Abstract
Objective
To estimate smoking prevalence during the year before pregnancy and during pregnancy and adverse outcomes among women who delivered infants with and without assisted reproductive technology (ART) using linked birth certificates (BC) and National ART Surveillance System (NASS) data.
Methods
Data were analyzed for 384,390 women and 392,248 infants born in Massachusetts and Michigan during 2008–2009. Maternal smoking prevalence was estimated using smoking indicated from BC by ART status. For ART users, to evaluate underreporting, prepregnancy smoking was estimated from BC, NASS, or both sources. Effect of prenatal smoking on preterm and mean birthweight (term only) for singleton infants were examined by ART status.
Results
Maternal smoking prevalence estimates were significantly lower for ART users than nonusers (pre-pregnancy = 3.2% vs. 16.7%; prenatal = 1.0% vs. 11.1%, p < 0.05). When combining smoking information from BC and NASS, prepregnancy smoking prevalence estimates for ART users could be as high as 4.4% to 6.1%. Adverse effects of smoking on infant outcomes in ART pregnancies were consistent with the effects seen in non-ART pregnancies, specifically decline in infant birthweight and increase in preterm delivery, although association between smoking and preterm was not significant.
Conclusion
A low, but substantial proportion of ART users smoked before and during pregnancy. As ART users are highly motivated to get pregnant, it should be clearly communicated that smoking can decrease fertility and adversely affect pregnancy outcomes. Continued efforts are needed to encourage smoking cessation and maintain tobacco abstinence among all women of reproductive age.
Introduction
Smoking can lead to reduced fertility, conception delays, and ectopic pregnancies.1 Maternal smoking during pregnancy can cause adverse outcomes in both mothers and infants, such as placental previa, placental abruption, preterm delivery, fetal growth restriction, and sudden infant death.1 Every year in the United States, about 1.4% of all infants born are conceived using assisted reproductive technology (ART), such as in vitro fertilization.2 A meta-analysis of 21 studies showed that smoking at the time of ART treatment was associated with significantly lower odds of clinical pregnancy per cycle, lower odds of live birth per cycle, higher odds of spontaneous miscarriage, and higher odds of ectopic pregnancy.3 Therefore, preventing and decreasing smoking could help to improve ART outcomes. However, population-based estimates of smoking among women undergoing ART and effect of smoking on infant outcomes are currently not available.
Starting in 2007, the National ART Surveillance System (NASS), which collects data on all ART procedures performed in the US, began collecting data on lifetime smoking and smoking in the 3 months before pregnancy. These data are collected at the start of a woman’s ART treatment cycle. The States Monitoring Assisted Reproductive Technology (SMART) Collaborative is a state-based data project that links ART surveillance data with birth certificates (BC) and other datasets, creating a population-based dataset of mother–infant pairs of ART.4 Thus, the SMART Collaborative data can be used to estimate the prevalence of smoking before and during pregnancy among women who deliver a live birth by ART status. These population-based estimates of prepregnancy and prenatal smoking can inform tobacco prevention and cessation efforts in women undergoing fertility treatment.
In general, the use of self-reported smoking to assess smoking status underestimates smoking prevalence.5 Smoking status obtained from the birth certificate may be especially problematic. For example, quality assessments of the smoking status collected on the BC have found a lower sensitivity (47%–62%) when compared to telephone interviews6 and other self-reported survey data.7 In contrast, specificity of smoking status on the birth certificate is believed to be high (97%).6 Reasons for this include that smoking status recorded on the BC is generally collected after delivery and so is subject to recall bias. Women may also underreport smoking status in this setting because of stigma. Combining smoking information from the BC with the information from NASS could identify more women who smoked before pregnancy than data from the BC alone.
In this study, using linked SMART data, we estimate the prevalence of smoking during the year before pregnancy and during pregnancy among women delivering live births by ART status and compare risk of smoking by select characteristics between ART users and nonusers using BC data. For ART users, we combined smoking information from BC with data from NASS to evaluate potential underreporting of prepregnancy smoking using BC alone. We also examine the association of smoking during pregnancy on adverse infant outcomes by ART status.
