Abstract
Objective
Limited data exist on child abuse-related immune variation during pregnancy, despite implications for maternal and infant health and extensive data showing that abuse history and depression are related to increased inflammation in other populations. This study examined associations among child abuse, depression, circulating levels of inflammatory markers, and perinatal health in pregnant adolescents, a group at high risk for childhood abuse and poor birth outcomes.
Methods
Pregnant teenagers (n=133; 14–19 years; 89.5% Latina) reported on abuse and depression and had two blood draws (24–27 and 34–37 gestational weeks, 2nd and 3rd trimesters, respectively) for Interleukin 6 (IL-6) and C-Reactive Protein (CRP); birth outcomes were collected.
Results
Abuse and depression interacted to predict higher IL-6 at 2nd trimester (B=.006, p=0.011) such that severely abused adolescents with high depression had higher IL-6 relative to severely abused adolescents with low depression; depression did not differentiate IL-6 levels for those with low abuse severity. Abuse and IL-6 also interacted to predict gestational age at birth (B=.004, p=0.040) such that those with low abuse and high IL-6 and those with high abuse and low IL-6 had infants with earlier gestational age at birth. Cortisol at the 2nd trimester mediated the association between IL-6 and gestational age at birth (Indirect effect estimate=−0.143, p<0.039).
Conclusion
Depression severity distinguished IL-6 levels among more severely abused pregnant Latina adolescents, but it was unrelated to IL-6 among less severely abused adolescents. Cortisol explained the relationship between IL-6 and earlier gestational age at birth. Multiple adversities and inflammation may influence birth outcomes and potentially affect intergenerational health.
Keywords: Interleukin-6, Cortisol, Pregnancy, Adolescents, Abuse, Depression
Introduction
Although the teen pregnancy rate is declining in the United States, it remains one of the highest among developed countries [1], and hence, presents a significant public health issue. Pregnant adolescents have elevated rates of childhood abuse [2, 3], abuse-related depression [4], and poor birth outcomes (e.g., younger gestational age at birth, low birth weight, and neonatal and infant mortality) [5–7]. More specifically, pregnant adolescents with histories of childhood abuse have higher levels of depression during pregnancy and give birth to smaller, less mature infants [8]. However, biological mechanisms that may explain the associations among abuse, depression, and poor birth outcomes are not well understood.
Inflammation has been identified as an important potential explanatory variable in the pathway from abuse to depression and poor health. Indeed, systemic inflammation, evidenced by elevated levels of Interleukin-6 (IL-6), C-Reactive Protein (CRP), and tumor necrosis factor-alpha (TNF-α), has been observed among adults [9–11] and adolescents [12–15] exposed to early adversity. Depression, a common sequela of childhood abuse [16–18], also has been associated with higher levels of cytokines such as IL-6 [19], and the association between depression and inflammation has been observed consistently among those exposed to childhood adversity [14, 20]. Furthermore, abuse and depression each have been related to glucocorticoid resistance [21], which may contribute to excessive inflammation [22]. Despite the potential influence of abuse and depression on prenatal immune activity [23], however, only one study has considered the impact of trauma history and psychiatric symptoms in relation to circulating levels of inflammatory markers during pregnancy [23]. Specifically, lifetime trauma exposure, but not current mood symptoms, was associated with higher levels of TNF-α, but not IL-6, in a sample of pregnant adults [23]. Similarly, higher levels of CRP (an acute phase reactant that is produced in response to IL-6 and other pro-inflammatory signals) have been associated with increased risk for preeclampsia and low birth weight [24] as well as preterm birth [25]. These associations have yet to be studied among pregnant adolescents, a population with heightened risk for childhood abuse and perinatal depression.
Healthy immune function is critical during pregnancy. The immune system strives to balance the pro-inflammatory responses needed to protect mothers from infectious disease with the immunosuppression necessary to prevent fetal rejection [26–28]. The cytokine IL-6, which has both pro- and anti-inflammatory properties, has multiple functions in fertility and pregnancy maintenance, including implantation, trophoblast invasion, and regulation of endometrial function [29]. Consequently, dysregulation of IL-6, up or down, can jeopardize a pregnancy [29–31]. Indeed, studies have shown that higher levels of IL-6 in peripheral blood are associated with increased risks for miscarriage [32], preterm birth [25], and gestational diabetes mellitus [33], as well as preeclampsia and low birth weight [24]. Additionally, IL-6 in local tissue has been associated with recurrent miscarriage [30, 31] and preterm birth [34]. However, this link between immune dysregulation and poor birth outcomes has not been studied in pregnant adolescents.
Pro-inflammatory cytokines also have been shown to activate the Hypothalamic Pituitary Adrenal (HPA) axis and contribute to heightened cortisol secretion [35], particularly in response to stressors. Heightened levels of cortisol have been linked with increased risk for poor birth outcomes, including pre-term birth [36]. Extended activation of glucocorticoid receptors has been shown to attenuate the production of pro-inflammatory cytokines. Among those exposed to chronic stress, however, the development of glucocorticoid resistance can lead to cytokine dysregulation, whereby the negative association between cortisol and immune activation is no longer observed [37]. To date, this phenomenon has yet to be studied in pregnant adolescents and it is unclear whether associations among trauma, neuroendocrine function, and inflammation vary by trimester.
The current study examined how child abuse severity and depression are related to circulating levels of immune markers (as assessed through IL-6 and CRP), cortisol, and birth outcomes in adolescent pregnancy. This study was unique for several reasons. First, it focused on associations among abuse severity, depression, and circulating levels of immune markers in pregnant Latina adolescents, an understudied population. Second, the study offered opportunities to examine associations between neuroendocrine function and circulating levels of immune markers separately by trimester. We hypothesized that: 1) adolescents’ abuse severity and depressive symptoms would be positively and independently associated with IL-6 and CRP; 2) abuse severity would interact with depression severity to predict higher levels of IL-6 and CRP compared to low abuse and depression severity; and 3) immune dysregulation would interact with abuse and depression to predict pregnancy complications (i.e. hypertension/preeclampsia, vascular complications, gestational diabetes mellitus), younger gestational age at birth, and lower birth weight. Because abuse, depression, and circulating levels of immune markers have been associated with increased glucocorticoid resistance in prior studies [21, 22], we also conducted post hoc analyses to examine whether cortisol mediates associations among abuse severity, depression, associated immune markers, and poor birth outcomes.
Methods
Study Design
Pregnant adolescents were recruited through the Departments of Obstetrics and Gynecology at Columbia University Medical Center (CUMC) and Weill Medical College of Cornell University between July 2009 and August 2011. Recruitment flyers also were posted in the CUMC neighborhood. This study included a subset (n=133) of a larger sample of 205 nulliparous pregnant adolescents, between 14 and 19 years of age. See Figure 1 for sample determination. Psychosocial and salivary cortisol assessments took place at 13–16, 24–27, and 34–37 gestational weeks (+/−1 week). Blood was collected at the latter two assessments. Birth outcome and pregnancy complication information was obtained from participants’ medical charts. Study procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute/CUMC and all participants under 18 provided assent with parental written informed consent, while those 18 and older provided written informed consent.
