Abstract
Background:
Khat (Catha edulis) is a controversial plant having a euphoretic effect, at the same time part of culture in many countries such as Africa and Arabian Peninsula. The presence of amphetamine-like substance, cathinone and cathine make this plant banned in many countries. Many neurological and other system related studies have been carried out in this plant, but the lack of toxicity studies are there especially the mechanism.
Objective:
In this study, Madin-Darby Bovine Kidney cell line was used as an in vitro model to study the cell death mechanism. Crude extract of fresh Khat plant leaves were prepared and exposed to cells.
Materials and Methods:
Trypan blue assay, phase-contrast microscopy, fluorescent microscopy, clonogenic assay, annexin-V assay, and hematoxylin and eosin (H and E) staining were employed to check the objectives.
Results:
Reductions in cellular viability were observed at concentrations above 1.25 mg/ml while using Trypan blue assay. The results of the clonogenic assay had shown that the untreated control with the highest number of colonies (100% survival) and the 0.1562 concentration could not prevent the colony formation significantly. The high concentrations reduced the colony formation at concentration dependent manner 27.4% and 24.9%, for 0.625 mg/ml and 1.25 mg/ml concentrations, respectively. The acridine orange/ethidium bromide experiment had observed the cells were intact with round nucleus while the apoptosis features such as blebbing and nuclear chromatin condensation were clearly observed in treatment. The shrinkage of cells was clearly observed in H and E staining.
Conclusion:
In addition, annexin-V binding confirmed the presence of apoptosis significantly on Khat treatment.
SUMMARY
Khat (Catha edulis) is a controversial plant having euphoretic effect
Reductions in cellular viability were observed at concentrations above 1.25 mg/ml while using Trypan blue assay
The high concentrations of khat extract had reduced the colony formation at concentration dependent manner
The acridine orange/ethidium bromide experiment had observed the apoptosis features such as blebbing and nuclear chromatin in treatment
Annexin-V binding confirmed the presence of apoptosis significantly on Khat treatment.
Abbreviation used: PS: Phosphatidylserine (PS); MDBK: Madin-Darby Bovine Kidney; DMEM: Dulbecco's modified Eagle's medium; PI: propidium iodide; EB: ethidium bromide; PBS: Phosphate Buffer saline; FITC: fluorescein isothiocyante; TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling.
Keywords: Apoptosis, Catha edulis, khat, kidney toxicity
INTRODUCTION
The most widespread recreational habits in Eastern Africa and the Arabian Peninsula are chewing of Khat leaves (Catha edulis). The plant has alkaloids mainly, cathinone, cathine, and others that have amphetamine similar action and give a diversity of pleasurable effects. A considerable figure of people chews khat leaves to practice the euphoric and stimulating effects.[1] In addition to its neurological effects, khat can also act systemically and has been linked with tachycardia, gastrointestinal disturbances, hypertension,[2] formation of micronuclei in human buccal and bladder mucosa,[3] and development of cancer as well as, the development of oral keratotic white lesions and signs of cytotoxic effects at the site of chewing.[4,5] In animals, there is rising proof signifying that khat is associated with cytotoxicity effect in lymphoid tissue, liver and kidney after per oral administration to white rabbits.[6] Histological examination of rat liver cells showed signs of apoptosis and fatty degeneration.[7]
Many plant derivative components cause apoptosis in mammalian cells.[8,9] Apoptosis is a programmed form of cell death that can be recognized from necrosis by its distinct morphological properties that comprise nuclear chromatin condensation, cytoplasmic shrinkage and plasma membrane blebbing,[10] and some biochemical changes including the external exposure of phosphatidylserine (PS) on the cell membrane,
DNA fragmentation.[11,12] Khat has been proven to induce apoptotic cell death in various leukemic cells,[13] as well as in normal human oral keratinocytes and fibroblasts.[14] In Jazan area, which shares a border with the Yemen, Khat has been cultivated for centuries and a considerable number of all sections of the community chewing khat is still common among inhabitants in this region.[15] With the rising facts of the damaging consequence of khat on the common health and the socioeconomic harms linked with its use, therefore, one of the significant priorities of the region and the country is to control and prevent the khat abuse. Due to deficient research work in our region about the effect of khat plant on oral tissues, Madin-Darby Bovine Kidney (MDBK) cell line was used as an in vitro model in this study to investigate the effect of different concentrations of khat extract on living cells. The crude extract of khat was used as a whole extract to mimic that occurs in chewing khat practice. The expected results can enhance existing public health programs for khat control by increasing public awareness with the adverse effects of khat.