Materials and Methods
Dataset
The SMART Collaborative project is coordinated by the Centers for Disease Control and Prevention’s (CDC) Division of Reproductive Health (DRH) in partnership with the Massachusetts, Florida, Michigan, and Connecticut state departments of health. These data can be used to monitor and study maternal and infant health outcomes related to ART. Details of the SMART methodology have been described previously.4,8 In brief, ART surveillance data are linked with state birth records, infant and fetal death records, and data from other surveillance systems and registries. For this study, we used the most recent and available linked 2008–2009 SMART data from Massachusetts and Michigan (births from January 1, 2008 to December 31, 2009) because we were able to calculate smoking before pregnancy from the BC. Florida and Connecticut were excluded because they did not collect information on smoking status before pregnancy on their BC (Florida was using a state-specific question and Connecticut was using the 1989 BC revision). The linkage methodology uses a probabilistic linkage method, using Link Plus software, with the following indirect linking variables: mother’s date of birth, infant’s date of birth, plurality, gravidity, and zip code. The average linkage rate for 2008–2009 was 90.4% for Massachusetts and 92.0% for Michigan. This study was approved by the institutional review boards in Massachusetts, Michigan, and CDC.
Variables
Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/jwh) shows the algorithm developed in consultation with state health department partners to define the maternal smoking measures used by each state and NASS. Information on smoking status before and during pregnancy is collected for the birth certificate during the delivery hospitalization using a maternal worksheet that is completed by the mother. NASS captures smoking at the time of the ART treatment cycle that is reported to the provider.
Prepregnancy smoking from BC
Prepregnancy smoking on the Massachusetts’ BC was defined by maternal report of smoking at least one cigarette daily in the year before pregnancy. Prepregnancy smoking on Michigan’s BC was defined as (1) maternal report of smoking before or during pregnancy, without report of quit date, or (2) report of smoking before or during pregnancy with a quit date occurring 2 years before the delivery year, as full birth date was not provided in the deidentified analytic dataset.
Prenatal smoking from the BC
Prenatal smoking was defined on Massachusetts’ BC by maternal report of smoking at least one cigarette daily during pregnancy and on Michigan’s BC by maternal report of smoking before or during pregnancy and without concomitant report of a quit date.
Prepregnancy smoking from NASS for ART users
Prevalence estimates of prepregnancy smoking from the BC were combined with data from NASS. In NASS, prepregnancy smoking was defined as report of smoking at least 100 cigarettes in her lifetime and smoking in the 3 months before the ART cycle. Because the time frame for smoking prevalence differs by data source (NASS and for each state’s BC), we calculated two estimates. For the first estimate, women who reported smoking on either BC or NASS or both BC and NASS were considered smokers in the year before pregnancy, including records with unknown smoking status in one data source and known smoking status in the other data source. For the second estimate, we used the first estimate and subtracted women who reported prepregnancy smoking on the BC and did not report prepregnancy smoking on NASS. The latter estimate takes into account the possibility that women who smoked in the prior year may have quit smoking in the 3 months before pregnancy. Prenatal smoking was not available in the NASS data.
We also explored maternal smoking by selected characteristics (maternal age, race/ethnicity, education, parity, and trimester of entry into prenatal care), state, and infant outcomes (preterm <37 weeks defined from clinical estimate of gestation age and infant birthweight, which were obtained from the BC).
Statistical analysis
First, we described the maternal characteristics of the study population by ART status, using frequencies and means with standard deviations (SD), as applicable. Prevalence and 95% confidence intervals (CI) of prepregnancy and prenatal smoking based on the BC were estimated by ART status, overall and by state. For prepregnancy smoking among ART users, prevalence was estimated separately for each data source (NASS vs. BC). Chi-squared tests were used to assess differences in smoking by ART status, and two-way t-tests were used to assess difference in prevalence estimates by data source (NASS vs. BC).
Adjusted prevalence ratios (aPR) and 95% CIs were estimated, to evaluate differences in prepregnancy smoking by maternal characteristics stratified by ART status, using method by Bieler et al.9 Model aPR were obtained from average marginal predictions in the fitted logistic regression model (using SUDAAN’s option ADJRR in PROC RLOGISTIC). APRs were adjusted for maternal age, race/ethnicity, education, parity, trimester of entry into prenatal care, and state. APRs were not estimated for prenatal smoking because the sample size of prenatal smokers who were ART users was low (n < 70).