Figure 1.
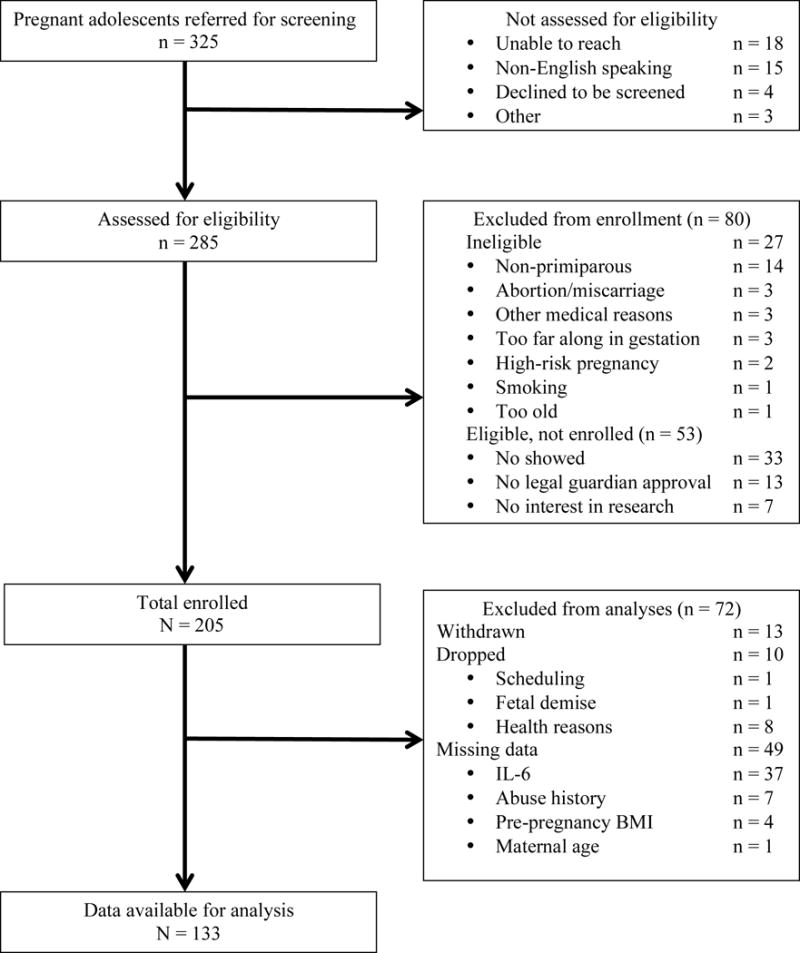
Flow chart showing participant enrollment and sample size determination.
Participants
All adolescents had a healthy pregnancy at the time of recruitment. Exclusion criteria included a lack of fluency in English, multiparity, or frequent use of any of the following: nitrates, steroids, beta blockers, triptans, and psychiatric medications. Participants also were excluded for cigarette smoking or use of recreational drugs as assessed through self-report and one random urine toxicology screen for the use of cannabinoids, amphetamines, benzodiazepines, opioids, and cotinine. The sample was predominantly Latina (89.5%). Participants included in our study did not differ from those who were excluded with respect to age (Wilcoxon test, p = 0.30) and ethnicity (Chi-square test, p = 0.079). Among participants with complete data for abuse history and depression symptoms, abuse history and depression symptoms did not differ between participants with and without IL-6 samples (Wilcoxon test, n.s.).
Measures
Demographic data were self-reported. Pre-pregnancy body mass index (BMI; kg/m2) was calculated using self-reported pre-pregnancy weight and height from medical records. Pregnancy weight gain was collected at each trimester, and complications, including infection, were coded from medical charts. Birth weight (corrected for gestational age [38]) was determined from the medical record. Gestational age at birth was determined through the medical record reporting of dating based on ultrasound examinations and last reported menstrual cycle.
Abuse was assessed through the Childhood Trauma Questionnaire (CTQ) [39], a widely used self–report measure [40] composed of 28 items that assesses lifetime exposure to five types of childhood maltreatment: emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect. Respondents indicate whether they experienced each type of childhood maltreatment based on a series of statements that are scored on a Likert-type scale from 1 (never true) to 5 (very often true). Response scores are summed to reflect the severity of each type of abuse or neglect. Following [41], we used a summary score reflecting the severity of emotional, physical, and sexual abuse.
Depressive symptoms were assessed using the depression subscale of the Symptom Checklist-90 (SCL-90-R), [42] which consists of 9 Likert-type items about dysphoric mood and affect, anhedonia, hopelessness, suicidal ideation, and other cognitive and somatic symptoms of depression. Items are rated from 0 (not at all) to 4 (extremely). The SCL-90 has good psychometric properties and acceptable concurrent validity with interview-based measures of depression [43]. The SCL-90 has been normed in an adolescent sample; therefore, in the current study, the adolescent-normed T-score for the depression subscale was used to reflect the severity of depression symptoms.
Consistent with previous studies assessing IL-6 through a single blood draw [37, 44, 45], 10 ml blood samples were collected in EDTA tubes. Samples were placed on ice, spun down, and frozen at −80°C within 60 minutes of collection. IL-6 and CRP were assayed using high sensitivity commercial ELISA kits (HS-IL-6: R+D Systems, Minneapolis, MN; Zymutest HS-CRP: Diapharma, West Chester, OH). IL-6 values increased slightly from the 2nd to 3rd trimesters (T2 and T3, respectively) (t(99) = −2.17, p = .033). Consistent with prior research suggesting that CRP is stable across the last two trimesters of pregnancy [46], there were no significant differences in average CRP values between study visits (t(99) = 1.63, p =.105). As described in [47], all samples were run in duplicate. Extraction efficiency was 70%. The detection limit was 20 pg/ml and intra-assay coefficient of variation was <10%. The inter-assay coefficients of variation were 4–11%.
Diurnal salivary cortisol was collected over 48 hours beginning at the time of the study session visit. Subsequent samples were collected on the following schedule: at waking; 45 min, 2.5 hours, 3.5 hours, and 8 hours after waking; and at 10 PM or before going to bed. Participants continued the collection schedule at the next collection point relative to their study visit. Salivette tubes (Sarstedt, Newton, NC) were used for cortisol collection. Cotton used for each sample was kept in a bottle with a Medication Event Monitoring System (MEMS) cap (Aardex, Union City, CA) to record the time of opening. Once used, the cotton was placed in a Salivette tube. After return to the lab, samples were kept frozen until assayed using a commercial ELISA kit (Salimetrics, State College, PA). Cortisol area under the curve (AUC) was calculated using samples from the second collection day only, as this provided the most uniform collection schedule. At least two cortisol samples were required: one needed to be the waking sample and at least one other needed to be collected 8 hours or more after the waking sample. Cortisol AUC was calculated from waking until the 8th hour [47]. Cortisol AUC increased from T2 to T3, (t(65) = −2.07, p = .043).
