Abstract
Objectives
Previous studies have found that depression predicts all-cause mortality in heart failure (HF), but little is known about its effect on long-term survival. This study examined the effects of depression on long-term survival in patients with HF.
Methods
Patients hospitalized with HF (n=662) at an urban academic medical center were enrolled in a prospective cohort study between January 1994 and July 1999. Depression was assessed on a structured interview during the index hospitalization and on quarterly interviews for one year after discharge. Patients were classified at index as having DSM-IV major depressive disorder (MDD; n=131), minor depression (MinDD; n=106), or no depression (ND; n=425). Clinical data and the National Death Index were used to identify date of death or last known contact through December 19, 2014, up to 20 years after the index hospitalization. The main outcome was time from enrollment to death from any cause.
Results
A total of 617 (94.1%) patients died during the follow-up period. MDD was associated with higher all-cause mortality compared to ND (adjusted HR, 1.64; 95%CI, 1.27–2.11; p=0.0001). This association was stronger than that of any of the established predictors of mortality that were included in the fully adjusted model. Patients with persistent or worsening depressive symptoms during the year after discharge were at greatest risk for death. The association between MinDD and survival was not significant.
Conclusions
Major depression is an independent risk factor for all-cause mortality in patients with heart failure. Its effect persists for many years after the diagnosis of depression.
MeSH Keywords: Depression, Depressive Disorder, Heart Failure, Mortality, Survival Analysis
INTRODUCTION
Depression is a common comorbidity in heart failure (HF). Approximately 17% to 37% of patients who are hospitalized with HF have major depressive disorder (MDD) and 16% to 22% have minor depression (MinDD) (1, 2). MDD is especially prevalent in patients younger than 60 and those in New York Heart Association (NYHA) Class III–IV (1). Depressed patients report dysphoric mood and/or loss of interest or pleasure in usual activities along with other symptoms such as insomnia or hypersomnia, fatigue, changes in appetite, psychomotor retardation or agitation, poor concentration, low self-esteem, excessive guilt, and recurrent thoughts of death or suicide (3). Depression is associated with a variety of physiological effects in patients with HF, such as elevated inflammation markers (4), nitric oxide dysregulation (5), and increased beta-adrenergic receptor sensitivity (6). Some cases of depression in patients with HF may also be associated with atrophy in brain regions that are involved in the regulation of mood states (7).
Depression predicts all-cause mortality (8–13), cardiovascular mortality (9, 14), and the composite end point of rehospitalization or death in patients with HF (15–17). Nine studies of these relationships in a total of 4,012 patients with HF were evaluated in a recent meta-analysis by Fan et al. (18). The pooled results showed that MDD significantly increases all-cause mortality (hazard ratio [HR], 1.98; 95% CI 1.23–3.19), but mild depression does not (HR, 1.04; 95% CI 0.75–1.45). In most of these studies, the follow-up has ranged from a few months to about 6 years. Very few studies have examined the effect of depression on long-term survival in HF. In this study, patients with HF were followed for up to 20 years to examine the long-term effect of depression on mortality.
METHODS
Study Cohort
Patients age 40 years or older who were hospitalized between January 1, 1994 and July 14, 1999 at Barnes-Jewish Hospital at Washington University Medical Center in St. Louis with an admitting diagnosis of HF, dyspnea, or acute myocardial infarction (MI) were screened for eligibility. Patients younger than 40 were excluded both because prevalence of HF is very low among individuals younger than 40 years old and because major adverse cardiac events are less common among younger than middle-aged and elderly HF patients (19).
Inclusion required the permission of the attending physician, evidence of HF on chest x-ray, and documentation of at least two of the following to fulfill a clinical diagnosis of HF: 1) dyspnea, 2) third heart sound, 3) jugular venous distention, 4) hepatojugular reflux, 5) pulmonary rales, 6) peripheral edema, or 7) symptomatic or clinical improvement in response to diuretics. Patients were excluded if they 1) were too medically unstable to participate (in the judgment of their attending physician); 2) had isolated right-sided HF; 3) had HF associated with valve disease for which surgical correction was pending; 4) had a terminal illness other than HF; or 5) had a neuropsychiatric condition or language barrier that would preclude informed consent or valid assessments. Participants signed an informed consent form approved by the institutional review board at Washington University School of Medicine. Participants (n=20; 2.9% of 682 enrolled patients) who died during the index hospitalization were excluded from this analysis.
Data Collection at Index
An experienced cardiac research nurse reviewed each patient’s hospital records and documented 1) whether there had been any previous hospital admissions for HF, 2) comorbid medical conditions, 3) routine laboratory test values, and 4) medications. The patient’s NYHA classification during the past 2 weeks was determined from an interview and the medical chart review. Oversight of the medical data collection was provided by a cardiologist with expertise in HF and geriatric cardiology (MWR).
Transthoracic 2D, Doppler, and color flow echocardiography was performed within 48 hours of hospital admission if logistically feasible. The images were stored and digitized for off-line analysis. All studies were performed by experienced cardiac sonographers. The left ventricular ejection fraction (LVEF) was calculated by the method of summation of disks (modified Simpson’s rule) from the apical 4-chamber view at end-diastole and end-systole at end-expiration. All measurements were confirmed by an expert echocardiographer (VGD).
