Abstract
Background:
Traditionally GS is used to treat diabetes mellitus. Drug-herb interaction of GS via cytochrome P450 enzyme system by substrate cocktail method using HLM has not been reported.
Objective:
To evaluate the in-vitro modulatory effects of GS extracts (aqueous, methanol, ethyl acetate, chloroform and n-hexane) and deacylgymnemic acid (DGA) on human CYP1A2, 2C8, 2C9, 2D6 and 3A4 activities in HLM.
Material and Methods:
Probe substrate-based LCMS/MS method was established for all CYPs. The metabolite formations were examined after incubation of probe substrates with HLM in the presence or absence of extracts and DGA. The inhibitory effects of GS extracts and DGA were characterized with kinetic parameters IC50 and Ki values.
Results:
GS extracts showed differential effect on CYP activities in the following order of inhibitory potency: ethyl acetate > Chloroform > methanol > n-hexane > aqueous > DGA. This differential effect was observed against CYP1A2, 2C9 and less on CYP3A4 and 2C8 but all CYPs were unaffected by aqueous extract and DGA. The ethyl acetate and chloroform extract exhibited moderate inhibition towards CYP1A2 and 3A4. The aqueous extract and DGA however showed negligible inhibition towards all five major human CYPs with very high IC50 values (>90μg/ml).
Conclusion:
The results of our study revealed that phytoconstituents contained in GS, particularly in ethyl acetate and chloroform extracts, were able to inhibit CYP1A2, 3A4 and 2C9. The presence of relatively small, lipophillic yet slightly polar compounds within the GS extracts may be attributed for inhibition activities. These suggest that the herb or its extracts should be examined for potential pharmacokinetic drug interactions in vivo.
Abbreviations used: GS: Gymnema sylvestre, GSE: Gymnema sylvestre extract, DGA: deacyl gymnemic acid, CYP: cytochrome P450, DMSO: dimethylsulphoxide, HLM: human liver microsomes, LC-MS/MS: liquid chromatography tandem mass spectroscopy, NADPH: reduced nicotinamide adeninedinucleotide phosphate, NRS: nicotinamide adeninedinucleotide phosphate regenerating system, CHE: chloroform extract, EAE: ethyl acetate extract, NHE- n-hexane extract, AE: aqueous extract, ME: methanol extract
Keywords: CYPs inhibition, drug–herb interaction, Gymnema sylvestre, human liver microsomes, LC-MS/MS
INTRODUCTION
Herbal medicines are often used with modern drugs with the aim to minimize side effects/toxicity or to obtain a synergistic/additive effect in terms of pharmacological activities. This is probably one of the major reasons for which herbal drugs are gaining popularity. However, it has been reported that herbal products containing a number of natural compounds may cause pharmacokinetic interaction with modern medicines particularly in combination.[1,2]
The human cytochrome P450s (CYPs) are a superfamilies of heme-containing mono-oxygenases, and their expression of individual P450s is regulated by both endogenous factors and foreign compounds including drugs and natural compounds.[3] There are many human CYP superfamiles identified, but enzymes namely from CYP1, CYP2 and CYP3 families are known to catalyze the biotransformation of most of the clinically accepted drugs.[4] The five major human CYPs e.g. CYP1A2, 2D6, 2C8, 2C9, and 3A4 which are well known for their important role in human drug metabolism were chosen for this study.