To examine birth outcomes by ART status, we limited the sample to singleton infants with plausible gestational ages (22–44 weeks) and birthweights (300–4600 g).10 Unadjusted and adjusted prevalence of preterm and mean birthweight difference (term only) were calculated for smokers and nonsmokers during pregnancy by ART status.
Analyses were conducted using SAS version 9.3 and SUDAAN version 11 to account for clustering of data by fertility clinic in each state.
Results
A total of 384,390 women who delivered live births were available for analysis in the two study states during 2008–2009, of which 1.7% (n = 6675) were births to ART users. Compared with nonusers, ART users were more likely to have more than a high school education (85.3% vs. 45.0%), be non-Hispanic White (86.6% vs. 72.3%), enter prenatal care in the first trimester (89.2% vs. 78.2%), and be nulliparous (54.0% vs. 42.4%) (all p < 0.001) (Table 1). The mean age of ART users was 35 years (SD = 5.0) compared to 28 years (SD = 6.1) for nonusers (p < 0.001). There were some differences by state. A significantly higher proportion of women with ART live births in Massachusetts had more than a high school education than women with ART live births in Michigan (91.0% vs. 73.5%, p = 0.02); also, Massachusetts ART users were slightly older than Michigan ART users (35.9 vs. 34.3 years, p < 0.01) (data not shown).
Table 1.
Characteristics of Women Who Delivered Live Births by Assisted Reproductive Technology Status, Massachusetts and Michigan (N = 384,390)
| ART user% (n) N = 6675 | Nonuser% (n) N = 377,715 | p-valuea | |
|---|---|---|---|
| Mean age (years) | 35.4 (SD = 5.0) | 28.1 (SD = 6.1) | <0.001 |
| Education (years) | |||
| High school or less | 14.7 (979) | 55.0 (206,767) | <0.001 |
| More than high school | 85.3 (5675) | 45.0 (169,098) | |
| Race/ethnicity | |||
| Non-Hispanic White | 86.6 (5770) | 72.3 (272,785) | <0.001 |
| Other | 13.4 (892) | 27.7 (104,609) | |
| Prenatal care | |||
| First trimester | 89.2 (5841) | 78.2 (286,128) | <0.001 |
| Second trimester or later | 10.8 (705) | 21.8 (79,851) | |
| Parity | |||
| 0 | 54.0 (3580) | 42.4 (159,442) | <0.001 |
| 1 or more | 46.0 (3052) | 57.6 (216,817) |
Statistical significance by ART status determined using t-test for maternal age and chi-square test for categorical variables (p < 0.05).
ART, assisted reproductive technology; SD, standard deviation.
Prepregnancy, prenatal smoking, and birth outcomes as reported on the birth certificate
Based on BC data for all live births, prepregnancy smoking prevalence was higher in births in nonusers than ART users overall (16.7% vs. 3.2%, p < 0.05) and by state (Fig. 1). After adjustment for maternal age, race/ethnicity, education, parity, trimester of entry into prenatal care, and state, the prepregnancy smoking prevalence in nonusers was 2.7 times higher than ART users (aPR = 2.67, 95% CI: 2.35–3.04). Similarly, prenatal smoking was higher in births in nonusers than ART users overall (11.1% vs. 1.0%, p < 0.05) and by state (Fig. 2). After adjustment for maternal age, race/ethnicity, education, parity, trimester of entry into prenatal care, and state, the prenatal smoking prevalence in nonusers was 4.3 times higher than ART users (aPR = 4.25, 95% CI: 3.25–5.39). Among those who smoked prepregnancy, the percentage who quit smoking during pregnancy was higher for births of ART users than nonusers (68.9% vs. 33.7%, p < 0.05).
FIG. 1.
