Abstract
Background:
Colon cancer (CC) is the third commonly diagnosed cancer and the second leading cause of mortality in the US when compared to India where prevalence is less. Possible reason could be the vegetarian diet comprising spices used in curry powders. Researchers believe that 70% of the cases are associated with diet. Spices have inherited a rich tradition for their flavor and medicinal properties. Researchers have been oriented towards spices present in food items for their antitumorigenic properties.
Objective:
We investigated the effects of sambar as a preventive measure for 1,2-dimethyl hydrazine (DMH)-induced CC in Wistar albino rats.
Materials and Methods:
The animals were divided into three groups (n = 6) namely control, DMH, and sambar. At the end of the experimental period, the animals were killed using anesthesia and the colons and livers were examined.
Results:
All the treatment groups exhibited a significant change in the number of aberrant crypt foci (ACF). Sambar group showed a significant change in the colonic GSH when compared to both normal and DMH groups. A significant reduction in the liver GSH was noted in the sambar group. Only sambar group showed a significant change in the liver catalase levels when compared to DMH. There was a significant reduction in the colonic nitrite in the sambar-treated group; 2.94 ± 0.29 when compared to DMH control at 8.09 ± 1.32. On the contrary, a significant rise in the liver nitrite levels was observed in the sambar-treated rats.
Conclusion:
Sambar may prevent the risk of CC when consumed in dietary proportions.
SUMMARY
Consumption of sambar significantly reduced aberrant crypt foci in DMH-induced colon cancer model
Sambar treatment prevented DMH-induced oxidative changes in the colonic tissue, indicating its antioxidant role
Sambar comprises a variety of spices that exhibited both pro- and antioxidant properties in different tissues, leading to its overall beneficial effect in this model.
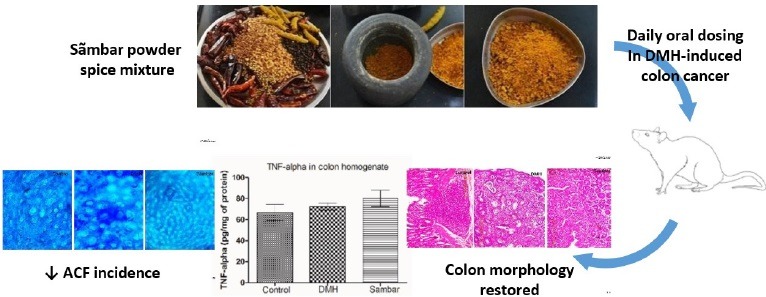
Abbreviations used: ACF: aberrant crypt foci, CC: colon cancer, DMH: 1,2-dimethyl hydrazine, GSH: glutathione, IL-6: Interleukin-6, TNF-α: Tumor necrosis factor-alpha.
Keywords: Aberrant crypt foci, dimethyl hydrazine, sambar, spices
INTRODUCTION
Cancer is a threat to the developed and developing nations and is the second most common cause of death. It results from abnormalities in normal mechanisms governing cell behavior. Cells being the smallest and most basic structural and functional unit of the human body undergo proliferation, differentiation, and several other biological processes to maintain the normal cycle of life. This whole phenomenon is controlled by various proteins and cell signaling mechanisms at the cellular and molecular level. Irregularity in any one step results in unregulated proliferation of cells. Thus, we can say that cancer represents a group of diseases that involves upregulated proliferation of cells leading to formation of tumor.
Sambar is a very popular South Indian dish that is eaten with rice and/or rice preparations like idlis, dosas, etc. Many versions of Sambar powder (ingredients differ) exist, varying from region to region. Indian spices that go into the making of this powder are coriander seeds, fenugreek seeds, turmeric rhizomes, black pepper, curry leaves, cumin seeds, and asafoetida. Spices are generally considered to be safe by the USFDA in the proportions used in food. In Asia, the intake of turmeric, a component of curry powder, has been reported to lower the incidence of colon cancer (CC).[1,2,3]
There are scientific reports stating the beneficial effects of Indian spices and condiments in cancer prevention.[4] CC is the third most commonly diagnosed cancer in the US, whereas in India the incidence is the lowest.[2,3] Almost 70% of the incidence of CC is linked to diet. This may also be attributed to lifestyle factors.[5] A possible explanation is the vegetarian diet comprising spices used in curry powders.[6] Hence, the study was proposed to investigate the activity of sambar in 1,2-dimethyl hydrazine (DMH)-induced CC models in albino rats.