CYP3A4 is the most abundant among human CYPs found in human liver (~40%) metabolizing more than 50% of the drugs marketed in the world,[5] which includes cancer chemotherapeutic drugs e.g. vinca alkaloids, irinotecan and also other widely used drugs such as atorvastatin, miodarone among others.[6] CYP2C subfamily accounting for approximately 16–20% of the CYP-mediated biotransformation.[7] Members of this subfamily include CYP2C8, 2C9, 2C18 and 2C19.[8] CYP2C9, the major member of this subfamily metabolizes more than 16% of the clinically used drugs, including hypoglycemic agents like tolbutamide, glipizide and so on. On the contrary, CYP2C8 has important role in the metabolic clearance of paclitaxel, amodiaquine, retinoic acid.[10] Although the hepatic expression levels of CYP2D6 is low still it is reported to be involved in the metabolism of the most commonly prescribed pharmaceuticals like the tricyclic antidepressants and selective serotonin re-uptake inhibitor (SSRI) e.g. fluoxetine.[11]
CYP1A2 is another important enzyme, which is responsible for first-stage detoxification of xenobiotics like ondansetron and amitryptyline.[12] Since CYPs play important roles in drug metabolism, co-administration of herbal preparations with conventional drugs which are otherwise CYP substrates may alter the metabolism of the latter.[1] Herbal preparations are usually considered to be safe because of their natural origin. Although, many studies have been reported for interaction between herbs and conventional medicines,[13] one such example is St. John's Wort, which is widely used to treat depression is known to decrease the efficacy of drugs such as cyclosporine and indinavir by inducing hepatic CYP3A4.[14] Many case reports claims events of irregular bleeding and unwanted pregnancies with St. John's Wort being taken simultaneously with ethinylestradiol.[15] Ginkgo biloba, has been found to inhibit CYP2C9 significantly and to a lesser extent, CYP1A2, CYP2E1 and CYP3A4 enzymes despite its claims for beneficial effects on the vascular system, memory, cognition, and gene regulation.[16] Similarly, other herbs like garlic (Allium sativum), liquorice (Glycyrrhiza glabra) and ginseng (Panax ginseng) are also reported to exhibit such CYP modulation. So far, a large number of literature and anecdotal reports suggest that concomitant administration of herbal products and pharmaceuticals may affect human drug metabolism and significantly increase the risk of serious adverse reactions.[17,18] A comprehensive summary of the clinical relevance of herb interactions has been reported in 2006 by Williamson, touching on interactions of Berberis, cinchona bark, Garlic, Ginkgo, Red clover, St. John's Wort among others and valerian with various CYP isoforms.[19] Measurement of CYP inhibition during drug discovery process thus provides an early warning with regards to potential safety issues.[20] Unfortunately, so far only a few programs have been taken up to establish the safety and efficacy of herbal medicines as was originally proposed by the WHO Guidelines[21] for better risk assessment of herbal medicines.
MATERIALS AND METHODS
Chemicals and reagents
Tacrine, diclofenac sodium, dextromethorphan hydrobromide monohydrate, midazolam, paclitaxel, NADPH tetra sodium salt, and MgCl2 hexahydrate and miconazole all were purchased from Sigma–Aldrich (St. Louis, MI, USA). Deacyl gymnemic acid from Natural Remedies (Bangalore, India), Human liver microsomes (HLM) from BD Gentest (California, USA) and all HPLC-grade solvents from JT Baker were purchased. Milli Q water (resistivity of 18M.Ω cm) has been generated from Milli Q water purification system.
Instrumentation and chromatographic conditions
The liquid chromatography (LC) system consisting of LC-20ADvp pump (Shimadzu, Kyoto, Japan), CTC PAL (HTS) autosampler, and the mass spectrometer composed of turboion spray with atmospheric pressure ionization source (API-4000) (AB Sciex Instruments, Foster CA, USA) and detection and integration of peaks were performed using Analyst 1.4.2.
Probe substrate-based liquid chromatography tandem mass spectrometry (LC-MS/MS) method was established for all CYPs. LC-MS/MS analysis was performed on a phenyl hexyl cartridge (2 × 10 mm, 5 μm) from phenomenex (CA, USA) with a flow rate of 0.8 mL/min. The mobile phases were 2 mM ammonium acetate in water (A) and a mixture 0.1% formic acid, 90% acetonitrile and 9.9% water (B). The gradient elution program was as follows: first 18 s only A for washing and next 36 s 100% B and cycle time was 1.5 min.