To assess depression, trained research interviewers administered a version of the National Institute of Mental Health Diagnostic Interview Schedule that had been used in previous studies of depression in patients with heart disease (20). The interview assessed the presence and duration of nine symptoms of unipolar depression (3). In addition to a depression symptom count (range, 0–9), the interview was used to determine whether the patient met the DSM-IV criteria (3) for a major or minor depressive episode during the index hospitalization. A diagnosis of major depressive episode required the presence of at least 5 of 9 symptoms during the same two-week period, and at least one of the symptoms had to be depressed mood or loss of interest or pleasure in usual activities. A diagnosis of minor depressive episode required between 2 and 4 symptoms, including depressed mood and/or loss of interest or pleasure.
The participants were contacted by telephone every three months for one year. The interview was re-administered during these calls to assess changes in the number of depression symptoms and in NYHA class. They were also asked to report any hospitalizations for any reason that had occurred during the past three months, and to sign a release of information form for the records of each hospitalization. If the participant was deceased, too ill to be interviewed, or unavailable for other reasons, a collateral informant was interviewed to identify recent hospital admissions.
Ascertainment of Deaths
Chart reviews, follow-up interviews, and contacts with collateral informants were used to document the vital status of each participant one year after enrollment and the date of death of those who died during this period. A National Death Index (NDI) search for the calendar years 2004–2011 was conducted in September 2014 for all participants who were alive at the end of the initial one-year follow-up period. A Social Security Death Index (SSDI) search was conducted in December 2014. The hospital’s electronic medical record system was also searched in December 2014 to identify the last known contact dates of patients who were not positively identified in the NDI and/or SSDI searches. The NDI, SSDI, and medical record searches were approved by the Human Research Protection Office at Washington University Medical Center. This study complies with the Declaration of Helsinki.
Statistical Analysis
Data that were plausibly missing at random were imputed with the multiple imputation procedure in SAS 9.3. One-hundred imputed datasets were generated from an imputation model that consisted of auxiliary and measured variables that were likely to be related to the missing data mechanism. In order to minimize bias, all variables that were used in the analysis models were also included in the imputation model.
Sixteen of the variables that were included in the multivariable model had missing values. Among these variables, the proportion of missing values ranged from 0.3% to 28%. The latter figure pertains to the data on the number of depressive symptoms and NYHA class at 12 months. The reason that most of these data points were missing was that the patients had died during the first year of follow-up. Among the baseline variables that were documented during the index hospitalization, the highest proportion of missing data was for LVEF (12%). This occurred because it was not always possible to obtain an echocardiogram, due primarily to logistical barriers (e.g., the patient was already scheduled for other tests or procedures).
Cox proportional hazards regression was used to model the effects of depression diagnosis during the index hospitalization on all-cause mortality. Depression symptom counts (range, 0–9) and NYHA class obtained from quarterly telephone interviews during the one-year follow-up period were included as time-dependent covariables. The model was also adjusted for factors that were documented during the index hospitalization and that have been shown to affect HF outcomes, including age, sex, race, HF etiology (ischemic or nonischemic), history of HF hospitalization, LVEF, NYHA class, diabetes, chronic obstructive pulmonary disease (COPD), atrial fibrillation or flutter, smoking history, systolic blood pressure (SBP), diastolic blood pressure (DBP), blood urea nitrogen (BUN), creatinine, hemoglobin, sodium, and current medications including antidepressants, beta blockers, calcium channel blockers, angiotensin-converting-enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), vasodilators, and aspirin (21–26). Squared terms for diastolic and systolic blood pressure, BUN, and creatinine were added along with the corresponding linear terms to the Cox model in order to improve compliance with the assumption of linearity. A nonsignificant interaction between depression and time indicated that the proportional hazards assumption was met, but another diagnostic procedure revealed disproportionately high dfbeta values for a small subgroup (n=6) of patients, indicating that their observations had undue influence on the Cox model parameter estimates. These six patients were excluded from the primary analysis (n=656), but a sensitivity analysis that included all study participants (n=662) was also performed. Based on differences in observed and expected mortality decile risk, the primary model fit the data better (LRT X2=5.7; df=9; p=.770) than the sensitivity analysis model (LRT X2=8.4; df=9; p=.49). No other significant violations of the Cox regression model were found. The Type I error rate was specified as .05. All analyses were conducted in SAS 9.3.
RESULTS
Baseline Characteristics
The enrolled sample (n=682) represented 26% of the 2,623 hospitalized patients with HF who completed study eligibility screening. Data on reasons for exclusion were presented in an earlier report (1). As shown in Table 1, the study cohort included approximately equal numbers of men and women, and approximately 40% of the participants were African-American. The mean age at enrollment was 66 years. Most were in NYHA Class II/III heart failure prior to the index admission, and approximately 70% had an LVEF below 45%. At index, 20% of the patients had major depression and 16% had minor depression.
Table 1.