MATERIALS AND METHODS
Chemicals
DMH was procured from Sigma-Aldrich Co. LLC, St. Louis, MO, USA. Glutathione, trichloroacetic acid, and 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB) were purchased from HiMedia Laboratories, Mumbai, Maharashtra, India. TNF-alpha kits (Catalog # KRC3011) were procured from Life Technologies, Carlsbad, CA, USA. Carboxy methyl cellulose was purchased from S D Fine-Chem Limited, Mumbai, Maharashtra, India. 5-fluorouracil was procured from Biochem Pharmaceuticals Industries Limited, Mumbai, Maharashtra, India. Potassium chloride and hydrogen peroxide (LR grade) were bought from Merck KGaA, Darmstadt, Germany. BCA reagent was from ThermoFisher Scientific India Pvt. Ltd., Mumbai, Maharashtra, India, and sodium nitrite was procured from LOBA Chemie Pvt. Ltd., Mumbai, Maharashtra, India.
Animals
Male Wistar rats inbred at Central Animal Research Facility, Manipal University, Manipal, were used in the study. Animals were acclimatized to the experimental room having temperature 23 ± 3°C, controlled humidity 75%, and 12:12 hour light and dark cycle. Rats were housed in sterile polypropylene cages containing sterile paddy husk as a bedding material. The animals were fed with autoclaved feed and water. Eight- to ten-weeks old male rats weighing 120 ± 10 g were used for the study. The animal care and handling was carried out in accordance to guidelines issued by Institutional Animal Ethics Committee (IAEC), Manipal University, Manipal. The protocol was approved by IAEC with reference no. IAEC/KMC/79/2014.
Grouping and dosing regimen
Animals were divided into three groups (n = 6) namely, normal control, DMH control, and sambar. The normal control received 0.25% CMC, p.o. CC in rats was induced using DMH as per our previously standardized protocol.[7] In brief, rats were pretreated with only sambar (6 mL/kg, p.o.) or vehicle (water; 6 mL/kg, p.o.) for an initial period of 8 weeks. After 8 weeks, DMH was administered weekly once for 20 weeks (dose of 20 mg/kg, i.p. for 10 weeks and thereafter 30 mg/kg, i.p. for next 10 weeks). Sambar/vehicle treatment was continued for an additional 20 weeks.
Composition of sambar
The ingredients present in sambar powder used for the study are listed below:
Dried long fruit of Capsicum annum var. annum (dried chilli pepper) = 1000 g
Dried seeds of Coriandrum sativum seeds (coriander seeds) = 1000 g
Cooked and dried unripe drupes of the Piper nigrum (black pepper) = 500 g
Dried seeds of Cuminum cyminum (cumin) = 400 g
Dried seeds of Trigonellafoenum graecum (fenugreek) = 30 g
Dried rhizome of Curcuma caesia (black turmeric) = 100 g
Oryza sativa var. ponni (rice) = 200 g
The above mentioned components were ground to a fine powder at Saraswathi Traders and Flour Mills, Chennai, Tamil Nadu, India (using Danish Type Flour Mill, Model No: 2059072, manufactured by Nilax Overseas, Rajkot, Rajasthan, India).
Next, 25 g of yellow pigeon peas cooked in pressure cooker for 15 min and 6 g of the sambar powder (Indian spices) were boiled for 10 min.
The sambar obtained was cooled and shaken well before administration to animals.
Parameters assessed in DMH-induced CC model
At the end of the study, the animals from each group were killed and the colons and livers dissected and perfused with an ice cold saline trans cardially. The tissues were blot dried, weighed, and 10% of homogenate was prepared with ice-cold (150 mM) potassium chloride solution using a homogenizer (RQ-127A/D, REMI House, Mumbai, Maharashtra, India). The homogenate was used for the following estimations, viz., GSH,[8] catalase,[9] nitrite,[10] and TNF-α.[11]
During the killing of animals, the entire colon was removed, opened longitudinally, and rinsed in saline. Cecum was excised. A piece of colon of 8 cm each was fixed on a filter paper in 10% buffered formalin for 24 hours and then stained with 0.1% methylene blue in phosphate buffer saline for 20 min for aberrant crypt foci (ACF) counting. Specimens were carefully examined, mucosal side uppermost, at 40x magnification under a compound light microscope and the number of ACF was determined. ACF incidence, polyps and ACF count, the colon length/weight ratio, and histopathological examinations were recorded.
Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) and significant differences among the treatment groups were evaluated by post-hoc Tukey's test. The results were considered significant at P < 0.05. All statistical analyses were done using fully functional demo of Prism version 5.03 (GraphPad Software Inc., San Diego, CA, USA).
RESULTS
The spleen and liver index
There were no significant changes in the liver index of the normal control, DMH, and sambar treatments [Figure 1A]. The same was true of the spleen index [Figure 1B]. These results indicate that DMH did not produce hepatomegaly or splenomegaly.
Figure 1.
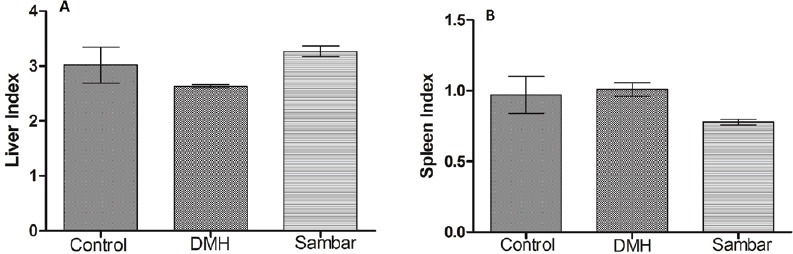
(A) Liver and (B) spleen indices of the normal, DMH, and pretreated sambar groups. Data is represented as mean ± SEM of six values
Colon L/W ratio
The normal control showed a significant change in the colon L/W ratio at 12.255 ± 0.718 when compared to DMH control at 9.476 ± 0.549 at P < 0.01. A significant change was noted in the colon L/W ratio of DMH control with respect to sambar group at 11.782 ± 0.320. No significant change was observed between normal and sambar treatments [Figure 2].
Figure 2.
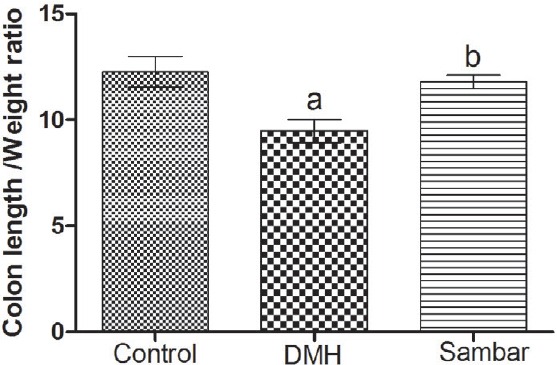
Colon L/W ratio of the normal, DMH, and pretreated sambar groups. aP < 0.01 versus control and bP < 0.05 versus DMH. Data is represented as mean ± SEM of six values
Number of ACF/5 cm2 and number of ACF/cm2
Treatment with DMH produced structural changes in colon, leading to the formation of ACF. Pretreatment with sambar along with DMH significantly (P < 0.01) reduced AFC [Figure 3A and B], indicating that sambar could protect against DMH-induced colonic changes.
Figure 3.
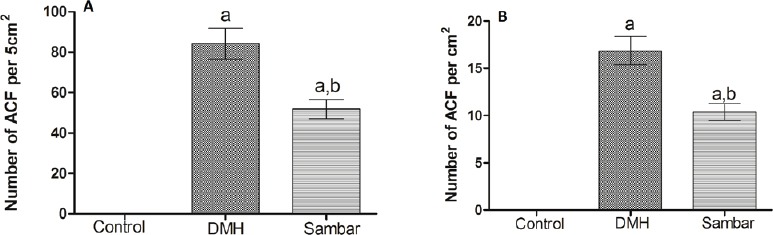
Number of aberrant crypt foci per (A) 5 cm2 and (B) cm2 in the normal, DMH, and pretreated sambar groups. aP < 0.001 versus control and bP < 0.01 versus DMH. Data is represented as mean ± SEM of six values
GSH in the liver and colon homogenate
There was no change in the colonic and liver GSH of normal control versus DMH control. Sambar group showed a significant change in the colonic GSH when compared to both normal and DMH control. A significant reduction in the liver GSH was noted at 0.98 ± 0.07 when compared to 7.229 ± 2.67 and 10.88 ± 0.93 of normal control and DMH controls, respectively [Figure 4A and D].