Preparation of gymnema sylvestre extract
The plant was identified from Botanical survey of India (BSI), WB and India. The voucher specimen number is CNH/80/2013/TechII/49 and a specimen was preserved for future reference. The leaves of the plant were dried under shade and powdered using a mechanical grinder. The various GS extracts were prepared following the procedure described below. Dried raw material of GS leaves was grounded and soaked in distilled water for 24 h. The homogenized suspension was then boiled in temperature controlled water bath at 37° C and filtered through a What-man No. 1 filter paper. The volume of the filtrate was then reduced by evaporation and later spray-dried to make the aqueous extract (AE). The yield of the extract was typically 3.9–4.2% (w/w) in terms of dried starting materials. Fresh dried leaves were grounded for successive extraction in different organic solvents. GS powdered leaves was extracted for 48 h successively with n-hexane, chloroform, ethyl acetate and methanol. More specifically, the extraction was first carried out by mixing the powdered leaves with n-hexane for 24 h. The extracts were filtered through a What-man No. 1 filter paper and the solvents from the filtrates were removed by rotary evaporator under reduced pressure at 45°C and typically the yield of the extract was 1.8–2.5% (w/w). The remaining residue from the plant following the n-hexane extraction was subsequently re-extracted with chloroform, later filtered and evaporated under reduced pressure to generate the chloroform extract (CHE). Residue remaining after chloroform extraction was again subjected to ethyl acetate extraction following the above procedure and finally the residue after filtration of ethyl acetate extract (EAE) was re-extracted with methanol. Typical yields obtained were 1.6–2.4%, 3.1–4.1% and 4.8–5.3% for chloroform, ethyl acetate, and methanol extracts, respectively. All the extracts were kept at 4°C until further use and characterized by LC-MS/MS for the presence of DGA after hydrolysis of extract containing gymnemic acids.
Preparation of solutions of authentic and analytic samples
Accurately weighed deacyl gymnemic acid was dissolved in DMSO to prepare 2 mg/mL stock. This primary stock was further diluted in methanol to prepare 1000 ng/mL which was injected in LC-MS/MS as a reference standard. Different extracts of GS was dissolved in methanol to prepare 2 mg/mL stocks of individual extract and then these were run in LC-MS/MS [Figure 1].
Figure 1.
MRM profiles of chloroform extract and reference standard (LC-MS/MS data)
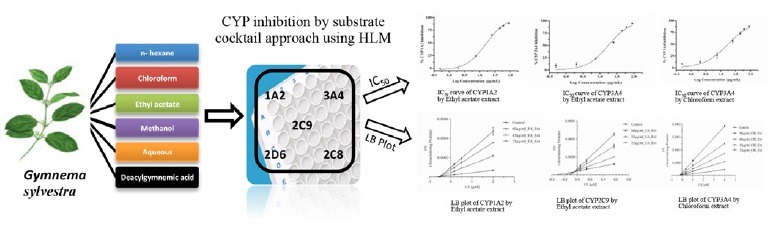
Enzyme assay by cocktail approach
In this study, a LC-MS/MS based CYP inhibition assay was developed to evaluate the modulatory activity of GS extracts on five major human CYPs. The assay protocol was similar to the previously published report[22,23] and the final concentration of organic solvent in incubation mixture was kept less than 1% v/v (>1% may cause false positive inhibition). In cocktail CYP inhibition assays, tacrine, midazolam, diclofenac, dextromethorphan, and paclitaxel were used as probe substrates for CYP1A2, 3A4, 2C9, 2D6, and 2C8 at 2, 1, 7, 5 and 5 μM concentrations respectively. The incubations were performed using U-bottom, 96 deep well plates with a volume of 100 μl in each well. Briefly a 100 μL of incubation mixture contained HLM (0.1 mg/mL), NAPDH (1.3 mM) and MgCl2 (3.3 mM) solution in 100mM phosphate buffer pH 7.4. All reactions were initiated by addition of NADPH solution in a temperature controlled water bath at 37°C for 8 min. The reaction was terminated by addition of 100 μL ice-cold acetonitrile. After proper mixing and centrifugation at 4000 rpm for 15 min at 15°C, a 20 μL aliquot of supernatant was injected to LC-MS/MS. The peaks of metabolites (2-OH-tacrine, 4-OH-midazola, 4-OH diclofenac, dextrorphan and 6α-OH-paclitaxel) were monitored in LC-MS/MS.