MATERIALS AND METHODS
Materials
Dulbecco's modified Eagle's medium (DMEM) was obtained from HyClone Laboratories (Logan, UT, USA). Fetal bovine serum was provided by Santa Cruz Biotechnology, Inc., (Santa Cruz, CA, USA). Phosphate buffered saline (PBS), Trypan blue dye, streptomycin, penicillin, amphotericin B, propidium iodide (PI), ethidium bromide (EB), hematoxylin and eosin (H and E) staining solutions, crystal violet, methanol, and glacial acetic acid were obtained from Sigma-Aldrich (St. Louis, MO, USA), trypsin/ethylenediaminetetraacetic acid from Life Technologies, Invitrogen™. The tissue culture plastic plates were obtained from Becton Dickinson. Acridine orange (AO) was obtained from DIFCO (West Molesey, Surrey, UK).
Khat extraction
Fresh khat sprout with soft stems, reserved wet and transferred at room temperature, were rinsed 3 times with distilled water, blotted efficiently with Whatmann paper. Then, the extraction was carried out using methanol technique because, in methanolic extraction, cathinone and the other alkaloids within the khat extract were preserved. The extraction was done as described by Lee[13,16,17] excluding alkaloid purification, to minimize acid or basic residues in the extract. Briefly, the khat shoots were quickly minced into small parts and put in an adequate quantity of methanol in a flask containing magnetic rod (Sigma-Aldrich). The combination, covered with aluminum foil to protect from light, was kept overnight at magnetic stirrer and filtered throughout an 11 mm filter (Grade 1, Whatman, Kent, UK). The coarse parts were re-extracted in new methanol. The mixture was filtered and mixed with the first one. The obtained liquid was then got rid from methanol entirely by evaporating at 60°C heat stirrer dried in preweighed conical flasks, giving a semi-solid substance that was dehydrated, collected as a powder and kept in the fridge. The khat solution used in the study was prepared by weighing appropriate amount, dissolving in double distilled water and sterilizing by filtration through 0.22 μm Millex® syringe filter units (Sigma-Aldrich, St. Louis, MO, USA).
Cell culture
Continuous cell line of MDBK cells were kindly gifted to the project by Veterinary Serum and Vaccine Research Institute, Egypt, which was originally purchased from American Type Culture Collection. Cells were seeded at concentration of 1 × 106 cells/ml, grown in monolayer culture with DMEM supplemented with 10% fetal calf serum, 100 IU/ml penicillin, 100 μg/ml streptomycin and amphotericin B (0.025 μg/ml) incubated at 37°C in 5% of CO2 for 24 h for confluence.
Morphological assessment of khat extraction on Madin-Darby Bovine Kidney cells by phase-contrast inverted microscope
MDBK cells were seeded at concentration of 1 × 106 cells/ml onto 96 well-plates and incubated for 16–18 h at 37°C in a 5% CO2 humidified incubator. The medium was removed and fresh DMEM medium containing 10, 5, 2.5, 1.25, 0.62, 0.31, and 0.15 mg/ml concentrations of khat extraction were added onto subconfluent cells in three replicates for each concentration of khat extract. Wells containing medium without khat extraction were also included as controls. The cell destruction and morphological changes that deviated from normal cells were evaluated under a Leica DMI 3000B phase-contrast inverted microscope at × 200 magnifications and photographed after 24, 48 and 72 h consecutively. Cytotoxicity was expressed as 50% cytotoxic concentration, that is, the concentration of khat extract required to produce cytopathogenicity for 50% of the examined cells.
Cell viability test
Trypan blue exclusion assay was conducted for determination of the number of viable cells (VI) using a method that formerly described by Ahmed et al.[18] In brief, the cells were grown at concentration of 1 × 106 cells/ml in 6 well-plates to 60–70% confluence by incubation for 16–18 h, and then inoculated by removal of the media and substituting it with media containing 2.5, 1.25, 0.62, 0.31, and 0 mg/ml of khat extract, and incubated for 16 h. Floating and adhering cells were collected, washed once with a (PBS, pH 7.4), centrifuged, suspended in a suitable amount of PBS and stained with 0.2% trypan blue for 5 min at room temperature. About 10 μl of the sample was loaded on hemocytometer chamber and numbers of viable and non-VI were counted under a light microscope (Olympus, Tokyo, Japan). The results were expressed as a percentage of the control.