Figure 4.
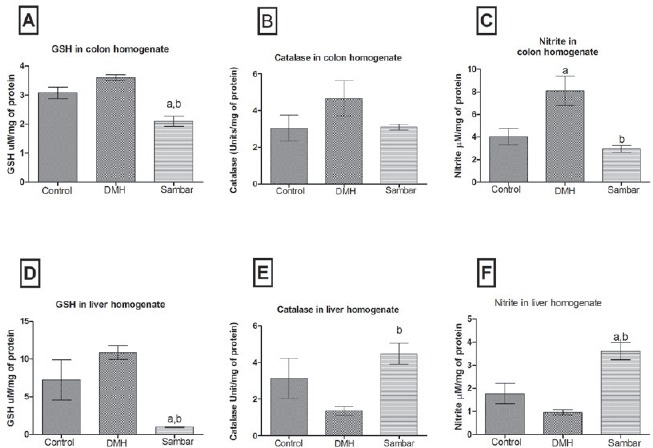
Glutathione (A), catalase (B), and nitrite (C) levels in the colon homogenate of the various groups. Glutathione (D), catalase (E), and nitrite (F) levels in the liver homogenate of the various groups. aP < 0.05 versus control and b P < 0.05 versus DMH. Data is represented as mean ± SEM of six values
Catalase in the liver and colon homogenate
No significant change was observed in the colonic catalase in any of the treatment groups. Only sambar group showed a significant (P < 0.05) change in the liver catalase levels when compared to DMH [Figure 4B and E].
Nitrite content in the liver and colon homogenate
There was a significant reduction in the colonic nitrite in the sambar-treated group; 2.94 ± 0.29 when compared to DMH control at 8.09 ± 1.32. On the contrary, a significant rise in the nitrite levels was observed in the sambar-treated rats; 3.61 ± 0.38 when compared to DMH group at 0.95 ± 0.12 [Figure 4C and F].
Measurement of TNF-α level
TNF-α levels in colonic tissue increased in DMH-treated group as compared to normal control group. Treatment with sambar resulted in a mild elevation of TNF-α levels as compared to DMH group. However, the changes are nonsignificant [Figure 5].
Figure 5.
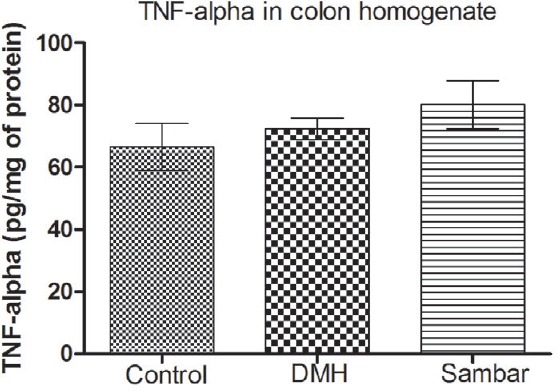
TNF-alpha levels in the colon homogenate of the normal, DMH, and pretreated sambar groups. Data is represented as mean ± SEM of six values
Histopathological results
In the normal rats, the epithelium was intact with no signs of dysplasia, adenoma, or carcinoma.
In the DMH group, mucosal polyps, ACF, and adenoma of colon were seen in 5 out of 6 rats. In all the rats severe dysplasia was observed in the DMH group. Mild hyperplasia with few crypts interspersed in the stromal tissues was observed in the sambar treatment group. There was also a reduced incidence of adenoma formation in the colon on treatment with sambar [Figure 6 and Figure 7].
Figure 6.