Data preparation
Prior to testing primary hypotheses, we examined possible covariates for inclusion in our models. Consistent with findings in the literature, in our sample, maternal pre-pregnancy BMI and age were positively correlated with IL-6 and CRP and were included as covariates in all analyses. Fifty–four participants (40.60%) reported having a minor infection (either a urinary tract or yeast infection) at some point during pregnancy; however, this was unrelated to abuse severity, depression symptoms, IL-6, and CRP levels and thus was excluded from analyses. We also examined sleep quality (using the Pittsburgh Sleep Quality Index [48]), and pregnancy specific distress (Revised Pregnancy Distress Questionnaire [49]) as potential covariates but these variables were not correlated with IL-6 or CRP and thus were not included in analyses. Finally, among the birth outcomes, gestational age was positively skewed and hence, a variable created by subtracting gestational age at birth from 42 weeks (based on maximum gestational age at birth in our sample) was used.
Data analytic plan
Analyses were conducted in PASW Statistics Version 21.0. To test the independent and interactive effects of abuse history and depression severity on IL-6 and CRP, linear regression analyses were conducted separately for each time point and each outcome controlling for age and BMI; additionally, at T3, we also controlled for T2 inflammatory markers. Abuse and depression were centered and entered on the first step along with covariates, and an interaction term reflecting the product of these centered variables was entered on the second step. To examine whether abuse or depression interacted with circulating levels of inflammatory markers to predict poor birth outcomes, separate linear regressions were conducted for gestational age at birth and birth weight and logistic regression was used for any birth complications. Separate models were estimated for the interactions between abuse and inflammatory markers and the interactions between depression and the inflammatory markers. For all birth outcomes, mode of delivery (0=spontaneous vaginal delivery, 1=vaginal delivery with induction/augmentation, or 2= cesarean section) was included as an additional categorical covariate. Finally, to examine whether cortisol at each time point mediated associations between inflammatory markers at that trimester and poor birth outcome, we tested indirect effects models with bootstrapped standard errors specifying that inflammatory markers predicted cortisol at the same occasion, which in turn predicted poor birth outcomes.
Results
Descriptives
Table 1 includes descriptive and sample size information of the key variables in the study. The majority of complications were related to labor and delivery (e.g., arrest of dilation) followed by infection (e.g., chorioamnionitis), and preeclampsia/hypertension; complications were unrelated to child abuse (F=.23, p=0.63) or depression (F=.76, p=0.39) severity. Of the sample, 10 (7.5%) participants had a baby born prior to 37 weeks, and 6 (4.5%) gave birth to a baby weighing less than 2500 grams (5 lbs, 8 ounces).
Table 1.
Descriptive Data
| N | Min. | Max. | Mean | S.D. | |
|---|---|---|---|---|---|
| Ethnicity | 133 | ||||
| Hispanic | 119 | ||||
| Non – Hispanic | 14 | ||||
| Income | 133 | ||||
| $0–$15,000 | 47 | ||||
| $16–$25,000 | 44 | ||||
| $26–$50,000 | 22 | ||||
| $51–$100,000 | 4 | ||||
| $101–$250,000 | 1 | ||||
| Above $250,000 | 0 | ||||
| Unknown | 15 | ||||
| CTQ Abuse Score | 133 | 15 | 55 | 21.12 | 7.56 |
| SCL-90 Depression T-Score @T2 | 102 | 29 | 81 | 53.30 | 9.18 |
| SCL-90 Depression T-Score @T3 | 101 | 34 | 82 | 53.40 | 9.22 |
| Interluekin-6 (pg/ml)* @T2 | 124 | 0.28 | 12.79 | 1.86 | 1.73 |
| Interluekin-6 (pg/ml)* @T3 | 109 | 0.57 | 18.22 | 2.20 | 1.96 |
| C-Reactive Protein @T2 | 124 | 0.25 | 49.56 | 8.32 | 9.44 |
| C-Reactive Protein @T3 | 109 | 0.01 | 29.06 | 7.31 | 6.13 |
| AUC Cortisol (μg/dl) @T2 | 98 | 0.74 | 5.64 | 2.26 | 0.77 |
| AUC Cortisol (μg/dl) @T3 | 89 | 1.20 | 5.33 | 2.56 | 0.84 |
| Gestational Age at Birth (weeks) | 119 | 27 | 42 | 39.01 | 2.27 |
| Corrected birth weight (grams) | 119 | 947 | 4525 | 3209.19 | 560.26 |
| Mode of Delivery | 120 | ||||
| Vaginal | 37 | ||||
| Vaginal (Induction/Augmentation) | 54 | ||||
| C-Section | 29 | ||||
| Pregnancy Complications | 122 | ||||
| Yes | 61 | ||||
| Infection (e.g., Chorioamnionitis) | 15 | ||||
| Preeclampsia/hypertension | 14 | ||||
| Vascular complications | 1 | ||||
| Other (e.g., arrest of dilation) | 31 | ||||
| No | 61 | ||||
| Maternal Age | 133 | 14 | 20 | 17.80 | 1.22 |
| Pre-pregnancy Body Mass Index | 133 | 17 | 48 | 25.67 | 6.10 |
CTQ Abuse score = Childhood Trauma Questionnaire score for physical, sexual, and emotional abuse; SCL-90 = Symptom Checklist 90; T2 = Trimester 2; T3 = Trimester 3; AUC = Area Under the Curve.
Correlations
Table 2 shows the correlations among the predictor, outcome, and covariate variables. IL-6 at T2 and T3 was not correlated with the CTQ abuse severity score, depression severity, or cortisol AUC at T3; however, IL-6 at T2 was positively associated with cortisol AUC at T2. Abuse was positively associated with depression symptoms, and depression at both trimesters was positively associated with age. IL-6 at both trimesters was positively associated with BMI. CRP at T2 and T3 was positively associated with IL-6 at T2 and T3 as well as with BMI, and CRP at T2 was positively associated with maternal age. Cortisol AUC at T2 was negatively associated with gestational age and birth weight.
Table 2.