Morphological assessment of khat extract on Madin-Darby Bovine Kidney by acridine orange ethidium bromide double staining
Khat-induced cell death in MDBK cells was quantified using AO and EB double-staining according to standard procedures and examined under a fluorescence microscope. Briefly, treatment was carried out in 6-well plates. Cells were plated at a concentration of 1 × 106 cells/ml for 3 h, the media was substituted with new media containing 5, 2.5, 1.25 and 0 mg/ml khat extract. The cells were incubated for 16 h. Floating and adhering cells were collected, centrifuged at 300 × g for 10 min, washed twice with PBS to remove the remaining media. Supernatant was discarded and the cells were stained with fluorescent dyes containing AO (10 mg/ml) and EB (10 mg/ml). Freshly stained cell suspension was dropped onto a glass slide and covered with a coverslip. Slides were observed under the ultraviolet-fluorescence microscope within 30 min before the fluorescent color starts to fade. The viable, early-dependent phospholipid-binding protein, detects the plasma membrane alterations, apoptotic, and late apoptotic cells were observed.
Annexin-V/propidium iodide staining assay
The effect of khat extract on the induction of early and late apoptosis (LA) was investigated in MDBK cells using the annexin-V conjugated with fluorescein isothiocyante (FITC) and PI staining assay. The cells (1 × 106 cells/ml) at the exponential phase of growth were seeded into a 6-well plate then incubated for 3 h at 37°C in presence of 5% of CO2, the media was substituted with another new media containing 5, 2.5, 1.25 and 0 mg/ml khat extract. The cells were harvested after 4 and 8 h by removing the supernatant and detached cells to separate tubes, then trypsinization of adherent cells, collecting them in tubes and stopping the trypsin with neutralizing solution and adding them to the previous corresponding tubes of detached cells. The tubes were centrifuged at 500 × g for 5 min, the supernatant was aspirated and discarded. The pellets were washed by gentle resuspension in cold PBS and centrifuged at 500 × g for 5 min followed by aspirating and discarding of the supernatant. The cells were resuspended at 1 × 106 cells/ml in annexin staining buffer gently and stained according to the manufacturer's protocol, shortly, an appropriate amount of fluorescently conjugated annexin-V stain was added and left for 15–20 min on ice in the dark at room temperature. Cells were washed twice with annexin staining buffer and then, PI (5 μg/ml) was added to the cells immediately before observation under fluorescent microscopy. The percentage of cells undergoing apoptosis was determined. The experiment was carried out in triplicates.
Clonogenic survival assay
This assay was used to measure the ability of MDBK single cells to retain their reproductive capability to survive cytotoxic effects of khat and grow to form clones or colonies. The assay was done according to Munshi et al.[19] Briefly, in 25 ml tissue culture flasks cells were plated at concentration of 1 × 106 cells/ml for 3 h, the media were substituted with new media containing 2.5, 1.25, 0.625, 0.3125, 0.15625, and 0 mg/ml of khat extract and left for 2–3 h. Then, the cells from each concentration were washed with PBS preheated to 37°C, trypsinized, counted and plated in 6 well-plates; at counts of 100 VI/well from 0 mg/ml of khat extract and suitable increasing amount of VI/well from each corresponding concentration, so, each single cell divides and forms a colony that could be easily counted after incubation. The test was performed in triplicates for each concentration, then, incubated at 37°C in 5% of CO2 in complete culture medium until the formation of new cell colonies consisting of at least four to six cells in cell control set (48 h). The colonies were fixed with 30% formalin in PBS and stained with 0.05% crystal violet, and the numbers of colonies were counted manually using inverted microscope using ×5 lens (Nikon, Japan). The results were expressed as survival curve between the different concentrations of khat extract and the cells retained their reproductive ability.
Hematoxylin and eosin staining
MDBK cells were seeded (106 cells/ml) onto glass coverslips in sterile screw caped 15 ml test tubes and incubated for 3 h at 37°C in 5% CO2, then the DMEM was replaced with DMEM containing 5, 2.5, 1.25, and 0 mg/ml khat extract, as triplicates. Negative controls were tubes containing DMEM with no additions. All tubes were incubated for 8 h at 37°C in 5% CO2. Then the media were discarded, cells were washed 3 times using PBS, fixed with 10% neutral formalin in PBS and processed for staining with hematoxylin and eosin. Photographs were captured by an Olympus DP25 camera of 10 evenly spaced fields per coverslip using a Zeiss Axioskop light microscope at ×20 magnifications.
Statistical analysis
All data were means ± standard deviation from three independent experiments in triplicate. Results were analyzed using Student's t-test. The values of P < 0.05 were considered to be statistically significant.
RESULTS AND DISCUSSION
Khat is an integral component of the socioeconomic traditions of few countries such as Yemen and some African regions.[20] Even though chewing the khat is the most common method of administration, sometimes it can be smoked for its euphoretic effect.[21] In both methods, the khat must be used as fresh as possible to keep the presence of the cathinone, the important phytochemical present in it.[22] Since the important factor associated with khat usage is an addiction, many researches focused in determining the underlying mechanisms of addiction and central nervous system effects.[23,24] However, risks of its toxicological effects on other various organs have been less reported.