Cyp inhibition studies by substrate-cocktail method
Five different substrates for corresponding five different major human CYPs were incubated with the HLM and various concentrations of DGA (0–90 μg/mL) and GS extracts (0–90 μg/mL) i.e. GS, n-hexane, ethyl acetate, chloroform, methanol, and aqueous extracts. Tacrine, midazolam, diclofenac, dextromethorphan, and paclitaxel were incubated for CYP1A2, 3A4, 2C9, 2D6 and 2C8 at 2, 2, 5, 5, and 5 μM concentrations, respectively. Different concentrations of GS extracts, DGA were tested and miconazole has been used as universal positive inhibitor for all CYPs. CYP inhibition (%) was calculated based on metabolite area in presence of test compound with respect to the negative control (solvent control where metabolite formation was maximum). Initially, concentration of extracts causing 50% inhibition of enzyme activity (IC50 values) were determined, followed by further determination of inhibition constants (Ki values) and mode of inhibition for those showing IC50 values of less than or equal to 50 μg/mL by Lineweaver–Burk plot.
Data analysis
Nonlinear regression analysis was employed to calculate IC50 values using the GraphPad Prism 5.0 (GraphPad Software, Inc. California, USA). The modes of inhibition were initially estimated graphically from the Lineweaver–Burk plots, and the apparent Ki values were later estimated using the secondary plots of the slopes of Lineweaver–Burk plots against individual inhibitor concentrations. These initial kinetics estimates were subsequently used to determine Ki values using nonlinear regression analysis by fitting different models of enzyme inhibition to the kinetic data using GraphPad Prism Enzyme Kinetics Module.
RESULTS
The inhibitory effect of five GS extracts and DGA on the human CYP1A2, CYP2D6, CYP2C9, CYP3A4 and CYP2C8 were investigated with their respective probe substrates. When the probe assays were established in our laboratory, they had been examined with regard to inhibition by the inhibitor probes, miconazole which served as positive control for all CYPs and Km for all these substrates were established. The obtained Km values for these probes were 2.41 ± 0.13, 5.03 ± 0.19, 4.54 ± 0.25, 2.02 ± 0.14 μM and 4.92 ± 0.31 for human CYP1A2, CYP2D6, CYP2C9, CYP3A4 and CYP2C8, respectively. These values were all comparable to the values reported by other investigators using the same probes.[23] This indicated that the assays can serve as valid markers to study the modulatory effects of GS extracts on the human CYPs. The activities of CYP2D6 and 2C8 were unaffected by all types of extracts and DGA except methanolic extract which was found to inhibit CYP2C8 activity (IC50, 5.20 ± 0.38 μg/mL). The IC50 values for the different GS extracts were determined as shown in Table 1 and IC50 curve of different extract and were represented in Figure 2a–d.
Table 1.
Inhibitory potencies (mean ± SEM) of GS extracts on human CYPs.
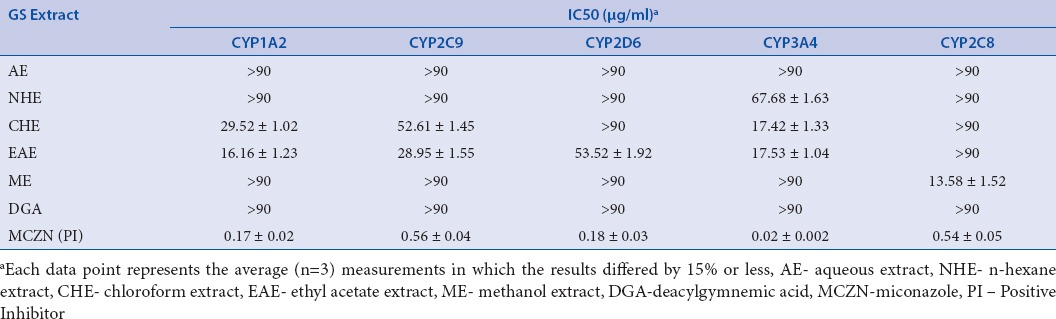
Figure 2.
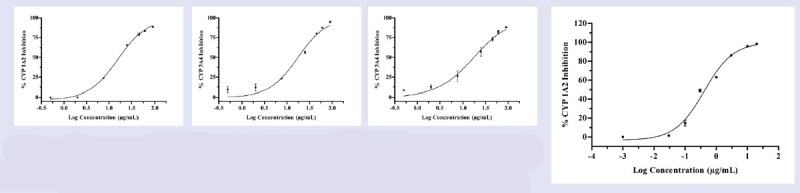
IC50 curve of CYP1A2 (Figure 2a), CYP3A4 (Figure 2b) inhibition by ethyl acetate extract and CYP3A4 (Figure 2c), and CYP1A2 (Figure 2d) inhibition by chloroform extract and miconazole, respectively.