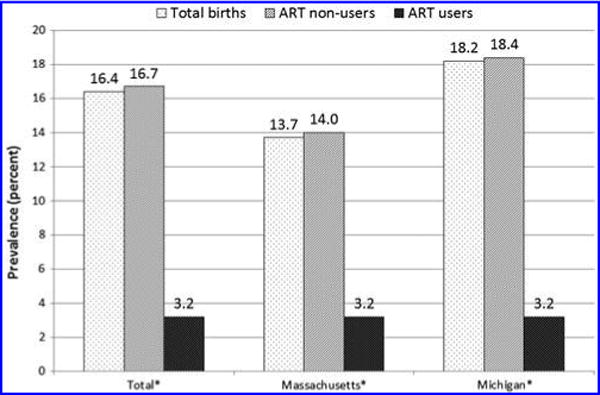
Prepregnancy smoking prevalence by ART status, Massachusetts and Michigan. Prepregnancy smoking prevalence defined as any smoking in the one year before pregnancy from the birth certificate only. *Difference in prevalence estimates between ART users and nonusers assessed by chi-square tests, overall, and by state (p < 0.05). ART, assisted reproductive technology.
FIG. 2.
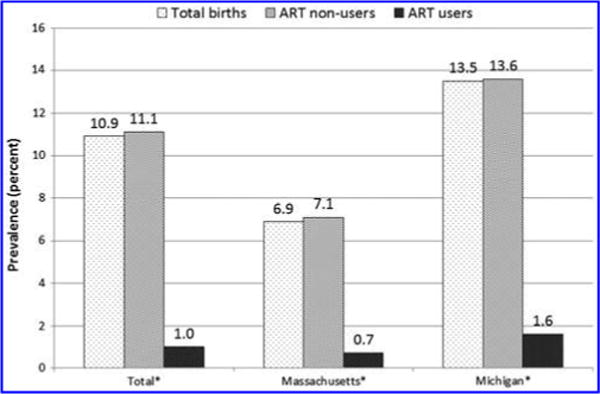
Prenatal smoking prevalence by ART status, Massachusetts and Michigan. Prenatal smoking prevalence defined as any smoking during pregnancy from the birth certificate only. *Difference in prevalence estimates (birth certificate only) between ART users and nonusers assessed by chi-square tests, overall, and by state (p < 0.05).
Of 392,248 infants, 364,974 (93.0%) were singletons with plausible gestational ages and birthweights. Prevalence of having a preterm infant was not statistically different between prenatal smokers and nonsmokers among ART users (14.8% vs. 11.0%, p = 0.37). Prevalence of having a preterm infant was significantly higher for prenatal smokers than for nonsmokers among ART nonusers (10.1% vs. 7.2%, p < 0.01) and remained significant after adjustment. Significant differences in mean birthweight among term singletons were seen for women who smoked during pregnancy compared to nonsmokers for both ART users (−210 g, p < 0.01) and ART nonusers (−193%, p < 0.01). This remained significant after controlling for maternal characteristics for ART users (−207 g, p < 0.01) and ART nonusers (−191%, p < 0.01).
Prepregnancy smoking reported on BC and/or NASS for ART users
Of the 6675 records of ART users, smoking information was missing on NASS alone (n = 1483), BC alone (n = 31), or both sources (n = 7) for a total of 23% of available records. In NASS, the percentage of unknown smoking status decreased each year, and the majority of unknowns (61%) were from fertility cycles started in 2007 when smoking was first being collected, compared with 31% missing for 2008 and 8% for 2009.
Of the 5168 ART users with known smoking status from NASS, 7.7% (n = 399) of women reported smoking at least 100 cigarettes in their lifetime based on NASS data. Prepregnancy smoking prevalence in the 12 months before pregnancy was 3.1% using BC and in the 3 months before pregnancy was 4.7% using NASS (Fig. 3), indicating that the NASS estimate was at least 52% higher than the BC estimate. Overall and in Michigan, prepregnancy smoking prevalence among ART users was significantly higher on NASS compared with the BC (t-tests, p < 0.05).
FIG. 3.