Since the C. edulis is a known cytotoxic agent,[25] in this study, we investigated the cytotoxic effect of khat on MDBK cells in vitro. Our results provide the first evidence for cytotoxicity of khat on MDBK cells. The study employed various morphological techniques to reveal the mode of cell death. Treatment with khat alter the cellular viability of MDBK cell lines, which was measured using trypan blue method at concentrations of 10, 5, 2.5, 1.25, 0.62, 0.31, and 0.15 mg/ml. Reductions in cellular viability were observed at concentrations above 1.25 mg/ml [Figure 1].
Figure 1.
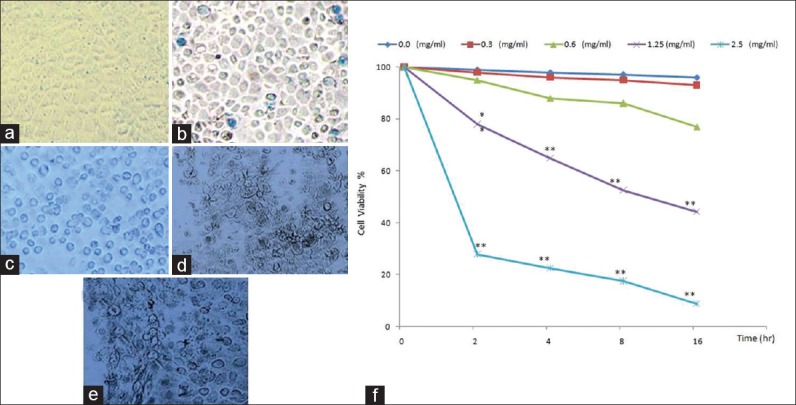
Trypan blue staining in Madin-Darby Bovine Kidney cells after exposure to khat extract for 16 h examined by inverted microscope; 0 mg/ml are viable (negative for trypan blue) (a), 0.6 mg/ml has nonsignificant very few dead cells (take trypan blue) (b), 1.25 mg/ml has significant dead cells (take trypan blue) (c), the number of dead cells are very marked in concentrations: 1.25 mg/ml (d) and 2. 5 mg/ml (e) (×200). The khat-treated group was compared to the control group (f) (*P < 0.05, **P < 0.01; n = 3)
The clonogenic assay is the method of choice to determine cell reproductive death after treatment with cytotoxic agents.[26] Because this assay has the potential to determine every cell in the population for its ability to undergo “unlimited” division, it has been carried out to check the ability of khat extract to inhibit the growth of MDBK cells, we have treated the cells with increasing concentrations and the colony formation was observed after 40 h [Figure 2]. The results showed that the untreated control showed the highest number of colonies (100% survival) and the 0.1562 concentration could not prevent the colony formation significantly. The high concentrations reduced the colony formation at concentration dependent manner 27.4% and 24.9%, for 0.625 mg/ml and 1.25 mg/ml concentrations, respectively. Only 15.9% cells were survived at 2.5 mg/ml concentration [Table 1]. This obviously indicates that khat actively inhibits the proliferation of MDBK cells. The inhibition of proliferation observed here is in concordance with what was detected to take place in animal and plant cells studies next inoculation to khat-specific alkaloids[27] or khat extract.[28,29]
Figure 2.
Representative colonies of Madin-Darby Bovine Kidney cells that survived exposure to different concentrations of khat extract; 0 mg/ml (untreated controls) (a), 0.1562 mg/ml (b), 0.3125 mg/ml (c), 0.625 mg/ml (d), 1.25 mg/ml (e) and 2.5 mg/ml (f) after 48 h in 6 well-plates that were inoculated with suitable viable cell counts in triplicates, fixed in 10% formaldehyde and counted at by inverted microscope (×5)
Table 1.
Survival percentage plotted using numbers derived from dilution sheet. Madin-Darby Bovine Kidney cells were exposed to different concentrations of khat extract in 25 ml flasks for 3 h, trypsinized, counted, then, reseeded for clonogenic assay in 6 well-plates by suitable viable cell counts and incubated. Colonies formed in untreated cultures (48 h)
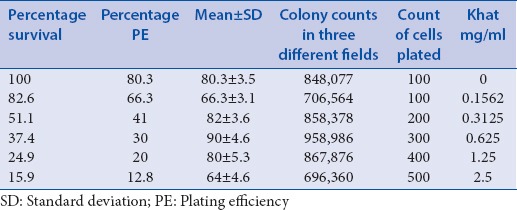
Among the various modes of cell death, apoptosis is the important one. It is generally characterized by distinct morphological characteristics and energy-dependent biochemical mechanisms.[30] Even though the detection of apoptosis shall employ various methods, morphological detection holds the gold standard.[31] It starts from phase contrast microscopy, till microscopy assisted with fluorescent images. Hence, first we used phase contrast microscopy to see the changes in the structure of MDBK cells with khat treatment. Cells were treated with khat extract at different concentrations; 1.25 mg/ml treatment showed a reduction of cells and the next concentrations 5 mg/ml and 10 mg/ml clearly showed the rounding up of cells significantly [Figure 3].