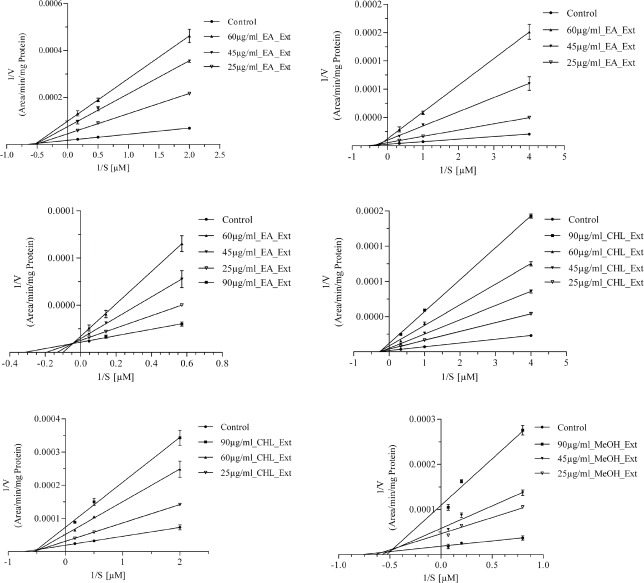
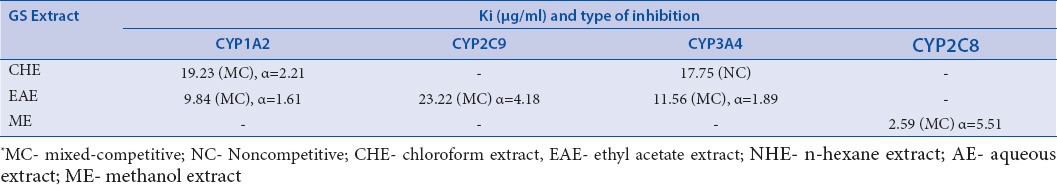
The type of inhibition were determined graphically from the Lineweaver–Burk plots [Figure 3a–f] and the apparent Ki values [Table 2] were later estimated using the slopes of Lineweaver–Burk plots against the concentrations of individual inhibitor.
Figure 3.
Lineweaver–Burk inhibition plots of CYP1A2 (Figure 3a), 3A4 (Figure 3b) and 2C9 (Figure 3c) by ethyl acetate extract and CYP3A4 (Figure 3d) and 1A2 (Figure 3e) by chloroform extract and CYP2C8 (Figure 3f) by methanol extract.
Table 2.
Inhibition constants and modes of inhibition (indicated in parenthesis) of CYP by GS extracts
Inhibition of human cyps by universal inhibitors
Miconazole known as positive inhibitor of CYPs (CYP1A2, 2C8, 2C9, 2D6 and 3A4) was used to confirm the selectivity of our assays at a range of 0.1–20 μg/mL. The IC50 values of positive inhibitor were found to be 0.89 μg/mL (1A2), 0.19 μg/mL (2C9), 0.56 μg/mL (2D6), 0.16 μg/mL (2C8) and less than 0.1 μg/mL (3A4). These values were all comparable to the values reported by other investigators using miconazole as positive inhibitor.[22,23]
DISCUSSION
The hepatic cytochrome P450 enzymes are prone to induction and inhibition by exposure to a wide variety of xenobiotics. Besides conventional drugs and chemicals, these enzymes are also affected by multiple active constituents of food and medicinal plants/herbs. Many compounds isolated from herbs have been identified as substrates, inhibitors and/or inducers of various CYP enzymes. Accordingly, we have taken up this study wherein various GS extracts and DGA have been investigated for their modulatory effects on the catalytic activities of CYP1A2, 3A4, 2C9, 2D6 and 2C8. This is particularly important as GS is widely and if not, frantically used by millions of diabetic sufferers where the herb alone or also in combination with conventional drugs is administered/self administered owing to the severity of the disease – diabetes. It can also be mentioned that limited information is currently available regarding the interactions of GS with clinically prescribed drugs. This is an issue of potential safety concern as GS is commonly consumed by hyperglycemic patients alone or in combination with other herbs/drugs. Small molecules or drugs commonly used by a diabetic patients are lovastatin, doxorubicin etc. (CYP3A4 substrates), diclofenac, tolbutamide (CYP2C9 substrates), phenacetin (CYP1A2 substrate), domperidone (CYP2C8 substrates), which are used clinically to treat different diseases. Furthermore, these patients may be inflicted with other comorbidities like hypertension and hypercholesterolemia that may require them to take drugs such as irbesartan, losartan, atorvastatin, simvastatine which are otherwise one or more CYP substrates. Common infectious diseases may necessitate the use of antimicrobials that modulate CYP activities such as isoniazid, rifampicin (for tuberculosis), and some protease inhibitors for HIV infection and macrolide antibiotics for respiratory and urogenital infections. Therefore, there may be potential risks of interaction when any or some of these drugs is taken together with GS as part of the remedies in the event of above-mentioned clinical emergencies.