Patient Characteristics at Baseline.
| Characteristic | DSM-IV Depression Diagnosis
|
||
|---|---|---|---|
| No Depression (N = 425) |
Minor Depression (N = 106) |
Major Depression (N = 131) |
|
| Sociodemographic factors | |||
| Age (in years) | 67.8 ± 11.8 | 64.5 ± 11.3* | 62.6 ± 11.7§ |
| Gender (male) ‡ | 227 (53.4) | 47 (44.3) | 46 (35.1) |
| African-American | 179 (42.1) | 42 (39.6) | 55 (42.0) |
| Married or partnered | 219 (51.5) | 53 (50.0) | 66 (50.4) |
| Education (< 12 years) | 143 (33.7) | 40 (37.7) | 53 (40.5) |
| Employed | 91 (21.4) | 21 (19.8) | 24 (18.3) |
| Prior history of major depression§ | 93 (21.9) | 42 (39.6) | 56 (42.8) |
| Family history of depression | 86 (21.1) | 25 (25.3) | 39 (31.5) |
| Medical characteristics | |||
| Ever smoked | 296 (69.7) | 70 (66.0) | 88 (67.2) |
| Body mass index (kg/m2) | 29.5 ± 8.1 | 31.9 ± 10.8* | 30.7 ± 9.3 |
| Mean # of major medical comorbidities | 2.6 ± 1.4 | 2.6 ± 1.5 | 2.7 ± 1.5 |
| NYHA class I–II prior to index admission§ | 284 (66.8) | 56 (52.8) | 45 (34.4) |
| LVEF (n=584) | |||
| Interval-scaled | 34.5 ± 17.3 | 36.1 ± 17.1 | 34.9 ± 15.6 |
| <45% | 262 (70.1) | 64 (68.1) | 84 (72.4) |
| Heart failure etiology (ischemic) | 241 (56.7) | 51 (48.1) | 69 (52.7) |
| Prior hospitalization for heart failure | 286 (67.3) | 67 (63.2) | 93 (71.0) |
| Blood Pressure (mmHg) | |||
| Systolic | 145.1 ± 34.0 | 142.7 ± 31.6 | 131.3 ± 32.2§ |
| Diastolic | 83.5 ± 19.5 | 82.0 ± 19.7 | 75.0 ± 19.1§ |
| History of cerebrovascular accident | 40 (9.5) | 8 (7.6) | 18 (13.7) |
| History of hypertension | 294 (69.7) | 75 (72.1) | 83 (63.9) |
| History of atrial fibrillation or flutter | 39 (29.3) | 15 (30.6) | 17 (29.3) |
| History of COPD† | 87 (20.6) | 24 (23.3) | 44 (33.9) |
| History of diabetes | 172 (40.5) | 51 (48.1) | 63 (48.1) |
| Medications | |||
| Antidepressant§ | 27 (6.4) | 17 (16.0) | 40 (30.5) |
| Aspirin | 253 (59.5) | 62 (58.5) | 67 (51.2) |
| Beta blocker | 85 (20.0) | 19 (17.9) | 16 (12.2) |
| ACE-I or ARB | 310 (72.9) | 79 (74.5) | 94 (71.8) |
| Calcium-channel blocker | 138 (32.5) | 43 (40.6) | 40 (30.5) |
| Vasodilator | 238 (56.0) | 61 (57.6) | 77 (58.8) |
| Laboratory values | |||
| Creatinine | 1.7 ± 1.3 | 1.9 ± 1.6 | 1.6 ± 1.3 |
| Hemoglobin | 12.0 ± 2.0 | 11.8 ± 1.9 | 11.5 ± 2.1* |
| Blood urea nitrogen | 27.6 ± 18.6 | 27.7 ± 16.7 | 25.8 ± 18.0 |
Values are mean ± SD or n (%). Tukey-adjusted p values are reported for comparisons of depressed vs. nondepressed groups on continuous variables. Omnibus p values are reported for chi-square tests differences of categorical variables among groups.
p < 0.050,
p < 0.010,
p < 0.001,
p < 0.0001.
ACE-I = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; COPD = chronic obstructive pulmonary disease; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association.
Mortality
A total of 617 (94.1%) of the patients died during the follow-up period. One hundred twenty-two (19.8%) of these deaths occurred during the first year of follow-up. Of the 45 patients who were not known to be deceased as of December 2014, 43 (96%) were found in the electronic medical record to have received clinical care at Washington University Medical Center after the one year follow-up phase ended. In 33 (77%) of these cases, the most recent clinical encounter had occurred within the past 2 years. The survival data for these observations were censored at the date of the last contact documented in the medical record. The searches produced no new information about 2 patients (0.3% of the entire cohort) who were known to be alive at the follow-up assessment one year after enrollment, so their survival data were censored at one year.
The median survival time was 3.8 years in the cohort as a whole, but the distribution of event times differed significantly by DSM-IV depression diagnosis (log-rank X2=9.0; df=2; p=0.010). The 25th, 50th, and 75th percentile survival times were considerably shorter in the MDD group (1.3, 3.2, 5.9 years) than in the MinDD (1.8, 3.9, 7.8 years) and ND (1.5, 4.1, 8.8 years) groups.
Long-term mortality was higher in patients with major depression at index compared to patients who were not depressed (adjusted HR, 1.64; 95% CI, 1.27 – 2.11; p=0.0001). Mortality did not differ between patients with minor depression at index and patients who were not depressed (adjusted HR, 1.23; 95% CI, 0.98 – 1.55; p=0.076).