Figure 3.
Inverted microscope micrographs of Madin-Darby Bovine Kidney treated with khat extract in a concentration-dependent manner in Dulbecco's modified Eagle's medium for 48 h; normal Madin-Darby Bovine Kidney cell cultures showing a confluent monolayer sheet of cells (a), Madin-Darby Bovine Kidney cells treated with 1.25 mg khat extract showing slight rounded cells (b), cells inoculated 5 mg/ml developed many rounding and gaps as a result of detaching (c) and in concentration of 10 mg/ml more many rounding, more gaps and clumping were observed that were considered as cytopathic effect (d) (×200)
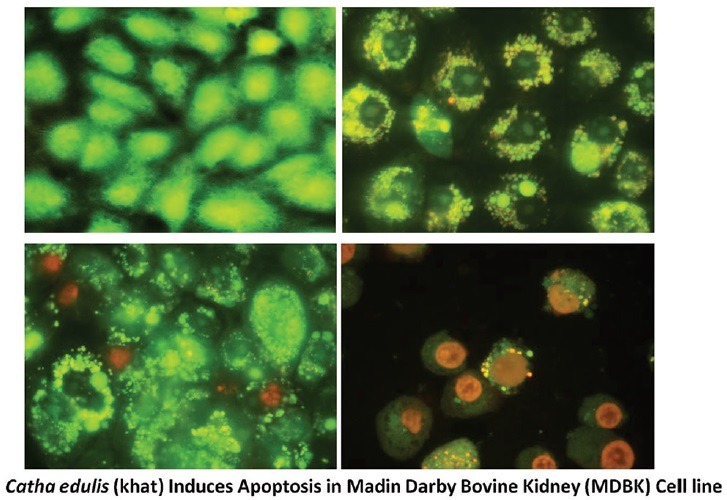
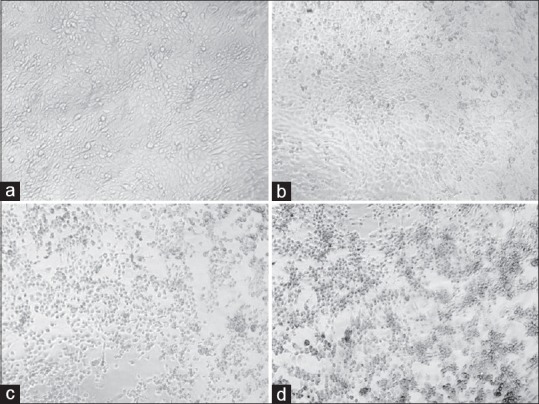
The confirmation phase contrast can be done using various intercalating dyes to differentiate various stages of apoptosis. One of these kinds is AO/EB assay. In this assay, the morphological changes happen during the cell death were initially assessed using phase-contrast microscopy and further, confirmed by fluorescent microscopy analysis. AO and EB double stain were used to see the different parameters of early and LA including death cells.[32] Under this experiment, we have observed that the control cells were intact with round nucleus while the apoptosis features such as blebbing and nuclear chromatin condensation was clearly observed later with higher concentrations. Moreover, the presence of reddish-orange color due to the binding of AO to denatured DNA was observed for late apoptotic cells [Figure 4]. H and E stain was employed for further confirming the apoptosis. In Figure 5, it was observed that cell shrinkage is prominent compared to control cells. Moreover, condensed chromatin [Figure 5c, darker than other cells] and fragmented nucleus were observed. In untreated control, numerous mitotic cells are clearly visible. Previously Dimba et al. had observed similar kind of observations using Hoechst 33,342 dyes.[33]
Figure 4.
Fluorescent micrographs of acridine orange/ethidium bromide double stained Madin-Darby Bovine Kidney cells treated with khat extract for 24 h. Untreated cells shows normal structure without prominent apoptosis and necrosis (bright green) (a). Early apoptosis features seen after 1.5 mg treatment (bright green) (b) and blebbing (bright green) were noticed in 2.5 mg treatment (orange color) (c and d). VI: Viable cells; BL: Blebbing of the cell membrane; CC: Chromatin condensation; LA: Late apoptosis (×400). Each experiment was performed in triplicate
Figure 5.