In this study, in-vitro experiments were performed to test the inhibitory effects of various extracts (polar to nonpolar) prepared from GS towards five major human CYPs. Extracts in different solvents were investigated because there might be compounds with different solubility present in GS that are able to modulate CYP activity. Hence, studying extracts in both polar (aqueous and methanol) and nonpolar (ethyl acetate and chloroform) solvents allows a comprehensive characterization of possible constituents involved in CYPs modulation. Our data showed that the extracts exhibited differential modulatory effects on the CYP enzymes. Nonpolar extracts (chloroform and ethyl acetate) exhibited potent inhibition of CYP 1A2 and 2C9 as compared to AE and DGA. Many constituents present in this herb are lipophillic in nature[24] and may account for the inhibitory effect observed ethyl acetate and chloroform extracts in this study. In fact, existing reports are of the opinion that many flavonoids and phenolics are inhibitors for CYP enzymes, where CYP isoforms like 2C9 and 3A4 are the most important two CYPs.[25,26]
Furthermore, the inhibitory effects on CYP2C8 by MeOH extract were intriguingly strong with mixed type inhibition showing Ki value of almost 2.59μg/ml and α = 5.51. Ki is the equilibrium constant for inhibitor binding to enzyme. Lower Ki value indicates higher level of inhibition due to higher affinity to enzyme and vice versa. Alpha (α) is the factor which denotes the effect (increase or decrease) on Km or Vmax or both parameters and it is inversely proportional to Ki. The observed variation in inhibition selectivity of the GS extracts towards different CYP subfamilies appears to be complicated. However, earlier reports in this context indicate that such variation might probably be determined by a combination of certain key structural features in the inhibitor molecules in GS extracts.[27] Binding to this combination of active site residues aligns the inhibitor compound(s) at the preferred site, resulting in inhibition. It is well known that CYP1A2 and CYP3A4 members have different binding preferences towards different ligands.[27] The CYP1A ligands are generally low or medium molecular weight molecules with a wide range of polarities whereas in that of CYP2C8 and CYP2C9, their ligands usually possess weak acidic properties with relatively high lipophilicity and come with multiple aromatic rings as well as one or two hydrogen bond-forming groups.[27,28] On the contrary, ligands for CYP3A4 with larger molecular weights which are mostly neutral lipophillic compounds characterized with aromatic ring systems.[28] It is thus likely that inhibitor compounds in GS extracts could possess structural features resembling the previously reported CYP1A2 and CYP2C9 ligands believed to be in favour of selective binding and inhibition of above stated CYPs rather than 2D6. The cavities of CYP1A2 and CYP2C9 are smaller than that of CYP3A4 and this has a major impact on the size of the ligands that could bind to the active site of CYPs.[29] Even though the cavity size of CYP2C8 is very nearer to CYP3A4, its enzyme pocket is much more sinuous and the binding space is much smaller compared with that of CYP3A4 and as a result, CYP2C8 has higher affinity towards large ligands.[29] On the basis of the discussion above, it is therefore likely that the interaction reported in this study would mainly involve hydrogen and hydrophobic binding interactions between the CYP active sites and relatively small, lipophillic yet slightly polar and/or nonpolar compounds within the GS extracts. These suggest that extracts of GS or different polyherbal formulations containing GS e.g. Baidyanath Madhumehari Churna, Shivayu Madhuhari Churna, Madhmehantak Churan etc.[30] should be investigated for unwanted herb-drug interactions clinically.