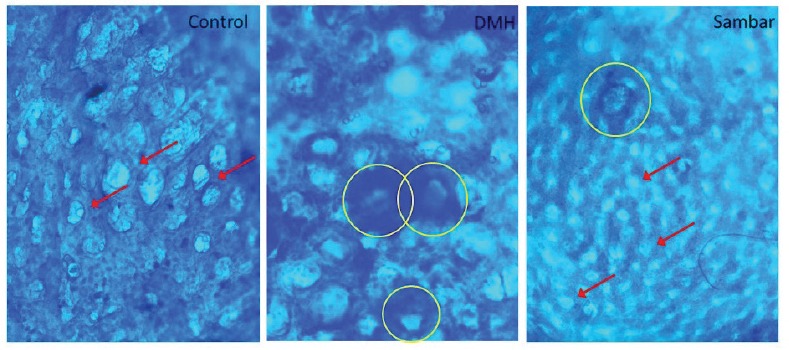
Surface view of unsectioned colonic mucosa stained with methylene blue (40×). In the control group, the crypts appeared normal. In the DMH group, yellow circles indicate methylene blue–stained ACF consisting of two large elliptical crypts. Sambar-treated group showed reduced number of ACF. Red arrows indicate the normal crypts
Figure 7.
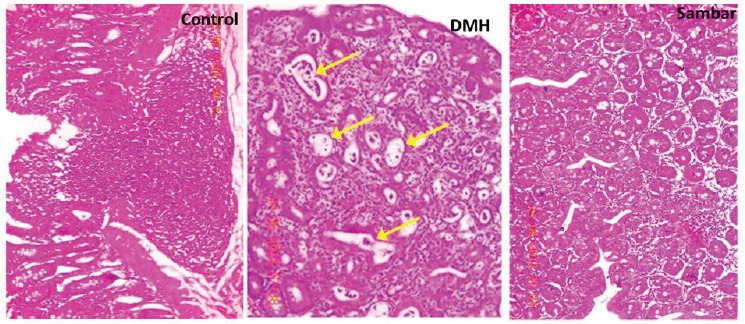
Histopathological analysis of the colonic mucosa with hematoxylin and eosin staining. Normal epithelium with no signs of dysplasia, adenoma, or carcinoma is noted in the control group. Severe dysplasia (arrows) was observed in the DMH group. Mild hyperplasia, crypts were well interspersed with the stromal tissues in the sambar treatment
DISCUSSION
The present study was undertaken to investigate the protective actions of sambar, a popular South Indian dish, in DMH-induced colon carcinogenesis in rodents.
CC is a complex process, which involves initiation, promotion, progression, and invasive phases from the development of a normal crypt to an adenoma.[12] Thus, an intervention into any one of these phases of colon carcinogenesis can be an effective strategy for the prevention of CC. Chronic inflammation is a well-recognized risk factor for the development of human cancer.[13] In the present study, the DMH control animals showed an initiation of ACF, promotion into adenoma, and a significant increase in inflammatory mediator levels of TNF-α.
DMH is a known carcinogen used to study sporadic forms of colon carcinoma resulting in a reproducible experimental in vivo model for pre-clinical screening of test compounds.[14] Metabolism of DMH leads to the formation of azoxymethane (AOM), methylazoxymethanol (MAM), and methyl diazonium ions, which involve alkylation of colonic mucosal DNA.[15] The primary metabolite of DMH, that is, AOM that is responsible for methylation at the O6 position of guanine occurs within 6–12 h of DMH injection. ACFs are considered to be the earliest hallmark of colon carcinogenesis. They are the focal lesions of colonic mucosa consisting of several enlarged/aberrant/elliptical shape crypts, which can be differentiated from normal crypts.[16] Considering this fact, we confirmed the induction of CC and grouped the animals by evaluating the colonic ACFs with methylene blue staining. The mutations in k-ras, hypo-Met-DNA in ACFs lead to the development of small and large adenoma, which will further develop into adenocarcinoma by the mismatch repair and p53 mutation.[17] We observed the initial lesions of ACF and development of small adenoma in DMH control group, whereas only ACFs and polyps, but not adenomas, were noted in rest of the groups.
No significant differences were noted in the liver and spleen indices of the pretreated sambar group, which suggests that the treatment is not harmful to the liver. The colon length/weight ratio of the various groups also indicated that sambar was at par with the control group. Colorectal carcinogenesis is associated with the adenoma-carcinoma sequence, wherein adenomas, spurred by acquired genetic mutations, evolve into CC.[17] Pretreatment with sambar in the DMH-induced CC significantly reduced the precursors of adenoma.