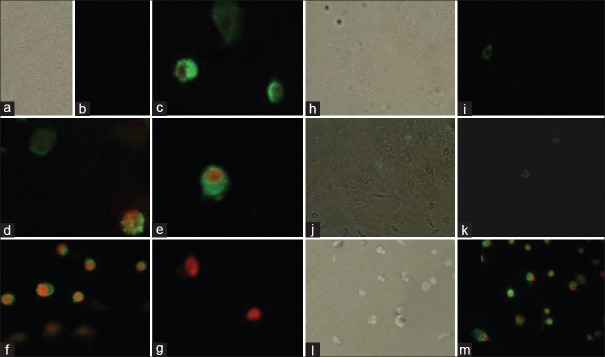
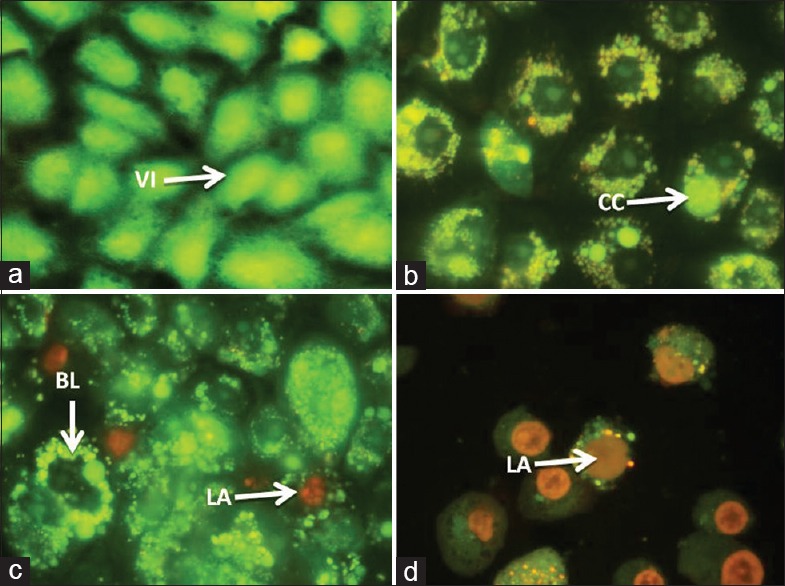
Annexin-V/propidium iodide assay. In control cells propidium iodide cannot go into the cell resulting in negative staining for both dyes; (0 mg/ml after 4 and 8 h) (a, b, h and i, respectively), annexin-V stain externalized phosphatidylserine on the cell surface (green) next the beginning of apoptosis (1.25 mg/ml after 8 h) (j and k), through the latter stages of apoptosis phosphatidylserine is exposed (green) and propidium iodide can enter the cell (red) due to the loss of membrane integrity of late apoptotic cell (2.5 mg/ml (c) and 5 mg/ml (d-f) after 4 h, 2.5 mg/ml (l and m) after 8 and dead cell (5 mg/ml after 8 h) (g)
Annexin-V is a methodology which detects apoptosis by targeting for the loss of phospholipid asymmetry of the plasma membrane.[34] It detects apoptosis earlier in the process than DNA-based assays such as TUNEL, PI secondary dye is included to differentiate apoptotic cells from viable and necrotic cells. It is more sensitive and authorizes the earlier results we obtained. The morphological examination result of annexin-V FITC staining assay attained from microscope images is shown in Figure 6. The MDBK cells PS externalization occurred with khat treatment was clearly detected and captured. As shown in figure, the control cells have not shown any significant annexin-V binding, but with khat treatment, significant increase in the number of annexin-V positive cells was observed. Further study will be intended to investigate the mode of apoptosis that has been occurred in our MDBK cell model.
Figure 6.