In conclusion, the results of our study revealed that phytoconstituents presented in GS, particularly in chloroform, n-hexane and ethyl acetate extracts were able to inhibit CYP1A2, 2C9 activities whereas moderate-to-mild modulatory activity was observed on CYP3A4 and 2C8. Therefore, the observed inhibition of CYP1A2 and CYP2C9 by the herb/herbal drug requires further detailed clinical correlation and perhaps necessary caution specifically in the events of concomitant use of this antidiabetic herb with known substrates of the said CYPs e.g. caffeine, clozapine, fluvastatin, glipizide, mefenamic acid and tolbutamide among others otherwise advised for chronic administration.
Financial support and sponsorship
Nil
Conflicts of interests
There are no conflicts of interest.
Acknowledgement
We express our sincere thanks to AICTE-RPS, New Delhi, India and UGC UPE-II for providing the financial support needed for this study.
REFERENCES
- 1.Ioannides C. Pharmacokinetic interactions between herbal remedies and medicinal drugs. Xenobiotica. 2002;32:451–78. doi: 10.1080/00498250210124147. [DOI] [PubMed] [Google Scholar]
- 2.Brazier NC, Levine MA. Drug–herb interaction among commonly used conventional medicines: a compendium for health care professionals. Am J Ther. 2003;10:163–32. doi: 10.1097/00045391-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Nebert DW, Nelson DR, Coon MJ, Estabrook RW, Feyereisen R, Fujii-Kuriyama Y, et al. The P450 superfamily: update on new sequences, gene mapping and recommended nomenclature. DNA Cell Biol. 1991;10:1–32. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- 4.Nebert DW, Russell DW. Clinical importance of the cytochromes P450. The Lancet. 2002;360:1155–32. doi: 10.1016/S0140-6736(02)11203-7. [DOI] [PubMed] [Google Scholar]
- 5.Danielson PB. The cytochrome P450 superfamily: biochemistry, evolution and drug metabolism in humans. Curr Drug Metab. 2002;3:561–32. doi: 10.2174/1389200023337054. [DOI] [PubMed] [Google Scholar]
- 6.Zhou S, Chan SY, Goh BC, Chan E, Duan W, Huang M, et al. Mechanism-based inhibition of cytochrome P4503A4 by therapeutic drugs. Clin Pharmacokinetics. 2005;44:279–32. doi: 10.2165/00003088-200544030-00005. [DOI] [PubMed] [Google Scholar]
- 7.Gray IC, Nobile C, Muresu R. A 2.4-megabase physical map spanning the CYP2C gene cluster on chromosome 10q24. Genomics. 1995;28:328–32. doi: 10.1006/geno.1995.1149. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein JA, de Morais SM. Biochemistry and molecular biology of the human CYP2C subfamily. Pharmacogenetics. 1994;4:285–32. doi: 10.1097/00008571-199412000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein JA. Clinical relevance of genetic polymorphisms in the human CYP2C subfamily. Br J Clin Pharmacol. 2001;52:349–32. doi: 10.1046/j.0306-5251.2001.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Totah RA, Rettie AE. Cytochrome P450 2C8: substrates, inhibitors, pharmacogenetics and clinical relevance. Clin. Pharmacol Ther. 2010;77:341–32. doi: 10.1016/j.clpt.2004.12.267. [DOI] [PubMed] [Google Scholar]
- 11.Jannetto PJ, Wong SH, Gock SB. Pharmacogenomics as molecular autopsy for postmortem forensic toxicology: genotyping cytochrome P450 2D6 for oxycodone cases. J Anal Toxicol. 2002;7:438–32. doi: 10.1093/jat/26.7.438. [DOI] [PubMed] [Google Scholar]
- 12.Otusaka Y, Kato RIE. Metabolism of Xenobiotic compound and cytochrome P4501A2. Foods Food Ingredients J Jpn. 2004;209:198–32. [Google Scholar]