Depression symptoms during the follow-up period did not independently predict mortality after accounting for depressive disorders at index. However, a post hoc examination of the subset of patients who survived at least 3 months (n=509) showed that those who had chronic or worsening depression were at higher risk than those whose depression improved (Table 3).
Table 3.
Effects of Chronic or Worsening Depression Over the First 3 Months After the Index Hospitalization on Subsequent Survival Time.
| DSM-IV Diagnosis at Baseline | <5 Depression Symptoms at 3 Months | ≥5 Depression Symptoms at 3 Months | ||
|---|---|---|---|---|
|
| ||||
| N | Median Survival Time (Years) | N | Median Survival Time (Years) | |
| Not depressed | 347 | 4.5 | 19 | 3.6 |
| Minor depression | 78 | 3.7 | 16 | 2.2 |
| Major depression | 63 | 3.5 | 46 | 2.9 |
Other significant predictors of reduced survival included older age, ischemic etiology, prior hospitalization for HF, LVEF, NYHA class, COPD, and high BUN. The same factors also predicted reduced survival in the sensitivity analysis of the entire cohort (n=662). The primary Cox model is presented in Table 2, and adjusted Kaplan-Meier curves are displayed in Figure 1.
Table 2.
Cox Proportional Hazards Regression Model of the Effects of Depression on Long-Term Survival.
| Parameter | HR (95% C.I.) | p |
|---|---|---|
| Major depression at index | 1.64 (1.27 – 2.11) | 0.0001 |
| Minor depression at index | 1.23 (0.98 – 1.55) | 0.080 |
| Depression symptom count during follow-up | 1.02 (0.98 – 1.07) | 0.290 |
| New York Heart Association class* | 1.12 (1.01 – 1.24) | 0.029 |
| Age (per 10 years) | 1.29 (1.19 – 1.41) | 0.0001 |
| Male | 1.21 (0.99 – 1.47) | 0.056 |
| African-American | 1.16 (0.95 – 1.42) | 0.148 |
| Ischemic heart failure | 1.38 (1.15 – 1.66) | 0.0006 |
| Prior hospitalization for heart failure | 1.34 (1.11 – 1.61) | 0.002 |
| Left ventricular ejection fraction, % | 0.99 (0.99 – 1.00) | 0.034 |
| Diabetes | 1.09 (0.91 – 1.30) | 0.367 |
| Chronic obstructive pulmonary disease | 1.29 (1.06 – 1.57) | 0.013 |
| Atrial fibrillation or flutter | 1.16 (0.96 – 1.41) | 0.133 |
| Ever smoked | 1.03 (0.85 – 1.24) | 0.796 |
| Systolic blood pressure | 0.99 (0.97 – 1.01) | 0.304 |
| Diastolic blood pressure | 0.98 (0.95 – 1.00) | 0.075 |
| Blood urea nitrogen | 1.04 (1.02 – 1.05) | 0.0001 |
| Creatinine | 1.06 (0.97 – 1.15) | 0.214 |
| Hemoglobin | 1.00 (0.71 – 1.40) | 0.997 |
| Antidepressant | 1.29 (0.98 – 1.69) | 0.069 |
| Beta blocker | 0.96 (0.77 – 1.20) | 0.725 |
| Calcium channel blocker | 0.85 (0.70 – 1.02) | 0.084 |
| ACE-I or ARB | 1.07 (0.87 – 1.30) | 0.522 |
| Vasodilator | 1.08 (0.90 – 1.29) | 0.402 |
| Aspirin | 0.85 (0.72 – 1.01) | 0.070 |
ACI-I = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; CI = confidence interval; HR = hazard ratio.
NYHA class is represented as a time-dependent covariable, including assessments at baseline and at each follow-up.
Figure 1. Covariate-adjusted Kaplan-Meier curves of patients with major, minor, or no depressive disorder during the index hospitalization.
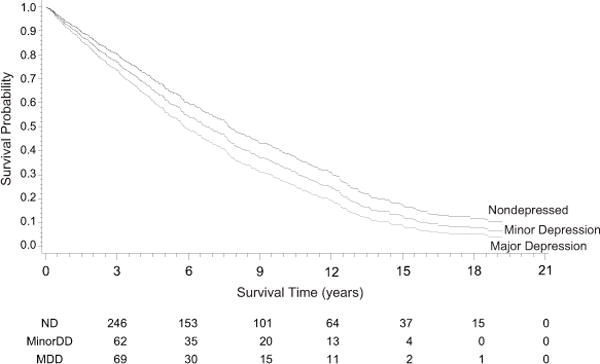
ND = not depressed; MinorDD = Minor Depressive Disorder; MDD = Major Depressive Disorder. The numbers of survivors within each diagnostic subgroup are listed by quarter below the graph.
