuki H, Shirohzu H, et al. Efficacy of a Novel Class of RNA Interference Therapeutic Agents. Zi X, ed. PLoS ONE. 2012;7:e42655. doi: 10.1371/journal.pone.0042655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaneko H, Dridi S, Tarallo V, et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471:325–330. doi: 10.1038/nature09830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damiani D, Alexander JJ, O'Rourke JR, et al. Dicer inactivation leads to progressive functional and structural degeneration of the mouse retina. J Neurosci. 2008;28:4878–4887. doi: 10.1523/JNEUROSCI.0828-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J-F, Murchison EP, Tang R, et al. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci USA. 2008;105:2111–2116. doi: 10.1073/pnas.0710228105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biswas S, Munier FL, Yardley J, et al. Missense mutations in COL8A2, the gene encoding the alpha2 chain of type VIII collagen, cause two forms of corneal endothelial dystrophy. Hum Mol Genet. 2001;10:2415–2423. doi: 10.1093/hmg/10.21.2415. [DOI] [PubMed] [Google Scholar]
- 34.Levy SG, Moss J, Sawada H, et al. The composition of wide-spaced collagen in normal and diseased Descemet's membrane. Curr Eye Res. 1996;15:45–52. doi: 10.3109/02713689609017610. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Taylor NE, Lu L, et al. Renal medullary microRNAs in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension. 2010;55:974–982. doi: 10.1161/HYPERTENSIONAHA.109.144428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christopher AF, Kaur RP, Kaur G, et al. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspect Clin Res. 2016;7:68–74. doi: 10.4103/2229-3485.179431. [DOI] [PMC free article] [PubMed] [Google Scholar]