- 13.Nicole CB, Mitchell AHL. Drug-herb interaction among commonly used conventional medicines: a compendium for health care professionals. Am J Ther. 2003;10:163–32. doi: 10.1097/00045391-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Henderson L, Yue QY, Bergquist C. St John's wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54:349–32. doi: 10.1046/j.1365-2125.2002.01683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwarz UI, Buschel B, Kirch W. Unwanted pregnancy on self medication with St. John's Wort despite hormonal contraception. Br J Clin Pharmacol. 2003;55:112–32. doi: 10.1046/j.1365-2125.2003.t01-1-01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaudineau C, Beckerman R, Welbourn S. Inhibition of human P450 enzymes by multiple constituents of the Ginkgo biloba extract. Biochem Biophys Res. 2004;318:1072–32. doi: 10.1016/j.bbrc.2004.04.139. [DOI] [PubMed] [Google Scholar]
- 17.Budzinski JW, Foster BC, Vandenhoek S. An in-vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomedicine. 2000;7:273–32. doi: 10.1016/S0944-7113(00)80044-6. [DOI] [PubMed] [Google Scholar]
- 18.Shord SS, Shah K, Lukose A. Drug-botanical interactions: a review of the laboratory, animal, and human data for 8 common botanicals. Integr Cancer. Ther. 2009;8:208–32. doi: 10.1177/1534735409340900. [DOI] [PubMed] [Google Scholar]
- 19.Williamson EM. Interactions between herb and conventional medicines: the role of cytochrome P450 enzymes and P-glycoprotein. Pharmacologyonline. 2006;2:200–32. [Google Scholar]
- 20.Kerns EH, Di Li. Drug-like properties: concepts, structure, design and methods from ADME to toxicity optimization. Boston: Academic Press, Amsterdam; 2008. [Google Scholar]
- 21.WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues. 2007 [Google Scholar]
- 22.Obach RS, Walsky RL, Venkatakrishnan K. The utility of in vitro cytochrome P450 inhibition data in the prediction of drug-drug interactions. J Pharmacol Exp Ther. 2006;316:336–32. doi: 10.1124/jpet.105.093229. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence HC, Michael JR, David R. In vitro drug interactions of cytochrome P450: An evaluation of fluorogenic to conventional substrates. Drug Metab Dispos. 2003;31:1005–32. doi: 10.1124/dmd.31.8.1005. [DOI] [PubMed] [Google Scholar]
- 24.Tiwari P, Mishra BN, Sangwan SN. Phytochemical and Pharmacological Properties of Gymnema sylvestre: An Important Medicinal Plant. Bio Med Res Int.Article ID. 2014 doi: 10.1155/2014/830285. 830285: 1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodek P, Tepla M, Krizkova J. Modulation of cytochrome P450 enzyme system by selected flavonoids. Neuroendocrinol Lett. 2009;30(Suppl 1):67–32. [PubMed] [Google Scholar]
- 26.Kimura Y, Ito H, Ohnishi R. Inhibitory effects of polyphenols on human cytochrome P4503A4 and 2C9 activity. Food Chem Toxicol. 2010;48:429–32. doi: 10.1016/j.fct.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 27.Lewis DFV. Human cytochromes P450 associated with the Phase-1 metabolism of drugs and other xenobiotics: a compilation of substrates and inhibitors of the CYP1, CYP2 and CYP3 families. Curr Med Chem. 2003;10:1955–32. doi: 10.2174/0929867033456855. [DOI] [PubMed] [Google Scholar]
- 28.Lewis DFV. Quantitative structure–activity relationships (QSARs) within human cytochromes P450 substrates of the CYP2 family. Toxicol in vitro. 2004;18:89–32. doi: 10.1016/s0887-2333(03)00134-6. [DOI] [PubMed] [Google Scholar]
- 29.Ma Q, Lu AYH. The challenges of dealing with promiscuous drug-metabolizing enzymes, receptors and transporters. Curr Drug Metab. 2008;9:374–32. doi: 10.2174/138920008784746337. [DOI] [PubMed] [Google Scholar]
- 30.Chandel HS, Pathak A K, Tailang M. Standardization of some herbal antidiabetic drugs in polyherbal formulation. Phcog Res. 2011;3:49–32. doi: 10.4103/0974-8490.79116. [DOI] [PMC free article] [PubMed] [Google Scholar]