In a series of post hoc analyses, interaction terms were added to the primary model to determine whether age, sex, race, or categorical LVEF moderate the effects of major or minor depression. Patients with an LVEF ≥45% were classified as having heart failure with preserved ejection fraction (HFpEF), and those with an LVEF <45% were classified as having heart failure with reduced ejection fraction (HFrEF). There were no significant interactions with age (MDD × age: HR, 1.00; 95% CI, 0.98 – 1.02; p=0.90; MinDD × age: HR, 0.98; 95% CI, 0.96 – 1.00; p=0.099), sex (MDD × sex: HR, 1.32; 95% CI, 0.85 – 2.06; p=0.21; MinDD × sex: HR, 0.96; 95% CI, 0.60 – 1.53; p=0.86), or LVEF category (MDD × LVEF: HR, 1.31; 95% CI, 0.80 – 2.140; MinDD × LVEF: HR, 1.08; 95% CI, 0.64 – 1.82; p=0.77). There was also no significant interaction between major depression and race (HR, 0.71; 95% CI, 0.46 – 1.09; p=0.11), but there was a significant interaction between minor depression and race (HR, 1.74; 95% CI, 1.08 – 2.79; p=0.023). Among African-Americans, the effect of MDD was not significant (HR, 1.31; 95% CI, 0.92 – 1.86; p=0.14) but the effect of MinDD was significant (HR, 1.76; 95% CI, 1.22 – 2.55; p=0.003). In contrast, among whites, the effect of MDD was significant (HR, 1.91; 95% CI, 1.40 – 2.60; p<0.0001) but the effect of MinDD was not significant (HR, 1.01; 95% CI, 0.75 – 1.36; p=0.95). These findings suggest that major depression affects survival in white but not in African-American patients, and that minor depression affects survival in African-American but not in white patients.
DISCUSSION
This study shows that major depression in patients hospitalized with HF is associated with a long-term increased risk of mortality. Although the patients with major depression tended to be more severely ill than the other patients, the adverse effect of major depression on survival remained after adjusting for established risk markers. Other significant predictors included ischemic etiology of HF, prior hospitalization for HF, LVEF, NYHA class, COPD, older age, and elevated BUN. As in other studies (27, 28), depression was more common in female than male patients, but the risk of mortality was slightly higher in men.
With a 20-year follow-up period, this is longest prospective study of depression and survival in HF conducted to date. Also, among the existing long-term follow-up studies, it is the only one to have examined the prognostic importance of major and minor depressive disorders. The two longest follow-up studies prior to this one were a Spanish study by Diez-Quevedo et al. (29) and an American study by Adams et al. (30) Diez-Quevedo et al. administered the Geriatric Depression Scale to 1,017 HF outpatients and followed them for up to 9 years. Depression was associated with increased all-cause mortality (adjusted HR, 1.39; 95% CI, 1.15–1.68). Adams et al. followed a cohort of 985 HF patients for up to 12 years. Depression during the index hospitalization, as indicated by a score higher than 10 on the Beck Depression Inventory, was associated with decreased survival (adjusted HR, 1.40; 95% CI, 1.16–1.68).
Comparison of the results of the present study to those of the Diez-Quevedo et al. and Adams et al. suggests that major depression, assessed by structured interview and diagnosed according to the DSM-IV criteria, has somewhat stronger effects on long-term mortality in HF than depression as assessed by self-report questionnaires. This study also shows that the effect of major depression on mortality persists for many years after hospitalization for HF. The Kaplan-Meier curves show that the survival times of patients with major depression start to diverge from those of nondepressed patients during the first year after hospitalization and that they continue to diverge over 20 years. Relatively few patients survived longer than 15 years, but among those who were still alive, the nondepressed patients continued to have a survival advantage over those who had been diagnosed with major depression at index.
Minor depression did not have a statistically significant effect on survival in the study population as a whole, although it did affect survival in African Americans. In addition, Figure 1 shows that through the entire follow-up period, the survival curve of patients with minor depression lies between those of the groups with no depression and major depression. Minor depression often progresses to major depression (31). It is possible that those who did progress to major depression had reduced survival compared to those who had persistent minor depression or whose minor depression remitted. Further research is needed to clarify the long-term prognostic implications of minor depression.
In a recent meta-analysis, Fan et al. (18) reported a pooled hazard ratio of 1.98 (95% CI, 1.23–3.19) for the effect of major depression on all-cause mortality in patients with HF. This is higher than the effect size that we found (adjusted HR, 1.68; 95% CI, 1.31–2.16) and suggests that major depression may have a stronger effect on short-term than long-term morality risk. Our finding that minor depressive disorder did not have a significant effect on all-cause mortality in the overall study population is consistent with the Fan et al. findings concerning mild depression.
Although 83 (13%) of the patients were taking an antidepressant at enrollment, antidepressant use did not explain the difference in the survival rates of the depressed and nondepressed patients. Previous studies of the effects of antidepressant use on survival in HF have yielded inconsistent results. Antidepressant use was associated with increased all-cause and cardiovascular mortality in a large (n=99,335) database study of patients who survived their first hospitalization for HF (32). However, antidepressant use was a proxy for depression in that study; independent data on the diagnosis or severity of depressive disorders were not available. In a study of outpatients with HF (n=1,017), patients who were prescribed fluoxetine were at higher risk for mortality, but other antidepressants were not associated with mortality risk (29). A smaller study (n=209) of outpatients with HF found no association between antidepressant use and mortality (15). Finally, the SADHART-CHF trial (n=469) was not designed to evaluate mortality, but it found no significant post-treatment difference between the sertraline and placebo arms in a composite cardiovascular status index (33). Based on these and other prognostic studies, the evidence that depression increases the risk of mortality in HF is stronger and more consistent than the evidence that antidepressants do so.