Correlations Among Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. CTQ Abuse | – | |||||||||||||
| 2. T2 Depression | .30** | – | ||||||||||||
| 3. T3 Depression | .31** | .80*** | – | |||||||||||
| 4. T2 IL-6 | .01 | .07 | .10 | – | ||||||||||
| 5. T3 IL-6 | −.15 | −.02 | −.02 | .44*** | – | |||||||||
| 6. T2 CRP | .11 | .14 | .18 | .39*** | .36*** | – | ||||||||
| 7. T3 CRP | −.01 | .09 | .05 | .27** | .29** | .83*** | – | |||||||
| 8. Age | .07 | .33*** | .36*** | .06 | .26** | .23* | .09 | – | ||||||
| 9. Pre-pregnancy BMI | .10 | .04 | .01 | .31*** | .28** | .44*** | .38*** | .14 | – | |||||
| 10. Gestational Age | .05 | .03 | −.08 | −.16 | −.10 | −.07 | −.13 | −.04 | .10 | – | ||||
| 11. Birth weight | .05 | .13 | .09 | −.02 | −.09 | −.05 | −.11 | −.01 | .15 | .76*** | – | |||
| 12. Complications | −.04 | .18 | .15 | .07 | .01 | −.03 | −.06 | .06 | .04 | −.14 | −.09 | – | ||
| 13. Cortisol AUC @T2 | −.13 | −.03 | −.13 | .21* | .02 | −.05 | .05 | −.13 | −.18 | −.35*** | −.34*** | .17 | – | |
| 14. Cortisol AUC @ T3 | −.001 | −.06 | .05 | −.03 | −.03 | −.003 | −.14 | .01 | −.19 | −.13 | −.19 | .05 | .21 | – |
p<.05,
p<.001
CTQ = Childhood Trauma Questionnair
Abuse and circulating levels of inflammatory markers across pregnancy
To test whether abuse history was associated with IL-6 or CRP after controlling for age and pre-pregnancy BMI, we regressed IL-6 and CRP at T2 and T3 on the abuse severity score controlling for significant demographic variables. Abuse was not significantly associated with IL-6 at T2 (B = −0.01, p=0.56) T3 (B = −0.05, p=0.074) or with CRP at T2 (B = .005, p = .39) or T3 (B = −.003, p = .57).
Depression and circulating levels of inflammatory markers across pregnancy
To test whether depression was associated with IL-6 and CRP after controlling for age and pre-pregnancy BMI, we regressed IL-6 and CRP at T2 and T3 on the depression score at the same trimester. Depression at T2 was not associated with IL-6 (B = 0.01; p=0.68) or CRP (B = .005, p = .37) at T2, and depression at T3 was not associated with IL-6 (B = −0.02; p=0.40) or CRP (B = .002, p = .77) at T3.
Interaction between abuse and depression on circulating levels of inflammatory markers during pregnancy
Consistent with other literature [20, 50, 51], we regressed IL-6 on the centered abuse and depression variables and their interaction controlling for age and pre-pregnancy BMI. As shown in Table 3, abuse and depression significantly interacted to predict IL-6 at T2 (B = 0.006, p=0.011), but not T3 (B = 0.002, p=0.63). Specifically, at T2, adolescents with high abuse and high depression had higher IL-6 compared to adolescents with high abuse and low depression (p=0.043). Depression did not differentially relate to IL-6 for adolescents with low abuse severity (p = 0.27; see Figure 2). Furthermore, post hoc tests comparing those above and below the mean on both abuse severity and depression revealed that IL-6 levels did not differ between those high on abuse and depression compared to those low on both (F = 0.36, p = 0.56). Finally, adolescents with a higher pre-pregnancy BMI also had higher IL-6 at T2 after controlling for abuse, depression, their interaction, and maternal age. Also shown in Table 3, abuse and depression did not interact to predict CRP at T2 or T3; however, pre-pregnancy BMI was significantly associated with CRP at both time points.
Table 3.
Interaction Between Abuse Severity and Depression on IL-6 and CRP
| Interleukin-6 at 2nd Trimester F(5,124) = 5.58, p<.001 |
Interleukin-6 at 3rd Trimester F(5,85) = 1.17, p = .34 |
|||||
|---|---|---|---|---|---|---|
|
| ||||||
| Estimate B | Standard Error | p-Value | Estimate B | Standard Error | p-Value | |
| Maternal Age | 0.01 | 0.12 | 0.92 | 0.27 | 0.18 | 0.15 |
| Body Mass Index | 0.08 | 0.02 | <0.01 | 0.04 | 0.03 | 0.11 |
| Abuse | −0.03 | 0.02 | 0.11 | −0.04 | 0.03 | 0.22 |
| Depression | 0.01 | 0.02 | 0.54 | −0.02 | 0.03 | 0.35 |
| Abuse * Depression | 0.006 | 0.002 | 0.01 | 0.002 | 0.003 | 0.65 |
| CRP at Trimester2 F(5,94) = 5.00, p<.001 |
CRP and Trimester 3 F(5,90) = 3.21, p=.011 |
|||||
| B | SE | p-value | B | SE | p-value | |
| Maternal Age | 0.06 | 0.04 | 0.13 | 0.02 | 0.04 | 0.70 |
| Body Mass Index | 0.03 | 0.01 | <.001 | 0.03 | 0.01 | <.001 |
| Abuse | 0.01 | 0.1 | 0.50 | −0.01 | 0.01 | 0.44 |
| Depression | 0.003 | 0.006 | 0.58 | 0.002 | 0.006 | 0.75 |
| Abuse * Depression | 0.001 | 0.001 | 0.58 | 0.0001 | 0.001 | 0.98 |
Bold indicates significant effects
Figure 2.
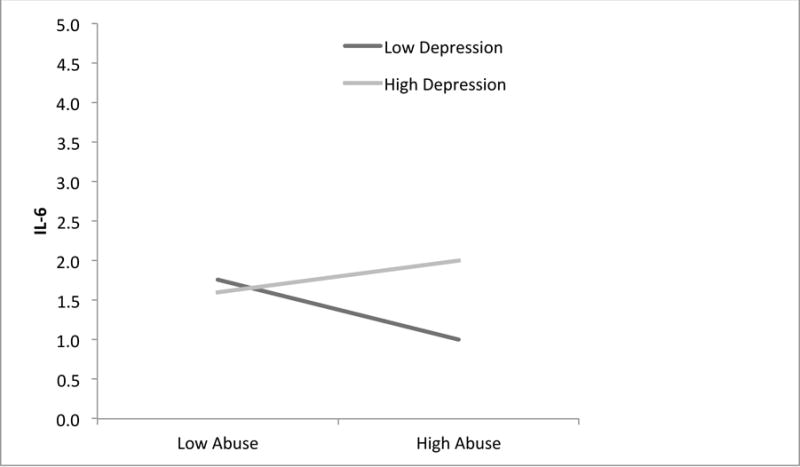
Interaction between Abuse Severity and Depression on IL-6 at 2nd Trimester. Continuous predictor variables are graphically depicted here; however, follow-up analyses using dichotomous predictors revealed that only 3 adolescents with severe abuse histories did not have clinically significant depression; therefore, this interaction should be interpreted cautiously.