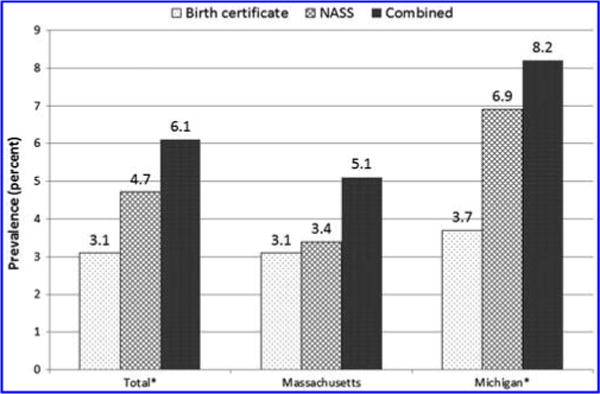
Prepregnancy smoking prevalence by state and data source among ART users, Massachusetts and Michigan 2008–2009. On Massachusetts’ birth certificate, prepregnancy smoking was determined from whether a woman reported smoking at least one cigarette daily in the year before pregnancy. On Michigan’s birth certificate, prepregnancy smoking was determined if a woman reported smoking before or during pregnancy and did not report a quit date or if a woman reported smoking before or during pregnancy and the quit date was 2 years before delivery year. In NASS, prepregnancy smoking was determined from whether a woman reported smoking at least 100 cigarettes in her lifetime and whether she smoked in the 3 months before pregnancy. The combined estimate of smoking was defined as women who reported smoking on BC, NASS, or both sources in the year before pregnancy, including records with unknown smoking status in one data source and known smoking status in the other data source. *Difference in prevalence estimates between birth certificate and NASS using two-way t-tests (p < 0.05). BC, birth certificates; NASS, National ART Surveillance System.
Among ART users who reported smoking in the year before pregnancy, 197 (55% of the total identified smokers) were identified using NASS only, 116 (32%) from BC only, and 46 women (13%) from both data sources (i.e., BC and NASS) (Supplementary Table S2). A higher proportion of women who reported smoking on the BC only were nulliparous compared with women who reported smoking on NASS only (p = 0.03) (data not shown). In addition, more women who reported smoking on the BC only smoked more than a half a pack a day compared with women who reported smoking on NASS only (23% vs. 0%) (based on Massachusetts data; not shown). The combined estimate of smoking prevalence (using smoking from NASS or BC) in the year before pregnancy was 6.1%, and it was higher in Michigan than Massachusetts (8.2% vs. 5.1%, p < 0.05). When accounting for women who may have quit smoking more than 3 months before pregnancy, smoking was 4.4%, and it was higher for Michigan than Massachusetts (6.5% vs. 3.4%, p < 0.05).
For ART users (using combined data from NASS and BC for ART users only), the prevalence of prepregnancy smoking was higher among women who had a high school education or less (13.5% vs. 4.9%), were non-Hispanic White (6.6% vs. 3.3%), or entered prenatal care in the second trimester or later (8.8% vs. 5.8%), compared to their counterparts (Table 2). For nonusers (BC only), the prevalence of prepregnancy smoking was higher among women <35 years (18.1% vs. 9.1%), had a high school education or less (24.6% vs. 7.0%), were non-Hispanic White (18.6% vs. 11.5%), or entered prenatal care in the second trimester or later (23.1% vs. 14.8%), compared to their counterparts. In the adjusted analyses, ART users who had a high school education or less (aPR = 2.62, 95% CI: 2.28–3.01) were non-Hispanic White (aPR = 2.08, 95% CI: 1.56–2.77), sought prenatal care later (aPR = 1.42, 95% CI: 1.06–1.90), and were more likely to smoke prepregnancy than their counterparts.
Table 2.
Prevalence and Adjusted Prevalence Ratios of Prepregnancy Smoking by Sociodemographic Characteristics and Assisted Reproductive Technology Status, Massachusetts and Michigan
|
ART users (N = 6675)a
|
ART nonusers (N = 377,715)
|
|||
|---|---|---|---|---|
| Prevalence% | aPR (95% CI) | Prevalence% | aPR (95% CI) | |
| Age (years)b | ||||
| <35 | 6.5 | Ref | 18.1 | Ref |
| 35+ | 5.9 | 1.05 (0.87–1.28) | 9.1 | 0.68 (0.67–0.70) |
| Educationb,c | ||||
| High school or less | 13.5 | 2.62 (2.28–3.01) | 24.6 | 3.64 (3.57–3.71) |
| More than high school | 4.9 | Ref | 7.0 | Ref |
| Race/ethnicityb,c | ||||
| Non-Hispanic White | 6.6 | 2.08 (1.56–2.77) | 18.6 | 2.08 (2.04–2.12) |
| Other | 3.3 | Ref | 11.5 | Ref |
| Prenatal careb,c | ||||
| First trimester | 5.8 | Ref | 14.8 | Ref |
| Second trimester or later | 8.8 | 1.42 (1.06–1.90) | 23.1 | 1.31 (1.29–1.33) |
| Parity | ||||
| 0 | 6.7 | 1.24 (0.92–1.68) | 16.6 | 0.98 (0.96–0.99) |
| 1 or more | 5.5 | Ref | 16.7 | Ref |
Adjusted for maternal age, education, race/ethnicity, trimester of entry into prenatal care, parity, and state.