Although mechanistic data were not collected for this study, previous studies have identified a variety of biobehavioral candidates that may help to explain the elevated mortality risk associated with depression in patients with heart failure. Possible biological mechanisms include elevated neurohormones, cardiovascular autonomic dysregulation, and inflammatory processes, and behavioral candidates include medication and dietary nonadherence, physical inactivity, and smoking.(34)
Major depression is a distressing and functionally disabling disorder that warrants clinical attention, and the results of this study contribute to a growing body of evidence that comorbid major depression in heart failure increases the risk of mortality. However, there have not been trials of treatments for depression in HF that have been large enough to target mortality as the primary endpoint, and two of the largest antidepressant trials in patients with HF have not even shown evidence of efficacy for depression (33, 35, 36). Nevertheless, antidepressants should be considered for patients with HF and comorbid MDD. In addition, the results of a recent trial showed that cognitive behavior therapy may be efficacious for these patients (37).
Limitations
This study has several limitations. First, the participants were enrolled in the mid- to late-1990s, and data on depression were collected only during the index hospitalization and quarterly for the first year thereafter. No other time-dependent data were available on depression, HF care, or any of the covariables that were included in the fully adjusted model. Consequently, any changes after one year in patient status that might have affected the long-term risk of mortality were omitted from the analysis. Because of subsequent advances in heart failure care and changes in health care policies and practices, depression may have weaker or stronger effects on rehospitalization rates and other HF outcomes in contemporary cohorts than in the study population. Second, only 26% of the patients who were screened for the study met all of the eligibility criteria and agreed to participate. In addition, 20 patients who died during the index hospitalization were excluded from the present analysis. Thus, the sample was not completely representative of the entire population of hospitalized patients with heart failure. Third, data on prognostic biomarkers (38) such as BNP (39) were not available. Inclusion of these biomarkers in the Cox model might have altered the predictive value of depression. Similarly, we lacked data on other psychological predictors of HF outcomes, such as anxiety (40). Inclusion of these factors in the model might also have altered the effect of depression. Fourth, patients under 40 years of age at index were excluded. Consequently, the results of the study are not generalizable to younger adults with heart failure. Finally, the primary outcome was all-cause mortality rather than cardiovascular-specific mortality, so it was not possible to determine whether depression increases the risk of any specific cause of death in patients with heart failure.
Conclusions
This study shows that major depression, diagnosed at index hospitalization, is associated with an increased risk of death for up to 20 years after hospitalization for heart failure. In fact, major depression has a stronger effect on long-term mortality than any of the established predictors that were included in the complete model. Those with chronic or worsening depression are at particularly high risk. Prospective studies are needed of the long-term effects of depression in contemporary heart failure patient cohorts.
Acknowledgments
Funding/Support: This study was funded by grant R01MH051419 from the National Institute of Mental Health (Kenneth E. Freedland, PhD, Principal Investigator). Additional funding for the National Death Index search was provided to Dr. Hesseler by an internal research grant from Washington University School of Medicine.
Abbreviations and Acronyms
- ACE-I
angiotensin converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- BUN
blood urea nitrogen
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- DBP
diastolic blood pressure
- DSM-IV
Diagnostic and Statistical Manual, 4th edition
- HF
heart failure
- HR
hazard ratio
- LVEF
left ventricular ejection fraction
- MDD
major depressive disorder
- MinDD
minor depressive disorder
- MI
myocardial infarction
- ND
not depressed
- NDI
National Death Index
- NYHA
New York Heart Association
- SBP
systolic blood pressure
Footnotes
Author Contributions: As the Principal Investigator, Dr. Freedland had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Carney, Skala, Dávila-Román, and Rich contributed to the development of the study protocol, the collection of data, and the interpretation of results. Dr. Hesseler contributed to the design of the follow-up and the collection of follow-up data. Dr. Steinmeyer was the study statistician and conducted all of the analyses. All co-authors contributed to the writing and revision of the manuscript.
Conflict of Interest Disclosures: None.