Birth outcomes
Child abuse severity, depression, IL-6, and CRP levels at T2 and T3 were not independently associated with gestational age at birth, birth weight, or pregnancy complications. Additionally, depression symptoms did not interact with IL-6 to predict birth outcomes. However, IL-6 at T2 and abuse severity interacted to predict gestational age at birth (B=.004, p <0.040) after controlling for age, pre-pregnancy BMI, and mode of delivery. Specifically, adolescents with lower abuse severity and higher IL-6 at T2 had babies born at an earlier gestational age than adolescents with lower abuse severity and lower IL-6 (p =0.050). However, among adolescents with higher abuse, having lower levels of IL-6 at T2 actually predicted younger gestational age compared to adolescents with higher abuse and higher IL-6 (p =0.046). Pre-pregnancy BMI was a positively associated with gestational age after accounting for cortisol and IL-6 at T2. Neither abuse nor depression interacted with CRP at either trimester to predict poor birth outcomes.
Post hoc analyses for HPA axis
Because glucocorticoid resistance has been linked with both maltreatment and inflammation [9, 22], we conducted post hoc analyses to assess whether HPA axis activation, measured by cortisol AUC, might mediate associations between IL-6 and poor birth outcomes. Cortisol and IL-6 were positively associated at T2 (r=0.21, p<0.041), although this association did not hold at T3 (r=−0.03, p=0.76). As shown in Table 4, an indirect effects model with bootstrapped standard errors revealed that higher IL-6 levels at T2 were related to younger gestational age at birth through heightened cortisol AUC at T2 (Indirect effect estimate = −0.143, SE = 0.07, p <0.039). This model accounted for 13% of the variance in gestational age at birth. BMI also contributed significantly to cortisol at T2 such that girls with a higher pre-pregnancy BMI had lower T2 cortisol AUC. Significant effects were not observed for IL6 and cortisol AUC at T3.
Table 4.
Cortisol AUC as an Indirect Path from IL-6 to Gestational Age
| Gestational Age on: | 2nd Trimester Predictors | 3rd Trimester Predictors | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Estimate B | Standard Error | p-Value | Estimate B | Standard Error | p-Value | |
| Maternal Age | −0.21 | 0.18 | 0.25 | −0.15 | 0.13 | 0.22 |
| Body Mass Index | 0.03 | 0.04 | 0.46 | 0.04 | 0.02 | 0.06 |
| Cortisol | −1.02 | 0.32 | <0.001 | −0.21 | 0.18 | 0.24 |
| IL-6 | −0.06 | 0.15 | 0.69 | −0.09 | 0.12 | 0.47 |
| Cortisol on: | ||||||
| Maternal Age | −0.09 | 0.06 | 0.14 | 0.001 | 0.07 | 0.99 |
| Body Mass Index | −0.03 | 0.01 | 0.03 | −0.03 | 0.01 | 0.07 |
| IL-6 | 0.14 | 0.04 | <0.001 | −0.01 | 0.04 | 0.87 |
| Indirect Effect: | 0.14 | 0.07 | 0.037 | 0.001 | 0.009 | 0.87 |
Bold indicates significant effects; Indirect effects were calculated using bootstrapped standard errors.
Discussion
To our knowledge, this is the first study to examine abuse-related immune variation in a sample of pregnant adolescents. Unlike other health research linking past trauma to elevated immune activity and poor health outcomes, in this sample, neither abuse nor depression was associated independently with IL-6 or CRP levels in the 2nd (24–27 weeks) or 3rd trimesters (34–37 weeks) in pregnancy. However, abuse and depression interacted to predict IL-6 such that adolescents with more severe abuse and depression had higher IL-6 at the 2nd trimester compared to adolescents with high abuse and low depression. Paradoxically, adolescents with low abuse and low depression had comparable levels of IL-6 relative to adolescents with high abuse and depression. That is, while two adversities— higher scores on childhood abuse and depression — compared to just one was associated with higher IL6, the relative increase was not significantly different from those with the healthiest profiles — low scores on abuse and depression. Finally, through its association with higher cortisol, higher IL-6 predicted a poor birth outcome, earlier gestational age at birth.
Given that numerous adult studies have related psychosocial adversities such as abuse and depression to higher levels of inflammation, it is curious that this sample of pregnant adolescents did not evidence the expected pattern of associations between abuse, depression, IL-6 and CRP. Though speculative, one explanation for these unexpected findings centers on developmental and ethnic differences in inflammation–promoting IL-6 gene expression. Specifically, the inflammation-related function of a polymorphism in the human IL-6 promoter (rs1800795; IL-6 – 174 G/C) may be moderated by developmental stage and exposure to stress such that older adult carriers of the G allele evidence chronic inflammation in the context of interpersonal stress [52] while adolescents with the G allele show resilience toward inflammation [12]. Notably, the distribution of the IL-6 genotypes differs significantly as a function of ethnicity, such that Latino populations are more likely to carry the G allele [12]. As our sample was comprised primarily of pregnant Hispanic adolescents, genetic and/or developmental factors may help explain the results: participants with high abuse and low depression showed resilience in inflammation relative to those with high abuse and high depression. However, in contrast to other studies using older, ethnically diverse samples, IL-6 was not significantly higher in the high abuse-high depression adolescents compared to low abuse-low depression adolescents [9, 10, 23].
Another explanation for the lack of a significant difference on IL-6 in the 2rd trimester between the high abuse-high depression and the low abuse-low depression adolescents may lie in the general good health of our sample. The severity scores for both abuse and depression were fairly low-level. Based on the larger study aims we only included adolescents with healthy pregnancies and deliveries, and we excluded those who were taking psychiatric medications. Thus, null findings may be a function of recruiting a relatively healthier sample where the abuse and depression were not severe enough to affect IL-6 levels in expected ways. As support for this notion, although IL-6 values in the current sample fell within the range of those reported in other studies with pregnant women, and we observed an increase in IL-6 from T2 to T3, which is consistent with prior work noting renewed inflammation in the 3rd trimester to stimulate labor and facilitate delivery [53], the means for our sample (M = 1.86 and 2.20) were slightly lower than the means observed in healthy adult pregnant women (e.g., M = 3; range = 0.4–7.6) [54], which may reflect the relative youth and health of the current sample. Alternatively, the overall lower IL-6 values compared to studies with adult pregnant women, and the lack of different levels between high and low risk participants may reflect an element of risk in this adolescent sample given that lower IL-6 levels are associated with poor birth outcomes.