Prepregnancy smoking prevalence of ART users is the combined prevalence that uses smoking from NASS or BC and includes records with unknown smoking status in one data source, but known smoking status in another data source.
Difference in smoking prevalence by characteristics among ART nonusers based on chi-square test (p < 0.05).
Difference in smoking prevalence by characteristics among ART users based on chi-square test (p < 0.05). aPR, adjusted prevalence ratio; NASS, National ART Surveillance System; Ref, reference group.
Discussion
In our study, we found that in two states, the maternal smoking prevalence among births of women undergoing ART based on BC data in the year before pregnancy was 3.2% and smoking during pregnancy was 1.0%. These estimates of maternal smoking in ART users were much lower than the maternal smoking prevalence that we found among nonusers (16.7% and 11.1%, respectively). ART users have demographic characteristics that put them at lower risk of smoking, such as being older and having more years of education.2 In addition, ART users are trying to get pregnant and may have more time and be more likely to adopt healthy behaviors, such as smoking cessation.11 We found that more than two-thirds of ART users who had live births were more likely to quit smoking during pregnancy compared with nonusers. Nonetheless, a low, but substantial proportion of ART users reported smoking before conception and should be counseled on the importance of tobacco cessation before starting the ART treatment cycle. Based on our study data, women who smoked before pregnancy and underwent ART had fewer years of education and sought prenatal care later, and these women could be targeted for tobacco cessation intervention (e.g., provider advice and counseling).
Although smoking prevalence during pregnancy was low, we found evidence for adverse effects of smoking on infant outcomes in ART births that were consistent with the effects seen in births in nonusers. Specifically, differences in birth-weight and preterm delivery between smokers and nonsmokers were similar, although the association between smoking and preterm delivery was not significant in births to ART users, perhaps because of the small sample size. This finding is consistent with a substantial body of evidence supporting causal relationships between smoking and increased risk of fetal growth restriction and preterm delivery.1 Considering the adverse impact of smoking on reproductive health and that ART users are highly motivated to get pregnant, efforts to educate all women of reproductive age that smoking can decrease fertility should be intensified. The Practice Committee of the American Society for Reproductive Medicine encourages all clinicians to educate women on the harms of tobacco and to provide cessation support whenever possible.12
As noted earlier, prepregnancy smoking prevalence ranged from 3.2% based on BC data alone to 6.1% when smoking information from both data sources—BC and NASS—are combined. Because the time frame for smoking prevalence differs by data source (NASS and for each state’s BC), these data should be interpreted with caution. When accounting for women who may have quit smoking at least 3 months before the ART cycle, prepregnancy smoking prevalence was 4.4%. We found 116 additional women identified as smokers in the year before pregnancy from the BC alone and 197 women who were identified as smokers in the 3 months prior on NASS. Women who reported smoking on the BC only were heavier smokers than those who reported on NASS, suggesting possible nondisclosure of the smoking prevalence in the NASS system. In Massachusetts, one health insurance policy did not cover ART for women who are actively smoking cigarettes and/or are using nicotine-containing products.13 However, we found that among ART patients, those who are lighter smokers reported smoking in the NASS data and not on the BC; this suggests that ART patients may be more likely to disclose smoking to fertility specialists, but are not being captured as smoking in the prior year on the BC. Thus, the contribution of instrument or recall bias may be considerable for the BC, such that the smoking prevalence in the year before pregnancy from the BC is likely an underestimate for women undergoing ART. These findings are useful in understanding the quality of the prepregnancy smoking data in NASS and BC. For studies examining associations of prepregnancy smoking among ART-conceived infants, combining smoking data from NASS and BC may help to identify more smokers. Additional validation studies may be needed to assess the quality of smoking in both data sources.