Previous reports have established that the spices like cumin and black pepper suppress colon carcinogenesis in the presence of the procarcinogen DMH.[18] The same spices may be responsible for the protective effect in the present study; however, for substantial evidence detailed studies are required to investigate the effects of the individual spices upon pretreatment. Black turmeric has been previously investigated for its anticarcinogenic properties in liver cancer induced by diethyl nitrosamine.[19] Based on this finding, the protective effect of sambar powder could also be attributed to black turmeric in DMH-induced CC. The incidence of colonic tumor has been seen to reduce to 16.6% on adding fenugreek to the diet of DMH-treated rats,[20] which confirms the potential of fenugreek in our study as well. However, the role of Capsicum annuum remains ambiguous, for which detailed studies are required, both for the prevention and treatment of CC.[21] Considering the properties of these individual spices, we can conclude that their combined presence in sambar could exert an overall protective effect against the development of CC when consumed in the diet.
The hepatic catalase levels rose significantly, which suggests the overall endogenous antioxidant potential. The enhanced levels of nitrite in the colon and not in the liver state that DMH is a specific toxicant to the colon only. The pretreated sambar group has significantly declined the colonic nitrite level when compared to DMH, which suggests that sambar could reduce the free radical–induced damage by DMH in the colonic tissue.
In the liver, DMH treatment did not significantly alter the oxidative markers like GSH, nitrite, and catalase. This indicates that the dose of DMH used for the induction of CC did not have any deleterious effect on the liver markers. Whereas, DMH caused oxidative stress in the colonic tissue as seen by an elevation in GSH, catalase, and nitrite levels. Treatment with sambar caused an elevation of catalase and nitrite levels and depletion of the GSH levels, indicating the presence of pro-oxidants in the spices present in sambar. Sambar treatment prevented DMH-induced oxidative changes in the colonic tissue indicating its antioxidant role. The constituents present in sambar exhibited both pro- and antioxidant properties in different tissues, which might have led to an overall beneficial effect in colonic tissue as is evident from the physiological and histological parameters assessed.
There are reports indicating DMH administration increases levels of TNF-α, IL-6, and causes oxidative stress.[22] The increase in ACF count and number of polyps were used to evaluate the extent of damage to the colon caused by DMH. DMH treatment showed an increase in ACF count with adenoma. The sambar-treated group maintained a lower number of ACF and polyps. However, there was no significant difference in the TNF-α levels across the groups. Thus, we could conclude that sambar was able to reduce oxidative stress in the colonic tissue without affecting the inflammatory mediators thereby preventing the development of CC.
Financial support and sponsorship
The authors acknowledge the funding provided by Manipal College of Pharmaceutical Sciences, Manipal University for this study.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Dr. K. Nandakumar
Dr. K. Nandakumar Started his career as a Pharmacist in 1999. He completed his doctoral degree in Pharmaceutical Sciences in 2006. He started his academic career as Senior Lecturer, later promoted as Assistant Professor and presently working as Associate Professor at Manipal College of Pharmaceutical Sciences, Manipal University. He is actively involved in research and has 61 Scopus-indexed publications, 2 awards and several Industry-academic research grants to his name. His area of research includes chemobrain, metabolic disorders and preclinical evaluation of drugs. He is a peer-reviewer for many leading journal and an editorial member of eCAM. He is also one of the leading reviewers in the field of Pharmacy and Pharmacology as per Publons
Acknowledgement
We are thankful to Manipal University for providing facilities to perform this study
REFERENCES
- 1.Mohandas KM, Desai DC. Epidemiology of digestive tract cancers in India. V. Large and small bowel. Indian J Gastroenterol. 1999;18:118–21. [PubMed] [Google Scholar]