Third, pregnancy-related factors also could explain the unexpected results. For instance, some studies show that the balance between Th1 and Th2 cytokines may provide a more comprehensive picture of immune functioning during pregnancy than an examination of IL-6 alone [55, 56]. An examination of interferon-gamma and the Th1/Th2 (interferon-gamma/interleukin-10) ratio [57] may provide a more nuanced understanding of maltreatment-related immune findings in pregnant adolescents, as well as pregnant women. Additionally, a previous study with pregnant adults found that trauma exposure was associated with higher levels of TNF-alpha but not IL-6; thus, future studies should consider including multiple indicators of inflammatory markers.
Cortisol AUC at T2 was positively associated with IL-6 at T2 and cortisol at T2 mediated the inverse association between IL-6 at T2 and younger gestational age at birth. At least one other study did not observe the typical inverse association between cortisol and IL-6 among low-income, minority pregnant women [37]. Since cortisol and other glucocorticoids are down-regulators of IL-6 gene expression, the positive association between cortisol and IL-6 evidenced at T2 may result from cortisol’s relation to other stress-related mediators such as catecholamine signaling, which is associated with a stimulatory effect on IL-6 gene expression [52]. In addition, abuse can lead to glucocorticoid receptor desensitization, resulting in complex alterations in typical regulatory dynamics [58]. In our sample, the positive associations between cortisol and IL-6 did not persist to T3, which is consistent with other studies showing that biomarkers in the 2nd trimester are associated with risk for younger gestational age. Specifically, elevated IL-6 and corticotropin releasing hormone levels detected in amniotic fluid during the 2nd trimester have been associated with risk for preterm birth [59, 60]. Further exploration of associations between neuroendocrine dysregulation and inflammation in the 2nd trimester and poor birth outcomes is warranted.
Abuse severity and IL-6 also interacted to predict gestational age at birth such that adolescents with high abuse and low IL-6 as well as adolescents with low abuse and high IL-6 had babies with lower gestational ages at birth. These findings fit with work showing that inflammation is implicated in preterm birth [25, 61–63], which can have negative downstream effects on later cognitive [64, 65] and health [66] outcomes. Findings are consistent with recent work suggesting that pregnancy is a carefully regulated inflammatory state where both high and low inflammation may be detrimental to a pregnancy [67], and contextual factors like abuse severity may increase the likelihood of dysregulation. It is important to note that our findings were specific to gestational age at birth, and we did not note associations with birth weight or pregnancy-related complications. These differential associations with various birth outcomes suggest that it will be important to extend this work with more thorough examinations of abuse and circulating levels of inflammatory markers in pregnancy.
Consistent with other studies emphasizing the importance of pre-pregnancy BMI in inflammation and pregnancy complications [68], adolescents with a higher pre-pregnancy BMI also had higher IL-6 at T2 and T3, and this relationship remained significant in the multivariate model for IL-6 at T2. Pre-pregnancy BMI also was a significant predictor of lower gestational age after accounting for cortisol and IL-6 at T2. This risk factor for inflammation is partially modifiable through diet and exercise; thus, it may be important to target these behaviors in high-risk adolescents to reduce risk for inflammation and associated poor birth outcomes.
Our findings should be considered within the context of limitations. First, consistent with other studies [37], we collected blood at a single time-point either in the late morning or early afternoon in T2 and T3 for IL-6 analysis and thus could not account for diurnal variation in IL-6. Because we were collecting multiple markers in addition to cytokines, we used EDTA tubes and thus could not rule out the possibility that the tubes interfered with cytokine production by removing Calcium. Second, we did not collect markers of inflammation during the 1st trimester and thus were unable to examine how inflammation across all three trimesters was related to abuse, depression, cortisol, and poor birth outcomes. Third, although participants reported a low severity of abuse on average, we did not assess abuse characteristics such as the frequency or duration of the abuse or the relationship to the perpetrator, and we have no data on whether the abuse was currently ongoing, which could have skewed results. We also lack data on other stressors or traumatic events that could be present and accounting for some of the similarities between adolescents with low and high abuse severity. Fourth, our assessment of depression was based on the SCL-90, a widely used measure of clinical depression that has adolescent norms; however, measures like the Edinburgh Depression Scale are commonly used to assess symptoms of perinatal depression and may be useful for drawing comparisons to other studies of pregnant girls and women. Fifth, we had relatively low power to detect interaction effects so findings should be replicated in larger samples with greater variability in abuse and depression severity.
In summary, for adolescents with more severe abuse, more severe depression was associated with higher levels of IL-6 compared to those with high abuse and low depression. Unexpectedly, adolescents with high abuse and high depression had statistically similar levels of IL-6 compared to adolescents with low abuse and low depression suggesting paradoxical physiological adaptation to adversity in this sample of young, pregnant, Latina women. Further, higher IL-6 levels were associated with younger gestational age at birth, and cortisol explained the relationship between IL-6 and younger gestational age at birth, suggesting that immune and HPA axis markers are important to examine in relation to pregnancy outcomes. The current report indicates that biomarkers at the 2nd trimester (weeks 24–27) were especially important to consider in risk for poor birth outcomes. The results suggest a pathway by which maternal factors such as abuse history and depression influence not only immune profiles during pregnancy but also the health of the next generation.