References
- 1.Freedland KE, Rich MW, Skala JA, Carney RM, Davila-Roman VG, Jaffe AS. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom Med. 2003;65(1):119–28. doi: 10.1097/01.psy.0000038938.67401.85. [DOI] [PubMed] [Google Scholar]
- 2.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527–37. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association, American Psychiatric Association, Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders (DSM-IV-TR) 4th. Washington, DC: American Psychiatric Association; 2000. text revision ed. [Google Scholar]
- 4.Xiong GL, Prybol K, Boyle SH, Hall R, Streilein RD, Steffens DC, Krishnan R, Rogers JG, O’Connor CM, Jiang W. Inflammation Markers and Major Depressive Disorder in Patients With Chronic Heart Failure: Results From the Sertraline Against Depression and Heart Disease in Chronic Heart Failure Study. Psychosom Med. 2015;77(7):808–15. doi: 10.1097/PSY.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mommersteeg PM, Schoemaker RG, Eisel UL, Garrelds IM, Schalkwijk CG, Kop WJ. Nitric oxide dysregulation in patients with heart failure: the association of depressive symptoms with L-arginine, asymmetric dimethylarginine, symmetric dimethylarginine, and isoprostane. Psychosom Med. 2015;77(3):292–302. doi: 10.1097/PSY.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 6.Redwine LS, Hong S, Rutledge T, Wentworth B, Pung M, Ziegler MG, Maisel A, Greenberg B, Mills PJ. Leukocyte ss-adrenergic receptor sensitivity and depression severity in patients with heart failure. Psychosom Med. 2014;76(9):726–31. doi: 10.1097/PSY.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almeida OP, Garrido GJ, Etherton-Beer C, Lautenschlager NT, Arnolda L, Alfonso H, Flicker L. Brain and mood changes over 2 years in healthy controls and adults with heart failure and ischaemic heart disease. Eur J Heart Fail. 2013;15(8):850–8. doi: 10.1093/eurjhf/hft029. [DOI] [PubMed] [Google Scholar]
- 8.Albert NM, Fonarow GC, Abraham WT, Gheorghiade M, Greenberg BH, Nunez E, O’Connor CM, Stough WG, Yancy CW, Young JB. Depression and clinical outcomes in heart failure: an OPTIMIZE-HF analysis. Am J Med. 2009;122(4):366–73. doi: 10.1016/j.amjmed.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 9.Frasure-Smith N, Lesperance F, Habra M, Talajic M, Khairy P, Dorian P, Roy D. Elevated depression symptoms predict long-term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation. 2009;120(2):134–40, 3p. doi: 10.1161/CIRCULATIONAHA.109.851675. [DOI] [PubMed] [Google Scholar]
- 10.Jiang W, Kuchibhatla M, Cuffe MS, Christopher EJ, Alexander JD, Clary GL, Blazing MA, Gaulden LH, Califf RM, Krishnan RR, O’Connor CM. Prognostic value of anxiety and depression in patients with chronic heart failure. Circulation. 2004;110(22):3452–6. doi: 10.1161/01.CIR.0000148138.25157.F9. [DOI] [PubMed] [Google Scholar]
- 11.Jiang W, Kuchibhatla M, Clary GL, Cuffe MS, Christopher EJ, Alexander JD, Califf RM, Krishnan RR, O’Connor CM. Relationship between depressive symptoms and long-term mortality in patients with heart failure. Am Heart J. 2007;154(1):102–8. doi: 10.1016/j.ahj.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor CM, Jiang W, Kuchibhatla M, Mehta RH, Clary GL, Cuffe MS, Christopher EJ, Alexander JD, Califf RM, Krishnan RR. Antidepressant use, depression, and survival in patients with heart failure. Arch Intern Med. 2008;168(20):2232–7. doi: 10.1001/archinte.168.20.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang B, Moser DK, Pelter MM, Nesbitt TS, Dracup K. Changes in Depressive Symptoms and Mortality in Patients With Heart Failure: Effects of Cognitive-Affective and Somatic Symptoms. Psychosom Med. 2015;77(7):798–807. doi: 10.1097/PSY.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson P, Dahlstrom U, Alehagen U. Depressive symptoms and six-year cardiovascular mortality in elderly patients with and without heart failure. Scand Cardiovasc J. 2007;41(5):299–307. doi: 10.1080/14017430701534829. [DOI] [PubMed] [Google Scholar]
- 15.Chung ML, Dekker RL, Lennie TA, Moser DK. Antidepressants do not improve event-free survival in patients with heart failure when depressive symptoms remain. Heart Lung. 2013;42(2):85–91. doi: 10.1016/j.hrtlng.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rumsfeld JS, Jones PG, Whooley MA, Sullivan MD, Pitt B, Weintraub WS, Spertus JA. Depression predicts mortality and hospitalization in patients with myocardial infarction complicated by heart failure. Am Heart J. 2005;150(5):961–7. doi: 10.1016/j.ahj.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 17.Sherwood A, Blumenthal JA, Trivedi R, Johnson KS, O’Connor CM, Adams KF, Jr, Dupree CS, Waugh RA, Bensimhon DR, Gaulden L, Christenson RH, Koch GG, Hinderliter AL. Relationship of depression to death or hospitalization in patients with heart failure. Arch Intern Med. 2007;167(4):367–73. doi: 10.1001/archinte.167.4.367. [DOI] [PubMed] [Google Scholar]
- 18.Fan H, Yu W, Zhang Q, Cao H, Li J, Wang J, Shao Y, Hu X. Depression after heart failure and risk of cardiovascular and all-cause mortality: a meta-analysis. Prev Med. 2014;63:36–42. doi: 10.1016/j.ypmed.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Morbidity & Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Diseases. Bethesda, MD: National Heart, Lung, and Blood Institute; 2012. [Google Scholar]
- 20.Carney RM, Rich MW, Tevelde A, Saini J, Clark K, Jaffe AS. Major depressive disorder in coronary artery disease. Am J Cardiol. 1987;60(16):1273–5. doi: 10.1016/0002-9149(87)90607-2. [DOI] [PubMed] [Google Scholar]