The strength of this study is that we assess the prevalence of smoking before pregnancy using two data sources and with more complete information on ART use for births in the population. This study is not without limitations. First, the smoking data in both data sources are self-reported and not biochemically verified; thus, our estimates may still underestimate the true smoking prevalence. The nondisclosure rate of self-reported smoking compared with biochemical verification has been estimated to be about 9% in nonpregnant women and 23% in pregnant women in the US.5 It is unclear how nondisclosure may differ in a population of women undergoing ART. In women with polycystic ovary syndrome undergoing fertility treatments, the nondisclosure rate was 10%.14 Although there was a high percentage missing when smoking status was first implemented in NASS, more smokers were ascertained in the 2008 birth year than the 2009 birth year (145 vs. 99 smokers) using NASS data. We attempted to address underreporting by combining self-reported smoking information from both NASS and BC. Second, as noted earlier, there were differences in smoking questions and time frame by state and NASS. Although we developed an algorithm to define the smoking measures used by each state and NASS, misclassification of smoking status may still exist. Third, our sample size of smokers offers limited power to test for differences in ascertainment by data source. Finally, these results are not generalizable outside of the two study states or to women who underwent ART and did not have a live birth.
In conclusion, a low, but substantial proportion of ART users reported smoking before conception. The combined smoking prevalence in the year before pregnancy among women who had a live birth after ART is at least 6% in our two study states. As expected, combining smoking information from NASS and BC identified more smokers than using either data source alone. Continued enhancements to data collection in NASS, such as better data collection instructions, are needed to increase ascertainment and accuracy of the smoking information. In the 2016 NASS data collection year, the number of smoking questions has been reduced to improve nonresponse and validity. Given that NASS has detailed information on all ART cycles and cycle outcomes in the US, future studies can explore the effects of smoking on ART outcomes, such as cycle cancellations, clinical pregnancies, and risk of adverse outcomes, such as ectopic pregnancies. A low, but substantial proportion of ART users reported smoking before conception, and these women should be counseled on the importance of smoking cessation before starting the ART treatment cycle. Considering the adverse impact of smoking on fertility, it should be clearly communicated to patients undergoing ART that smoking can decrease fertility, as well as adversely affect pregnancy outcomes, and patients should be provided effective cessation support to help them quit smoking and maintain tobacco abstinence.
Supplementary Material
Acknowledgments
We would like to acknowledge the SMART Collaborative: Karyn Backus, MPH (Connecticut Department of Public Health, Hartford, Connecticut), Lloyd Mueller, PhD (Connecticut Department of Public Health, Hartford, Connecticut), Carol Stone, PhD, MPH, MA (Connecticut Department of Public Health, Hartford, Connecticut), Dana Bernson, (MPH Massachusetts Department of Public Health, Boston, Massachusetts), Bruce Cohen, PhD (Massachusetts Department of Public Health, Boston, Massachusetts), Hafsatou Diop, MD (Massachusetts Department of Public Health, Boston, Massachusetts), Glenn Copeland, MBA (Michigan Department of Health and Human Services, Lansing, Michigan), Patricia McKane, DVM, MPH (Michigan Department of Health and Human Services, Lansing, Michigan), Michael Mersol-Barg, MD (Center for Reproductive Medicine and Surgery, Birmingham, Michigan), Russell Kirby, PhD (University of South Florida, Tampa), William Sappenfield, PhD (University of South Florida, Tampa), Marie Bailey, MA, MSW (Florida Department of Health, Tallahassee, Florida), Dmitry Kissin, MD, MPH (Centers for Disease Control and Prevention, Atlanta, Georgia), Sheree Boulet, DrPH, MPH (Centers for Disease Control and Prevention, Atlanta, Georgia), Jeani Chang, MPH (Centers for Disease Control and Prevention, Atlanta, Georgia), Sara Crawford, PhD (Centers for Disease Control and Prevention, Atlanta, Georgia), Denise Jamieson, MD, MPH (Centers for Disease Control and Prevention, Atlanta, Georgia), Aniket Kulkarni, MBBS (Centers for Disease Control and Prevention, Atlanta, Georgia), Saswati Sunderam, PhD (Centers for Disease Control and Prevention, Atlanta, Georgia), and Yujia Zhang, PhD (Centers for Disease Control and Prevention, Atlanta, Georgia).