Abbreviations
- IL-6
Interleukin-6
- TNF−
tumor necrosis factor-alpha
- CRP
C-Reactive Protein
- HPA
Hypothalamic Pituitary Adrenal
- CTQ
Childhood Trauma Questionnaire
- AUC
Area Under the Curve
- BMI
Body Mass Index
- T2
Trimester 2
- T3
Trimester 3
- CUMC
Columbia University Medical Center
References
- 1.Darroch JE, Singh S, Frost JJ. Differences in teenage pregnancy rates among five developed countries: The roles of sexual activity and contraceptive use. Family planning perspectives. 2001;33:244–250, 281. [PubMed] [Google Scholar]
- 2.Herrenkohl EC, Herrenkohl RC, Egolf BP, Russo MJ. The relationship between early maltreatment and teenage parenthood. Journal of adolescence. 1998;21:291–303. doi: 10.1006/jado.1998.0154. [DOI] [PubMed] [Google Scholar]
- 3.Noll JG, Shenk CE, Putnam KT. Childhood sexual abuse and adolescent pregnancy: A meta-analytic update. Journal of pediatric psychology. 2009;34:366–378. doi: 10.1093/jpepsy/jsn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meltzer-Brody S, Bledsoe-Mansori SE, Johnson N, Killian C, Hamer RM, Jackson C, Wessel J, Thorp J. A prospective study of perinatal depression and trauma history in pregnant minority adolescents. American journal of obstetrics and gynecology. 2013;208:211. e211–211. e217. doi: 10.1016/j.ajog.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X-K, Wen SW, Fleming N, Demissie K, Rhoads GG, Walker M. Teenage pregnancy and adverse birth outcomes: A large population based retrospective cohort study. International journal of epidemiology. 2007;36:368–373. doi: 10.1093/ije/dyl284. [DOI] [PubMed] [Google Scholar]
- 6.Straube S, Voigt M, Jorch G, Hallier E, Briese V, Borchardt U. Investigation of the association of apgar score with maternal socio-economic and biological factors: An analysis of german perinatal statistics. Archives of gynecology and obstetrics. 2010;282:135–141. doi: 10.1007/s00404-009-1217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khashan AS, Baker PN, Kenny LC. Preterm birth and reduced birthweight in first and second teenage pregnancies: A register-based cohort study. BMC pregnancy and childbirth. 2010;10:36. doi: 10.1186/1471-2393-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens-Simon C, McAnarney ER. Childhood victimization: Relationship to adolescent pregnancy outcome. Child abuse & neglect. 1994;18:569–575. doi: 10.1016/0145-2134(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 9.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Archives of general psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gouin J-P, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK. Childhood abuse and inflammatory responses to daily stressors. Annals of Behavioral Medicine. 2012;44:287–292. doi: 10.1007/s12160-012-9386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole SW, Arevalo JM, Manu K, Telzer EH, Kiang L, Bower JE, Irwin MR, Fuligni AJ. Antagonistic pleiotropy at the human il6 promoter confers genetic resilience to the pro-inflammatory effects of adverse social conditions in adolescence. Developmental psychology. 2011;47:1173. doi: 10.1037/a0023871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller G, Chen E. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosomatic medicine. 2007;69:402–409. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- 14.Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological psychiatry. 2012;72:34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreier HM, Roy LB, Frimer LT, Chen E. Family chaos and adolescent inflammatory profiles: The moderating role of socioeconomic status. Psychosomatic medicine. 2014;76:460–467. doi: 10.1097/PSY.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 16.Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of affective disorders. 2004;82:217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. The Lancet. 2012;379:1056–1067. doi: 10.1016/S0140-6736(11)60871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewinsohn PM, Allen NB, Seeley JR, Gotlib IH. First onset versus recurrence of depression: Differential processes of psychosocial risk. Journal of abnormal psychology. 1999;108:483. doi: 10.1037//0021-843x.108.3.483. [DOI] [PubMed] [Google Scholar]
- 19.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biological psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nature reviews Immunology. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from hpa axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain, behavior, and immunity. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blackmore ER, Moynihan JA, Rubinow DR, Pressman EK, Gilchrist M, O’Connor TG. Psychiatric symptoms and proinflammatory cytokines in pregnancy. Psychosomatic medicine. 2011;73:656. doi: 10.1097/PSY.0b013e31822fc277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guven MA, Coskun A, Ertas IE, Aral M, Zencırcı B, Oksuz H. Association of maternal serum crp, il-6, tnf-α, homocysteine, folic acid and vitamin b12 levels with the severity of preeclampsia and fetal birth weight. Hypertension in pregnancy. 2009;28:190–200. doi: 10.1080/10641950802601179. [DOI] [PubMed] [Google Scholar]
- 25.Coussons-Read ME, Lobel M, Carey JC, Kreither MO, D’Anna K, Argys L, Ross RG, Brandt C, Cole S. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain, behavior, and immunity. 2012;26:650–659. doi: 10.1016/j.bbi.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elenkov IJ, Wilder RL, Bakalov VK, Link AA, Dimitrov MA, Fisher S, Crane M, Kanik KS, Chrousos GP. Il-12, tnf-α, and hormonal changes during late pregnancy and early postpartum: Implications for autoimmune disease activity during these times. The Journal of Clinical Endocrinology & Metabolism. 2001;86:4933–4938. doi: 10.1210/jcem.86.10.7905. [DOI] [PubMed] [Google Scholar]
- 27.Makhseed M, Raghupathy R, Azizieh F, Al-Azemi M, Hassan N, Bandar A. Mitogen-induced cytokine responses of maternal peripheral blood lymphocytes indicate a differential th-type bias in normal pregnancy and pregnancy failure. American journal of reproductive immunology. 1999;42:273–281. doi: 10.1111/j.1600-0897.1999.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 28.Sykes L, MacIntyre DA, Yap XJ, Teoh TG, Bennett PR. The th1: Th2 dichotomy of pregnancy and preterm labour. Mediators of inflammation. 2012:2012. doi: 10.1155/2012/967629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markert UR, Morales-Prieto DM, Fitzgerald JS. Understanding the link between the il-6 cytokine family and pregnancy: Implications for future therapeutics. 2011 doi: 10.1586/eci.11.60. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez G, Sarto A, Berod L, Canellada A, Gentile T, Pasqualini S, Margni R. Regulation of interleukin-6 fetoplacental levels could be involved in the protective effect of low-molecular weight heparin treatment on murine spontaneous abortion. American journal of reproductive immunology. 2004;51:160–165. doi: 10.1046/j.8755-8920.2003.00131.x. [DOI] [PubMed] [Google Scholar]
- 31.Jasper MJ, Tremellen KP, Robertson SA. Reduced expression of il-6 and il-1α mrnas in secretory phase endometrium of women with recurrent miscarriage. Journal of reproductive immunology. 2007;73:74–84. doi: 10.1016/j.jri.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Galazios G, Tsoulou S, Zografou C, Tripsianis G, Koutlaki N, Papazoglou D, Tsikouras P, Maltezos E, Liberis V. The role of cytokines il-6 and il-8 in the pathogenesis of spontaneous abortions. Journal of Maternal-Fetal and Neonatal Medicine. 2011;24:1283–1285. doi: 10.3109/14767058.2011.575482. [DOI] [PubMed] [Google Scholar]
- 33.Kuzmicki M, Telejko B, Szamatowicz J, Zonenberg A, Nikolajuk A, Kretowski A, Gorska M. High resistin and interleukin-6 levels are associated with gestational diabetes mellitus. Gynecological endocrinology. 2009;25:258–263. doi: 10.1080/09513590802653825. [DOI] [PubMed] [Google Scholar]
- 34.Wei S-Q, Fraser W, Luo Z-C. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: A systematic review. Obstetrics & Gynecology. 2010;116:393–401. doi: 10.1097/AOG.0b013e3181e6dbc0. [DOI] [PubMed] [Google Scholar]