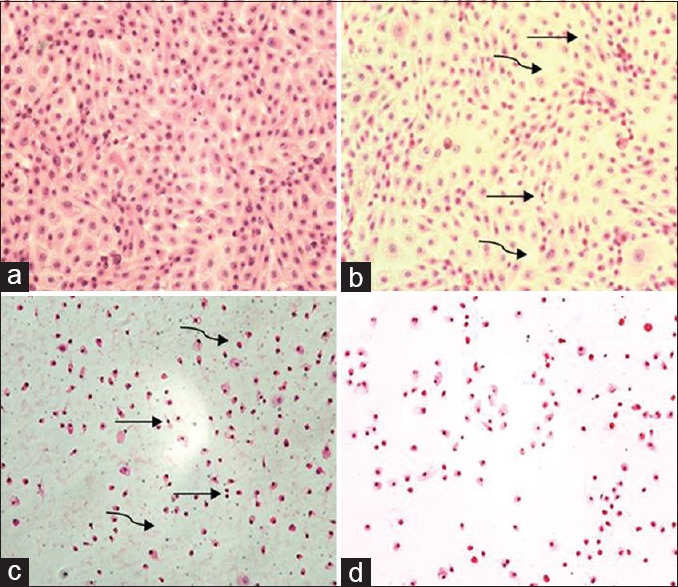
H and E stain of Madin-Darby Bovine Kidney cells incubated for 8 h with khat extract; 0.0 mg/ml, Madin-Darby Bovine Kidney cells show complete confluent healthy monolayer cells (a), 1.5 mg/ml, very few cells show small, hyperchromic fragmented nuclei (curved arrow) and very few gaps (straight arrow) between cells (b), 2.5 mg/ml, many cells show small, hyperchromic, fragmented nuclei, many gaps between cells and cytoplasmic and nuclear dust were found (c) and in 5 mg all fields lack cells, with hyperchromic naked nuclei, cytoplasmic and nuclear stages of destruction, with detachment of most of the cells from adherent surface (d) (×100)
CONCLUSION
This study clearly showed that the C. edulis extract has the potential of exerting cytopathogencity in MDBK. In addition, the study also proves that the mode of cell death is via programmed cell death. Further studies are in progress to explain the effect of khat in vivo.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Dr. Syam Mohan
Dr. Syam Mohan, is an Associate Professor at the Medical Research Center, Jazan University, Saudi Arabia. He is currently serves as Director, Central Laboratory and Head of Bio-Medical Research Unit. His research interest is in the area of drug discovery and pharmacological and toxicological evaluation of Herbal plants.
REFERENCES
- 1.Kalix P, Geisshusler S, Brenneisen R, Koelbing U, Fisch HU. Cathinone, a phenylpropylamine alkaolid from khat leaves that has amphetamine effects in humans. NIDA Res Monogr. 1990;105:289–90. [PubMed] [Google Scholar]
- 2.Al-Habori M. The potential adverse effects of habitual use of Catha edulis (khat) Expert Opin Drug Saf. 2005;4:1145–54. doi: 10.1517/14740338.4.6.1145. [DOI] [PubMed] [Google Scholar]
- 3.Kassie F, Darroudi F, Kundi M, Schulte-Hermann R, Knasmüller S. Khat (Catha edulis) consumption causes genotoxic effects in humans. Int J Cancer. 2001;92:329–32. doi: 10.1002/ijc.1195. [DOI] [PubMed] [Google Scholar]
- 4.Halbach H. Medical aspects of the chewing of khat leaves. Bull World Health Organ. 1972;47:21–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Soufi HE, Kameswaran M, Malatani T. Khat and oral cancer. J Laryngol Otol. 1991;105:643–5. doi: 10.1017/s0022215100116913. [DOI] [PubMed] [Google Scholar]
- 6.al-Meshal IA, Qureshi S, Ageel AM, Tariq M. The toxicity of Catha edulis (khat) in mice. J Subst Abuse. 1991;3:107–15. doi: 10.1016/s0899-3289(05)80011-2. [DOI] [PubMed] [Google Scholar]
- 7.Ageely HM, El-Nagar MM, Abouelmagd A, Abou-Elhamd AS, Kelany ME, Patil BR. Khat extract mediated morphological and histochemical alterations in rat liver. Int J Adv Res. 2014;2:971–80. [Google Scholar]
- 8.Horie N, Hirabayashi N, Takahashi Y, Miyauchi Y, Taguchi H, Takeishi K. Synergistic effect of green tea catechins on cell growth and apoptosis induction in gastric carcinoma cells. Biol Pharm Bull. 2005;28:574–9. doi: 10.1248/bpb.28.574. [DOI] [PubMed] [Google Scholar]
- 9.Lai KC, Lee TC. Genetic damage in cultured human keratinocytes stressed by long-term exposure to areca nut extracts. Mutat Res. 2006;599:66–75. doi: 10.1016/j.mrfmmm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–56. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimba EA, Gjertsen BT, Bredholt T, Fossan KO, Costea DE, Francis GW, et al. Khat (Catha edulis)-induced apoptosis is inhibited by antagonists of caspase-1 and -8 in human leukaemia cells. Br J Cancer. 2004;91:1726–34. doi: 10.1038/sj.bjc.6602197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukandu OM, Costea DE, Neppelberg E, Johannessen AC, Vintermyr OK. Khat (Catha edulis) induces reactive oxygen species and apoptosis in normal human oral keratinocytes and fibroblasts. Toxicol Sci. 2008;103:311–24. doi: 10.1093/toxsci/kfn044. [DOI] [PubMed] [Google Scholar]