- 21.Cowburn PJ, Cleland JG, Coats AJ, Komajda M. Risk stratification in chronic heart failure. Eur Heart J. 1998;19(5):696–710. doi: 10.1053/euhj.1997.0820. [DOI] [PubMed] [Google Scholar]
- 22.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290(19):2581–7. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 23.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113(11):1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 24.O’Connor CM, Abraham WT, Albert NM, Clare R, Gattis SW, Gheorghiade M, Greenberg BH, Yancy CW, Young JB, Fonarow GC. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Am Heart J. 2008;156(4):662–73. doi: 10.1016/j.ahj.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor CM, Whellan DJ, Wojdyla D, Leifer E, Clare RM, Ellis SJ, Fine LJ, Fleg JL, Zannad F, Keteyian SJ, Kitzman DW, Kraus WE, Rendall D, Pina IL, Cooper LS, Fiuzat M, Lee KL. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF-ACTION predictive risk score model. Circ Heart Fail. 2012;5(1):63–71. doi: 10.1161/CIRCHEARTFAILURE.111.963462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, Ostergren J, Michelson EL, Pieper KS, Granger CB. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27(1):65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 27.Lesman-Leegte I, Jaarsma T, Sanderman R, Hillege HL, van Veldhuisen DJ. Determinants of depressive symptoms in hospitalised men and women with heart failure. Eur J Cardiovasc Nurs. 2008;7(2):121–6. doi: 10.1016/j.ejcnurse.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Faller H, Stork S, Schowalter M, Steinbuchel T, Wollner V, Ertl G, Angermann CE. Depression and survival in chronic heart failure: does gender play a role? Eur J Heart Fail. 2007;9(10):1018–23. doi: 10.1016/j.ejheart.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Diez-Quevedo C, Lupon J, Gonzalez B, Urrutia A, Cano L, Cabanes R, Altimir S, Coll R, Pascual T, de Antonio M, Bayes-Genis A. Depression, antidepressants, and long-term mortality in heart failure. Int J Cardiol. 2013;167(4):1217–25. doi: 10.1016/j.ijcard.2012.03.143. [DOI] [PubMed] [Google Scholar]
- 30.Adams J, Kuchibhatla M, Christopher EJ, Alexander JD, Clary GL, Cuffe MS, Califf RM, Krishnan RR, O’Connor CM, Jiang W. Association of depression and survival in patients with chronic heart failure over 12 Years. Psychosomatics. 2012;53(4):339–46. doi: 10.1016/j.psym.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuijpers P, Smit F. Subthreshold depression as a risk indicator for major depressive disorder: a systematic review of prospective studies. Acta Psychiatr Scand. 2004;109(5):325–31. doi: 10.1111/j.1600-0447.2004.00301.x. [DOI] [PubMed] [Google Scholar]
- 32.Fosbol EL, Gislason GH, Poulsen HE, Hansen ML, Folke F, Schramm TK, Olesen JB, Bretler DM, Abildstrom SZ, Sorensen R, Hvelplund A, Kober L, Torp-Pedersen C. Prognosis in heart failure and the value of {beta}-blockers are altered by the use of antidepressants and depend on the type of antidepressants used. Circ Heart Fail. 2009;2(6):582–90. doi: 10.1161/CIRCHEARTFAILURE.109.851246. [DOI] [PubMed] [Google Scholar]
- 33.O’Connor CM, Jiang W, Kuchibhatla M, Silva SG, Cuffe MS, Callwood DD, Zakhary B, Stough WG, Arias RM, Rivelli SK, Krishnan R. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692–9. doi: 10.1016/j.jacc.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kop WJ, Synowski SJ, Gottlieb SS. Depression in heart failure: biobehavioral mechanisms. Heart Fail Clin. 2011;7(1):23–38. doi: 10.1016/j.hfc.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Angermann CE, Gelbrich G, Stork S, Fallgatter A, Deckert J, Faller H, Ertl G. Rationale and design of a randomised, controlled, multicenter trial investigating the effects of selective serotonin re-uptake inhibition on morbidity, mortality and mood in depressed heart failure patients (MOOD-HF) Eur J Heart Fail. 2007;9(12):1212–22. doi: 10.1016/j.ejheart.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Pellicori P, Clark AL. Clinical trials update from the European Society of Cardiology-Heart Failure meeting 2015: AUGMENT-HF, TITRATION, STOP-HF, HARMONIZE, LION HEART, MOOD-HF, and renin-angiotensin inhibitors in patients with heart and renal failure. Eur J Heart Fail. 2015;17(9):979–83. doi: 10.1002/ejhf.340. [DOI] [PubMed] [Google Scholar]
- 37.Freedland KE, Carney RM, Rich MW, Steinmeyer BC, Rubin EH. Cognitive Behavior Therapy for Depression and Self-Care in Heart Failure Patients: A Randomized Clinical Trial. JAMA Intern Med. 2015;175(11):1773–82. doi: 10.1001/jamainternmed.2015.5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaggin HK, Januzzi JL., Jr Biomarkers and diagnostics in heart failure. Biochim Biophys Acta. 2013;1832(12):2442–50. doi: 10.1016/j.bbadis.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 39.Kociol RD, Horton JR, Fonarow GC, Reyes EM, Shaw LK, O’Connor CM, Felker GM, Hernandez AF. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail. 2011;4(5):628–36. doi: 10.1161/CIRCHEARTFAILURE.111.962290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garfield LD, Scherrer JF, Hauptman PJ, Freedland KE, Chrusciel T, Balasubramanian S, Carney RM, Newcomer JW, Owen R, Bucholz KK, Lustman PJ. Association of anxiety disorders and depression with incident heart failure. Psychosom Med. 2014;76(2):128–36. doi: 10.1097/PSY.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]