- 35.Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK. Il-6 enhances plasma il-1ra, il-10, and cortisol in humans. American Journal of Physiology-Endocrinology And Metabolism. 2003;285:E433–E437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- 36.Giurgescu C. Are maternal cortisol levels related to preterm birth? Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2009;38:377–390. doi: 10.1111/j.1552-6909.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- 37.Corwin EJ, Guo Y, Pajer K, Lowe N, McCarthy D, Schmiege S, Weber M, Pace T, Stafford B. Immune dysregulation and glucocorticoid resistance in minority and low income pregnant women. Psychoneuroendocrinology. 2013;38:1786–1796. doi: 10.1016/j.psyneuen.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spicer J, Werner E, Zhao Y, Choi CW, Lopez-Pintado S, Feng T, Altemus M, Gyamfi C, Monk C. Ambulatory assessments of psychological and peripheral stress-markers predict birth outcomes in teen pregnancy. Journal of psychosomatic research. 2013 doi: 10.1016/j.jpsychores.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernstein D, Fink L. Manual for the childhood trauma questionnaire. The Psychological Corporation; New York: 1998. [Google Scholar]
- 40.Scher CD, Stein MB, Asmundson GJ, McCreary DR, Forde DR. The childhood trauma questionnaire in a community sample: Psychometric properties and normative data. Journal of traumatic stress. 2001;14:843–857. doi: 10.1023/A:1013058625719. [DOI] [PubMed] [Google Scholar]
- 41.McLaughlin KA, Sheridan MA, Alves S, Mendes WB. Child maltreatment and autonomic nervous system reactivity: Identifying dysregulated stress reactivity patterns by using the biopsychosocial model of challenge and threat. Psychosomatic medicine. 2014;76:538–546. doi: 10.1097/PSY.0000000000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Derogatis LR. Scl-90-r: Administration, scoring of procedures manual-ii for the r (evised) version and other instruments of the psychopathology rating scale series. Clinical Psychometric Research Incorporated. 1992 [Google Scholar]
- 43.Schmitz N, Kruse J, Heckrath C, Alberti L, Tress W. Diagnosing mental disorders in primary care: The general health questionnaire (ghq) and the symptom check list (scl-90-r) as screening instruments. Social psychiatry and psychiatric epidemiology. 1999;34:360–366. doi: 10.1007/s001270050156. [DOI] [PubMed] [Google Scholar]
- 44.Smerieri A, Petraroli M, Ziveri MA, Volta C, Bernasconi S, Street ME. Effects of cord serum insulin, igf-ii, igfbp-2, il-6 and cortisol concentrations on human birth weight and length: Pilot study. PLoS One. 2011;6:e29562. doi: 10.1371/journal.pone.0029562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greig PC, Murtha AP, Jimmerson CJ, Herbert WN, Roitman-Johnson B, Allen J. Maternal serum interleukin-6 during pregnancy and during term and preterm labor. Obstetrics & Gynecology. 1997;90:465–469. doi: 10.1016/s0029-7844(97)00294-9. [DOI] [PubMed] [Google Scholar]
- 46.Watts D, Krohn M, Wener M, Eschenbach D. C-reactive protein in normal pregnancy. Obstetrics & Gynecology. 1991;77:176–180. doi: 10.1097/00006250-199102000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Doyle C, Werner E, Feng T, Lee S, Altemus M, Isler JR, Monk C. Pregnancy distress gets under fetal skin: Maternal ambulatory assessment & sex differences in prenatal development. Developmental psychobiology. 2015 doi: 10.1002/dev.21317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 49.Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychology. 2008;27:604. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- 50.Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological psychiatry. 2012;72:34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Archives of general psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cole SW, Arevalo JM, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, Sheridan JF, Seeman TE. Computational identification of gene–social environment interaction at the human il6 locus. Proceedings of the National Academy of Sciences. 2010;107:5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mor G, Cardenas I. Review article: The immune system in pregnancy: A unique complexity. American journal of reproductive immunology. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma A, Satyam A, Sharma JB. Leptin, il-10 and inflammatory markers (tnf-α, il-6 and il-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. American Journal of Reproductive Immunology. 2007;58:21–30. doi: 10.1111/j.1600-0897.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 55.Calleja-Agius J, Schembri-Wismayer P, Calleja N, Brincat M, Spiteri D. Obstetric outcome and cytokine levels in threatened miscarriage. Gynecological Endocrinology. 2011;27:121–127. doi: 10.3109/09513590.2010.487614. [DOI] [PubMed] [Google Scholar]
- 56.Aris A, Lambert F, Bessette P, Moutquin JM. Maternal circulating interferon-γ and interleukin-6 as biomarkers of th1/th2 immune status throughout pregnancy. Journal of Obstetrics and Gynaecology Research. 2008;34:7–11. doi: 10.1111/j.1447-0756.2007.00676.x. [DOI] [PubMed] [Google Scholar]
- 57.Groer MW, Davis MW. Cytokines, infections, stress, and dysphoric moods in breastfeeders and formula feeders. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2006;35:599–607. doi: 10.1111/j.1552-6909.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- 58.Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holzman C, Jetton J, Siler-Khodr T, Fisher R, Rip T. Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstetrics & Gynecology. 2001;97:657–663. doi: 10.1016/s0029-7844(00)01209-6. [DOI] [PubMed] [Google Scholar]
- 60.Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, DuBard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. American journal of obstetrics and gynecology. 1998;178:546–550. doi: 10.1016/s0002-9378(98)70436-3. [DOI] [PubMed] [Google Scholar]
- 61.Christian LM. Psychoneuroimmunology in pregnancy: Immune pathways linking stress with maternal health, adverse birth outcomes, and fetal development. Neuroscience & Biobehavioral Reviews. 2012;36:350–361. doi: 10.1016/j.neubiorev.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Christian LM. Psychoneuroimmunology in pregnancy: Immune pathways linking stress with maternal health, adverse birth outcomes, and fetal development. Neuroscience and biobehavioral reviews. 2012;36:350–361. doi: 10.1016/j.neubiorev.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coussons-Read ME, Lobel M, Carey JC, Kreither MO, D’Anna K, Argys L, Ross RG, Brandt C, Cole S. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain, behavior, and immunity. 2012;26:650–659. doi: 10.1016/j.bbi.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noble KG, Fifer WP, Rauh VA, Nomura Y, Andrews HF. Academic achievement varies with gestational age among children born at term. Pediatrics. 2012;130:e257–264. doi: 10.1542/peds.2011-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brumbaugh JE, Hodel AS, Thomas KM. The impact of late preterm birth on executive function at preschool age. American journal of perinatology. 2014;31:305–314. doi: 10.1055/s-0033-1348950. [DOI] [PubMed] [Google Scholar]
- 66.Arpi E, Ferrari F. Preterm birth and behaviour problems in infants and preschool-age children: A review of the recent literature. Dev Med Child Neurol. 2013;55:788–796. doi: 10.1111/dmcn.12142. [DOI] [PubMed] [Google Scholar]
- 67.Shelton MM, Schminkey DL, Groer MW. Relationships among prenatal depression, plasma cortisol, and inflammatory cytokines. Biological research for nursing. 2014:1099800414543821. doi: 10.1177/1099800414543821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bodnar LM, Ness RB, Harger GF, Roberts JM. Inflammation and triglycerides partially mediate the effect of prepregnancy body mass index on the risk of preeclampsia. American journal of epidemiology. 2005;162:1198–1206. doi: 10.1093/aje/kwi334. [DOI] [PubMed] [Google Scholar]