- 15.Ageely HM. Health and socio-economic hazards associated with khat consumption. J Family Community Med. 2008;15:3–11. [PMC free article] [PubMed] [Google Scholar]
- 16.Lee MM. The identification of cathinone in khat (Catha edulis): A time study. J Forensic Sci. 1995;40:116–21. [Google Scholar]
- 17.Kimani ST, Patel NB, Kioy PG. Effect of single and daily khat (Catha edulis) extract on locomotor behaviour in CBA mice. Sci Res Essays. 2008;3:187–96. [Google Scholar]
- 18.Ahmed AE, Aronson J, Jacob S. Induction of oxidative stress and TNF-alpha secretion by dichloroacetonitrile, a water disinfectant by-product, as possible mediators of apoptosis or necrosis in a murine macrophage cell line (RAW) Toxicol In Vitro. 2000;14:199–210. doi: 10.1016/s0887-2333(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 19.Munshi A, Hobbs M, Meyn RE. Clonogenic cell survival assay. Methods Mol Med. 2005;110:21–28. doi: 10.1385/1-59259-869-2:021. [DOI] [PubMed] [Google Scholar]
- 20.Klein A. Fountain, Jane, Korf, Dirk J. Drugs in Society: European Perspectives. Oxford: Radcliffe Publishing Ltd; 2007. Khat and the creation of tradition in the Somali diaspora. ISBN 9781846190933. [Google Scholar]
- 21.Kebede Y. Cigarette smoking and khat chewing among college students in North West Ethiopia. Ethiop J Health Dev. 2002;16:9–17. [Google Scholar]
- 22.Brenneisen R, Fisch HU, Koelbing U, Geisshüsler S, Kalix P. Amphetamine-like effects in humans of the khat alkaloid cathinone. Br J Clin Pharmacol. 1990;30:825–8. doi: 10.1111/j.1365-2125.1990.tb05447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel NB. Mechanism of action of cathinone: The active ingredient of khat (Catha edulis) East Afr Med J. 2000;77:329–32. doi: 10.4314/eamj.v77i6.46651. [DOI] [PubMed] [Google Scholar]
- 24.Kalix P. Cathinone, a natural amphetamine. Pharmacol Toxicol. 1992;70:77–86. doi: 10.1111/j.1600-0773.1992.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 25.Al-Ahdal MN, McGarry TJ, Hannan MA. Cytotoxicity of Khat (Catha edulis) extract on cultured mammalian cells: Effects on macromolecule biosynthesis. Mutat Res. 1988;204:317–22. doi: 10.1016/0165-1218(88)90105-x. [DOI] [PubMed] [Google Scholar]
- 26.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–9. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 27.Al-Meshal IA. Mitodepressive effect of (-)-cathinone, from Catha edulis (khat), on the meristematic region of Allium cepa root tips. Toxicon. 1987;25:451–4. doi: 10.1016/0041-0101(87)90079-1. [DOI] [PubMed] [Google Scholar]
- 28.de Hondt HA, Fahmy AM, Abdelbaset SA. Chromosomal and biochemical studies on the effect of kat extract on laboratory rats. Environ Mutagen. 1984;6:851–60. doi: 10.1002/em.2860060611. [DOI] [PubMed] [Google Scholar]
- 29.Qureshi S, Tariq M, Parmar NS, al-Meshal IA. Cytological effects of khat (Catha edulis) in somatic and male germ cells of mice. Drug Chem Toxicol. 1988;11:151–65. doi: 10.3109/01480548808998219. [DOI] [PubMed] [Google Scholar]
- 30.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Cruchten S, Van Den Broeck W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol. 2002;31:214–23. doi: 10.1046/j.1439-0264.2002.00398.x. [DOI] [PubMed] [Google Scholar]
- 32.Abdelwahab SI, Abdul AB, Alzubairi AS, Mohamed Elhassan M, Mohan S. In vitro ultramorphological assessment of apoptosis induced by zerumbone on (HeLa) Biomed Res Int. 2009. Article ID 769568, 10 pages. http://dx.doi.org/10.1155/2009/769568 . [DOI] [PMC free article] [PubMed]
- 33.Dimba E, Gjertsen BT, Francis GW, Johannessen AC, Vintermyr OK. Catha edulis (Khat) induces cell death by apoptosis in leukemia cell lines. Ann N Y Acad Sci. 2003;1010:384–8. doi: 10.1196/annals.1299.070. [DOI] [PubMed] [Google Scholar]
- 34.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]