- 2.Mohandas KM. Colorectal cancer in India: controversies, enigmas and primary prevention. Indian J Gastroenterol. 2011;30:3–6. doi: 10.1007/s12664-010-0076-2. [DOI] [PubMed] [Google Scholar]
- 3.Pathy S, Lambert R, Sauvaget C, Sankaranarayanan R. The incidence and survival rates of colorectal cancer in India remain low compared with rising rates in East Asia. Dis Colon Rectum. 2012;55:900–6. doi: 10.1097/DCR.0b013e31825afc4e. [DOI] [PubMed] [Google Scholar]
- 4.Kaefer CM, Milner JA. The role of herbs and spices in cancer prevention. J Nutr Biochem. 2008;19:347–61. doi: 10.1016/j.jnutbio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal B, Prasad S, Sung B, Krishnan S. Prevention and treatment of colorectal cancer by natural agents from Mother Nature. Curr Colorectal Cancer Rep. 2013;9:37–56. doi: 10.1007/s11888-012-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad VG, Kawade S, Jayashree BS, Reddy ND, Francis A, Nayak PG, et al. Iminoflavones combat 1,2-dimethyl hydrazine-induced aberrant crypt foci development in colon cancer. Biomed Res Int. 2014 doi: 10.1155/2014/569130. Article ID 569130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 9.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 10.Guevara I, Iwanejko J, Dembiñska-Kieć A, Pankiewicz J, Wanat A, Anna P, et al. Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin Chim Acta. 1998;274:177–88. doi: 10.1016/s0009-8981(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 11.Habig WH, Jakoby WB. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- 12.Wali RK, Stoiber D, Nguyen L, Hart J, Sitrin MD, Brasitus T, et al. Ursodeoxycholic acid inhibits the initiation and post-initiation phases of azoxymethane induced colonic tumor development. Cancer Epidemiol Biomarkers Prev. 2002;11:1316–21. [PubMed] [Google Scholar]
- 13.Tanaka T. Development of an inflammation-associated colorectal cancer model and its application for research on carcinogenesis and chemoprevention. Int J Inflam. 2012 doi: 10.1155/2012/658786. Article ID 658786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaMont JT, O’Gorman TA. Experimental colon cancer. Gastroenterol. 1978;75:1157–69. [PubMed] [Google Scholar]
- 15.Fiala ES. Investigations into the metabolism and mode of action of the colon carcinogens 1,2-dimethyl hydrazine and azoxymethane. Cancer. 1977;40:2436–45. doi: 10.1002/1097-0142(197711)40:5+<2436::aid-cncr2820400908>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues MA, Silva LA, Salvadori DM, De Camargo JL, Montenegro MR. Aberrant crypt foci and colon cancer: comparison between a short- and medium-term bioassay for colon carcinogenesis using dimethyl hydrazine in Wistar rats. Braz J Med Biol Res. 2002;35:351–5. doi: 10.1590/s0100-879x2002000300010. [DOI] [PubMed] [Google Scholar]
- 17.Sakai E, Takahashi H, Kato S, Uchiyama T, Hosono K, Endo H, et al. Investigation of the prevalence and number of aberrant crypt foci associated with human colorectal neoplasm. Cancer Epidemiol Biomarkers Prev. 2011;20:1918–24. doi: 10.1158/1055-9965.EPI-11-0104. [DOI] [PubMed] [Google Scholar]
- 18.Nalini N, Manju V, Menon VP. Effect of spices on lipid metabolism in 1,2 dimethyl hydrazine-induced rat colon carcinogenesis. J Med Food. 2006;9:237–45. doi: 10.1089/jmf.2006.9.237. [DOI] [PubMed] [Google Scholar]
- 19.Hadem KH, Sharan RN, Kma L. Inhibitory potential of methanolic extracts of Aristolochia tagala and Curcuma caesia on hepatocellular carcinoma induced by diethylnitrosamine in BALB/c mice. J Carcinog. 2014;13:7. doi: 10.4103/1477-3163.133520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devasena T, Venugopal MP. Fenugreek seeds modulate 1,2-dimethyl hydrazine induced hepatic oxidative stress during colon carcinogenesis. Ital J Biochem. 2007;56:28–34. [PubMed] [Google Scholar]
- 21.Anonymous Final report on the safety assessment of capsicum annuum extract, capsicum annuum fruit extract, capsicum annuum resin, capsicum annuum fruit powder, capsicum frutescens fruit, capsicum frutescens fruit extract, capsicum frutescens resin, and capsaicin. Int J Toxicol. 2007;26:3–106. doi: 10.1080/10915810601163939. [DOI] [PubMed] [Google Scholar]
- 22.Umesalma S, Sudhandiran G. Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-êB, iNOS, COX-2, TNF-α, and IL-6 in 1,2 dimethyl hydrazine-induced rat colon carcinogenesis. Basic Clin Pharmacol Toxicol. 2010;107:650–5. doi: 10.1111/j.1742-7843.2010.00565.x. [DOI] [PubMed] [Google Scholar]