Financial support for this analysis was provided by the Centers for Disease Control and Prevention, Department of Health and Human Services.
Footnotes
Disclaimer
The findings and conclusions are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.CDC. The Health Consequences of Smoking: 50 Years of Progress. Atlanta, GA: US Department of Health and Human Services, CDC; 2014. [Google Scholar]
- 2.Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Barfield WD. Assisted reproductive technology surveillance—United States, 2011. MMWR Surveill Summ. 2014;63:1–28. [PubMed] [Google Scholar]
- 3.Waylen AL, Metwally M, Jones GL, Wilkinson AJ, Ledger WL. Effects of cigarette smoking upon clinical outcomes of assisted reproduction: A meta-analysis. Hum Reprod Update. 2009;15:31–44. doi: 10.1093/humupd/dmn046. [DOI] [PubMed] [Google Scholar]
- 4.Mneimneh AS, Boulet SL, Sunderam S, et al. States Monitoring Assisted Reproductive Technology (SMART) Collaborative: Data collection, linkage, dissemination, and use. J Womens Health (Larchmt) 2013;22:571–577. doi: 10.1089/jwh.2013.4452. [DOI] [PubMed] [Google Scholar]
- 5.Dietz PM, Homa D, England LJ, et al. Estimates of nondisclosure of cigarette smoking among pregnant and nonpregnant women of reproductive age in the United States. Am J Epidemiol. 2011;173:355–359. doi: 10.1093/aje/kwq381. [DOI] [PubMed] [Google Scholar]
- 6.Kharrazi M, Epstein D, Hopkins B, et al. Evaluation of four maternal smoking questions. Public Health Rep. 1999;114:60–70. doi: 10.1093/phr/114.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong VT, Dietz PM, Farr SL, D’Angelo DV, England LJ. Estimates of smoking before and during pregnancy, and smoking cessation during pregnancy: Comparing two population-based data sources. Public Health Rep. 2013;128:179–188. doi: 10.1177/003335491312800308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Cohen B, Macaluso M, Zhang Z, Durant T, Nannini A. Probabilistic linkage of assisted reproductive technology information with vital records, Massachusetts 1997–2000. Matern Child Health J. 2012;16:1703–1708. doi: 10.1007/s10995-011-0877-7. [DOI] [PubMed] [Google Scholar]
- 9.Bieler GS, Brown GG, Williams RL, Brogan DJ. Estimating model-adjusted risks, risk differences, and risk ratios from complex survey data. Am J Epidemiol. 2010;171:618–623. doi: 10.1093/aje/kwp440. [DOI] [PubMed] [Google Scholar]
- 10.Talge NM, Mudd LM, Sikorskii A, Basso O. United States birth weight reference corrected for implausible gestational age estimates. Pediatrics. 2014;133:844–853. doi: 10.1542/peds.2013-3285. [DOI] [PubMed] [Google Scholar]
- 11.Tong VT, Dietz PM, Morrow B, et al. Trends in smoking before, during, and after pregnancy—Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000–2010. MMWR Surveill Summ. 2013;62:1–19. [PubMed] [Google Scholar]
- 12.Smoking and infertility: A committee opinion. Vol. 98. Fertil Steril; 2012. pp. 1400–1406. [DOI] [PubMed] [Google Scholar]
- 13.Tufts Health Plan. Medical Necessity Guidelines: Infertility Services-Massachusetts. Available at: www.tuftshealthplan.com/providers/pdf/mng/infertility_services_ma.pdf Accessed December 11, 2014.
- 14.Legro RS, Chen G, Kunselman AR, et al. Smoking in infertile women with polycystic ovary syndrome: Baseline validation of self-report and effects on phenotype. Hum Reprod. 2014;29:2680–2686. doi: 10.1093/humrep/deu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


